Abstract
Patients with Alzheimer's disease (AD) frequently have difficulties with spatial orientation in their day-to-day life. Although AD is typically preceded by amnestic mild cognitive impairment (MCI), spatial navigation has not yet been studied in MCI. Sixty-five patients were divided into five groups: probable AD (n = 21); MCI, further classified as amnestic MCI single domain (n = 11); amnestic MCI multiple domain (n = 18), or nonamnestic MCI (n = 7), and subjective memory complaints (n = 8). These patients, together with a group of healthy control subjects (n = 26), were tested by using a four-subtests task that required them to locate an invisible goal inside a circular arena. Each subtest began with an overhead view of the arena showed on a computer monitor. This was followed by a real navigation inside of the actual space, an enclosed arena 2.9 m in diameter. Depending on the subtest, the subjects could use the starting position and/or cues on the wall for navigation. The subtests thus were focused on allocentric and egocentric navigation. The AD group and amnestic MCI multiple-domain group were impaired in all subtests. The amnestic MCI single-domain group was impaired significantly in subtests focused on allocentric orientation and at the beginning of the real space egocentric subtest, suggesting impaired memory for allocentric and real space configurations. Our results suggest that spatial navigation impairment occurs early in the development of AD and can be used for monitoring of the disease progression or for evaluation of presymptomiatic AD.
Keywords: allocentric navigation, Alzheimer's disease, egocentric navigation, spatial memory, biomarker
The neurodegenerative processes in Alzheimer's disease (AD) precede the onset of clinical manifestations. Affected individuals already may have objectively detectable cognitive impairment before clinical onset while still maintaining their social and occupational functioning (1).
Impairment of medial temporal lobe structures has been demonstrated even in the early stages of AD, and this finding clarifies why declarative memory impairment is one of the earliest hallmarks of the disease (2–4). AD is in nearly all subjects preceded by a presymptomatic stage called mild cognitive impairment (MCI) (5, 6), with the rate of conversion estimated at 15% every year (7).
MCI encompasses patients with and without memory impairment. Of those with memory loss, some have memory impairment as their only deficit [amnestic MCI single domain (aMCIs)], whereas others have impairments of memory loss plus changes in other cognitive domains [amnestic MCI multiple domain (aMCImd)] (5). Multiple-domain MCI is more common than pure amnestic type MCI and is characterized by slight impairment in more than one cognitive domain but of insufficient severity to constitute dementia (8, 9). Of those without any memory loss, some patients have deficits in one domain only, such as executive functions, apraxia or aphasia. Or they may have deficits in several domains, excluding memory. These prodromal states may progress to non-AD dementias, such as vascular dementia, frontotemporal dementia, Lewy body dementia, primary progressive aphasia, or corticobasal degeneration (10).
Patients with AD and some patients with MCI frequently have difficulties with spatial orientation in everyday activities (11). Patients may fail to find their way in unfamiliar environments when facing entirely new spatial settings during traveling or shopping. In advanced stages of the disease, they may be disoriented even within their familiar neighborhood or inside their own flat. Disorientation and spatial memory were studied in AD with tests consisting of navigation inside a hospital (12–14), orientation in a circular arena (15), and remembering object position (16). In contrast, only one study addressed spatial orientation in MCI (17), correlating motion flow perception with results in a table-top Money Road Map test.
The discovery of place-specific firing in the hippocampus (18) and spatial navigation impairment after hippocampal lesion in the water maze (19) gave strong support to the theory of a cognitive map. This theory dissociates hippocampal navigation, based on a configuration of distal landmarks, from navigation to and from landmarks. This concept has evolved into the dissociation between allocentric navigation, using flexible representation of an ensemble of distal landmarks and independent of actual subject positions, and egocentric navigation, using distances and angles to or from individual landmarks. In humans, the allocentric mode of navigation was shown to be connected with the hippocampal function in analogues of the Morris water maze (MWM) (20, 21), in place navigation inside a virtual town (22), and in remembering the location of objects on a table (23).
The aim of this study was to characterize spatial navigation deficits in MCI and early AD and to assess how spatial navigation impairment could distinguish MCI from healthy subjects. Allocentric and egocentric navigation were investigated in an analogue of the MWM. The subject had to locate an unmarked goal inside of a circular arena by using its relation to two landmarks on the arena wall and/or by the memory of the start-goal trajectory.
Results
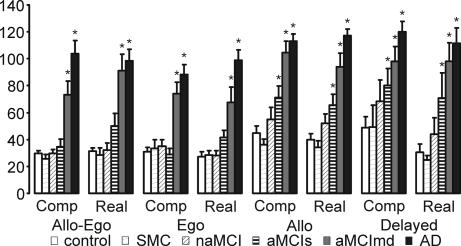
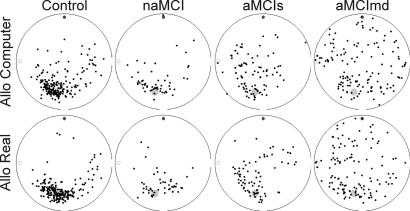
The impairment of the AD group was evident in all subtests (see Fig. 1). The differences between the MCI subtypes, however, were obvious predominantly in the third allo subtest: although the results of the nonamnestic MCI (naMCI) group were similar to the control group, on the figure, of all hits in the allo subtest, the hits of both amnestic MCI groups (aMCIs and aMCImd) were less clustered around the goal and more distributed over the arena (see Fig. 2).
