Abstract
Tumor-infiltrating cytotoxic T lymphocytes (TILs), including CD8 TILs, have been associated with favorable clinical outcomes in multiple tumor types. Tumor-infiltrating CD8 T cells and major histocompatibility complex (MHC) class I expression in urothelial carcinoma (UC) have not been previously reported. Most immune responses are mediated by local cytotoxic lymphocytes (CD8 T cells), which can eradicate tumor cells by recognizing tumor-associated antigens presented by MHC class I molecules. Here we analyzed the presence of intratumoral CD8 T cells, the expression of MHC class I antigen, and the expression of the NY-ESO-1 tumor antigen in UC samples and correlated our findings with clinical outcome. Immunohistochemical staining for intratumoral CD8 T cells in tissue samples from 69 patients with UC showed that patients with advanced UC (pT2, pT3, or pT4) and higher numbers of CD8 TILs within the tumor (≥8) had better disease-free survival (P < 0.001) and overall survival (P = 0.018) than did patients with similar-staged UC and fewer intratumoral CD8 TILs. We conclude that the extent of intratumoral CD8 TILs is an important prognostic indicator in advanced UC.
Keywords: CD8 T cell, major histocompatibility complex, cancer testis antigen, NY-ESO-1, bladder
Carcinoma of the urinary bladder is the only malignant neoplasm for which immunotherapy is often included as part of standard care. Intravesical application of the immunomodulator Bacillus Calmette Guerin in selected patients with superficial urothelial carcinoma (UC) reduces the risk of local recurrence by ≈60% and can lead to 5-year survival rates of ≈90% in certain cancer patients with unifocal disease (1). Although induction of a local T cell-mediated immune response seems to be the most likely cause, little is known about the basis for the effectiveness of Bacillus Calmette Guerin therapy in urinary bladder carcinoma (2). Interestingly, the presence of tumor-infiltrating cytotoxic T lymphocytes (TILs) correlates with improved disease outcome in various other tumors, including esophageal, ovarian, renal, and colon carcinoma (3–8).
To be immunologically active, CD8 cytotoxic T lymphocytes require that antigens be presented in the context of major histocompatibility complex (MHC) class I molecules. Down-regulation of MHC class I molecules in cancer is thought to be an important mechanism of tumor escape from immune surveillance (9). However, knowledge of MHC class I expression patterns in malignant tumors is limited because few serologic reagents are available for the immunohistochemical analysis of MHC class I molecule expression in formalin-fixed, paraffin-embedded (FFPE) tissue specimens (10). Class I MHC antibodies, such as the one used in this study and generated against the consensus region of the MHC class I protein present in all MHC class I haplotypes, provide additional reagents for the assessment of MHC class I expression in FFPE samples.
Cancer-testis antigens (CTAs) are tumor-associated antigens expressed in various types of cancer and in testicular germ cells in adult males (11). We previously documented high expression of CTAs in UC (12, 13). Because of the essentially tumor-restricted expression pattern of CTAs, these antigens are regarded as viable targets in immunotherapy for cancer (14). CTAs were first identified by their ability to elicit T and B cell responses in the autologous host; the first CTA, MAGE-1 (now known as MAGE-A1), was isolated by T cell epitope cloning (15). Subsequently, other methods such as serologic analysis of recombinant expression libraries and database mining (16–18) have revealed several families of CTAs. To date, >44 distinct CTA gene or antigen families, such as MAGE, GAGE, BAGE, and NY-ESO-1, have been identified (19, 20), and several CTAs have been used as target antigens in vaccine clinical trials for various types of tumors, including UC (21–25). Our own work has shown that high-grade UC expresses high levels of the CTA NY-ESO-1, and that NY-ESO-1 is capable of eliciting T cell responses in patients (12). Expression of the NY-ESO-1 antigen in UC tumor samples could potentially correlate with increased antigen-specific TILs.
In the present study on UC samples, we analyzed the presence of CD8 TILs, expression of MHC class I protein, and expression of tumor antigen NY-ESO-1 and correlated the findings with clinical variables such as survival time and tumor recurrence. Prevalent CD8 TILs and concurrent NY-ESO-1 expression in a tumor sample from one patient (who also provided peripheral blood for additional studies) prompted us to investigate for NY-ESO-1 specific T cells and led us to generate an NY-ESO-1-specific T cell clone from this particular patient. Our results show that the presence of tumor-infiltrating CD8 T cells, which could represent a response to specific tumor antigens, correlated with better patient prognosis, thereby implicating these cells in local tumor control.
Results
Patterns of T Cell Infiltration in UC.
