Abstract
Adenosine is a purine nucleoside that regulates cell function through G protein-coupled receptors that activate or inhibit adenylyl cyclase. Based on the understanding that cAMP regulates alveolar epithelial active Na+ transport, we hypothesized that adenosine and its receptors have the potential to regulate alveolar ion transport and airspace fluid content. Herein, we report that type 1 (A1R), 2a (A2aR), 2b (A2bR), and 3 (A3R) adenosine receptors are present in rat and mouse lungs and alveolar type 1 and 2 epithelial cells (AT1 and AT2). Rat AT2 cells generated and produced cAMP in response to adenosine, and micromolar concentrations of adenosine were measured in bronchoalveolar lavage fluid from mice. Ussing chamber studies of rat AT2 cells indicated that adenosine affects ion transport through engagement of A1R, A2aR, and/or A3R through a mechanism that increases CFTR and amiloride-sensitive channel function. Intratracheal instillation of low concentrations of adenosine (≤10−8M) or either A2aR- or A3R-specific agonists increased alveolar fluid clearance (AFC), whereas physiologic concentrations of adenosine (≥10−6M) reduced AFC in mice and rats via an A1R-dependent pathway. Instillation of a CFTR inhibitor (CFTRinh-172) attenuated adenosine-mediated down-regulation of AFC, suggesting that adenosine causes Cl− efflux by means of CFTR. These studies report a role for adenosine in regulation of alveolar ion transport and fluid clearance. These findings suggest that physiologic concentrations of adenosine allow the alveolar epithelium to counterbalance active Na+ absorption with Cl− efflux through engagement of the A1R and raise the possibility that adenosine receptor ligands can be used to treat pulmonary edema.
Keywords: active sodium transport, adenosine receptors, cystic fibrosis transmembrane conductance regulator
Pulmonary edema is due to increased fluid flux into the airspace and impairment of the active Na+ transport that clears it (1–4). A variety of approaches to improve alveolar epithelial cell active Na+ transport for purposes of accelerating alveolar fluid clearance (AFC) have been explored in experimental systems. Of particular interest are receptor–ligand interactions that increase cAMP production in alveolar epithelial cells. Adenosine is a purine nucleoside that signals through four distinct G protein-coupled receptors, type 1 (A1R), type 2a (A2aR), type 2b (A2bR), and type 3 (A3R). In most cell systems, the A1R and A3R receptors inhibit adenylyl cyclase and/or lead to signaling through inositol-3-phosphate and phospholipase C. Engagement of type 2 receptors activates adenylyl cyclase by means of Gsα and increases cAMP levels. The ability of adenosine receptors (ARs) to couple to adenylyl cyclase led us to hypothesize that ARs might participate in regulation of alveolar epithelial active Na+ transport. We approached this hypothesis in rats and mice by testing whether adenosine and its receptors are present in the distal airspace and whether they affect AFC in vivo and vectorial Na+ transport in rat alveolar epithelial type 2 cells (AT2).
Herein, we report the presence of functional A1R, A2aR, and A3R in alveolar epithelial cells and micromolar concentrations of adenosine in the distal lung. Measurements of ion transport by rat AT2 cells revealed that adenosine stimulates vectorial ion transport by increasing the function of apical Na+ and Cl− channels. In vivo measurements showed that physiologic doses of adenosine decrease AFC, probably by means of an A1R-dependent mechanism that causes Cl− efflux through CFTR, whereas lower doses increase AFC via the A2aR and/or A3R.
Results and Discussion
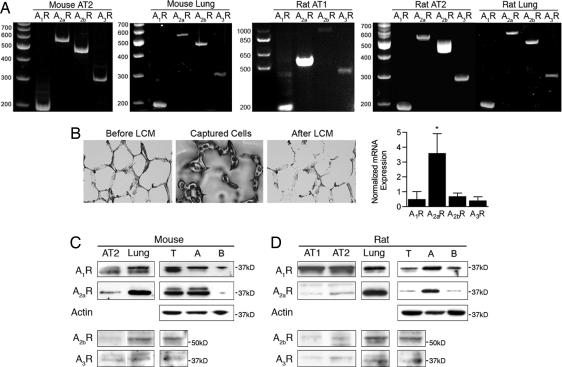
Messenger RNA for all four AR subtypes has been identified in whole lung tissue (5, 6); however, data regarding AR in alveolar epithelial cells is limited to reports of a type 2 receptor on cultured rat AT2 cells (7, 8). RT-PCR using total RNA from freshly harvested rat AT1 cells, mouse and rat AT2 cells, and mouse and rat lung tissue (Fig. 1A) yielded mRNA for all four receptor subtypes. Quantitative real-time RT-PCR using RNA from a mixed population of alveolar cells collected by laser capture microdissection (LCM) showed a 5- to 9-fold greater expression of A2aR than A1R, A2bR, or A3R (Fig. 1B). Western blot analysis using 10 μg of protein per lane revealed A1R and A2aR signals in whole-cell homogenates produced from freshly isolated rat AT1 and AT2 cells and peripheral rat and mouse lung tissue (Fig. 1 B and C). Faint bands at the expected migration positions for A2bR and A3R were detected by using 20 μg per lane of protein. Western blot analysis of cell membranes from peripheral lung tissue of rats and mice revealed the presence of all four receptors. Enrichment of whole cell membrane fractions for apical or basolateral membrane domains showed ≈3-fold greater relative expression of both A1R and A2aR in apical membranes as compared with basolateral membranes in rats and mice. A2bR and A3R were not detected in membrane subfractions from rats or mice.
