Abstract
Population profiles of industrialized countries show dramatic increases in cardiovascular disease with age, but the molecular and genetic basis of disease progression has been difficult to study because of the lack of suitable model systems. Our studies of Drosophila show a markedly elevated incidence of cardiac dysfunction and arrhythmias in aging fruit fly hearts and a concomitant decrease in the expression of the Drosophila homolog of human KCNQ1-encoded K+ channel α subunits. In humans, this channel is involved in myocardial repolarization, and alterations in the function of this channel are associated with an increased risk for Torsades des Pointes arrhythmias and sudden death. Hearts from young KCNQ1 mutant fruit flies exhibit prolonged contractions and fibrillations reminiscent of Torsades des Pointes arrhythmias, and they exhibit severely increased susceptibility to pacing-induced cardiac dysfunction at young ages, characteristics that are observed only at advanced ages in WT flies. The fibrillations observed in mutant flies correlate with delayed relaxation of the myocardium, as revealed by increases in the duration of phasic contractions, extracellular field potentials, and in the baseline diastolic tension. These results suggest that K+ currents, mediated by a KCNQ channel, contribute to the repolarization reserve of fly hearts, ensuring normal excitation-contraction coupling and rhythmical contraction. That arrhythmias in both WT and KCNQ1 mutants become worse as flies age suggests that additional factors are also involved.
Keywords: cardiac dysfunction, fibrillation, heart, long-QT syndrome, longevity
Despite recent advances in preventing deaths related to cardiac disorders, cardiovascular disease (CVD) remains the leading cause of death in industrialized countries (1). As life expectancy increases, the population profiles in these countries are changing to include increasing numbers of middle-aged and elderly individuals. In industrialized countries and countries that are becoming industrialized, a number of other factors, such as altered life styles, urbanization, and as-yet-unidentified genetic and environmental factors, combine to compound the effects of aging on CVD (2). Cardiac arrhythmias are common in patients with cardiac dysfunction, and some forms of arrhythmias, such as atrial fibrillations, increase with age (3). However, the mechanisms underlying arrhythmias and heart failure have remained elusive, in part because heart diseases in humans are associated with a complex array of hormonal, physiological, genetic, and biochemical abnormalities. In addition, vertebrate heart structure is very complex, as is the process of its embryological development from a simple tube-like structure. Because heart function is so essential for survival, it is difficult to study very deleterious heart abnormalities in vertebrate systems. Recent insights into the molecular genetics of CVD suggest that the genetic heterogeneity underlying heart disease is very high (4). Because the basic mechanisms of heart development and function are conserved between Drosophila and vertebrates (5–10), we have begun to use the fly heart and the power of Drosophila genetics to understand the genetic and molecular mechanisms underlying the aging of cardiac tissue and their contributions to cardiac disorders and arrhythmias.
A number of genetic defects that contribute to arrhythmogenic disorders have been identified in humans. Many encode K+ channels, such as the Human Ether-a-go-go Related Gene (HERG), which encodes a channel underlying the rapid phase of cardiac repolarization (IKr), and the KCNQ1 gene, which encodes a subunit of a K+ channel responsible for the slower repolarizing current (IKs) (for reviews, see refs. 11–13). Mutations in these K+ channels commonly lead to a loss or decrease in channel function, resulting in reduced cardiac repolarization and prolonged cardiac action potentials that increase the risk of early after-depolarization. In humans, this prolonged repolarization phase manifests as a prolonged QT interval on the surface electrocardiograms with increased risk of Torsades des Pointes ventricular arrhythmias, which would cause recurrent syncope or sudden cardiac death, and is known as long QT syndrome (LQTS). Age, environmental stressors, exercise, genetic modifiers, and some commonly prescribed drugs have also been shown to produce arrhythmic disorders such as LQTS, but the complex interactions between these acquired and inherited factors for arrhythmogenesis remain to be determined (14, 15). A systematic genetic analysis will be required if we are to identify genetic variations (polymorphisms) in known and novel genes and in gene products that influence the risk of arrhythmias. Because susceptibility to drug-induced LQTS is likely to have a genetic basis, a functional assessment of genetic mutations and identification of interactions between genes that contribute to arrhythmias would permit more appropriate drug administration to patients with cardiovascular disease (16). The relatively long lifespan of mammalian systems precludes a simple approach to elucidate the aging-related factors contributing to the genesis and facilitation of arrhythmic disorders. More importantly, hereditary and/or acquired arrhythmic disorders in mammalian hearts usually lead to sudden death, making the study of genetic interactions or polygenetic disorders extremely difficult in mammals (15).