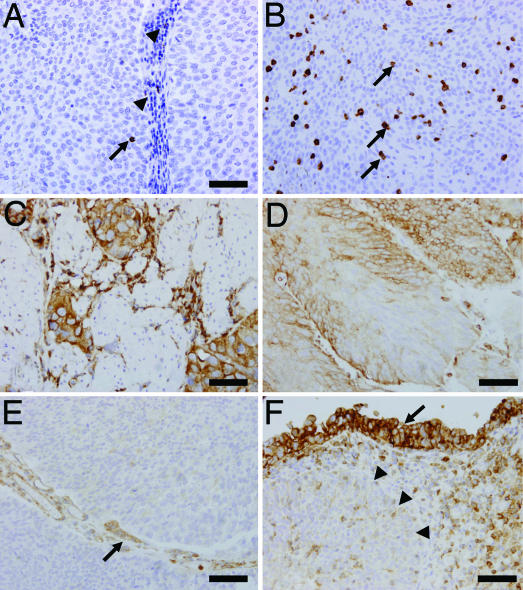
CD8 TILs were detected in both the stromal areas and intratumorally within the epithelial tumor nests (Fig. 1 A and B). For our purposes, we considered only the intratumoral TILs [see supporting information (SI) Table 3 for mean numbers of each subject]. Only intratumoral TILs were considered in this study because our previous study on ovarian cancer demonstrated that intratumoral TILs, as opposed to stromal TILs, correlated with favorable clinical outcomes (3).
Fig. 1.
TILs and expression of MHC class I antigen in representative UC tumor samples. (A) An intratumoral CD8 TIL (arrow) accompanied by stromal CD8 lymphocyte infiltration (arrowheads). (B) Abundant CD8 TILs (arrows) infiltrating a solid tumor with no stromal component. (C) Muscle-invasive case showing heterogeneous expression of class I MHC. (D) Homogeneous expression of class I MHC in another tumor. (E) Class I MHC are not expressed by cancer cells, but are expressed by endothelial cells (arrow). (F) Down-regulation of class I MHC expression associated with cancer invasion. Class I MHC is expressed in the mucosal component (arrow), but not in the invasive component (arrowheads). (Scale bar, 50 μm.)
Human Lymphocyte Antigen (HLA) Expression.
For these analyses, we generated a monoclonal antibody capable of recognizing the consensus sequence of MHC class I molecules in FFPE tissues. The cutoff value for expression versus no expression was 10% (i.e., samples in which >10% of tumor cells that expressed the antigen were considered positive). Although immunostaining was present in both cell membrane and cytoplasm, we evaluated only cell membrane expression as a surrogate of functional protein. Endothelial cells known to express MHC class I served as an internal control (HLA expression in endothelial cells adjacent to tumor nests is illustrated in Fig. 1E). HLA expression varied from case to case, with some samples demonstrating complete lack of expression, others heterogeneous expression, and still others homogeneous expression (Fig. 1 C and D). In some cases, HLA seemed to be expressed at lower levels by cancer cells within the invasive component of the tumor, but at higher levels within the less invasive compartment (Fig. 1F).
Median disease-free survival (DFS) time was 20 months [95% confidence interval (C.I.), 8.5, 100+] for patients whose tumors expressed low HLA (≤10% of tumor cells) versus 57 months (95% C.I., 31.6, 100+) for cases with high HLA expression (>10% of cells); this difference was not considered statistically significant (P = 0.097) in a univariate Cox model (Table 1). Overall survival (OS) time also did not differ according to HLA expression in that model (P = 0.230; Table 2).
Table 1.
Predictors of disease-free survival: Univariate and multivariate analyses
| Factor | Coefficient | Hazard ratio | 95% C.I. | P value |
|---|---|---|---|---|
| Univariate Cox model | ||||
| No. of CD8+ TILs (continuous) | −0.06 | 0.94 | (0.90, 0.99) | 0.018 |
| No. of CD8+ TILs (binary) | ||||
| <8 | 1 | reference | ||
| ≥8 | −0.8 | 0.45 | (0.21, 0.96) | 0.034 |
| Age | 0.01 | 1.01 | (0.98, 1.05) | 0.40 |
| Disease stage | ||||
| T1 or Ta | 1 | reference | ||
| T2+ | 0.79 | 2.21 | (1.09, 4.49) | 0.025 |
| Carcinoma in situ | ||||
| No | 1 | reference | ||
| Yes | −0.19 | 0.83 | (0.37, 1.87) | 0.650 |
| Sex | ||||
| Female | 1 | reference | ||
| Male | −0.13 | 0.88 | (0.41, 1.85) | 0.730 |
| Chemotherapy | ||||
| No | 1 | reference | ||
| Yes | 0.37 | 1.44 | (0.66, 3.14) | 0.360 |
| MHC class I (HLA) expression | ||||
| ≤10 | 1 | reference | ||
| >10+ | −0.64 | 0.53 | (0.25, 1.12) | 0.097 |
| Multivariate Cox model | ||||
| Disease stage T2+ (vs. T1/Ta) | 1.66 | 5.28 | (2.32, 13.62) | <0.001 |
| No. of CD8+ TILs ≥8 (vs. <8) | −0.24 | 0.79 | (0.27, 2.33) | 0.670 |
| Stage*CD8+ | −1.59 | 0.2 | (0.04, 0.90) | 0.040 |
Table 2.