Fig. 1.
Adenosine receptor expression in lung and alveolar epithelial cells. (A) RT-PCR for A1R, A2aR, A2bR, and A3R in total RNA harvested from (left to right) mouse AT2 cells, mouse distal lung, rat AT1 cells, rat AT2 cells, rat distal lung. (B) LCM. Photomicrographs show alveoli before and after microdissection. A photo of representative cells collected is shown (Center). Graph is A1R, A2aR, A2bR, and A3R mRNA expression (n = 6 rats) measured by using real-time, quantitative RT-PCR, and normalized to GAPDH mRNA. (C) Western blot analysis of AR expression (left to right) mouse AT2 cell homogenates, peripheral lung homogenates, whole-cell membranes from peripheral lung (T), peripheral lung membrane enriched for apical (A), and basolateral (B) membrane domains. Blots for A1R and A2aR are 10 μg of protein per lane, and A2bR and A3R are 20 μg of protein per lane. (D) Western blot analysis of AR expression (left to right) rat AT1 and AT2 cell homogenates, peripheral lung homogenates, whole cell membranes from peripheral lung (T), peripheral lung membrane enriched for apical (A) and basolateral (B) membrane domains. Blots for A1R and A2aR used 10 μg of protein per lane, and A2bR and A3R used 20 μg of protein per lane.
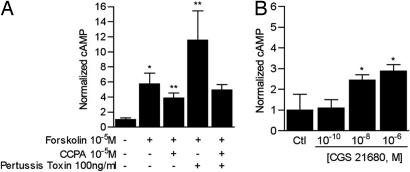
To test for A1R function in vitro, rat AT2 cells were pretreated with forskolin (10−5 M for 4 h) before addition of the A1R-specific agonist 2-chloro-N6-cyclopentyladenosine (CCPA; 10−5 M for 10 min; Fig. 2A). Forskolin was used to increase adenylyl cyclase (AC) activity above low basal levels in unstimulated epithelial cells; doing so facilitates detection of a signaling mechanism that decreases AC function. CCPA reduced cellular cAMP levels by 20% but not to baseline levels. Pretreatment with pertusis toxin before CCPA did not bring cellular cAMP levels to basal values either; thus, the A1R modulates cAMP levels via Gi-dependent and independent mechanisms. Treatment of rat AT2 cells with the A2aR-specific agonist CGS 21680 (Fig. 2B) increased cellular cAMP levels up to 3-fold. Concomitant treatment of rat AT2 cells with the nonspecific A2 agonist NECA and the A2bR-specific antagonist MRS 1706 (Tocris Bioscience, Ellisville, MO) (9) did not yield evidence of A2bR function in these cells (data not shown).
Fig. 2.
A1R and A2aR function in rat AT2 cells. (A) Change in whole-cell cAMP concentration in response to the A1R agonist CCPA (10−5 M for 10 min). Cells were pretreated with the adenylyl cyclase activator forskolin (2 μM for 10 min) with and without pertussis toxin (100 ng/ml for 4 h) before addition of CCPA for 10 min. ∗, P = 0.03 vs. cells treated with vehicle only (Ctl), ∗∗, P < 0.01 vs. cells treated with forskolin only. (B) Whole-cell cAMP concentration in rat AT2 cells treated with incremental concentrations of the A2aR agonist CGS 21680 (10 min). ∗, P < 0.02 vs. vehicle-treated controls (Ctl).
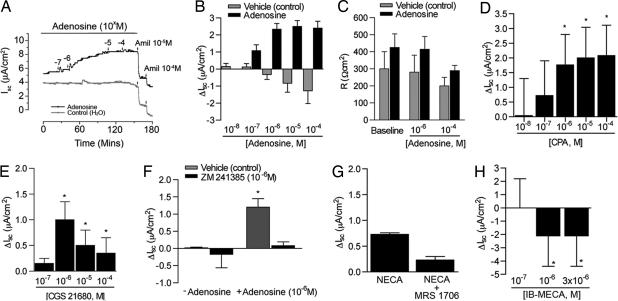
The effect of adenosine on vectorial ion transport in vitro was assessed by measuring short circuit currents (Isc) across high-resistance AT2 cell monolayers mounted in Ussing chambers containing symmetrical NaCl solutions in the apical and basolateral compartments. Adenosine concentrations ≥10−6 M added to the apical (airspace) compartment in an incremental fashion increased Isc up to 35% (7.55 ± 0.7 to 10.17 ± 0.85 μA/cm2, Fig. 3 A and B) without diminution of monolayer resistance (Fig. 3C). Addition of the Na+ channel inhibitor amiloride (10−4 M) to the apical compartment of vehicle-treated control cells reduced Isc by 91% (8.35 ± 1.57 to 0.6 ± 0.22 μA/cm2). Amiloride had less of an effect on Isc in cells treated with 10−4 M adenosine, reducing Isc by only 58% (7.55 ± 0.7 to 4.15 ± 0.83 μA/cm2, P = 0.02 vs. control).