We have developed a number of assays and quantitative measures that allow us to characterize the physiology of the myogenic Drosophila heart. Because oxygen distribution in the fruit fly is carried out by an independent tracheal system, genetic manipulation of cardiac-expressed genes that compromise heart function are not immediately lethal (17), which permits the characterization of severe abnormalities in heart physiology. Our findings suggest that an important aspect of age-related cardiac dysfunction in fly heart tissue is a decrease in the efficacy of cardiac relaxation/repolarization due to a concomitant decrease in ion channel gene expression. This system will allow us to identify and characterize genes that contribute to normal heart function, analyze the effects of genetic aberrations, perform structure-function studies in a functioning organ, and examine the genetic basis for functional deterioration with age.
Results
Fruit flies have an open circulatory system, with a linear heart tube located along the dorsal midline in the abdomen and an aorta that extends anteriorly into the head region [supporting information (SI) Movie 1] (17–20). Four sets of internal valves divide the abdominal heart into an anterior conical chamber and three posterior compartments (21). Each of the four heart compartments also contains a pair of valves to the exterior, called ostia, that permit hemolymph to enter and leave the heart (18).
KCNQ Gene Expression in Fly Hearts.
To study the heart-related function of the Drosophila KCNQ gene, deletion mutants were generated by the imprecise excision of a transposable element (EP2074) (SI Fig. 5A). Two alleles (KCNQ186 and KCNQ370) that delete all transmembrane domains, including the potassium selective pore region of the KCNQ channel, were used. A precise excision of the inserted EP2074 element (KCNQ97) served as the WT control. Both deletion alleles are homozygous-viable and are fertile without any visible defects, except that mutant larvae take 1–2 days longer (compared with KCNQ97) to develop, fewer eclose, and the mean lifespan in females is reduced by 20–30% (data not shown). KCNQ transcripts are first expressed in the embryo at mid-embryonic stages, primarily in the nervous system, but not yet in the heart (SI Fig. 5B). In the adult, KCNQ is expressed at high levels in the head and also in other tissues, including the anterior and posterior portion of the heart (SI Fig. 5 C–E). RT-PCR of adult flies and isolated hearts shows absence of 5′ (and transmembrane) KCNQ RNA in KCNQ186 and KCNQ370 mutants, compared with KCNQ97 WT control or compared with transcripts corresponding to the 3′ KCNQ region (SI Fig. 5C).
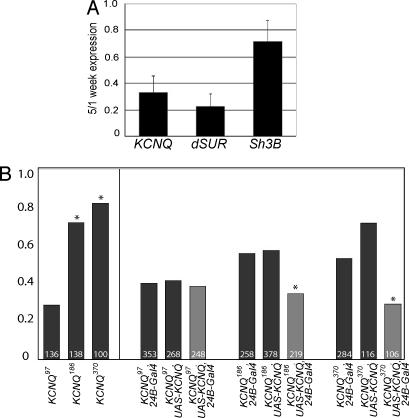
A recent report shows that KATP channel associated dSUR RNA levels of the adult heart decline dramatically with age (22). To explore whether KCNQ expression changes with age, we assessed the KCNQ RNA levels of isolated hearts with qRT-PCR at 1 and 5 weeks of age. We find that with age, KCNQ RNA declines dramatically to 33% of the levels seen at 1 week (Fig. 1A, SI Materials and Methods, and SI Fig. 5F).
Fig. 1.
Cardiac KCNQ levels are reduced with age, and KCNQ mutants are susceptible to pacing-induced failure. (A) KCNQ RNA levels isolated from hearts dissected at 5 weeks are decreased to 33% relative to the 1-week levels (mean ± SD of three independent experiments; see also SI Fig. 5E). A control gene SH3β was only reduced to 72% of 1-week levels. (B) (Left) One-week-old KCNQ mutant flies (KCNQ186 and KCNQ370) exhibit significantly elevated heart-failure rates (∗, P < 0.01, χ2 analysis) in response to electrical pacing compared with WT controls (KCNQ97). (Right) Mesodermal overexpression of the WT KCNQ cDNA (light bars) reduces the failure rate to control levels (dark bars).
Pacing-Induced Cardiac Dysfunction Is Elevated in KCNQ Mutants.
In humans, the effects of loss of function mutations in the KCNQ gene are more pronounced under conditions of physical or emotional stress (23, 24). To gage cardiac performance in Drosophila, we used an external electrical pacing paradigm to physically stress the fly heart (17, 25). Immediately after the pacing regime, we visually assessed heart performance; heart dysfunction manifests as temporary or permanent heart arrest (reminiscent of human sudden death syndromes) or uncoordinated twitching. The fraction of young, 1-week-old WT, and KCNQ97 control hearts that exhibit such cardiac dysfunction is relatively low (20–30% “failure rate”; Fig. 1B Left), as reported in ref. 17. By comparison, the incidence of pacing-induced cardiac dysfunction in age-matched KCNQ186 and KCNQ370 mutants is drastically increased (70–80% failure rate). These elevated rates, seen in young KCNQ mutant flies, were as high as those observed in 5-week-old KCNQ97 control flies and other WT strains (17). The elevated failure rates seen in young KCNQ mutant flies did not increase further with age suggesting that that cardiac performance in these flies is already significantly compromised because of the absence of KCNQ channel function.
