Abstract
A major component of burn injury is caused by additional local damage from acute inflammation. Using a scald burn model in mice, we find that this part of the injury is dependent on recognition of self-antigen by specific natural IgM, leading to activation of the complement system. We propose that the depth of a burn wound is a sum of the thermal energy applied and of the degree of host inflammatory response.
Keywords: complement, natural IgM, trauma, wound physiology
With >2 million patients in the United States seeking care for burns each year, the limited repertoire of therapies that reduce burn wound depth represents a significant contributor to deformity and death in burned patients. Of these 2 million, 20% require hospitalization, and as many as 7,000 die because of burn injury complications (1). The difference between a superficial second-degree burn, where tissue loss does not extend to the deepest projection of the hair follicles, and a deep second-degree burn, where tissue loss extends deeper than the skin appendages (but still within the dermis), is crucial clinically. Superficial second-degree burns heal without scarring, as the skin is repopulated from epithelium in the hair follicles. A deep second-degree burn usually requires skin grafting and heals with deforming and disabling scars, just as if it had been a third-degree or full-thickness burn. Smaller area burns can develop a tenuous epidermal layer; larger burns ulcerate. The ability to restrict the progression of thermal injury by even 1 mm to prevent extension beyond the hair follicles would be an important therapeutic advance. Amplification of burn injury depth has been attributed to the acute inflammatory response that follows wounding. Studies in man (2) and animal models of burn (3–7) identify activation of neutrophils as one component of this inflammatory response. Indeed, the limited reduction in burn depth accomplished in patients in a phase II clinical trial using an antibody to the adhesion receptor intercellular adhesion molecule 1 (ICAM-1) supports potential benefit of reducing burn wound inflammation (8).
Results
To examine the early events of inflammation in cutaneous burn injury, a scald burn wound model was developed in various strains of mutant mice. The standard scalding condition (54°C for 25 sec) was selected after an initial titration to produce a wound of moderate severity that heals with ulceration and wound contracture. Because mice rapidly heal a deep second- or third-degree burn by scar contraction, we tracked the wound size by tattooing the corners of the original wound with serial photography for wound size, shape, and presence/absence of hair. No burns that healed by contracture ever regrew hair or re-epithelialized.
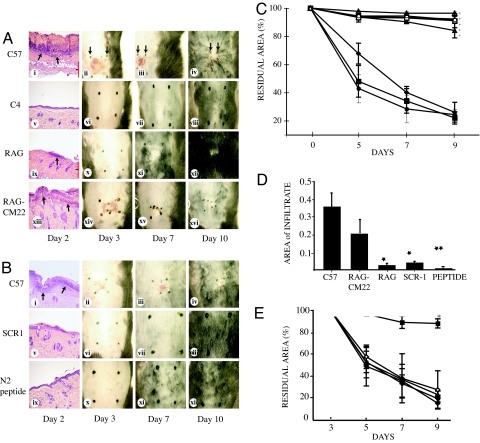
To determine whether the complement system was involved in the amplification of burn depth, mice deficient in complement component C4 (C4−/−) were compared with parental strain C57BL/6 in the scald burn model. As expected, the wounds on C57BL/6 mice healed by contracture with scarring and hair loss (Fig. 1 Ai–Aiv). Furthermore, the site of burn on these mice demonstrated a pronounced inflammatory response as seen by the histology of the wounds on day 2 with the presence of an infiltrate across the surface of the wound (Fig. 1 Ai and D). By contrast, the site of burn injury on C4−/− mice healed without contracture, scarring, hair loss, or appreciable infiltration of neutrophils (Fig. 1 Av–Aviii). To further test the importance of an intact complement pathway in burn injury, C57BL/6 mice were pretreated with a complement inhibitor, sCR1. This approach transiently blocks activation of the complement system at the C3 cleavage step (9). Notably, burn injury was negligible in sCR1-treated mice. Thus, in the absence of complement activation, the severity of the burn wound was dramatically reduced (Fig. 1 Bv–Bviii and D).
Fig. 1.