Fig. 1.
The distance errors. The errors averaged across subtests are depicted (mean ± SEM). The asterisks represent significant differences (P < 0.05) from the control group. Please note the significant impairment of the aMCIs in both the allo and delayed subtests and the impairment of the aMCImd group in all subtests.
Fig. 2.
Example of the hits pattern in the subtest allo computer and subtest allo real, demonstrating the differences between the control and MCI subjects. In this subtest, only two cues on the wall of the arena, here represented by the small circle and disk, could be used as orientation cues. The position of the goal (larger gray disk) was not constant relative to the starting position (which therefore is not shown). Hits are represented by small black dots.
Average Distance Errors.
Significant differences across these groups were found in average distance errors in all subtests [analysis of covariance (ANCOVA), all F > 5.201, P < 0.001]. The contrast analysis relative to controls showed impaired performance of AD and aMCImd groups in all subtests (ANCOVA, all P < 0.001) (see Fig. 1). No differences were found in the performance of the subjective memory complaints (SMC) (all P > 0.382) and naMCI groups (all P > 0.127). Only the aMCIs group showed differential impairment depending on the subtest: in the first and second subtest (allo-ego and ego) in which navigation by starting position could be used, it did not significantly differ from the control group (all P > 0.157). In the third and fourth subtests (allo and delayed), where only two orientation cues on the wall could be used for navigation, the aMCIs group showed at least 1.5-fold worse estimates of the goal position than the control group and was significantly impaired (allo computer, P = 0.015; allo real, P = 0.016; delayed computer, P = 0.047; delayed real, P = 0.021).
The impairment of the aMCIs and aMCImd groups showed significant differences. The chart in Fig. 1 suggests the aMCImd group is closer to the AD group than the aMCIs group. We tested this hypothesis with the AD group as a reference. The aMCIs group performed similar to the AD group in the delayed computer subtest (P = 0.051) and was slightly better than the AD group in the delayed real subtest (P = 0.042). In all other (not-delayed) subtests, the aMCIs group scored considerably better than AD (all P < 0.003). In contrast, the aMCImd group performed better than the AD group only in the first computer subtest (allo-ego computer, P = 0.028) and in two real space subtests (ego real, P = 0.018; allo real, P = 0.045). This group was similar to the AD group in the first real space subtest allo-ego real, as well as in the two following computer subtests, ego computer and allo computer, along with both 30-min delayed subtests delayed computer and real (all P > 0.509).
Individual Trials.
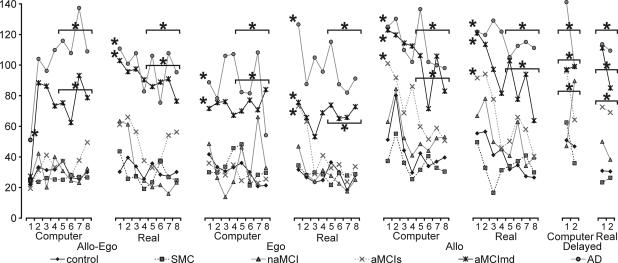
The correct position of the goal was shown to the subjects after each single trial. Consequently, learning was expected to occur during the trials of each subtest. Because the position of the goal was constant relative to the starting position and/or cues throughout the test, the first trial in each subtest assesses the subject's ability to use the information from the previous subtests. We therefore were interested in comparing the results from these first trials with the averages of the whole subtests and evaluating learning during each subtest by examining group differences in the averages of the second half of each subtest (trials 5–8).
From the chart showing all trials of the test (Fig. 3), it is obvious, that the AD group was largely impaired throughout the test and exhibited no apparent learning. This observation was confirmed by significant differences across the groups in all subtests (all F > 2.984, P < 0.017), and the AD group's impairment in both the first trial and the average of trials 5–8 within each subtest (all P < 0.004). Similar general impairment was found in the aMCImd group with the exception being the allo-ego computer subtest. The aMCImd group was similar to controls in the first trial of this subtest (P = 0.722), possibly reflecting this trial requires only recalling the correct goal position on the computer screen without any delay or rotation, testing simple short-term visual memory. This view was supported by the impairment of this group in the average of trials 5–8 (ANCOVA, P < 0.001). The impairment of the aMCImd group was highly significant in all other subtests (P < 0.007), except for borderline differences in the first trial of ego computer (P < 0.031). Similarly to the allo-ego computer subtest, this subtest assesses simple visual memory but after a delay. The aMCImd group was impaired in the second half of this subtest (P < 0.001).
Fig. 3.
The average distance errors in all trials of each subtest of the Hidden Goal Task. The standard errors are not included because of clarity. The asterisks represent significant differences (P < 0.05) from the control group. These significant differences were analyzed in the first trial of each subtest and in the average of trials 5–8 during each subtest. The horizontal line above several trials means that the significance applies for the average of trials 5–8. Please note the learning curves of the aMCIs and aMCImd (and other) groups, which are most distinct in the allo subtest.