Because the heart rate in Drosophila can be modulated by neuronal input (26), and because KCNQ is also expressed in the nervous system, we examined whether the increased incidence in cardiac dysfunction seen in mutant flies could be rescued by overexpressing the WT KCNQ gene specifically in the mesoderm, which includes the heart. Control and KCNQ deletion mutants were combined with either the 24B-Gal4 mesodermal driver (27) or an upstream activating sequence (UAS)-KCNQ gene and crossed together. The incidence of pacing-induced cardiac dysfunction in KCNQ deletion mutants expressing the WT KCNQ cDNA was similar to the control KCNQ97 combinations and was significantly reduced compared with KCNQ mutant flies (Fig. 1B Right). Similar results were obtained for a duplicate set of crosses with an independent UAS-KCNQ insertion (data not shown). These results suggest that KCNQ is required autonomously within the heart muscle to establish normal cardiac performance.
Increased Incidence of Heart Arrhythmias in KCNQ Mutant Flies.
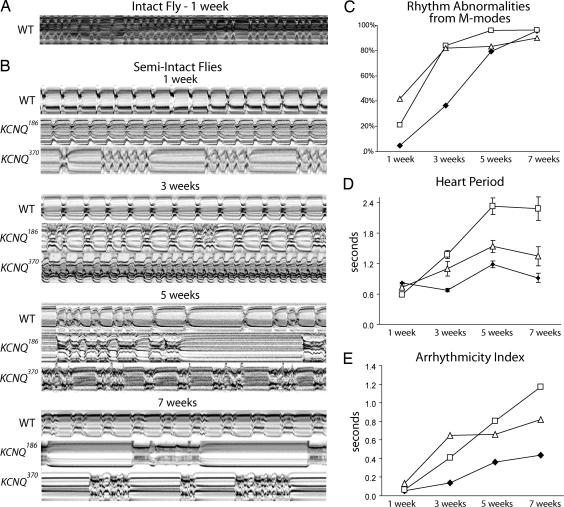
Pacing-induced cardiac failure is indicative of cardiac dysfunction, but it does not provide information about the specific underlying causes. To further characterize the heart's contractile properties, we captured heart wall movements in individual flies, using a high speed digital video camera. Movies of normally beating hearts taken through the cuticle of intact flies were used to generate M-mode traces that display the dynamics of heart tube contraction (Fig. 2A). These traces show the position of the heart wall edges (y axis) over time (x axis). Heart beat frequencies in intact flies showed alternations between faster and slower rates, probably because of neuronal and hormonal input, as has been previously described (26, 28, 29). To study the inherent myogenic contraction parameters without confounding influences from neuronal input, we developed a semiintact fly preparation in which the heart is surgically exposed and most neuronal inputs to the heart are disrupted. M-modes from semiintact heart preparations from young, 1-week-old flies (yw, w1118 and KCNQ97, all considered WT) show regular rhythmic contractions lasting for over an hour in oxygenated supplemented artificial hemolymph (Fig. 2B, WT 1–3 weeks, SI Fig. 5G, and SI Movie 1). The highly rhythmic beating pattern deteriorates as flies age, and by 5–7 weeks, a majority of WT flies exhibit nonrhythmical heart contraction patterns, including asystoles/bradycardias and fibrillations/tachyarrhythmias (Fig. 2B, WT 5–7 weeks).
Fig. 2.
Cardiac arrhythmias in old and KCNQ mutant flies. (A) M-mode traces (10 s) prepared from high-speed movies of intact flies. (B) Representative M-mode traces from semiintact Drosophila preparations. Arrhythmic heartbeats are evident in KCNQ mutants as early as 1 week of age. (C) Visual quantification of aberrant heartbeats in random, 10-s M-mode traces. KCNQ mutants have an elevated incidence of arrhythmia at young ages compared with WT flies. (D) Automated analysis of heart period obtained from 10-s high-speed video images. Mean (± SEM) heart period increased significantly with age in most KCNQ mutant flies compared with age-matched controls (∗, P < 0.05). Genotype-by-age analysis of the mutant versus WT curves also shows significant differences (∗, P < 0.05). (E) SD of the heart periodicity was used as an overall measure of arrhythmicity (arrhythmia index ± SEM). All time points (except 1 week) and overall curves for both KCNQ mutants were significantly different from the WT controls (∗, P < 0.01, analysis of covariance regression analysis). Age-by-genotype analysis also shows a significant difference between WT and mutants (∗, P < 0.01). For C–E, ◇, WT; □, KCNQ186; ▵, KCNQ370; n = 20–30 flies per data point.