Predictors of overall survival: Univariate and multivariate analyses
| Factor | Coefficient | Hazard ratio | 95% C.I. | P value |
|---|---|---|---|---|
| Univariate Cox model | ||||
| No. of CD8+ TILs (continuous) | −0.05 | 0.95 | (0.88, 1.02) | 0.140 |
| No. of CD8+ TILs (binary) | ||||
| <8 | 1 | reference | ||
| ≥8 | −0.82 | 0.44 | (0.14, 1.39) | 0.152 |
| Age | 0.03 | 1.03 | (0.98, 1.08) | 0.290 |
| Sex | ||||
| Female | 1 | reference | ||
| Male | 0.46 | 1.58 | (0.44, 5.61) | 0.480 |
| Carcinoma in situ | ||||
| No | 1 | reference | ||
| Yes | 0.28 | 1.32 | (0.44, 3.96) | 0.499 |
| Chemotherapy | ||||
| No | 1 | reference | ||
| Yes | 0.86 | 2.36 | (0.83, 6.70) | 0.110 |
| Disease stage | ||||
| T1 or Ta | 1 | reference | ||
| T2+ | 2.03 | 7.62 | (2.13, 27.3) | <0.001 |
| MHC class I (HLA) expression | ||||
| ≤10% | 1 | reference | ||
| >10% | −0.666 | 0.514 | (0.18, 1.50) | 0.230 |
| Multivariate Cox model | ||||
| Disease stage T2+ (vs. T1/Ta) | 2.2 | 9.4 | (2.60, 33.96) | <0.001 |
| No. of CD8+ TILs ≥8 (vs. <8) | −1.2 | 0.3 | (0.09, 0.96) | 0.042 |
CD8 TILs Are Prognostic in Muscle-Invasive Disease.
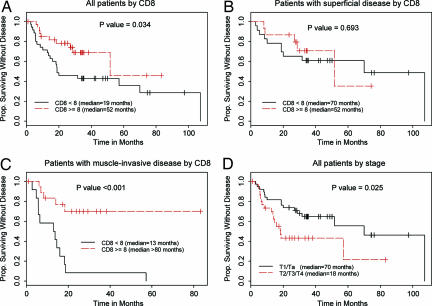
DFS time was longer for patients whose tumors expressed CD8 TILs (median, 52 months; 95% C.I., 52, 100+ months) than for those whose tumors did not express CD8 TILs (median, 19 months; 95% C.I., 19, 100+ months) (P = 0.034, log-rank test) (Fig. 2A). In terms of disease stage, the median DFS time was 70 months (95% C.I., 52, 100+) for patients with early superficial (Ta or T1) disease versus 18 months (95% C.I., 14, 100+) for those with advanced, muscle-invasive (T2, T3, or T4) disease (P = 0.025, log-rank test) (Fig. 2D). Interestingly, the presence of CD8 TILs did not influence DFS time among patients with superficial UC (P = 0.693) (Fig. 2B), but had a substantial influence among patients with muscle-invasive disease (P < 0.001) (Fig. 2C).
Fig. 2.
Kaplan–Meier plots of DFS according to CD8 TILs and disease stage. P values are from log-rank tests. (A) DFS in all patients according to CD8 TILs <8 (n = 35) vs. CD8 cells ≥8 (n = 34). (B) DFS in patients with early stage (pT1/pTa) disease by CD8 cells <8 (n = 23) vs. CD8 cells ≥8 (n = 15). (C) DFS in patients with advanced (pT2/pT3/pT4) disease by CD8 cells <8 (n = 12) vs. CD8 cells ≥8 (n = 19). (D) DFS in all patients according to disease stage: early stage (T1/Ta; n = 38) vs. advanced (T2/T3/T4; n = 31).
Univariate and multivariate analyses of the influence of CD8 TILs, disease stage, and other risk factors of interest on DFS indicated that only pathologic disease stage and the presence of CD8 TILs were significantly associated with DFS; the presence of carcinoma in situ (CIS), prior chemotherapy, and sex of the patient were not associated with DFS. When CD8 TILs was treated as a continuous variable, the risk of recurrence or death was found to decrease as the number of CD8 TILs increased (P = 0.018). In a multivariate Cox proportional hazards model, CD8 TILs and pathologic disease stage remained significant factors for predicting DFS, with a statistically significant interaction between the two factors (P = 0.04) (Table 1). This significant interaction between pathologic disease stage and CD8 TILs further confirms the importance of CD8 as a predictor of DFS in advanced disease (Fig. 2C), but not in early stage disease (Fig. 2B).