The aMCIs group was impaired relative to controls in the first trial of the two allocentric subtests (allo computer, P < 0.008; allo real, P < 0.042). The curve of the individual trials in Fig. 3, however, suggests that this group could reach the level of controls in these subtests. This observation was supported by comparing the groups in the second half of the two subtests, where aMCIs performed similarly to controls (allo computer, P = 0.165; allo real, P = 0.054). Although these differences in learning were distinct in the computer version, they were only slight in the real version. The aMCIs group also was impaired in the first trial of the ego real subtest (P < 0.038), but they performed similarly to controls in the second half of this subtest (P = 0.760). This contrasted with the lack of impairment of aMCIs group in the ego computer subtest, both in its first trial (P = 0.751) and its second half (P = 0.760). No impairment was found in the first trial of the allo-ego subtest in both the computer (P = 0.669) and real versions (P = 0.150).
Test Components.
To evaluate the structure of the subtests, we analyzed the common factors explaining the variability in our results by using principal component analysis with varimax rotation. Both the averages of the individual subtests and the first trials of each subtest were included in the analysis. The eigenvalue >1 revealed a two-factor solution. Together, these two factors explained 65% of the variance. Factor 1 explained 57% of the variance, correlating most with the delayed real (0.834) and allo real (0.804) subtests. Correlation with other variables was only slightly lower, but the six highest correlation coefficients (range 0.648– 0.834) belonged to the six variables from the allocentric subtests (allo and delayed, both computer and real). Factor 2 explained 8% of the variance, correlating most with the ego computer (0.775) and allo-ego computer (0.757) subtests. Similarly to factor 1, correlation coefficients of other variables were only slightly lower but among the nine variables with highest correlation (range 0.439–0.775), eight of them were from the egocentric subtests (allo-ego and ego, both computer and real). This suggests that at least the allocentric and egocentric components are dissociable in HGT.
Navigational Strategies.
We further analyzed several types of errors made by the subjects' during the task to investigate which of them contributed to their impairment. From Fig. 2, which pictures all hits of the groups in individual subtests, we can guess that many aMCIs subjects confused the two cues in the allo real subtest because the hits form two symmetrical clusters. Similarly, we can assume that the subjects from the aMCImd group generally remembered the correct side of the arena because their hits are more clustered near the goal than on the opposite side of the arena. Thus, in addition to the previously analyzed distances between position given by the subject and the correct goal position, two other variables reflecting this observation were analyzed. The correct side variable estimated whether the subject recognized the side of the arena with the goal and the side error variable was used to estimate how much confusion of the arena side contributed to the error in estimating goal position.
There was no difference in correct side between the aMCIs group, and controls in any subtest (Mann–Whitney test, all P > 0.081) and the aMCIs group was better in determining the side than the AD group in all subtests (all P < 0.028), except for the delayed subtests (both computer P = 0.389 and real P = 0.475). The impairment of the aMCIs group in side error was different in the computer and real subtests: the group was impaired relative to controls in the allo computer (ANCOVA, P < 0.004) and delayed computer subtests (P < 0.007), but it was similar to controls in the allo real (P = 0.084) and delayed real subtests (P = 0.108). The group also performed similar to controls in ego computer (P = 0.824) and ego real (P = 0.166) subtests. These results confirm our observation that the aMCIs group confused the sides of the arena in the allo real and allo delayed subtests.
The aMCImd group was impaired in correct side in all subtests (Mann–Whitney test, all P < 0.017) but remembered the side better than the AD group in the ego real (P < 0.010) and allo real (P < 0.021) subtests. The group also was impaired relative to controls in all subtests in side error (ANCOVA, all P < 0.001) and performed similarly to the AD group in all subtests (all P > 0.196) except for the ego real subtest (P < 0.018).
Discussion
The results of this paper indicate strong differences in spatial navigation impairment among the subtypes of MCI. The AD and aMCImd groups were impaired in all subtests, whereas the naMCI and SMC groups were similar to controls.
Pronounced differences appeared between the two amnestic types of MCI, aMCIs and aMCImd. The aMCImd subjects were impaired in the first trial as well as in the second half of all subtests, indicating they could not learn how to find the goal in any of the subtests. They were impaired not only in the distance error during all subtests, but even in the recall of the correct side of the arena, suggesting serious impairment in spatial orientation. The only trial where they performed similar to controls was the first trial of the allo-ego computer subtest.
In contrast, the aMCIs group was impaired only in several specific parts of the test: namely in the first trial of the ego real subtest, the first trials of both the allo computer and allo real subtests, and the averages of both the delayed computer and delayed real subtests. This pattern of impairment suggests two factors contributing to impairment of this group: (i) allocentric navigation by two cues independent of the starting position (in both allo and delayed subtests), and (ii) navigation in real space (in all real subtests except for the allo-ego real subtest). The first factor was more contributing to the impairment, as indicated by the high significance of the impairment in the allo computer subtest. This also corresponds with the results of the principal component analysis, where most variance-explaining factors correlated with the allocentric subtests. The impairment of the aMCIs group was not found in the second half of the subtests, indicating difficulties with remembering the goal position for longer periods of time (from the previous subtest), but not an inability to learn it during the subtest.