Acute inflammation following burn is dependent on specific natural IgM. (A) Shown are 1-cm2 scald burns in mice viewed over time with postburn day 2 histology. (ii and iii) Black arrows mark wound area on days 3 and 7 demonstrating wound ulceration and contraction. (i–iv) C57BL/6 mice. (v–viii) Complement C4−/− mice. (ix–xii) RAG1−/− antibody-deficient mice. (xiii–xvi) RAG1−/− mice pretreated i.v. with the IgM clone that initiates reperfusion injury, CM22. (i, v, ix, and xiii) Representative wound histologic sections where arrows indicate infiltration of leucocytes. (Magnification: ×10.) (B) Shown are 1-cm2 scald wounds in C57BL/6 mice pretreated with i.v. sCR1 (10 mg/kg, i.v.) complement inhibitor or N2 peptide (40 μM, i.v.) reperfusion injury inhibitor over time with postburn day 2 histology. (i–iv) C57BL/6 mice. (v–viii) Pretreated with sCR-1. (ix–xii) Pretreated with N2 peptide. (C) Residual wound area as the percentage of baseline vs. time in mice after scalding. ⧫, C57BL/6; ■, RAG1−/− pretreated i.v. with C57BL/6 IgM; ◊, RAG1−/− pretreated i.v. with C57BL/6 IgG; ●, RAG1−/− pretreated i.v. with CM22; ▴, RAG1−/−; ○, C57BL/6 pretreated i.v. with sCR-1; ▵, C57BL/6 pretreated i.v. with N2 peptide; □, C4−/− knockout mice. ∗, P < 0.01 for RAG1−/− and sCR-1-pretreatments compared with untreated C57BL/6; P < 0.001 for C4−/− and N2 pretreated C57BL/6, and RAG1−/− pretreated with IgG, compared with untreated C57BL/6. Bars portray SEM. (D) Area of inflammatory cell infiltrate in mice on day 2 after scalding (in millimeters). ∗, P < 0.01; ∗∗, P < 0.001, compared with infiltrate in C57BL/6. C57, C57BL/6 mice; RAG-CM22, RAG1−/− pretreated with CM22; RAG, RAG1−/−; sCR1, C57BL/6 pretreated with sCR1; Peptide, C57BL/6 pretreated with N2 peptide. (E) Residual wound area vs. time in C57BL/6 treated i.v. with N2 peptide (40 μM) at various time points after scalding. ◊, No drug; ▴, 5 min; ●, 30 min; ⧫, 60 min; ■, 90 min. n > 6 for all groups. ∗, P < 0.01 compared with no drug.
The finding that C4 was a factor in determining the final depth of the burn wound suggested that either the classical or the lectin pathways of complement activation were the initiators of inflammation (10–13), both of which can be activated by antibody. To determine whether antibody triggered this complement activation following burn, mice (RAG1−/−) totally deficient in Ig were subjected to scalding as above. Significantly, RAG1−/− mice were protected from scarring and ulceration to the same degree as that observed in C4−/− mice (Fig. 1 Aix–Axii). Thus, Ig is important in induction of injury. Reconstitution of the Ig-deficient mice i.v. with either IgM or IgG (400 μg of each) identified IgM as the active antibody fraction in this model (Fig. 1C). Together, these findings suggest that this postburn inflammation might be mediated by the same pathway that had been identified in ischemia and reperfusion (IR) injury (9, 12, 14). Ischemia with reperfusion represents a general pathologic condition in which restoration of blood flow to hypoxic tissue leads to acute, severe inflammation (15). Although the precise mechanism for IR injury is unknown, studies in man (16), rodents (9, 12, 17–23), and pigs (24) support a critical role for the complement system. One current hypothesis is that the complement system is activated by IgM deposited in the injured tissues, following the expression of neoepitopes on ischemic tissue for which circulating natural IgM has specificity (25). One specific clone of natural IgM that mediates IR injury in murine intestinal and skeletal muscle models is known to be IgMCM-22 (25). To determine whether it also was involved in the evolution of burns, RAG1−/− mice were reconstituted with IgMCM-22 (100 μg i.v.) before scalding. The results indicated that this single clone restored the full extent of thermal injury to RAG1−/− mice (Fig. 1 Axiii–Axvi; see also Fig. 1 C and D). Reconstitution of RAG1−/− mice with a murine natural IgM clone of irrelevant specificity, IgMCM-75 (100 μg i.v.) before scalding did not restore any extent of thermal injury [see supporting information (SI) Fig. 4]. Thus, as observed in IR injury, specific natural IgM is required for the full evolution of a burn injury. This finding that cutaneous burns induced a similar pathway to inflammation as had been identified in IR injury suggested also that blood flow might be reduced locally following thermal treatment, a phenomenon also suggested by others (26–28).