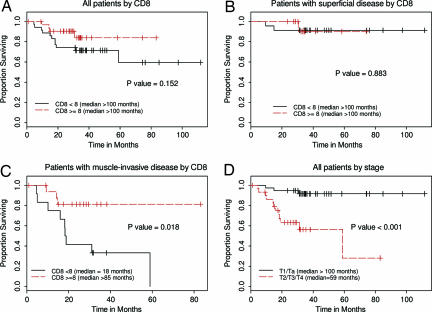
In analyses of OS that included all patients (regardless of disease stage), those whose tumors expressed CD8 TILs seemed to have better OS than those whose tumors did not; however, this apparent difference was not statistically significant (P = 0.152) (Fig. 3A). Further analyses revealed that the presence of CD8 TILs was associated with OS among patients with muscle-invasive disease (P = 0.018) (Fig. 3C), but not among patients with superficial UC (P = 0.883) (Fig. 3B). As expected, disease stage was associated with OS time (P < 0.001) (Fig. 3D).
Fig. 3.
Kaplan–Meier plots of OS according to CD8 TILs and disease stage. P values are from log-rank tests. (A) OS in all patients according to CD8 TILs <8 (n = 35) vs. CD8 cells ≥8 (n = 34). (B) OS in patients with early stage (T1/Ta) disease by CD8 cells <8 (n = 23) vs. CD8 cells ≥8 (n = 15). (C) OS in patients with advanced (T2/T3/T4) disease by CD8 cells <8 (n = 12) vs. CD8 cells ≥8 (n = 19). (D) OS in all patients according to disease stage: early stage (T1/Ta; n = 38) vs. advanced (T2/T3/T4; n = 31).
Univariate analyses indicated that pathologic disease stage was the only risk factor associated with OS (P < 0.001). However, in the multivariate Cox proportional hazards model, the presence of CD8 TILs and disease stage were both significant prognostic indicators of OS (Table 2). Specifically, the hazard ratio for CD8 TILs(+) versus CD8 TILs (−) was 0.3 (95% C.I., 0.09, 0.96), and the hazard ratio for advanced (pT2/pT3/pT4) versus early stage (pT1/pTa) disease was 9.4 (95% C.I., 2.60, 33.96) (Table 2). Although results from the univariate analyses and Fig. 3 B and C suggest different effects of CD8 TILs on OS for patients with advance and early stage diseases, the interaction term is not statistically significant in the multivariate model (P = 0.360). A possible reason for the interaction term to lack statistical significance in this setting may be the small number of deaths that were documented in the limited follow-up of this small cohort of patients.
We also explored the relationship between disease stage and CD8 TILs. χ2 tests showed a marginal association between disease stage and CD8 TILs (P = 0.07). Among patients with early stage disease, most [23 of 38 (61%)] had CD8 TILs(−) tumors. In contrast, most patients with advanced-stage disease [19 of 31 (61%)] had CD8 TILs(+) tumors. Hence, the presence of CD8 TILs seemed to influence DFS and OS for patients with advanced (muscle-invasive) disease, but not for patients with early stage disease. No significant association was found between CIS and CD8 TILs [P (from χ2 test) = 0.733], but CIS was associated with disease stage (P = 0.017) (data not shown). Eighteen percent of patients with early stage disease had CIS, whereas 48% of patients with advanced-stage disease had CIS. However, the presence of CIS was not associated with DFS or OS (Tables 1 and 2). Further, no strong association was noted between the presence of CD8 TILs and HLA expression: The Spearman rank correlation between CD8 TILs and HLA expression (as continuous variables) was 0.232 (P = 0.056). No correlation was found between CD8 TILs and NY-ESO-1 expression when NY-ESO-1 expression was considered as a binary variable (P = 0.589, Wilcoxon test), and no association was noted between NY-ESO-1 and HLA expression (P = 0.09, Wilcoxon test). Finally, no difference was found in DFS (P = 0.745, log-rank test) or OS (P = 0.754, log-rank test) by NY-ESO-1 expression in an analysis of all patients (data not shown).
Recognition of the NY-ESO-1 Tumor Antigen by Peripheral T Cells.
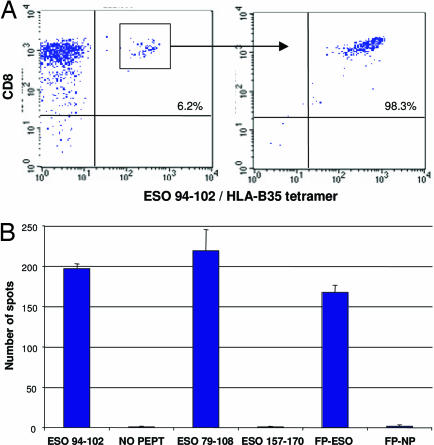
We previously reported that ≈30-40% of high-grade UCs express the CTA NY-ESO-1 (12); in the present sample, we found that 22 (32%) of the 69 UC tumor specimens expressed NY-ESO-1 (SI Table 3). To ascertain whether NY-ESO-1 tumor antigen can be recognized by T cells, we analyzed peripheral blood lymphocytes from patient 17, whose tumor showed large numbers of CD8 TILs (mean, 19.44 CD8 cells) and was immunopositive for the mAb E978, indicating expression of the NY-ESO-1 protein. We chose peripheral blood lymphocytes as a source of circulating T cells for these experiments because we could not obtain TILs from the FFPE specimens. We found evidence of a T cell response to the NY-ESO-1 peptide 94–102 in the context of HLA-B*3501 as detected by tetramer staining (Fig. 4A). To further characterize this response, we sorted the tetramer-stained cells by flow cytometry and cloned them by limiting dilution. Several clones were obtained and showed specificity for NY-ESO-1 peptide 94–102. Moreover, these cells could also secrete IFN gamma in response to a 30-amino acid peptide overlapping with the minimal epitope required for processing by the proteasome (26), as well as to full-length NY-ESO-1 processed from a recombinant fowlpox vector (Fig. 4B). Collectively, these findings indicate the presence of systemic CD8 T cell responses against naturally processed NY-ESO-1 in this patient. These results also suggest that circulating T cells may recognize expressed tumor antigens within the tumor microenvironment.