Difficulties with spatial orientation have been associated with the damage of several brain areas, including the medial temporal lobe, ventral occipitotemporal, posterior parietal, and restrosplenial cortex (24). Most studies on spatial disorientation in AD focused on its connection with optic flow discrimination deficit, which possibly reflects posterior parietal cortical dysfunction in integrating multisensory cues on self-movement. This theory was documented by a significant correlation of optic flow discrimination thresholds with several measures of spatial navigation, such as disorientation in a hospital lobby (14), a score in the Money Road Map (MRM) test and the ability to respect lane boundaries during sustained driving in an on-the-road driving test (25). Further studies described results consistent with this theory, such as poor incidental landmark learning instead of proper recognition of landmarks mentioned during the walk (12), or a lack of relationship between disorientation with memory tests and a failure to use spatial architectural information (13). The AD patients probably are limited by their memory deficits in using spatial navigation strategies based on visual perceptual analyses (13). A hypothesis that this visual perceptual deficit can at least partially apply to MCI was suggested by Mapstone et al. (17). In this study, approximately half of MCI patients were impaired in radial motion perception, suggesting a visuospatial subtype of MCI based on spatial perception. The motion perception thresholds correlated significantly with the results of the MRM test, requiring subjects to follow a path through a city on a map and indicate left and right turns, but not with figural and verbal memory. However, the MCI subjects were not impaired in the MRM test. The study, therefore, does not document any spatial navigation deficit.
Although memory deficit is a defining and important diagnostic feature of AD, its impact on spatial disorientation in AD and MCI is not clear. The selective impairment in aMCIs in all allocentric and most real space subtests suggests a hippocampal deficit. Disrupted allocentric navigation after medial temporal lobe damage was described in analogues of the MWM (21, 26), an invisible sensor task in a hospital room after a 30-min delay (27), and in a virtual reality shifted-viewpoint spatial memory test (28). Temporal lobe damage also disrupted topographical orientation in a real (29) environment. On the contrary, optic flow perception activates right posterior parietal cortex (30). Therefore, impairment in both allocentric mode of navigation and memory for configurations in the real space are consistent with the medial temporal lobe damage found in MCI (7, 31), but not with a parietal dysfunction connected with optic flow discrimination deficit.
Although we can hypothesize about the nature of impairment in aMCIs patients because of its selectivity, we are not able to specify the cognitive domains influencing bad results in our AD and aMCImd patients because they were impaired in all subtests. Presumably, both perceptual and memory deficit had a significant impact. The more global defect in the aMCImd and AD groups could be explained by the disease spreading beyond the hippocampus (4, 32) with affection of other nonmemory domains. The early episodic memory deficit in AD (33) is followed by the early impairment of executive functions with later involvement in constructional praxis, language, and sustained attention (34). Our findings are consistent with other papers suggesting that multidomain MCI is similar to AD in many domains of cognition as well as in behavioral and psychological symptoms (35).
The similarity of spatial navigation impairment in the aMCImd and AD groups demonstrated by our results is prominent and consistent with the contemporary view of aMCImd as a prodromal stage of AD. aMCImd has a less favorable prognosis with a higher proportion of conversion to AD and may represent a more advanced prodromal stage of dementia than aMCIs (10). Bozoki et al. (36) showed that patients exhibiting impairment in other cognitive areas beyond memory loss have a higher risk of developing dementia than those with memory loss alone. Our results might suggest that the aMCIs represents an earlier stage of AD than aMCImd. At the same time, memory impairment is a presymptomatic stage of AD because the early nonmemory domain deficit precedes other non-AD dementias (37). Yaffe et al. (38) proved that the subtype of MCI influences the rates of progression toward dementia and death and has a major influence on future diagnosis of dementia type. Among patients who progressed to AD, 76% had prior amnestic MCI; of the patients who progressed to vascular dementia, 50% had prior amnestic MCI; and all patients who progressed to a frontal dementia syndrome had single nonamnestic MCI.
Intact spatial memory in the SMC group is consistent with other studies evaluating other kinds of declarative memory (39). This group was placed in our study because SMC individuals form a large proportion of clients in memory clinics and should be monitored because some of these patients may convert into a MCI group.
Our study shows that spatial navigation impairment is not limited to AD, but is, instead, detectable earlier in MCI and therefore can be expressed in a more complex or novel environment. According to our results, the disorientation in MCI detected in our subjects tested by an analogue of the MWM is due to impaired spatial memory. If spatial navigation begins to decline early in the disease process, presymptomatic measures of spatial navigation should predict the onset of clinical symptoms. The occurrence of spatial navigation impairment in amnestic MCI, and the similarity of deficits in multidomain MCI with those of early AD, suggests that these manifestations may assist in identifying patients in the earlier stages of AD distinguishing them from patients with MCI of other aetiologies. This fact makes it a potential biomarker of AD. Similar computer tests can serve as an inexpensive, but reliable, proof to the degree of impairment of critical brain structures in AD.
Methods
Subjects.