Fig. 3.
Electrophysiologic studies in rat AT2 cell monolayers. (A) Typical tracing of short circuit current (Isc) before and after addition of adenosine and amiloride. (B) Change in short circuit current (ΔIsc) across high-resistance monolayers of rat AT2 cells after 4 d in culture in the presence or absence of adenosine. Change is calculated as adenosine-induced current − baseline current measured in the presence of vehicle (water). ∗, P < 0.009 vs. baseline current. (C) Monolayer resistance of adenosine- or vehicle (control)-treated AT2 cells. Baseline values for all Isc measurements were obtained after stabilization of Isc and before addition of adenosine or vehicle. (D) Change in short circuit current (ΔIsc) across monolayers of rat AT2 cells treated with the doses shown of the A1R agonist CPA. ∗, P < 0.01 vs. control (n = 3). (E) ΔIsc produced by AT2 cells treated with the doses shown of the A2aR agonist CGS 21680. ∗, P < 0.04 vs. baseline (n = 3). (F)(ΔIsc produced by rat AT2 cells concomitantly treated with (adenosine, 10−6 M) and the A2aR antagonist ZM 241385 (10−6 M). ∗, P < 0.01 vs. vehicle (water)-treated control (n = 3). (G) ΔIsc in rat AT2 cells treated with the nonspecific type 2 receptor agonist NECA (3 × 10−6 M) with and without the A2bR-specific antagonist MRS 1706 (10−5 M). n = 3 filters. (H) ΔIsc from baseline in rat AT2 cells treated with the A3R-specific agonist MECA at the doses shown. n = 3 filters. ∗, P < 0.02 vs. baseline Isc.
To define the receptor(s) responsible for adenosine's effect on ion transport in vitro, cells were treated with AR subtype-specific agonists and antagonists. Treatment of rat AT2 cells with the A1R agonist CPA increased Isc in a dose-dependent fashion (Fig. 3D). The A2aR-specific agonist CGS 21680 increased Isc by >1 μA/cm2 above baseline values (5–6 μA/cm2, Fig. 3E). Likewise, addition of the A2aR-specific antagonist ZM 241385 (Tocris Bioscience) (10) to adenosine (10−6 M) pretreated AT2 cells reduced Isc to baseline levels (Fig. 3F), supporting the presence of A2aR function in these cells. The nonspecific A2 agonist NECA (Fig. 3G) increased Isc by 0.7 μA/cm2; however, addition of the A2bR antagonist MRS 1706 (10−6 M; Tocris Bioscience) had no statistically significant effect on Isc. The A3R agonist IB-MECA produced a complete reversal of Isc (Fig. 3H) at all doses tested. Cumulatively, these data support the presence of functional A1R, A2aR, and A3R in rat AT2 cells in vitro.
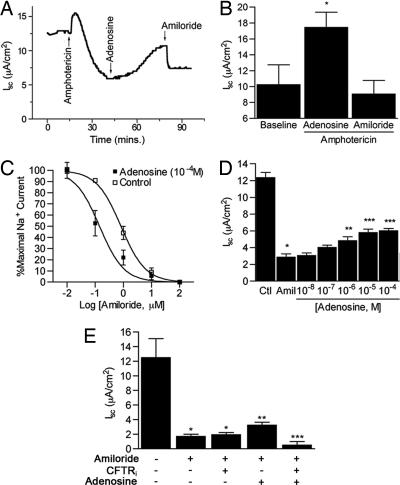
The observation of differential sensitivity to amiloride between control and adenosine-treated cells (91% vs. 58%) implies that adenosine decreases the sensitivity of Na+ channels to amiloride, increases Na+ flux via amiloride-insensitive Na+ pathways, or stimulates anion (e.g., Cl−) efflux. To differentiate among these possibilities, we performed three sets of experiments. First, Isc was measured across monolayers in which the basolateral membrane was permeabilized with amphotericin B (10−5 M). Application of the Na, K-ATPase inhibitor ouabain (1 mM) to the basal compartment of amphotericin B-treated cells had no effect on Isc, confirming effective permeabilization of the basal membrane. In the presence of a 145:25 mM apical/basal Na+ gradient, apical adenosine (10−6 M) increased Isc. This effect was completely blocked by amiloride (10−5 M; Fig. 4 A and B), indicating that adenosine affects primarily amiloride-sensitive apical Na+ entry pathways and that there is little paracellular Na+ flux in our system. In a second set of experiments, Isc was measured in intact cells treated with adenosine (10−4 M) and incremental concentrations of amiloride (10−8 to 10−4 M) in the apical compartment. Adenosine treatment shifted the amiloride dose–response curve to the left (Fig. 4C). The corresponding IC50s (i.e., the concentration of amiloride inhibiting Isc by 50%) for adenosine-treated and control cells were 0.143 ± 0.11 and 0.825 ± 0.02 μM, respectively, indicative of increased sensitivity to amiloride. This change implies that there may be more heterotrimeric Na+ channels (as opposed to monomeric or dimeric channels) in the cell membrane, as suggested by Canessa and colleagues (11) in their initial description of ENaC. Finally, AT2 cells were treated with amiloride (10−5 M) before adenosine (Fig. 4D). High-dose adenosine (10−5 to 10−4 M) partially reversed the amiloride-mediated inhibition of Isc. To test whether this reversal is linked to Cl− transport, cells were treated with amiloride (10−5 M) and then adenosine (10−5 M) before the application of the CFTR inhibitor CFTRinh-172 (Fig. 4E). This inhibitor had no effect on Isc in amiloride-treated controls in the absence of adenosine; conversely, CFTR blockade eliminated the adenosine-driven increase in Isc, implying that adenosine up-regulates Cl− movement by means of CFTR. Similar results were obtained after addition of glibenclamide (0.3 mM). These data indicate that adenosine-mediated increases in Isc occur by up-regulation of both cation and anion channels in the apical membrane of rat AT2 cells. The demonstration of AR in AT1 cell homogenates (Fig. 1C) and the recent appreciation that AT1 cells transport both Cl− and Na+ (12) suggest that adenosine has the potential of regulating ion transport in both AT1 and AT2 cells.