In contrast to WT flies, M-mode records from KCNQ mutant hearts exhibit severely nonrhythmic beating patterns already at young ages (Fig. 2B, 1–3 weeks), and the incidence of arrhythmia increased more rapidly with age in mutants (Fig. 2C). In addition, there appeared to be an age-dependent increase in the severity of the rhythmicity defects in mutant flies beyond those seen in aged WT flies (Fig. 2B, 5–7 weeks). We developed an image-analysis program that allowed us to objectively measure a number of heart parameters (see Materials and Methods; M.F., K.O., W.G., R.B., unpublished data). In semiintact WT heart preparations, the average heart period, defined as the length of time between the ends of two consecutive diastolic intervals, increases moderately with age (Fig. 3D), as reported in refs. 17 and 30. In contrast, KCNQ mutant flies exhibit a dramatic age-dependent increase of the heart period compared with age-matched controls (Fig. 3D; P < 0.05), and both diastolic intervals (D.I.) and systolic intervals (S.I.) show age-dependent increases in KCNQ mutants (Fig. 3 A and D and SI Figs. 6A and 7A).
Fig. 3.
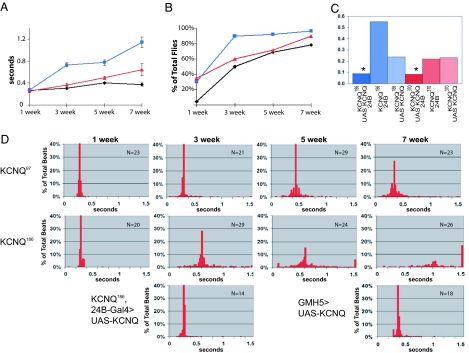
KCNQ mutants exhibit early onset of prolonged, fibrillatory contractions. (A) Systolic interval lengths for all heartbeats in each record were computed and averaged for each fly (∗, P < 0.05, analysis of covariance regression analysis and independent samples t test). (B) Incidence of fibrillation, plotted as a percentage of total flies (∗, P < 0.05, Pearson's χ2 test). For A and B, ◇, WT; □, KCNQ186; ▵, KCNQ370; n = 20–30 flies per data point. (C) Arrhythmicity index from semiintact fly heart preparations in 3-week-old flies. Overexpression of the WT UAS-KCNQ in KCNQ mutant flies (dark blue/red bars) significantly reduced the arrhythmicity index compared with control flies with the UAS-KCNQ construct or the 24B driver alone (light blue/red bars; ∗, P < 0.05). (D) Combined data, showing the distribution of systolic intervals for control and KCNQ mutant flies. Systolic intervals lasting >1.5 s were grouped together and are shown in the last bar of each histogram. The bottom row of the histograms shows rescue of the 3-week-old KCNQ186 mutant phenotype by overexpression of KCNQ transgene in all mesoderm (on the left) and improvement in the cardiac status of 7-week-old WT flies by overexpression of KCNQ transgene specifically in all cardiomyocytes (right) using the GMH5 driver (17).
We explored whether the irregularities observed in the mutant heart rhythms were reflected in an increased variability of the heart periodicity. Using the standard deviation of the heart period as an “arrhythmia index,” we find that this quantitative measure of rhythmicity reflects well the heart-rhythm disturbances observed in M-mode traces (compare Fig. 2 B, C, and E). In control flies, the cardiac arrhythmia index increases progressively with age, from ≈0.1 at 1 week to 0.4 at 7 weeks of age. The arrhythmia index for KCNQ mutant hearts is approximately double that of the controls at most ages examined. Thus, arrhythmias increase with age in both WT and mutants but much more rapidly in KCNQ flies (Fig. 3E).
To quantify the incidence of unsustained fibrillation/tachyarrhythmia, we measured the number of S.I. that were unusually long (>0.5 s, indicative of sustained contractions) as well as the number of very short D.I. (<0.06 s, indicative of incomplete relaxations; see Materials and Methods). Using these criteria, we computed a significant elevation in the incidence of fibrillation events in both KCNQ mutants compared with controls, especially in young flies (Fig. 3B), reflecting the increase in unsustained fibrillations observed when visually inspecting in the M-mode records (Fig. 2B). The mean D.I. also tended to be longer in KCNQ flies when compared with age-matched controls (SI Fig. 6 A and B).
To further illustrate the differences in rhythmicity, we plotted the distribution of S.I. and D.I. for individual flies in histogram format (SI Fig. 7A). The majority of WT flies show relatively tight clustering of both D.I. and S.I. that persists until 5 weeks of age, when the distributions broaden. KCNQ mutant flies, however, show a much more variable distribution at both young and old ages. To represent the increased incidence of unsustained fibrillation for all flies in a genotype, we normalized the data for individual flies to the average median value (Fig. 3D). The median S.I. length in KCNQ mutant flies increases dramatically with age relative to WT, and the incidence of very long S.I., which indicate episodes of fibrillation, is higher in KCNQ mutant flies at all ages. Similar results are seen in plots of heart periodicity (SI Fig. 7B).