All subjects were recruited at Motol Hospital's Memory Disorders Clinic in Prague, Chech Republic, and signed standard informed consent. All patients underwent standard protocol and were examined by MRI, neurological, medical, and laboratory evaluation, a semistructured interview, and the following neuropsychological tests: Clinical Dementia Rating (CDR), Activities of Daily Living, Hachinski Ischemic Scale, Geriatric Depression Scale, Mini Mental State Examination, Clock Drawing Test, Auditory Verbal Learning Test, 16 words Grober and Buschke Test, Benton's Visual Retention Test, digit span forward and reversed, Category Fluency, Initial Letter Fluency, Trail Making Tests, A and B, and Rey complex figure.
Patients were classified into groups (Table 1) based on the results of the psychological tests mentioned above, subjectively reported memory problems, and information provided by the patients' informants:
Table 1.
Demographic characteristics of the groups
| Control | SMC | naMCI | aMCIs | aMCImd | AD | |
|---|---|---|---|---|---|---|
| Men/women | 8/18 | 5/3 | 5/2 | 4/7 | 13/5 | 5/16 |
| Age | 69.4 (1.3) | 65.6 (4.0) | 70.6 (3.0) | 71.7 (2.0) | 72.9 (2.4) | 75.8 (1.2) |
| Years of education | 15.5 (0.6) | 16.4 (0.6) | 14.3 (1.1) | 15.5 (0.7) | 13.9 (0.8) | 12.4 (0.7) |
Values are mean (SEM).
(i) Mild to moderate probable AD (n = 21). subjects were included when meeting the Diagnostic and Statistical Manual of Mental Disorders IV criteria for dementia and National Institute of Neurological and Communicative Disorders and Stroke and Alzheimer Disease and Related Disorders Association criteria for probable AD (40). Patients with dementia had an impairment of memory and other cognitive domain. They had their Activities of Daily Living impaired, and their CDR was ≥1.0.
(ii) Patients with MCI met the Petersen's criteria (41) by impairment in at least one cognitive domain (Table 2). They were further classified in the following groups: patients with naMCI (n = 7) or amnestic MCI, which included pure aMCIs (n = 11) and aMCImd (n = 18). All amnestic MCI patients had memory complaints and scored >1.5 of SD lower than the control group in memory tests, either verbal or nonverbal (verified by Auditory Verbal Learning Test, Grober and Buschke, or Benton's Visual Retention Test). Of the 29 broadly defined amnestic MCI cases, only 11 had pure amnesia (all of the other tests were within the normal range), whereas the rest, labeled as aMCImd, suffered from other subtle semantic and/or attention-executive function deficits (>1.5 SD). Patients with naMCI had impairment only in the nonmemory cognitive domains, manifesting as attentional-executive deficits, language, praxis, or visuospatial deficits. These domains were assessed by other cognitive tests (Trail Making Tests, digit span, Clock Drawing test, Initial Letter Fluency, Category fluency, or Rey figure). All MCI groups had a normal Activities of Daily Living and a CDR of maximum 0.5 (42).
Table 2.
Neuropsychological characteristics of the groups
| Control | SMC | naMCI | aMCIs | AMCImd | AD | |
|---|---|---|---|---|---|---|
| MMSE | 29.3 (0.9) | 29.8 (0.4) | 29.0 (1.0) | 28.6 (1.5) | 27.1 (2.3) | 23.1 (4.0) |
| AVLT1–6 | 60.4 (14.5) | 61.8 (8.9) | 53.4 (9.4) | 38.8 (10.9) | 32.3 (9.7) | 22.7 (5.4) |
| AVLT30 | 10.7 (4.1) | 12.0 (3.1) | 8.4 (2.8) | 3.9 (3.6) | 1.9 (2.4) | 0.4 (0.7) |
| TMT A | 18.4 (4.4) | 17.8 (7.0) | 21.1 (7.7) | 18.0 (6.0) | 33.8 (15.0) | 42.7 (26.8) |
| TMT B | 76.1 (23.2) | 85.8 (25.1) | 179.3 (42.8) | 100.2 (31.1) | 212.4 (106.1) | 369.0 (260.3) |
| FAS | 43.2 (10.4) | 51.4 (12.9) | 41.3 (9.1) | 42.0 (12.0) | 26.6 (6.3) | 26.1 (12.8) |
| BVLT A errors | 3.9 (2.8) | 4.0 (1.4) | 7.0 (3.2) | 6.9 (3.2) | 10.8 (4.2) | 15.1 (3.1) |
| BVLT C errors | 0.5 (1.2) | 0.0 (0.0) | 1.2 (1.5) | 0.7 (1.1) | 2.1 (2.7) | 2.8 (2.6) |
| Digit span | 6.4 (1.1) | 6.2 (1.3) | 5.6 (1.1) | 6.4 (1.5) | 6.4 (3.5) | 5.6 (1.3) |
| Reversed digit span | 4.7 (1.1) | 5.2 (1.3) | 4.0 (0.8) | 5.2 (0.9) | 4.1 (1.1) | 3.6 (1.4) |
| Buschke spont. | 10.7 (2.4) | 9.8 (1.6) | 9.0 (2.2) | 6.7 (3.0) | 4.9 (3.4) | 2.2 (1.5) |
| Buschke total | 16.0 (0.0) | 16.0 (0.0) | 16.0 (0.0) | 14.6 (2.3) | 13.4 (3.2) | 8.7 (3.4) |
Values are mean (SD). SD is used here to allow direct comparison of the groups based on the diagnostic criteria. An impairment of at least 1.5 SD from the control group defined the subtypes of MCI. ALVT1–6, average of AVLT 1 to 6 words presentation; AVLT30, word recall after 30 minutes; Buschke spont., spontaneous (non-cued) recall; Buschke total, total recall after cuing.