Fig. 4.
Effect of adenosine on Na+ and Cl− transporter. (A) Tracing from a representative study of cells with basal membranes permeabilized with amphotericin B (10 μM) before application of adenosine (10−6 M). (B) Net effect of amiloride (10 μM) on Isc in permeabilized, adenosine (10−6 M)- treated cells. Data are peak Isc of basally permeabilized AT2 cells in the presence of an apical-to-basal Na+ gradient (145:25 mM). n = 3 (adenosine) or 4 (control and amiloride) filters per condition. ∗, P = 0.04 vs. baseline Isc. (C) Isc across monolayers of AT2 cells treated with a fixed dose of adenosine (10−4 M) before and during application of incremental concentrations of amiloride. Data are normalized to maximal Isc for each condition. n = 4 (control) and 5 (adenosine). (D) Iscs in AT2 cells treated with amiloride (10 μM) before application of the indicated concentrations of adenosine. n = 5 filters. Base, baseline current measured after stabilization and before addition of amiloride. ∗, P = 0.001 vs. baseline, ∗∗, P = 0.0.04 vs. amiloride-treated cells; ∗∗∗, P < 0.005 vs. amiloride-treated cells. (E) Effect of CFTR inhibition on Isc in rat AT2 cells. Baseline Isc was measured before addition of amiloride (10−5 M), followed by adenosine (10−5 M) and then CFTRinh-172 (CFTRi, 50 μM) or just CFTRinh-172. Iscs shown were measured after stabilization of Isc. n = 5 filters. ∗, P = 0.05 vs. baseline; ∗∗, P < 0.05 vs. amiloride and CFTRinh-172; ∗∗∗, P < 0.05 vs. all other groups.
To test for the presence of adenosine in the distal airspace, bronchoalveolar lavage (BAL) was performed in spontaneously breathing C57Bl6 mice. Adenosine concentrations in BAL fluid (BALF) were 0.68 ± 0.44 μM. The ratio of serum to BALF urea in untreated mice was 73.4; multiplying measured BALF levels by this dilution factor implies that alveolar concentrations of adenosine are in the range of 60–70 μM, which is consistent with concentrations measured in human BALF (13). To test whether alveolar epithelial cells might be a source of adenosine, AT2 cells were maintained in defined medium (Opti-MEM; Invitrogen, Carlsbad, CA) with the adenosine deaminase inhibitor EHNA for 30 min. No adenosine was detected in medium collected under these conditions. Adenosine is a metabolite of AMP that accrues in the extracellular space when cAMP production and/or ATP utilization are high. Thus, rat AT2 cells were treated with the β2-adrenergic receptor agonist procaterol (10−6 M for 30 min) to increase cAMP production and ATP utilization (e.g., increase Na, K-ATPase activity) to provide substrate for adenosine production. Procaterol treatment resulted in significant accumulation of adenosine (6 μM). Thus, AT2 cells are a potential source of airspace adenosine.