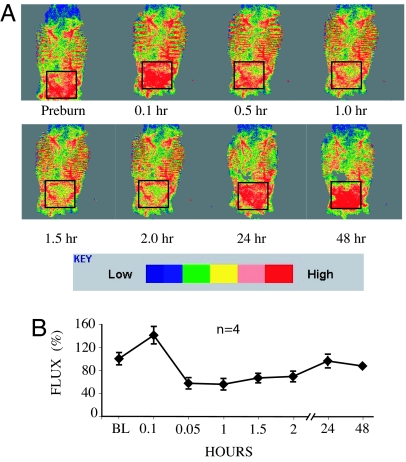
Mice were analyzed by laser Doppler preburn and at various time points postburn to visualize any changes in cutaneous burn that accompany burn injury. The results indicated that within minutes after injury, blood flow within the region is limited for at least 2 h (Fig. 2). Further support for transient local ischemia caused by burning was obtained by administration of a solution of 1% Evans blue dye i.v. before scalding for monitoring of capillary leakage at various time points. We observed an immediate blanching of vessels underlying the region of burn with leakage of dye into the surrounding tissues by 60 min postburn (SI Fig. 5). Thus, scald burn appears to induce vasospasm of blood vessels within the site of injury leading to a distinct period of relative ischemia followed by capillary leak.
Fig. 2.
Scald burn induces vasospasm and transient block of blood flow. (A) Representative laser Doppler images from the entire dorsum of one C57BL/6 mouse followed sequentially after scald burn. The Doppler scan demonstrates that the distribution of blood flow along the shaved dorsum is not uniform. Red indicates presence of blood flow. The black box indicates the area of scalding. There is hyperemia of the injury at 5 min postburn that then becomes vasospasm with reduced blood flow. By 24 h, blood flow is returned to baseline. (B) Plot of relative Doppler red cell flux measured from the 1-cm2 burn wound site as a percentage of baseline (BL) flow vs. time. Bars portray SEM. n = 4 mice per group.
Recent studies in the intestinal model of IR injury identified nonmuscle myosin heavy chain II (NMHC-II) as a self-antigen target of IgMCM-22 in intestinal tissue (29). Significantly, pretreatment of WT mice with a synthetic peptide representing the NMHC-II target (N2 peptide) or a mimotope of the natural ligand before reperfusion gave full protection from IR injury in both intestinal (29) and skeletal muscle (30) models. To test whether similar treatment is protective in the cutaneous burn model, N2 peptide at the optimal dose for IR injury (40 μM) was administered to C57BL/6 mice at various time points before and after scalding. As expected, mice administered only saline i.v. preburn developed the full extent of injury (Fig. 1 Bi–Biv and D). By contrast, mice administered N2 peptide i.v. preburn were protected from injury and never developed scarring, contracture, or ulceration (Fig. 1 Bix–Bxii and D). Postburn i.v. treatment with peptide was protective 90 min after scalding but was not protective at earlier postburn time points, (5, 30, or 60 min). (Fig. 1E). This unusual timing of effective administration is supported by the findings from laser Doppler (Fig. 2) that blood flow is blocked immediately after burn. Peptide administered i.v. in advance of wound perfusion by blood and pathogenic IgM may have been degraded, allowing the pathogenic IgM to act without impediment. Pretreatment of C57BL/6 mice with i.v. 40 μM 12-mer scrambled peptide had no effect on the evolution of the burn (SI Fig. 6).