The alterations in rhythmicity observed for the KCNQ mutant flies can be rescued by supplying WT KCNQ channel function transgenically. We analyzed hearts from KCNQ mutant flies containing both a WT UAS-KCNQ construct and the mesodermal 24B driver. In such “mesodermal rescue” KCNQ flies, the arrhythmia index is much reduced compared with UAS or 24B controls (Fig. 3C and Fig. 3D, bottom of 3 week column) and is even below the WT level at 3 weeks of age (see Fig. 2E). These results suggest that supplying functional KCNQ channels to the heart restores a regular heart rhythm in KCNQ mutants. In addition, heart-specific KCNQ overexpression in old WT flies reverses the age-dependent increase in arrhythmias (Fig. 3D, bottom right; compare with top row). These findings are consistent with the idea that the age-dependent decrease in KCNQ expression within the heart (Fig. 1A) contributes to the increased incidence of arrhythmia observed with age.
Electrophysiological Analysis of Fly Heart Function.
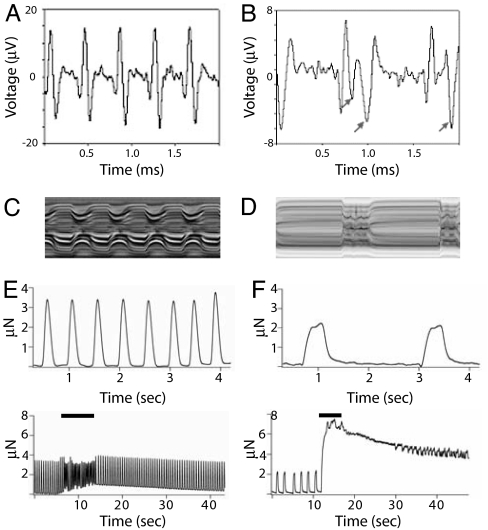
We used a multielectrode array system (SI Materials and Methods) to monitor field potentials from dissected heart preparations. Recordings from beating hearts in control flies showed positive deflections followed immediately by negative deflections that correlated with heart contraction and relaxation, respectively (Fig. 4A). In KCNQ mutants, the negative deflections did not immediately follow the initial positive potentials and usually occurred with significant delay compared with field potentials from WT hearts (Fig. 4B, arrows), consistent with the increases in S.I. observed in KCNQ mutants (Fig. 4 C and D; see also Figs. 2B and 3 A and D). This result is consistent with a reduced ability of the heart to repolarize, presumably because of the decrease in the repolarizing K+ currents of the cardiomyocytes in mutant flies.
Fig. 4.
Field potential and tension recordings from KCNQ mutant hearts. (A) Spontaneous field potential recordings from semiintact fly heart preparations. WT heart (3 weeks) exhibits regular upward depolarizing potentials and downward repolarizing potentials. (B) Depolarizing potentials from KCNQ null mutant heart (3 weeks) are similar to WT in appearance, but the repolarizing potentials (arrows) are relatively smaller and/or delayed compared with WT. (C) M-mode trace from a WT fly with the same 2-s time base as for the field potential recordings. (D) Two-second M-mode from a KCNQ186 mutant fly. (E) (Upper) Baseline tension recordings across an individual WT heart tube show rhythmic heart contractions. (Lower) Tension recordings before, during, and after a 5-Hz electrical stimulation signal (solid bar). (F) (Upper) Baseline tension development in KCNQ mutant heart showed more prolonged contractions and slower heart rate than WT. (Lower) Tension recordings from a KCNQ mutant heart before, during, and after 5-Hz electrical stimulation (solid bar).
Muscle Tension Measurements.
Muscle tension was measured as an indicator of muscle function in semiintact fly hearts (SI Materials and Methods). Baseline tension measurements from control hearts showed spontaneous regular contractions (Fig. 4E, top trace). Electrical stimulation (5–10 ms at 5 Hz) induced tachycardia and elevated diastolic tension (Fig. 4E, bottom trace). In WT flies, the heart rate and diastolic tension quickly decreased to baseline levels after cessation of stimulation. In contrast, contractions in KCNQ mutant hearts occurred at lower rates and with longer S.I. (Fig. 4F, top trace, and Table 1). In response to electrical stimulation, KCNQ hearts exhibited fibrillation, markedly elevated diastolic tension, and extremely delayed recovery to baseline diastolic tension relative to WT hearts (Fig. 4F, bottom trace). These results indicate that the heart muscle fibers from KCNQ mutant flies have a markedly reduced ability to relax after contractions, consistent with the “diastolic dysfunctions” commonly found in various types of cardiomyopathy in humans (31).
Table 1.