Prior studies have reported that adenosine, its analogs, or an A1R antagonist attenuate lung water accumulation and/or histologic injury in experimental models of lung injury (14–17). None of these studies considered whether adenosine affected alveolar active Na+ transport. To address this possibility, Na+-dependent AFC was measured by using an isolated, fluid-filled, perfused rat lung preparation with and without adenosine in the alveolar airspace. Concentrations of adenosine between 10−14 and 10−8 M increased AFC up to 45%; conversely, doses ≥10−6 M reduced AFC up to 35% [see supporting information (SI) Fig. 6A]. No flux of FITC-albumin from the vascular compartment to the airspace was noted, implying that adenosine does not markedly alter alveolar permeability. To pharmacologically define the AR responsible for these effects, A1R- or A2aR-specific agonists and antagonists were added to the alveolar instillate. The highest tested dose (10−4 M) of the A1R-specific agonist CPA abolished net AFC, whereas 10−8 M reduced it by 35% (SI Fig. 6B). The A1R antagonist DPCPX produced the opposite effect and increased AFC by 80% (SI Fig. 6D). The A2aR agonist CGS 21680 (Sigma, St. Louis, MO) increased AFC 135% (SI Fig. 6C), whereas the A2aR antagonist CSC [8-(3-chlorostyryl)] caffeine (Tocris) caused a small but significant reduction in AFC (SI Fig. 6E). These findings suggest that, in rats, the inhibitory effects of physiologic concentrations (micromolar) of adenosine are mediated by the A1R (which inhibits adenylyl cyclase) and that the A2aR (which increases cAMP production) accelerates AFC. Similar differential, dose-dependent effects have been observed in other experimental systems (pig trachealis muscle) (18). The mechanism(s) responsible for these opposing, dose-dependent effects are not yet known. The finding that blockade of the A2aR unmasks A1R function, and vice versa, without supplemental adenosine, suggests that A1R and A2aR functionally coexist in rat alveolar epithelium and that they tonically regulate alveolar ion transport in rats.
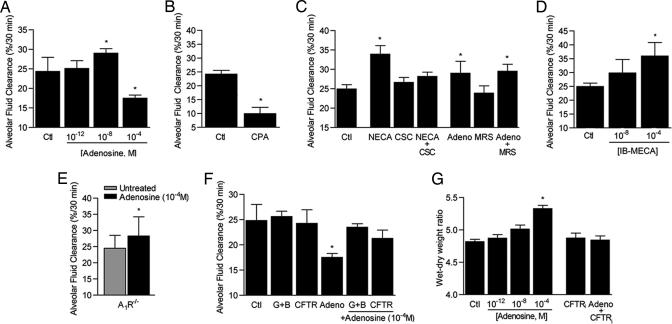
To determine whether adenosine affects alveolar active Na+ transport in mice, we measured AFC using an established, mechanically ventilated, live mouse model (19). High-dose adenosine (10−4 M) reduced AFC, whereas low-dose (10−8 M) increased it up to 20% (Fig. 5A). Inclusion of the A1R agonist CPA (10−6 M) in the airspace reduced AFC ≈40%. The A2aR-specific agonist CGS 21680 increased clearance by 12% to a level that was not significantly different from untreated controls; however the nonspecific A2 agonist NECA (10−4 M) increased AFC by 33%. The stimulatory effect of NECA was completely blocked by concomitant instillation of the A2aR antagonist CSC-caffeine (10−6 M) but not by the A2bR antagonist MRS 1706 (10−6 M). Neither CSC-caffeine nor MRS 1706 affected AFC in the absence of adenosine or NECA. Together these findings reveal a role for the A2aR but not the A2bR in regulation of alveolar ion transport in the intact lung. CSC-caffeine is an 8-styrylxanthine that is ≈500-fold more specific for the A2aR than A1R; its affinity for A2bR is not known (20). To test for A3R function, mice were treated with IB-MECA, which increased AFC up to 35%. A priori A3R would be expected to reduce AFC by means of its inhibitory effects on cAMP production. However, the A3R also signals through inositol-3-phosphate and phospholipase C to raise intracellular calcium, a pathway that increases AFC in mice and could explain the increased AFC noted in IB-MECA-treated mice (27).
Fig. 5.
AFC in C57bl6 mice. (A) Adenosine effects on AFC. n = 8 mice per group. ∗, P = 0.03 vs. vehicle-treated controls (Ctl). (B) AFC in mice treated with the A1R agonist CPA (10−6 M, n = 5). ∗, P = 0.001 vs. vehicle-treated controls (Ctl). (C) AFC in the presence of the nonspecific A2 agonist NECA (10−4 M) with and without the A2aR antagonist CSC-caffeine (10−6 M). A2bR function was assessed by using a dose of adenosine that increases AFC (10−8 M) with and without the A2bR antagonist MRS 1706 (10−6 M). n = 8 mice per group. ∗, P < 0.02 vs. vehicle-treated control (Ctl). (D) A3R function was assessed by using the A3R agonist IB-MECA at the doses shown. n = 8 mice per group. ∗, p = 0.001 vs. vehicle-treated controls (Ctl). (E) Alveolar fluid clearance in mice with targeted deletions of the A1R (A1R−/−) in the presence and absence of a dose of adenosine that decreases AFC (10−4 M). n = 8 mice per group. P = 0.04 vs. untreated A1R−/− mice. (F) Effect of Cl− transport inhibitors glibenclamide (10−6 M) with bumetinide (10−6 M) (G+B) or CFTRinh-172 (CFTRi,10−6 M) on adenosine (10−4 M) induced reduction of AFC. n = 6 mice. ∗, P = 0.03 vs. controls treated with vehicle only (Ctl). (G). Wet–dry weight ratios of mice treated with the doses of adenosine shown (in 50 μl of 0.9% NaCl, intratracheal) or an equal volume of vehicle (0.9% NaCl) for 30 min (Ctl). The role of CFTR in this model was assessed by instillation of the CFTR inhibitor CFTRinh-172 (CFTRi, 10−6 M) with and without adenosine (10−4 M). n = 4 mice. ∗, P = 0.002 vs. vehicle-treated control (Ctl).