Fig. 4.
Clonal isolation of CD8 T cells against NY-ESO-1 from the peripheral blood of patient 17. (A) CD8 T cells were stimulated with NY-ESO-1 peptide 79–108 (GARGPESRLLEFYLAMPFATPMEAELARRS), and the resulting polyclonal line was stained with tetramers of synthetic HLA-B*3501/NY-ESO-1 94–102 complexes and with anti-CD8 antibody (Left). Percentages indicate double-positive cells, which were sorted by flow cytometry; several clones were derived and confirmed by tetramer staining (Right). (B) ELISPOT assay showing number of spots corresponding to IFN-γ secretion by cells specific for NY-ESO-1 94–102. These cells also reacted against the 30-mer peptide NY-ESO-1 79–108 and the full-length NY-ESO-1 encoded from recombinant fowlpox virus (FP-ESO), but not against control peptide (FP) or vector (NO PEPT).
Discussion
In this study, we found that the presence of intratumoral CD8 TILs was significantly associated with clinical outcome among patients with muscle-invasive UC. Specifically, in a sample of 31 patients with muscle-invasive UC, the presence of intratumoral CD8 TILs correlated with favorable DFS. We also found a significant association between CD8 TILs and OS in multivariate analyses. These findings underscore the importance of the host immune system in the clinical outcome of patients with cancer. Improved clinical outcome has been associated with the presence of intratumoral T cells in ovarian (3, 4), esophageal (5), colorectal, and renal (6–8) carcinoma; we have now demonstrated that intratumoral CD8 T cells are a significant predictor of clinical outcome in UC.
Our findings also suggest that the presence of CD8 TILs is associated with favorable survival for patients with muscle-invasive UC, but not for patients with superficial UC. According to the concept of immunoediting (27), invasion and progressive disease in cancer are carried out mostly by tumor cells that “escaped” immune recognition. However, our findings strongly indicate that immune recognition of cancer cells via an immunosurveillance mechanism exists even in advanced cancer, as revealed by the increased numbers of CD8 TILs in muscle-invasive UC. Our findings further suggest that, in the setting of muscle-invasive UC, the existence of CD8 TILs within the primary tumor points to an ongoing immune response that may be able to suppress tumor recurrence. Previously, Chiba et al. (28) hypothesized that intratumoral CD8 TILs suppress micrometastatic disease in colorectal cancer. They presented results indicating that the number of CD8 TILs was not prognostic during short follow-up (i.e., <2 years), but was prognostic during longer follow-up. Our observations from patients with UC seem to parallel these findings by demonstrating a significant association between CD8 TILs and OS.
To assess T cell recognition of tumor antigens, we analyzed peripheral blood lymphocytes from a patient with muscle-invasive UC and large numbers of intratumoral CD8 T cells, as well as expression of the CTA NY-ESO-1. In this patient, we demonstrated a clonal T cell population with specificity for the NY-ESO-1 antigen. This observation suggests that peripheral T cells, which are specific for tumor-associated antigens such as NY-ESO-1, may potentially participate in immune responses within the tumor microenvironment, including primary tumors and metastasized foci. Collectively, these results highlight the importance of CD8 T cells in UC and the importance of effective immunotherapy strategies that can evoke CD8 T cell responses.
To address the effect of HLA expression on the presence of T cells in the tumor microenvironment, we generated a monoclonal antibody. With this antibody, we were able to detect HLA expression in FFPE tumor samples by immunohistochemical staining. We were also able to demonstrate the absence and down-regulation of HLA expression by cancer cells within the various UC samples. We observed that HLA down-regulation occurred heterogeneously and the expression level of HLA was not associated with the presence of CD8 TILs. Detailed in situ and ex vivo analyses of larger numbers of patients would be needed to clarify the association between down-regulation of HLA expression by cancer cells and intratumoral CD8 TILs.