Tension development in heart muscle
| Time-to-peak, s | 50% relaxation | 75% relaxation | |
|---|---|---|---|
| KCNQ97 | 0.110 ± 0.011 | 0.062 ± 0.016 | 0.089 ± 0.022 |
| KCNQ186 | 0.266 ± 0.157 | 0.106 ± 0.031 | 0.147 ± 0.042 |
| P value | 0.03 | 0.004 | 0.005 |
The time-to-peak contraction and relaxation times were calculated from digitized tension records in semiintact fly preparations. Traces were made from eight wild-type and eight KCNQ mutant hearts; five consecutive contractions were averaged from each trace. Data were analyzed for significance by using a two-tailed t test.
Discussion
Outward currents passing through KCNQ channels contribute to the waveform and rhythmic contractions in at least two types of vertebrate muscle cells, heart muscle (32), and portal vein smooth muscle (33). Repolarization in the Drosophila heart also appears to depend on outward K+ currents that are mediated, in part, by KCNQ channels. Mutations in the pore-forming region of this channel result in an increase in heart arrhythmias, which are manifest as an increased variation of the heart period (arrhythmia index), including episodes of unsustained fibrillation/tachyarrhythmia in young flies. This increased incidence in arrhythmias also correlates with an increase in pacing-induced cardiac dysfunction (heart failure) in young KCNQ mutant flies. It is likely that both the increased incidence of arrhythmias and the decreased ability to withstand (pacing-induced) stress arise from the reduced ability of the myocardium to repolarize. That hearts from old WT flies behave similarly to the hearts of young KCNQ mutants suggests that a decrease in repolarization reserve due to decreased K+ channel function may be one of the molecular changes underlying age-related heart dysfunction. Our observations that KCNQ expression decreases with age and that replenishing KCNQ function in old flies apparently rejuvenates cardiac function are consistent with this hypothesis. Interestingly, clinical studies in humans and a recent study in flies suggest that activation of the ATP-sensitive K+ channel in heart muscle is also attenuated with age (22, 34). However, this channel is thought to have a cardioprotective role against hypoxia by slowing the heart rate, and does not appear to function during the normal heart contraction (35, 36).
The progressively arrhythmic M-mode patterns seen in aging flies are reminiscent of the increased incidence of cardiac arrhythmic activities, e.g., atrial fibrillation, observed in aging humans (ref. 37; reviewed in refs. 1, 2, and 38). Our findings that the incidence of cardiac arrhythmia in the hearts of KCNQ mutants occurs earlier than in WT flies and increases with age suggest that alterations in these and other channels involved in heart muscle repolarization may be responsible for some of the effects of aging on heart function. Furthermore, the ability to rescue these age-related defects by overexpressing WT KCNQ channels in mutant flies suggests that increases in the repolarization capacity of aging hearts may exert a protective effect against other age-related changes.
The conservation of genes and gene function between Drosophila and vertebrates, including humans, has repeatedly been documented. This similarity is likely to extend to heart function because the complement of ion channels that are expressed in the heart includes many of the same channels found in the vertebrate heart (10, 22, 39). Remarkably, the regularity of the heart rhythm critically depends on properly functioning KCNQ channels in flies and humans, and these channels seem to play equivalent roles in basic cardiac myocyte physiology. Homologs of other ion channel genes associated with human arrhythmias, such as HERG, also appear to produce arrhythmia in fly hearts. Mesodermal RNAi knockdown of seizure (the fly homolog of HERG) and mutants in ether-a-go-go (eag1) produce nonsustained and sustained fibrillations/tacharrhythmias in semiintact preparations (K.O. and R.B., unpublished data).
At least one other human heart condition, dilated cardiomyopathy, has also been observed in flies (40). Here, we report that a second cardiac condition, arrhythmias of the heartbeat, also exists in flies and that aberrant KCNQ function contributes to this condition. Arrhythmias in flies increase dramatically with age, as does the incidence in atrial fibrillation in the elderly (37), suggesting that the age-dependent changes in molecular constituents within the fly heart may also be conserved. The fly heart promises to provide clues as to the roles of (new) genes in aging and disease and to provide a physiological model that is more complex than cultured myocytes and simpler and easier to study than is the vertebrate heart. The ability to combine genetic manipulation and physiological assays in a system that has a short life span makes Drosophila an attractive model in which to study the genetics of age-related changes in heart function and heart disease.
Materials and Methods
Semiintact Drosophila Preparation.
Flies were anesthetized with fly nap for 2–5 min, and the head, ventral thorax, and ventral abdominal cuticle were removed, exposing the abdomen. All internal organs except the heart and any abdominal fat were removed. Dissections were done under oxygenated artificial hemolymph (see SI Materials and Medthods). These semiintact preparations were allowed to equilibrate with oxygenation for 15–20 min before filming. All procedures were done at room temperature (18–22°C). Hearts exposed by this procedure typically beat rhythmically for up to 4 h and have been observed beating as long as 8 h after dissection (see SI Fig. 5G). Analysis of flies that express GFP specifically in neuronal membranes showed that the peripheral neural input to the conical chamber and the portion of the heart tube in the third abdominal segment was consistently disrupted by the dissection procedure (data not shown).