(iii) The subjects with SMC (n = 8) complained about everyday memory problems (any memory, not only spatial) but did not display any objective memory impairment, as defined by deviation from results in the control group. These subjects received an overall CDR of 0.5 and had the following characteristics: memory complaints, normal Activities of Daily Living, normal general cognitive function, and no dementia.
(iv) Subjects in the control group (n = 26) denied having any memory problems, which was confirmed by neuropsychological testing, and their CDR was 0.0. These individuals were recruited from relatives of staff and patients. These subjects were selected to have a similar age, education, and sex ratio as the other groups.
The CDR score, central to the categorization of the subjects, was derived from the semistructured interview administered to each subject and the subject's collateral source (42). All subjects completed the Geriatric Depression Scale and were excluded if they scored >5 points. The Hachinski scale was up to 4 points. Unlike MCI and SMC, all patients with AD were treated by cholinesterase inhibitors.
Hidden Goal Task.
The Hidden Goal Task is a human analogy of the MWM and has been described (15). It is designed to separate two different modes of navigation, allocentric and egocentric. The real space navigation setting called the Blue Velvet Arena has been described (15, 43). It consists of a fully enclosed cylindrical arena 2.9 m in diameter surrounded by a 2.8-m-high dark-blue velvet curtain. A TV camera above the center of the arena enables recording of the position of an infrared LED, which sits on top of a standing pole (1.6 m high). Eight large digital numerical displays, hung at 45° intervals 1.5 m above the floor, are used as orientation cues. They are invisible to the subject unless they are turned on with a pattern of two horizontal or three vertical bars. The decimal point sign on the numerical display is similarly controlled by the computer and was used as the starting location. The computer version of the tests was performed on a 17-inch LCD monitor.
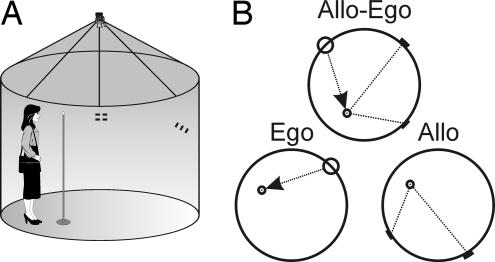
The subject was required to locate an invisible goal in four different subtests described below, each consisting of a computer version, followed by a real space version (see Fig. 4). In the computer version (labeled “computer” in the text), a large circle (280 pixels in diameter on a 640 × 480 pixel screen) represented the overhead view of the arena. The starting point was indicated by a circle on the arena contour, orientation cues were indicated by a red and a green line parallel to the arena contour, and the goal was indicated by a small red circle inside the arena. The subject was instructed to remember the location of the goal by using its relationship to the starting position as well as the cues. Then, the goal disappeared and the subject had to identify its position with a mouse pointer. There were eight trials in each subtest. The start-cues-goal configuration remained the same during the whole test and, during the eight trials, it assumed eight equally spaced rotations around the arena in a fixed order. The subject was reminded before each trial that he or she should locate the goal in a position relative to the start and orientation cues similar to the previous trials. There was no time limit to locate the goal. After the subject indicated the supposed goal position, the correct position was shown and the subject again was encouraged to notice its relative position to the starting position and cues.
Fig. 4.
The Hidden Goal Task. (A) In-scale diagram of the real space testing environment. (B) The scheme of the individual subtests. The task was to navigate to a goal (small circle) inside of a circular arena. The invisible goal could be identified either by its position relative to the start (larger circle) as in the ego subtest, relative to two landmarks (short lines on the border of the arena) as in the allo subtest, or relative to both start and landmarks as in the first subtest allo-ego.
In the real space version (labeled “real” in the text), the starting position was marked by a small red dot on the arena wall and the orientation cues were shown as two and three red lines, as described above. The subject was not shown the correct position of the goal before the first trial and therefore had to transfer this information from the preceding computer part. He or she was required to start from the starting point and mark the assumed goal location by the long pole. The correct location then was shown, marked by a small red circle on the arena floor. The real space test also consisted of eight trials with the start-cue-goal configuration in eight different rotations. As in the computer version, the subject was reminded of the constant position of the goal relative to the starting point and/or orientation cues before and after each trial as well.
The individual subtests assessed allocentric and/or egocentric mode of navigation. Each one consisted of the computer and real version. In the first “allo-ego” subtest (allocentric + egocentric), both the start-goal relationship and the relative positions of the two orientation cues could be used to locate the goal. In the second “ego” subtest (egocentric), only the starting position could be used to locate the goal. In the third “allo” subtest (allocentric), the two orientation cues at the arena periphery could be used for navigation. This time, however, each trial started from a different starting location relative to the goal. The aim of the fourth “delayed” subtest was to measure the effect of the time delay. It was similar to the allo subtest but consisted of only two trials administered 30 minutes after the end of the allo subtest. During this delay, other tests from our spatial navigation battery were administered. In the delayed subtest, the correct goal position was not shown so as to prevent the subjects from learning.