To complement these pharmacologic studies, the effects of adenosine on AFC were measured in mice with targeted deletions of the A1R (A1R−/−). Baseline clearance in A1R−/− mice was not different from wild-type controls (Fig. 5E). Treatment of A1R−/− mice with high-dose adenosine (10−4 M) produced a significant increase in AFC (Fig. 5E). Increased clearance in these mice is likely due to loss of the inhibitory influence of the A1R, lending additional support for the conclusion that high-dose adenosine reduces AFC by means of theA1R.
To probe how adenosine effects alveolar Cl− transport in vivo, AFC was measured after coinstillation of adenosine (10−4 M) and either the Cl− transport inhibitor CFTRinh-172 (10−6 M; provided by M. Matthay and A. Verkman, University of California, San Francisco, CA) or the combination of glibenclamide (10−6 M) and bumetinide (10−6 M) into the alveolar airspace (Fig. 5F). In both sets of experiments, blockade of Cl− transport prevented the inhibitory effects of adenosine on AFC. These findings raise the possibility that adenosine causes Cl− efflux into the alveolar airspace by means of CFTR. We further tested this hypothesis by measuring lung wet-to-dry weight ratios in mice after intratracheal instillation of adenosine (10−12 to 10−4 M for 30 min). High-dose adenosine (10−4 M) produced significant increases in this index of total lung water. Inclusion of the CFTR inhibitor CFTRinh-172 (10−6 M) with adenosine blocked changes in total lung water. Lower doses of adenosine had no effect on lung water content. These findings provide supportive evidence that high-dose adenosine causes ion efflux through CFTR (Fig. 5G).
In summary, our data reveal the expression of all four ARs in the distal lung of rodents and in AT1 and AT2 cells. We also noted that rat AT2 cells produce adenosine when stimulated and that the distal airspace contains micromolar concentrations of adenosine. The presence of these receptors and their ligand in the alveolus offers the possibility of paracrine/autocrine regulation of AFC by adenosine. We also noted that A2aR and A3R activation increases AFC, creating the possibility that agonists for these receptors or A1R antagonists might be useful for treatment of pulmonary edema. Our data do not support a role for the A2bR in regulation of alveolar fluid balance; however, these studies must be considered in the context of the absence of a highly specific A2bR agonist.
A key finding of this study is that physiologic concentrations (micromolar) of adenosine reduce the lung's ability to clear alveolar fluid in rats and mice via an A1R-mediated pathway. Our isolated rat lung studies (SI Fig. 6), the work of others (14), and our in vitro studies demonstrate no adenosine-associated reduction of barrier function or active Na+ transport (Fig. 3 A and B). Rather, our data suggest that physiologic doses of adenosine reduce net AFC by causing Cl− efflux through CFTR. Maintenance of electrical neutrality requires parallel cation (Na+) movement, the result of which would be reduced net Na+ absorption and diminished AFC. Thus, the milieu of the uninjured airspace includes a nucleotide that modulates apical Na+ and Cl− transporter function, allowing the epithelium to regulate alveolar fluid content through Cl− efflux.
Our initial hypothesis that adenosine could modulate alveolar active ion transport was based on the simple paradigm that AR effects on adenylyl cyclase function alter cellular cAMP levels with parallel changes in vectorial ion transport. Our data regarding A1R and A2aR support this paradigm but also reveal that the A3R, a receptor that reduces cAMP production, increases AFC. This unexpected finding supports the growing understanding that adenosine signaling is complex, redundant and cell type-specific and that alveolar ion transport is regulated by cAMP-dependent and -independent signal transduction pathways.
Comparison of the effects of adenosine on AFC (in vivo) and Isc (in vitro) reveal diametric effects of A1R and A3R ligands. The in vitro systems used in these studies measure ion transport in dedifferentiating AT2 cells, thereby excluding contributions from AT1 cells. Additionally, Ussing chambers evaluate ion transport with nearly symmetric, protein-free solutions in the apical and basal chambers, eliminating ion (e.g., Cl−) gradients that may be present in vivo. These differences could explain the disparity between our lung models and Ussing chamber data.
Prior in vivo studies of alveolar active ion transport support a model where adrenergic agonists accelerate AFC in fluid-filled lungs. Our data add to this paradigm by suggesting that the alveolar epithelium can transition between an adenosine-regulated surface capable of Cl− secretion and an absorptive surface that can be activated by adrenergic agonists to actively clear airspace fluid when in excess. To our knowledge, this is a previously unreported role for adenosine in regulation of alveolar fluid content. Our findings raise the possibility of AR ligands to accelerate AFC in patients with pulmonary edema.
Materials and Methods
A complete description of the methods and materials used in these studies is provided in http://www.pnas.org/cgi/content/full/0601117104/DC1SI Materials and Methods. Unless otherwise stated, adenosine receptor agonists and antagonists and other reagents were obtained from Sigma–Aldrich.