Image Analysis.
Image analysis of heart contractions was performed by using high-speed movies of semiintact Drosophila preparations. Movies were taken at rates of 100–200 frames per second by using a Hamamatsu (McBain Instruments, Chatsworth, CA) EM-CCD digital camera on a Leica (McBain Instruments, Chatsworth, CA) DM LFSA microscope with a ×10 immersion lens. To get a random sampling of the heart function from the flies, a single 10-s recording was made for each fly without previewing. All images were acquired and contrast enhanced by using Simple PCI imaging software (Compix, Sewickley, PA). M-modes were generated by using a MatLab-based image analysis program written by M.F. (unpublished work). Briefly, a 1-pixel-wide region is defined in a single frame of the movie that encompasses both edges of the heart tube; identical regions are then cut from all of the frames in the movie and aligned horizontally, which provides an edge trace that documents the movement of the heart tube edges in the y axis over time in the x axis.
Measurements of diastolic and systolic diameters and D.I. and S.I. were obtained as output from the MatLab-based program (Mathworks, Natick, MA). The incidence of fibrillation in flies was automatically detected by quantifying S.I. >0.5 s or D.I. <0.06 s. The systolic value was chosen because systoles that have clearly definable contraction and relaxation phases were never seen to last >0.4 s, and the maximal average S.I. in WT flies was 0.4 s (in 5-week-old flies; see Fig. 3A). The D.I. cutoff was approximately half the shortest regularly occurring D.I. observed in all of the semiintact preparations, which was 0.13 s. Thus, any detected relaxations lasting <0.06 s were most likely incomplete. The incidence of asystoles was determined by quantifying all D.I. lasting >1.3 s, a value approximately twice the average D.I. for all WT flies examined and longer than any of the individual D.I. measured for 1- and 3-week-old WT flies. Significant differences were determined by analysis of covariance and a two-tailed independent-samples t test where appropriate; P values <0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank Lynsey Hamilton for technical assistance with early versions of the M-mode traces and Grant Hogg and Frances Cho for technical assistance. This work was supported by National Institutes of Health grants (to R.B., J.M.M., and J.W.P.), a Bechtel Trusts & Foundation grant, and an American College of Cardiology Foundation award (to H.-S.V.C.).
Abbreviations
- D.I.
diastolic intervals
- S.I.
systolic intervals
- UAS
upstream activating sequence.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0609278104/DC1.
References
- 1.Thom TNH, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, Zheng Z, Flegal K, O'Donnell C, Kittner S, Lloyd-Jones D, et al. Circulation. 2006;113:e85–e151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 2.Lakatta EG. Heart Fail Rev. 2002;7:29–49. doi: 10.1023/a:1013797722156. [DOI] [PubMed] [Google Scholar]
- 3.Lakatta EG, Levy D. Circulation. 2003;107:346–354. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- 4.Priori SG, Napolitano C. Ann NY Acad Sci. 2004;1015:96–110. doi: 10.1196/annals.1302.008. [DOI] [PubMed] [Google Scholar]
- 5.Bodmer R. Trends Cardiovasc Med. 1995;5:21–27. doi: 10.1016/1050-1738(94)00032-Q. [DOI] [PubMed] [Google Scholar]
- 6.Harvey RP. Dev Biol. 1996;178:203–216. doi: 10.1006/dbio.1996.0212. [DOI] [PubMed] [Google Scholar]
- 7.Bodmer R, Frasch M. In: Heart Development. Rosenthal N, Harvey RP, editors. New York: Academic; 1999. pp. 65–90. [Google Scholar]
- 8.Cripps RM, Olson EN. Dev Biol. 2002;246:14–28. doi: 10.1006/dbio.2002.0666. [DOI] [PubMed] [Google Scholar]
- 9.Seidman JG, Seidman C. J Clin Invest. 2002;109:451–455. doi: 10.1172/JCI15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bodmer R, Wessells R, Johnson EC, Dowse H. In: Comprehensive Molecular Insect Science. Gilbert L, Latrau K, Gill S, editors. Vol 2. Amsterdam: Elsevier; 2005. pp. 199–250. [Google Scholar]
- 11.Jentsch TJ. Nat Rev Neurosci. 2000;1:21–30. doi: 10.1038/35036198. [DOI] [PubMed] [Google Scholar]