Data Analysis.
Original software created in MS-DOS Quick-Basic was used to track the LED-diode position during the test and to control the cues and starting point signs position in the arena. For analysis, the diameters of the real and computer circular arena were divided into 280 pixel units to enable direct comparison of errors made by the subjects.
Several measures of the subject's performance were used. The distance errors, in pixels, between the subject's choice and the correct goal location were used in most of the analysis (marked as “distance error”). The navigational strategies were analyzed by using two other measures. The first one (marked “correct side”) estimated whether the subject knew at least the approximate location of the goal. The arena was divided into two equal parts by a line going through the start position in the ego subtest or by a line going in the middle between the two cues in the allo and delayed subtest. The measure then was computed as the number of positions given by the subject that were lying in the same half of the arena as the goal. The second measure (marked “side error”) was used to estimate how much confusion of the side of the arena contributed to the error in estimating the goal position. The sides of the arena were determined as in the previous measure. The measure then was computed as the distance between the position given by the subject and the goal position, but regardless of the side. The first allo-ego subtest was excluded from this analysis, because the side of the arena that should be taken as reference was ambiguous.
ANCOVA was used to evaluate the group differences, controlling for the effect of covariates sex, years of education, and age. Simple contrasts with control and AD as reference groups were used to compare individual groups. The group differences in the correct side measure were evaluated by the Mann–Whitney U test. The significance level used throughout the analysis was 0.05. All statistical analysis was run using SPSS 13.0 for Windows (SPSS, Inc., Chicago, IL).
Acknowledgments
This work was supported by Grant Agency of the Czech Republic Grants 309/05/0693 and 309/06/1231 and Ministry of Education, Youth, and Sports Grant CR 1M0517 and research project AV0Z50110509.
Abbreviations
- ANCOVA
analysis of covariance
- AD
Alzheimer's disease
- MCI
mild cognitive impairment
- aMCIs
amnestic MCI single domain
- aMCImd
amnestic MCI multiple domain
- naMCI
nonamnestic MCI
- CDR
Clinical Dementia Rating
- MWM
Morris water maze
- SMC
subjective memory complaints.
Footnotes
The authors declare no conflict of interest.
References
- 1.Touchon J, Ritchie K. Int J Geriatr Psychiatry. 1999;14:556–563. doi: 10.1002/(sici)1099-1166(199907)14:7<556::aid-gps982>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 2.Squire LR, Zola-Morgan S. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- 3.Cummings JL, Mendez MF. Dementia: A Clinical Approach. Woburn, MA: Butterworth-Heinemann; 2003. [Google Scholar]
- 4.Braak H, Braak E. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 5.Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 6.Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, Berg L. Arch Neurol. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 7.Grundman M, Petersen RC, Ferris SH, Thomas RG, Aisen PS, Bennett DA, Foster NL, Jack CR, Galasko DR, Doody R, et al. Arch Neurol (Chicago) 2004;61:59–66. doi: 10.1001/archneur.61.1.59. [DOI] [PubMed] [Google Scholar]
- 8.Alladi S, Arnold R, Mitchell J, Nestor PJ, Hodges JR. Psychol Med. 2006;36:507–515. doi: 10.1017/S0033291705006744. [DOI] [PubMed] [Google Scholar]
- 9.Lopez OL, Becker JT, Jagust WJ, Fitzpatrick A, Carlson MC, Dekosky ST, Breitner J, Lyketsos CG, Jones B, Kawas C, et al. J Neurol Neurosurg Psychiatry. 2006;77:159–165. doi: 10.1136/jnnp.2004.045567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alexopoulos P, Grimmer T, Perneczky R, Domes G, Kurz A. Dement Geriatr Cogn Disord. 2006;22:27–34. doi: 10.1159/000093101. [DOI] [PubMed] [Google Scholar]
- 11.Pai MC, Jacobs WJ. Int J Geriatr Psychiatry. 2004;19:250–255. doi: 10.1002/gps.1081. [DOI] [PubMed] [Google Scholar]
- 12.Cherrier MM, Mendez M, Perryman K. Neuropsychiatry Neuropsychol Behav Neurol. 2001;14:159–168. [PubMed] [Google Scholar]