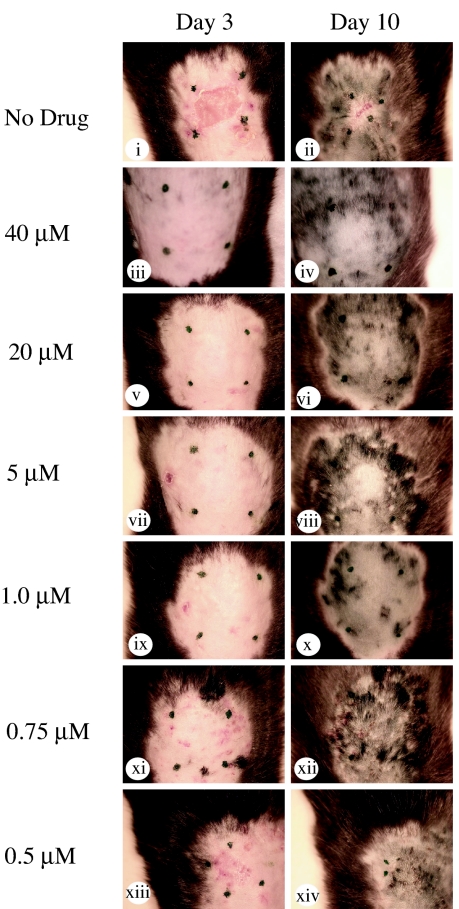
A consequence of a cutaneous burn is increased permeability of the skin and dermis, allowing uptake of small molecules into the area of potential injury. To determine whether topical application of N2 peptide protected the animals from scalding, 40 μM N2 peptide (or saline control) was administered four times to the site of thermal injury in C57BL/6 mice over a 60-min period. Evaluation of mice at days 3 and 10 indicated full extent of injury in mice given saline topically, as expected (Fig. 3 i and ii). On the other hand, mice receiving optimal N2 peptide topically during the immediate postburn period (Fig. 3 iii and iv) did not develop injury, as had been the case for animals receiving N2 peptide i.v. before burning (Fig. 1 Bix–Bxii and D). To evaluate the optimal dose of N2 peptide applied topically, C57BL/6 mice were given decreasing concentrations of N2 in a range of 40 to 0.30 μM per cm2 over the first 60-min postburn period. Analysis of injury based on wound contracture and extent of ulceration demonstrated full protection from injury in doses at >1 μM per cm2 (Fig. 3 i–x). At doses <1 μM, limited ulceration and wound contracture was apparent, suggesting the peptide had lost its protective effect (Fig. 3 xi–xiv). Thus, topical administration of N2 peptide at a dose of as low as 1 μM per cm2 is sufficient to protect from inflammatory amplification of burn injury.
Fig. 3.
Scald burns in C57BL/6 mice viewed over time. Mice were treated topically either with saline-soaked gauze or saline-soaked gauze with differing concentrations of N2 peptide. Application of N2 peptide topically is protective. The peptide prevented scarring, ulceration, and contracture when applied at a concentration range of 1–40 μM (iii–x), but protection was reduced at concentrations <1 μM (xi–xiv). n = 3 mice per group.
Discussion
The deep scald burn is a serious health problem for which there is no immediate cure. Studies in animal models indicate that deep second-degree burns are potentially nonscarring in the absence of inflammation and can heal spontaneously. However, acute inflammation significantly amplifies injury, resulting in extensive ulceration leading to wound contracture in mice or permanent scarring in humans. Using a cutaneous scald burn model in mice, we found that injury was mediated by a local effect of a specific natural IgM with subsequent complement activation and that injury can be blocked by pretreatment i.v. with a complement inhibitor or a synthetic peptide (N2) with sequence derived from nonmuscle myosin heavy chain, a sequence known to bind to the pathogenic IgM. Moreover, topical administration of peptide doses as low as 1 μM/cm2 postburn is sufficient to significantly limit acute inflammation and ensuing wound ulceration and contracture.
Our results also identify an immediate diminution of blood flow after burning, most likely due to intense vasospasm by underlying vessels. Subsequent restoration of circulation and induction of inflammation may explain the similarity of a deep second-degree cutaneous burn to a reperfusion injury. Alternatively, the properties of vasospasm and intense inflammatory amplification could be a feature of all injuries, of which reperfusion and burns are common examples. Blocking of specific natural IgM suggests a potential therapeutic approach for reducing severe inflammation following burn. It is also the case that vasospasm could be so profound in more severe burns that reversal of these inflammatory mechanisms are unable to improve the outcome.
Materials and Methods
Animals.
C57BL/6 and RAG1−/− (on C57BL/6 background) mice were purchased (The Jackson Laboratory, Bar Harbor, ME) and bred under specific pathogen-free conditions. C4−/− (on C57BL/6 background) mice were supplied by the M.C.C. laboratory (CBR Institute for Biomedical Research). Animals in this study were maintained in accordance with the guidelines of the Committee on Animals of Harvard Medical School and those prepared by the Committee on the Care and Use of Laboratory Animals of the Institute of Laboratory Resources, National Research Council (32).