- 12.Robbins J. Pharmacol Ther. 2001;90:1–9. doi: 10.1016/s0163-7258(01)00116-4. [DOI] [PubMed] [Google Scholar]
- 13.Sanguinetti MC. Nature. 2006;440:463–469. doi: 10.1038/nature04710. [DOI] [PubMed] [Google Scholar]
- 14.Priori SG. Circ Res. 2004;94:140–145. doi: 10.1161/01.RES.0000115750.12807.7E. [DOI] [PubMed] [Google Scholar]
- 15.Roberts R. J Am Coll Cardiol. 2006;47:9–21. doi: 10.1016/j.jacc.2005.08.059. [DOI] [PubMed] [Google Scholar]
- 16.Grunnet M, Behr ER, Calloe K, Hoffman-Bang J, Till J, Christiansen M, McKenna WJ, Olesen SP, Schmitt N. Heart Rhythm. 2005;2:1238–1249. doi: 10.1016/j.hrthm.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 17.Wessells RJ, Fitzgerald E, Cypser JR, Tatar M, Bodmer R. Nat Genet. 2004;36:1275–1281. doi: 10.1038/ng1476. [DOI] [PubMed] [Google Scholar]
- 18.Rizki T. In: The Genetics and Biology of Drosophila. Wright TRF, Ashburner M, editors. London: Academic; 1978. pp. 397–452. [Google Scholar]
- 19.Curtis NJ, Ringo JM, Dowse HB. J Morphol. 1999;240:225–235. doi: 10.1002/(SICI)1097-4687(199906)240:3<225::AID-JMOR2>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 20.Molina MR, Cripps RM. Mech Dev. 2001;109:51–59. doi: 10.1016/s0925-4773(01)00509-3. [DOI] [PubMed] [Google Scholar]
- 21.Monier B, Astier M, Semeriva M, Perrin L. Development (Cambridge, UK) 2005;132:5283–5293. doi: 10.1242/dev.02091. [DOI] [PubMed] [Google Scholar]
- 22.Akasaka T, Klinedinst S, Ocorr K, Bustamante EL, Kim SK, Bodmer R. Proc Natl Acad Sci USA. 2006;103:11999–12004. doi: 10.1073/pnas.0603098103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz PJ, Zaza A, Locati E, Moss AJ. Circulation. 1991;83:1171–1180. [PubMed] [Google Scholar]
- 24.Gligorova S, Agrusta M. Cardiovasc Ultrasound. 2005;3:36. doi: 10.1186/1476-7120-3-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wessells RJ, Bodmer R. BioTechniques. 2004;37:58–60. doi: 10.2144/04371ST01. [DOI] [PubMed] [Google Scholar]
- 26.Dulcis D, Levine RB. J Neurosci. 2005;25:271–280. doi: 10.1523/JNEUROSCI.2906-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brand AH, Perrimon N. Development (Cambridge, UK) 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 28.Johnson E, Ringo J, Dowse H. J Insect Physiol. 2000;46:1229–1236. doi: 10.1016/s0022-1910(00)00043-3. [DOI] [PubMed] [Google Scholar]
- 29.Dulcis D, Levine RB, Ewer J. J Neurobiol. 2005;64:259–274. doi: 10.1002/neu.20136. [DOI] [PubMed] [Google Scholar]
- 30.Paternostro G, Vignola C, Bartsch DU, Omens JH, McCulloch AD, Reed JC. Circ Res. 2001;88:1053–1058. doi: 10.1161/hh1001.090857. [DOI] [PubMed] [Google Scholar]
- 31.Maurer MS, Spevak D, Burkhoff D, Kronzon I. J Am Coll Cardiol. 2004;44:1543–1549. doi: 10.1016/j.jacc.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 32.Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL, Keating MT. Nature. 1996;384:80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- 33.Yeung SY, Greenwood IA. Br J Pharmacol. 2005;146:585–595. doi: 10.1038/sj.bjp.0706342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee TM, Su SF, Chou TF, Yuan-Teh Lee YT, Tsai CH. Circulation. 2002;105:334–340. doi: 10.1161/hc0302.102572. [DOI] [PubMed] [Google Scholar]
- 35.Zingman LV, Hodgson DM, Bast PH, Kane GC, Perez-Terzic C, Gumina RJ, Pucar D, Beinengraeber M, Dzeja PP, Miki T, et al. Proc Natl Acad Sci USA. 2002;99:13278–13283. doi: 10.1073/pnas.212315199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Senio S, Miki T. Prog Biophys Mol Biol. 2003;81:133–176. doi: 10.1016/s0079-6107(02)00053-6. [DOI] [PubMed] [Google Scholar]
- 37.Wolf PA, Abbot RD, Kannel WB. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 38.Lakatta EG. Heart Lung Circ. 2002;11:76–91. doi: 10.1046/j.1444-2892.2002.00126.x. [DOI] [PubMed] [Google Scholar]
- 39.Lalevee N, Monier B, Senatore S, Perrin L, Semeriva M. Curr Biol. 2006;16:1502–1508. doi: 10.1016/j.cub.2006.05.064. [DOI] [PubMed] [Google Scholar]
- 40.Wolf MJ, Amrein H, Izatt JA, Choma MA, Reedy MC, Rockman HA. Proc Natl Acad Sci USA. 2006;103:1394–1399. doi: 10.1073/pnas.0507359103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.