Limitations of our study include the lack of long-term follow-up and the small number of patients studied. Therefore, one should interpret the results with caution, especially the multivariate survival analyses. Given the exploratory nature of this study, further investigation is necessary to verify our findings. Another limitation is related to the heterogeneity of the patient population, especially with regard to differences attributable to surgery (e.g., the extent of lymph node dissection) and differences attributable to disease pathology (e.g., absence or presence of lymphovascular invasion).
In summary, the presence of high numbers of CD8 intratumoral T cells in UC correlated with improved DFS and OS. Prospective studies are warranted to validate the sentinel findings of this hypothesis-generating study. The presence of CD8 TILs in UC implies an immunologically protective mechanism that confers a survival benefit, and thus TIL infiltration may have prognostic value in UC patients. Our observations require validation in a larger cohort of patients with UC treated in a more homogeneous manner, such as in a prospective study with an independent group controlled for pathologic disease features, extent of lymph node dissection, and type of perioperative chemotherapy. Our findings, along with confirmatory data, suggest that immunotherapeutic studies aimed at expanding intratumoral CD8 T cells in patients with UC may be a useful strategy for the development of more effective treatment programs.
Materials and Methods
Patients and Specimens.
FFPE tissue specimens from 69 patients with UC were retrieved from the archives of the Department of Pathology of Memorial Sloan–Kettering Cancer Center. The series consisted of 51 male and 18 female patients treated between 1996 and 2001 at the Memorial Sloan–Kettering Cancer Center. Thirty-eight cases involved superficial tumors (pT1 or pTa) and 31 muscle-invasive disease (pT2, pT3, or pT4). Adjuvant cisplatin-based chemotherapy had been administered to 19 patients with pT3 or pT4 disease. At the time of the analyses, 33 of the 69 patients had recurrent disease, and 15 had died from the disease.
Anti-HLA Monoclonal Antibody.
To detect expression of MHC class I antigen in tumor samples, we generated a monoclonal antibody (clone no. 212–445). To ensure the applicability of this antibody to standard paraffin-stored material, BALB/c mice were immunized with a degenerated protein resembling the consensus region of the MHC class I protein present in all human MHC class I haplotypes. The protein was treated at 25°C with 10% neutral-buffered formalin for 2 h, followed by 100% ethanol for 2 h, and then incubated at 70°C for 2 h and vacuum-dried. Splenocytes from immunized BALB/c mouse were fused with SP0/2 cells, and hybridoma supernatant was screened by ELISA against the degenerated protein that was previously used for immunization of the mice.
Immunohistochemical Analysis: Quantification of Antigen Expression and CD8 T Cell Infiltration.
Immunohistochemical staining was done as previously described (12, 13). Briefly, paraffin sections were deparaffinized and rehydrated in xylene and a series of graded alcohols. Antigens were retrieved by incubating slides in a household vegetable steamer at 95°C for 20 min in a high-pH solution (Dako North American, Inc., Carpinteria, CA). Primary incubation was done overnight at 4°C. For the detection of CD8 cells, murine clone C8/144B (Neomarkers, Fremont, CA) was used. The presence of MHC class I molecules was tested with our mAb (clone no. 212–445). NY-ESO-1 protein expression was assessed with mAb E978 as previously described (12). Endogenous peroxidase activity was blocked by a 20-min incubation in PBS containing 0.3% hydrogen peroxide and 0.1% sodium azide. For the detection of primary antibodies, EnVision Plus (Dako) was used for the anti-CD8 and anti-HLA mAbs. For mAb E978, a biotinylated horse anti-mouse secondary antibody (1:200; Vector Laboratories, Burlingame, CA) followed by an avidin-biotin system (ABC-Elite Kit, Vector Laboratories) was used. The chromogen was 3, 3′-diaminobenzidine (BioGenex, San Ramon, CA), and the counterstain was Gill's hematoxylin. For each specimen, three independent, 0.0625-mm2 areas with the most abundant CD8 tumor infiltrates were selected and digitally imaged with a Nikon Coolpix 990 camera with standard commercial software (Nikon, Melville, NY). TILs were counted manually from the digital images displayed on a monitor. All counts were repeated three times by the same investigator (E.S.), and the average of the repeat counts was used for statistical analyses. For HLA expression, cases with positive signal in >10% of the cancer cells were considered high expressing, whereas tumors with HLA immunostaining in ≤10% of tumor cells were regarded as low expressing. For NY-ESO-1 expression, cases in which 5% or more cancer cells showed staining were defined as positive and graded as follows: Focal, <5% of tumor cells stained; +, 5-25% of cells stained; ++, >25-50% of cells stained; +++, >50-75% of cells stained; and ++++, >75% of cells stained.
Isolation of CD8 T Cells Specific for NY-ESO-1.