AT1 and AT2 Cell Isolation.
The use of animals for this study was approved by the institutions of the participating investigators. Type 1 and 2 cells were isolated from rats (22, 23) and mice as described (24); purity was assessed by morphologic analysis and immunostaining for AT1 (RTI40) and AT2 (RTI70) cell markers. Electrophysiologic studies of rat AT2 cells were performed in Ussing chambers as described (21, 25).
Alveolar Fluid Clearance Measurement.
The method of measurement used for mice was modified from Hardiman et al. (19). The rat isolated lung preparation used has been extensively described (22). In both models, change in concentration of a slowly absorbed marker (Evan's blue-tagged albumin) instilled into the airspace is used to calculate change in airspace fluid volume over a 30-min (mouse) or 60-min (rat) period.
Adenosine Receptor Function in rat AT2 Cells.
Adenosine type 1 receptor function was measured as percent reduction of whole-cell cAMP concentration in cells treated with forskolin (2 μM for 10 min) before the addition of the A1R agonist 2-chloror-N6-CPA (10−5 M for 10 min). A2aR function was quantified by treating cells with the A2aR agonist CGS 21680 (10−6 M) for 10 min before measurement of whole-cell cAMP concentration. cAMP concentrations were determined by using a commercial enzyme immunoassay (Cayman Chemical, Ann Arbor, MI) (A2aR) (26) or a column chromatography approach as described (A1R) (27, 28).
HPLC Measurement of Adenosine Levels.
The lungs of anesthetized mice were lavaged three times with PBS containing the adenosine deaminase inhibitor erythro-9-(2-hydroxy-3-nonyl)adenine hydrochloride (EHNA; 2.5 μM) and the nucleotide transport inhibitor dipyridamole (250 μM) (stopping solutions). Lavage fluid was cleared of cells and analyzed by using HPLC for adenosine concentrations.
RT-PCR from QT2 Cells and Lung Tissue.
Total RNA from cells and tissues was isolated with RNAzol B. DNase-treated RNA was used for first-strand cDNA synthesis by using oligo-dT12–16. The sequences of primer used are provided in SI Materials and Methods.
Western Blot Analysis.
Lung and alveolar epithelial cell membrane proteins were produced from peripheral lung tissue as described in ref. 29 and SI Materials and Methods. Protein was separated with SDS/PAGE, transferred to nitrocellulose, and probed with rabbit polyclonal antibodies against rodent A1R (Affinity BioReagents, Golden, CO), A2aR (Santa Cruz Biotechnology, Santa Cruz, CA), and A2bR and A3R (Chemicon International, Temecula, CA).
LCM and Real-Time Quantitative RT-PCR (QRT-PCR).
Frozen sections (15 μM) were prepared from rat lung tissue embedded in optimal cutting temperature (OCT) compound. Cells (≈2,000 per specimen) were isolated immediately after staining with hematoxylin by LCM using the PixCell system and CapSure LCM caps (Arcturus; Molecular Devices, Sunnyvale, CA). RNA was extracted with the PicoPure RNA isolation kit (Arcturus). Transcript levels were determined by using QRT-PCR normalized to GAPDH (TaqMan; Applied Biosystems, Foster City, CA). Copy numbers were calculated for each PCR target from the same cDNA and normalized to copy numbers of GAPDH.
Statistical Analysis.
All values are reported as means ± SD. Statistical significance was defined by using Student's t test and one-way ANOVA with P < 0.05 defined as statistically significant.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health (NIH) Grants HL-66211, HL-79094, and HL-71042 (to P.F.), the American Heart Association, the American Lung Association, the American Lung Association of Metropolitan Chicago, and NIH Grants ES015024 (to G.M.M.) and HL-31197, HL-51173, and HL-72871 (to S.M.).
Abbreviations
- AFC
alveolar fluid clearance
- AR
adenosine receptor
- AT1
alveolar epithelial type 1 cells
- AT2
alveolar epithelial type 2 cells
- BAL
bronchoalveolar lavage
- BALF
BAL fluid
- CCPA
2-chloro-N6-cyclopentyladenosine
- CPA
cyclopentyladenosine
- Isc
short circuit current
- ΔIsc
change in Isc
- LCM
laser capture microdissection.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0601117104/DC1.