- 13.Monacelli AM, Cushman LA, Kavcic V, Duffy CJ. Neurology. 2003;61:1491–1497. doi: 10.1212/wnl.61.11.1491. [DOI] [PubMed] [Google Scholar]
- 14.Tetewsky SJ, Duffy CJ. Neurology. 1999;52:958–965. doi: 10.1212/wnl.52.5.958. [DOI] [PubMed] [Google Scholar]
- 15.Kalova E, Vlcek K, Jarolimova E, Bures J. Behav Brain Res. 2005;159:175–186. doi: 10.1016/j.bbr.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 16.Kessels RP, Feijen J, Postma A. Dement Geriatr Cogn Disord. 2005;20:184–191. doi: 10.1159/000087233. [DOI] [PubMed] [Google Scholar]
- 17.Mapstone M, Steffenella TM, Duffy CJ. Neurology. 2003;60:802–808. doi: 10.1212/01.wnl.0000049471.76799.de. [DOI] [PubMed] [Google Scholar]
- 18.O'Keefe J, Dostrovsky J. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- 19.Morris RG, Garrud P, Rawlins JN, O'Keefe J. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 20.Astur RS, Taylor LB, Mamelak AN, Philpott L, Sutherland RJ. Behav Brain Res. 2002;132:77–84. doi: 10.1016/s0166-4328(01)00399-0. [DOI] [PubMed] [Google Scholar]
- 21.Feigenbaum JD, Morris RG. Neuropsychology. 2004;18:462–472. doi: 10.1037/0894-4105.18.3.462. [DOI] [PubMed] [Google Scholar]
- 22.Maguire EA, Burgess N, Donnett JG, Frackowiak RS, Frith CD, O'Keefe J. Science. 1998;280:921–924. doi: 10.1126/science.280.5365.921. [DOI] [PubMed] [Google Scholar]
- 23.Abrahams S, Pickering A, Polkey CE, Morris RG. Neuropsychologia. 1997;35:11–24. doi: 10.1016/s0028-3932(96)00051-6. [DOI] [PubMed] [Google Scholar]
- 24.Aguirre GK, Zarahn E, D'Esposito M. Proc Natl Acad Sci USA. 1998;95:839–846. doi: 10.1073/pnas.95.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Brien HL, Tetewsky SJ, Avery LM, Cushman LA, Makous W, Duffy CJ. Cereb Cortex. 2001;11:1083–1092. doi: 10.1093/cercor/11.11.1083. [DOI] [PubMed] [Google Scholar]
- 26.Holdstock JS, Mayes AR, Cezayirli E, Isaac CL, Aggleton JP, Roberts N. Neuropsychologia. 2000;38:410–425. doi: 10.1016/s0028-3932(99)00099-8. [DOI] [PubMed] [Google Scholar]
- 27.Bohbot VD, Kalina M, Stepankova K, Spackova N, Petrides M, Nadel L. Neuropsychologia. 1998;36:1217–1238. doi: 10.1016/s0028-3932(97)00161-9. [DOI] [PubMed] [Google Scholar]
- 28.King JA, Burgess N, Hartley T, Vargha-Khadem F, O'Keefe J. Hippocampus. 2002;12:811–820. doi: 10.1002/hipo.10070. [DOI] [PubMed] [Google Scholar]
- 29.Maguire EA, Burke T, Phillips J, Staunton H. Neuropsychologia. 1996;34:993–1001. doi: 10.1016/0028-3932(96)00022-x. [DOI] [PubMed] [Google Scholar]
- 30.Morrone MC, Tosetti M, Montanaro D, Fiorentini A, Cioni G, Burr DC. Nat Neurosci. 2000;3:1322–1328. doi: 10.1038/81860. [DOI] [PubMed] [Google Scholar]
- 31.Pennanen C, Kivipelto M, Tuomainen S, Hartikainen P, Hanninen T, Laakso MP, Hallikainen M, Vanhanen M, Nissinen A, Helkala EL, et al. Neurobiol Aging. 2004;25:303–310. doi: 10.1016/S0197-4580(03)00084-8. [DOI] [PubMed] [Google Scholar]
- 32.Brun A, Gustafson L. Arch Psychiatr Nervenkr. 1976;223:15–33. doi: 10.1007/BF00367450. [DOI] [PubMed] [Google Scholar]
- 33.Nagy Z, Hindley NJ, Braak H, Braak E, Yilmazer-Hanke DM, Schultz C, Barnetson L, King EM, Jobst KA, Smith AD. Dement Geriatr Cogn Disord. 1999;10:115–120. doi: 10.1159/000017111. [DOI] [PubMed] [Google Scholar]
- 34.Baudic S, Barba GD, Thibaudet MC, Smagghe A, Remy P, Traykov L. Arch Clin Neuropsychol. 2006;21:15–21. doi: 10.1016/j.acn.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Cummings JL. CNS Spectr. 2005;10:22–25. doi: 10.1017/s1092852900014206. [DOI] [PubMed] [Google Scholar]
- 36.Bozoki A, Giordani B, Heidebrink JL, Berent S, Foster NL. Arch Neurol. 2001;58:411–416. doi: 10.1001/archneur.58.3.411. [DOI] [PubMed] [Google Scholar]
- 37.Petersen RC. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 38.Yaffe K, Petersen RC, Lindquist K, Kramer J, Miller B. Dement Geriatr Cogn Disord. 2006;22:312–319. doi: 10.1159/000095427. [DOI] [PubMed] [Google Scholar]
- 39.Jonker C, Geerlings MI, Schmand B. Int J Geriatr Psychiatry. 2000;15:983–991. doi: 10.1002/1099-1166(200011)15:11<983::aid-gps238>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 40.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 41.Petersen RC. Neurologia. 2000;15:93–101. [PubMed] [Google Scholar]
- 42.Morris JC. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 43.Stepankova K, Pastalkova E, Kalova E, Kalina M, Bures J. Behav Brain Res. 2003;147:95–105. doi: 10.1016/s0166-4328(03)00141-4. [DOI] [PubMed] [Google Scholar]