Peripheral blood samples from one patient (ID 17, SI Table 3) whose tumor cells expressed NY-ESO-1 and were infiltrated by CD8 TILs were used to clone NY-ESO-1-specific T cells as follows. This patient was the only one in our cohort who had stored blood available for T cell analyses. CD8 T cells were stimulated with NY-ESO-1 peptide 79–108 (GARGPESRLLEFYLAMPFATPMEAELARRS), and the resulting polyclonal line was stained with tetramers of synthetic HLA-B*3501/NY-ESO-1 peptide 94–102 complexes and with an anti-CD8 antibody (Caltag Laboratories, Burlingame, CA). Cells specific for NY-ESO-1 peptide 94–102 were sorted with a FACSVantage flow cytometer (BD Biosciences, San Jose, CA) by using fluorescent tetrameric complexes of synthetic HLA-B*3501-purified molecules and NY-ESO-1 peptide 94–102. The cells were subsequently cloned by limiting dilutions in 96-well plates in the presence of allogeneic peripheral blood lymphocytes and 1 μg/ml phytohemagglutinin L (Sigma–Aldrich, St. Louis, MO). Individual clones were expanded in complete RPMI medium supplemented with 10% Benchmark FCS (Gemini Bio-Products, West Sacramento, CA) and 150 units/ml interleukin-2 (Chiron Mimotypes, Clayton, Australia) and tested for specificity by using tetramers and ELISPOT to detect IFN-γ secretion (12). Briefly, presensitized or cloned CD8 T cells (5 × 104 or 2 × 103) and 5 × 104 Epstein–Barr virus-transformed B cells that had been pulsed with peptide or infected with recombinant fowlpox encoding NY-ESO-1 (FP-ESO) or control influenza nucleoprotein (FP-NP) were added to plates precoated with IFN-γ mAb (2 μg/ml, 1-D1K; Mabtech, Stockholm, Sweden) and incubated for 20 h in RPMI. After washes, another IFN-γ mAb (0.2 μg/ml, 7-B6–1-biotin; Mabtech) was added to each well for 2 h at 37°C, followed by streptavidin-alkaline phosphatase (1 μg/ml; Roche Diagnostics, Indianapolis, IN) for 1 h at room temperature. After washes, substrate (5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium; Sigma–Aldrich) was added for 10 min. Spots, indicating IFN-γ secretion by cells specific for NY-ESO-1 94–102, were counted with an automated Immunospot device (Cellular Technology Limited, Cleveland, OH).
Statistical Analyses.
In the statistical analyses, pathologic disease stage was stratified as a binary variable, with early superficial disease (pT1 or pTa) compared with advanced, muscle-invasive disease (pT2, pT3, or pT4). The presence of CD8 TILs was defined as a binary variable, with <8 CD8 TILs designated CD8 TILs(−) and compared with ≥8 CD8 TILs designated CD8 TILs(+). The value of 8 was selected because it was the median number of CD8 TILs among all patients analyzed. χ2 tests were used to test the association between two categorical variables. DFS and OS distributions were calculated by the Kaplan–Meier method. The Cox proportional hazards model was used to evaluate the effect of CD8 TILs on DFS and OS distributions with or without adjustment for disease stage. Relative risks of DFS and OS and their C.I.s were calculated and compared between CD8 TILs(+) cases and CD8 TILs(−) cases. Two-sided P values <0.05 were considered significant. S-PLUS version 6.0 (Insightful Corp., Seattle, WA) software was used for the analyses.
Supplementary Material
Acknowledgments
This work was supported in part by a Physician-Scientist Program Award from the University of Texas M. D. Anderson Cancer Center and a Career Development Award from the American Society of Clinical Oncology (both to P.S.).
Abbreviations
- C.I.
confidence interval
- CIS
carcinoma in situ
- CTA
cancer-testis antigen
- DFS
disease-free survival
- FFPE
formalin-fixed, paraffin-embedded
- HLA
human lymphocyte antigen
- MHC
major histocompatibility complex
- OS
overall survival
- TIL
tumor-infiltrating cytotoxic T lymphocyte
- UC
urothelial carcinoma.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0611618104/DC1.