Reconstitution of Antibody-Deficient Mice.
RAG1−/− mice were reconstituted with either i.v. administration of 400 μg of murine IgG (Calbiochem, San Diego, CA) or IgM isolated from pooled C57BL/6 serum, or 100 μg of IgMCM-22 clone, or 100 μg of IgMCM-75 clone 15–20 min before scald burn.
Reconstitution of WT Mice.
C57BL/6 mice were reconstituted i.v. with sCR1 (10 mg/kg; Avant, Needham, MA) (9) 15–20 min before scald burn in a total volume of 100 μl.
Scald Burn Model.
Ten-week-old male mice were anesthetized with i.p. ketamine (10 mg/kg) and xylazine (20 mg/kg) followed by intrascapular hair removal. A burn template was created from a high-impact, non-heat-conducting plastic container (Belkin Components, Compton, CA) to produce a 1-cm2 wound on the dorsum of a mouse between the scapulae (2.5% body surface area). With the template held in position, shaved skin was scalded in a circulating water (54°C) bath for 25 sec. Scalding resulted in a histologically confirmed deep second-degree burn to the exposed dorsum. This is an adaptation of the model used in the laboratory of John Mannick (31). Mice were removed from the burn template and placed on a heating pad to maintain body temperature. Mice were allowed to recover on fresh bedding with access to food and water without any intervention for 72 h after burn. At this time, wounds were either biopsied on postburn day 3 or sequentially photographed until healed. All animal groups had a minimum n = 3. Pain was accessed twice daily, and buprenorphine (0.05 mg/kg) SQ/IM was given as needed for the first 72-h postburn period. For the experiments described, every animal group was treated twice daily for 3 days.
Histology of Burn Tissue.
For permanent sections, the burn tissue was fixed in 4% paraformaldehyde in PBS for 18 h and changed to PBS (Sigma, St. Louis, MO) and kept at 4°C. Sections were stained by hematoxylin/eosin (H&E) and examined by microscopy.
Quantification of Inflammation and Wound Contraction.
The zone of inflammation and wound contraction area measurements were attained by using the National Institutes of Health J-Image program, which calculates the number of pixels within a circumscribed area.
For H&E histology samples of the burn, the top of the histology sample to base of the neutrophil infiltrate was measured as the depth of inflammation.
Evans Blue Experiment.
Mice were anesthetized with i.p. ketamine (10 mg/kg) and xylazine (20 mg/kg). At times (10, 20, 30, 60, and 90 min) before and after burn, 100 μl of 1% Evans blue (MW = 960) was administered i.v. to visualize skin vasculature.
Laser Doppler Scanning.
A laser Doppler imager (Moor Instruments, Wilmington, DE) was used to assess blood flow. The laser Doppler source was mounted on a movable rack exactly 20 cm above the back of the mouse after the animal was anesthetized and restrained on the underlying table. The laser beam (780 nm) reflected from moving red blood cells in nutritional capillaries, arterioles, and venules was detected and processed to provide a computerized, color-coded image. By using image analysis software (Laser Doppler Perfusion Measure, Version 3.08; Moor Instruments), mean flux values representing blood flow were calculated from the relative flux units for the areas corresponding to the dorsum of the mice. Baseline images were obtained from each mouse before burning. Mice were burned, and serial laser Doppler images were obtained postburn. The entire procedure was done under warm ischemic conditions.
Administration of Peptide.
The N2 peptide and the 12-mer scrambled peptide (H2N-AGCMPYVRIPTA-OH) were prepared in sterile deionized water as a stock and then diluted in normal saline for use. For i.v. injection, mice received a final concentration of 40 μM (assuming 2-ml volume of blood) (29). For topical application, four doses at a known concentration were applied over the first 60-min postburn period in a damp gauze cut to fit the burn.
Statistical Analysis.