References
- 1.Alexandroff AB, Jackson AM, O'Donnell MA, James K. Lancet. 1999;353:1689–1694. doi: 10.1016/S0140-6736(98)07422-4. [DOI] [PubMed] [Google Scholar]
- 2.Ponticello A, Perna F, Maione S, Stradolini M, Testa G, Terrazzano G, Ruggerio G, Malerba M, Sanduzzi A. Respir Med. 2004;98:509–514. doi: 10.1016/j.rmed.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Sato E, Olson SH, Ahn J, Kepper J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K, et al. Proc Natl Acad Sci USA. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang L, Conejo-Garcia JR, Katsaros D, Gimott PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, et al. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 5.Schumacher K, Haensch W, Roefzaad C, Schlag PM. Cancer Res. 2001;61:3932–3936. [PubMed] [Google Scholar]
- 6.Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, Ohtani H. Cancer Res. 1998;58:3491–3494. [PubMed] [Google Scholar]
- 7.Nakano O, Sato M, Naito Y, Suzuki K, Orikasa S, Aizawa M, Suzuki Y, Shintaku I, Nagura H, Ohtani H. Cancer Res. 2001;61:5132–5136. [PubMed] [Google Scholar]
- 8.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, et al. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 9.Mendez R, Serrano A, Jager E, Maleno I, Ruiz-Cabello F, Knuth A, Garrido F. Tissue Antigens. 2001;57:508–519. doi: 10.1034/j.1399-0039.2001.057006508.x. [DOI] [PubMed] [Google Scholar]
- 10.Stam NJ, Vroom TM, Peters PJ, Pastoors EB, Ploegh HL. Int Immunol. 1990;2:113–125. doi: 10.1093/intimm/2.2.113. [DOI] [PubMed] [Google Scholar]
- 11.Boon T, Coulie PG, Van den Eynde B. Immunol Today. 1997;18:267–268. doi: 10.1016/s0167-5699(97)80020-5. [DOI] [PubMed] [Google Scholar]
- 12.Sharma P, Gnjatic S, Jungbluth AA, Williamson B, Herr H, Stockert E, Dalbagni G, Donat SM, Reuter VE, Santiago D, et al. Cancer Immun. 2003;3:19. [PubMed] [Google Scholar]
- 13.Sharma P, Shen Y, Wen S, Bajorin D, Reuter V, Old L, Jungbluth A. Clin Cancer Res. 2006;12:5442–5447. doi: 10.1158/1078-0432.CCR-06-0527. [DOI] [PubMed] [Google Scholar]
- 14.Boon T, Old LJ. Curr Opin Immunol. 1997;9:681–683. doi: 10.1016/s0952-7915(97)80049-0. [DOI] [PubMed] [Google Scholar]
- 15.van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, Knuth A, Boon T. Science. 1991;254:1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 16.Chen YT, Old LJ. Cancer J Sci Am. 1999;5:16–17. [PubMed] [Google Scholar]
- 17.Sahin U, Tureci O, Schmitt H, Cochlovius B, Johannes T, Schmits R, Stenner F, Luo G, Schobert I, Pfreundschuh M. Proc Natl Acad Sci USA. 1995;92:11810–11813. doi: 10.1073/pnas.92.25.11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scanlan MJ, Gordon CM, Williamson B, Lee SY, Chen YT, Stockert E, Jungbluth A, Ritter G, Jager D, Jager E, et al. Int J Cancer. 2002;98:485–492. doi: 10.1002/ijc.10276. [DOI] [PubMed] [Google Scholar]
- 19.Scanlan MJ, Simpson AJ, Old LJ. Cancer Immun. 2004;4:1. [PubMed] [Google Scholar]
- 20.Simons MA, Hayward AR, Gathings WE, Lawton AR, Young-Cooper GO, Cooper MD, Mage RG. Eur J Immunol. 1979;9:887–891. doi: 10.1002/eji.1830091110. [DOI] [PubMed] [Google Scholar]
- 21.Davis ID, Chen W, Jackson H, Parente P, Shackelton M, Hopkins W, Chen Q, Dimopoulus N, Luke T, Murphy R, et al. Proc Natl Acad Sci USA. 2004;101:10697–10702. doi: 10.1073/pnas.0403572101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jager E, Jager D, Knuth A. Onkologie. 2000;23:410–415. doi: 10.1159/000027202. [DOI] [PubMed] [Google Scholar]
- 23.Nishiyama T, Tachibana M, Horiguchi Y, Nakamura K, Ideda Y, Takesako K, Murai M. Clin Cancer Res. 2001;7:23–31. [PubMed] [Google Scholar]
- 24.Sadanaga N, Nagashima H, Mashino K, Tahara K, Yamaguchi H, Ohta M, Fujie T, Tanaka F, Inoue H, Takesako K, Akiyoshi T, Mori M. Clin Cancer Res. 2001;7:2277–2784. [PubMed] [Google Scholar]
- 25.Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. Nat Rev Cancer. 2005;5:615–625. doi: 10.1038/nrc1669. [DOI] [PubMed] [Google Scholar]
- 26.Gnjatic S, Atanackovic D, Matsuo M, Jager E, Lee SY, Valmori D, Chen YT, Ritter G, Knuth A, Old LJ. J Immunol. 2003;170:1191–1196. doi: 10.4049/jimmunol.170.3.1191. [DOI] [PubMed] [Google Scholar]
- 27.Dunn GP, Ikeda H, Bruce AT, Koebel C, Uppaluri R, Bui J, Chan R, Diamond M, White JM, Sheehan KC, et al. Immuol Res. 2005;32:231–246. doi: 10.1385/ir:32:1-3:231. [DOI] [PubMed] [Google Scholar]
- 28.Chiba T, Ohtani H, Mizoi T, Naito Y, Sato E, Nagura H, Ohuchi A, Ohuchi K, Shiiba K, Kurokawa Y, et al. Br J Cancer. 2004;91:1711–1717. doi: 10.1038/sj.bjc.6602201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.