Statistical comparisons between groups were made by Student's t test. Standard error of the mean (SEM) was used to evaluate significance. P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Avant Immunotherapeutics (Needham, MA) for the sCR1; Dr. Elisabeth Alicot-Carroll (CBR Institute for Biomedical Research) for providing the IgMCM22; Drs. M. T. Watkins and H. Albadawi (Vascular Surgery Laboratory, Massachusetts General Hospital) for use of the laser Doppler scanner; Yveta Masarova for assistance in assembling and proofing the manuscript; and Jessica Carroll for help with an Evans blue experiment. F.S. thanks Drs. H. Hechtman and K. F. Austen for advice and mentorship throughout his research fellowship and review of the manuscript. This work was supported by National Institutes of Health Grant P50 52585 (to M.C.C. and F.D.M.).
Abbreviation
- IR
ischemia and reperfusion.
Footnotes
Conflict of interest statement: M.C.C. and F.D.M. declare a potential financial interest. M.C.C. and F.D.M. are cofounders of DecImmune, Inc. DecImmune is licensed to develop therapeutics from N2 peptide and IgMCM-22.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0609026104/DC1.
References
- 1.Alexander AS, Adzick NS. In: The Surgical Review. Krupnick KD, Kaiser AS, editors. Philadelphia: Lippincott; 2001. pp. 123–138. [Google Scholar]
- 2.Moore FD, Davis D, Rodrick M, Mannick JA, Fearon DT. N Engl J Med. 1986;314:948–953. doi: 10.1056/NEJM198604103141503. [DOI] [PubMed] [Google Scholar]
- 3.Mileski W, Borgstrom D, Lightfoot E, Rothlein R, Faanes R, Lipsky P, Baxter C. J Surg Res. 1992;52:334–339. doi: 10.1016/0022-4804(92)90112-d. [DOI] [PubMed] [Google Scholar]
- 4.Mileski W, Gates B, Sigman A, Sikes P, Atiles L, Lightfoot E, Lipsky P, Baxter C. J Burn Care Rehabil. 1993;14:610–616. doi: 10.1097/00004630-199311000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Mileski WJ, Rothlien R, Lipsky P. Eur J Pediatr Surg. 1994;4:225–230. doi: 10.1055/s-2008-1066110. [DOI] [PubMed] [Google Scholar]
- 6.Nwariaku FE, Mileski WJ, Lightfoot E, Sikes PJ, Lipsky PE. J Trauma. 1995;39:285–288. doi: 10.1097/00005373-199508000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Horton JW, Mileski WJ, White DJ, Lipsky P. J Surg Res. 1996;64:49–56. doi: 10.1006/jsre.1996.0305. [DOI] [PubMed] [Google Scholar]
- 8.Mileski WJ, Burkhart D, Hunt JL, Kagan RJ, Saffle JR, Herndon DN, Heimbach DM, Luterman A, Yurt RW, Goodwin CW, Hansborough J. J Trauma. 2003;54:950–958. doi: 10.1097/01.TA.0000030626.84680.11. [DOI] [PubMed] [Google Scholar]
- 9.Weisman HF, Bartow T, Leppo MK, Marsh HC, Carson GR, Concing MF, Boyle MP, Roux KH, Weisfeldt ML, Fearon DT. Science. 1990;249:146–151. doi: 10.1126/science.2371562. [DOI] [PubMed] [Google Scholar]
- 10.Reid KBM, Porter RR. Annu Rev Biochem. 1981;50:433–464. doi: 10.1146/annurev.bi.50.070181.002245. [DOI] [PubMed] [Google Scholar]
- 11.Holmskov U, Theil S, Jensenius JC. Annu Rev Immunol. 2003;21:547–578. doi: 10.1146/annurev.immunol.21.120601.140954. [DOI] [PubMed] [Google Scholar]
- 12.Weiser MR, Williams JP, Moore FD, Kobzik L, Ma M, Hechtman HB, Carroll MC. J Exp Med. 1996;183:2343–2348. doi: 10.1084/jem.183.5.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang M, Takahashi K, Alicot EM, Vorup-Jensen T, Kessler B, Thiel S, Jensenius JJ, Ezekowitz AB, Moore FD, Carroll MC. J Immunol. 2006;177:4727–4734. doi: 10.4049/jimmunol.177.7.4727. [DOI] [PubMed] [Google Scholar]
- 14.Williams JP, Pechet TT, Weiser MR, Reid R, Kobzik L, Moore FD, Carroll MC, Hechtman HB. J Appl Physiol. 1999;86:938–942. doi: 10.1152/jappl.1999.86.3.938. [DOI] [PubMed] [Google Scholar]
- 15.Contran RS. Robbins Pathologic Basis of Disease. St Louis: Saunders; 1991. pp. 7–12. [Google Scholar]
- 16.Schafer PJ, Mathey D, Hugo F, Bhadki S. J Immunol. 1986;137:1945–1949. [PubMed] [Google Scholar]
- 17.Hill J, Lindsay TF, Ortiz F, Yeh CG, Hechtman HB, Moore FD. J Immunol. 1992;149:1723–1728. [PubMed] [Google Scholar]
- 18.Chan RK, Ibrahim S, Takahashi K, Ezekowitz A, Moore FD. J Am Col Surg. 2004;139:105. [Google Scholar]
- 19.Chan RK, Austen WG, Ibrahim S, Ding GY, Verna N, Hechtman HB, Moore FD. J Surg Res. 2004;122:54–60. doi: 10.1016/j.jss.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Reid RR, Woodcock S, Shimabukuro-Vornhagen A, Austen WG, Kobzik L, Zhang M, Hechtman HB, Moore FD, Carroll MC. J Immunol. 2002;169:5433–5440. doi: 10.4049/jimmunol.169.10.5433. [DOI] [PubMed] [Google Scholar]
- 21.Lindsay TF, Hill J, Ortiz F, Rudolph A, Valeri CR, Hechtman HB, Moore FD. Ann Surg. 1992;216:677–683. doi: 10.1097/00000658-199212000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hill JH, Ward PA. J Exp Med. 1971;133:885–900. doi: 10.1084/jem.133.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fleming SD, Shea-Donohue T, Guthridge JM, Kulik L, Waldschmidt TJ, Gipson MG, Tsokos GC, Holers VM. J Immunol. 2002;169:2126–2133. doi: 10.4049/jimmunol.169.4.2126. [DOI] [PubMed] [Google Scholar]
- 24.Chai PJ, Nasser R, Oakeley AE, Craig DM, Quick G, Jaggers J, Sanders SP, Ungerleider RM, Anderson PA. Circulation. 2000;101:541–546. doi: 10.1161/01.cir.101.5.541. [DOI] [PubMed] [Google Scholar]
- 25.Zhang M, Austen WG, Chiu I, Alicot EM, Hung R, Ma M, Verna N, Xu M, Hechtman HB, Moore FD, Carroll MC. Proc Natl Acad Sci USA. 2004;101:3886–3891. doi: 10.1073/pnas.0400347101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Machens HG, Pabst A, Dreyer M, Gliemroth J, Gorg S, Bahlmann L, Klaus S, Kaun M, Kru Ger S, Mailander P. Surgery. 2006;139:550–555. doi: 10.1016/j.surg.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Radke A, Mottaghy K, Goldmann C, Khorram-Sefat R, Kovac B, Jansssen A, Klosterhalfen B, Hafemann B, Pallua N, Kirschfink M. Crit Care Med. 2000;28:3224–3232. doi: 10.1097/00003246-200009000-00018. [DOI] [PubMed] [Google Scholar]
- 28.Ravage ZB, Gomez HF, Czermak BJ, Watkins SA, Till GO. Inflammation. 1998;22:619–629. doi: 10.1023/a:1022366514847. [DOI] [PubMed] [Google Scholar]
- 29.Zhang M, Alicot EM, Chiu I, Li J, Verna N, Vorup-Jensen T, Kessler B, Shimaoka M, Chan RK, Friend D, et al. J Exp Med. 2006;203:141–152. doi: 10.1084/jem.20050390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan RK, Verna N, Afnan J, Zhang M, Ibrahim S, Carroll MC, Moore FD. Surgery. 2006;139:236–243. doi: 10.1016/j.surg.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 31.O'Sullivan ST, Lederer JA, Horgan AF, Chin DH, Mannick JA, Rodrick ML. Ann Surg. 1995;222:482–490. doi: 10.1097/00000658-199522240-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Committee on the Care and Use of Laboratory Animals of the Institute of Laboratory Resources. Guide for the Care and Use of Laboratory Animals. Bethesda: Natl Inst Health; 1985. NIH Publ No 85-23. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.