References
- 1.Ware LB, Matthay MA. Am J Respir Crit Care Med. 2001;163:1376–1383. doi: 10.1164/ajrccm.163.6.2004035. [DOI] [PubMed] [Google Scholar]
- 2.Olivera W, Ridge K, Sznajder J. Am J Respir Crit Care Med. 1995;152:1229–1234. doi: 10.1164/ajrccm.152.4.7551375. [DOI] [PubMed] [Google Scholar]
- 3.Saldias FJ, Azzam ZS, Ridge KM, Yeldandi A, Rutschman DH, Schraufnagel D, Sznajder JI. Am J Physiol Lung Cell Mol Physiol. 2001;281:L591–L597. doi: 10.1152/ajplung.2001.281.3.L591. [DOI] [PubMed] [Google Scholar]
- 4.Modelska K, Matthay M, Brown L, Deutch E, Lu L, Pittet J. Am J Physiol Lung Cell Mol Physiol. 1999;276:L844–L857. doi: 10.1152/ajplung.1999.276.5.L844. [DOI] [PubMed] [Google Scholar]
- 5.Chunn JL, Young HW, Banerjee SK, Colasurdo GN, Blackburn MR. J Immunol. 2001;167:4676–4685. doi: 10.4049/jimmunol.167.8.4676. [DOI] [PubMed] [Google Scholar]
- 6.Fan M, Qin W, Mustafa SJ. Am J Physiol Lung Cell Mol Physiol. 2003;284:L1012–L1019. doi: 10.1152/ajplung.00353.2002. [DOI] [PubMed] [Google Scholar]
- 7.Griese M, Gobran LI, Douglas JS, Rooney SA. Am J Physiol. 1991;260:L52–L60. doi: 10.1152/ajplung.1991.260.2.L52. [DOI] [PubMed] [Google Scholar]
- 8.Griese M, Gobran LI, Rooney SA. Am J Physiol. 1991;261:L140–L147. doi: 10.1152/ajplung.1991.261.2.L140. [DOI] [PubMed] [Google Scholar]
- 9.Kim YC, Ji X, Melman N, Linden J, Jacobson KA. J Med Chem. 2000;43:1165–1172. doi: 10.1021/jm990421v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poucher SM, Keddie JR, Brooks R, Shaw GR, McKillop D. J Pharm Pharmacol. 1996;48:601–606. doi: 10.1111/j.2042-7158.1996.tb05981.x. [DOI] [PubMed] [Google Scholar]
- 11.Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC. Nature. 1994;367:463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- 12.Johnson MD, Bao HF, Helms MN, Chen XJ, Tigue Z, Jain L, Dobbs LG, Eaton DC. Proc Natl Acad Sci USA. 2006;103:4964–4969. doi: 10.1073/pnas.0600855103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Driver AG, Kukoly CA, Ali S, Mustafa SJ. Am Rev Respir Dis. 1993;148:91–97. doi: 10.1164/ajrccm/148.1.91. [DOI] [PubMed] [Google Scholar]
- 14.Jolin A, Myklebust R, Olsen R, Bjertnaes LJ. Acta Anaesthesiol Scand. 1994;38:75–81. doi: 10.1111/j.1399-6576.1994.tb03841.x. [DOI] [PubMed] [Google Scholar]
- 15.Neely CF, Jin J, Keith IM. Am J Physiol. 1997;272:L353–L361. doi: 10.1152/ajplung.1997.272.2.L353. [DOI] [PubMed] [Google Scholar]
- 16.Kutzsche S, Lyberg T, Bjertnaes LJ. Crit Care Med. 2001;29:2371–3276. doi: 10.1097/00003246-200112000-00021. [DOI] [PubMed] [Google Scholar]
- 17.Sakamaki F, Ishizaka A, Urano T, Sayama K, Nakamura H, Terashima T, Waki Y, Soejima K, Tasaka S, Sawafuji M, et al. J Lab Clin Med. 2003;142:128–135. doi: 10.1016/S0022-2143(03)00105-7. [DOI] [PubMed] [Google Scholar]
- 18.Ghai G, Zimmerman MB, Hopkins MF. Life Sci. 1987;41:1215–1224. doi: 10.1016/0024-3205(87)90199-8. [DOI] [PubMed] [Google Scholar]
- 19.Mutlu GM, Dumasius V, Burhop J, McShane PJ, Meng FJ, Welch L, Dumasius A, Mohebahmadi N, Thakuria G, Hardiman K, et al. Circ Res. 2004;94:1091–1100. doi: 10.1161/01.RES.0000125623.56442.20. [DOI] [PubMed] [Google Scholar]
- 20.Jacobson KA, Nikodijevic O, Padgett WL, Gallo-Rodriguez C, Maillard M, Daly JW. FEBS Lett. 1993;323:141–144. doi: 10.1016/0014-5793(93)81466-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swystun V, Chen L, Factor P, Siroky B, Bell PD, Matalon S. Am J Physiol Lung Cell Mol Physiol. 2005;288:L820–L830. doi: 10.1152/ajplung.00396.2004. [DOI] [PubMed] [Google Scholar]
- 22.Factor P, Saldias F, Ridge K, Dumasius V, Zabner J, Jaffe HA, Blanco G, Barnard M, Mercer R, Perrin R, Sznajder JI. J Clin Invest. 1998;102:1142–1150. doi: 10.1172/JCI3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson MD, Widdicombe JH, Allen L, Barbry P, Dobbs LG. Proc Natl Acad Sci USA. 2002;99:1966–1971. doi: 10.1073/pnas.042689399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardiman KM, McNicholas-Bevensee CM, Fortenberry J, Myles CT, Malik B, Eaton DC, Matalon S. Am J Respir Cell Mol Biol. 2003;30:720–728. doi: 10.1165/rcmb.2003-0325OC. [DOI] [PubMed] [Google Scholar]
- 25.Guo Y, DuVall MD, Crow JP, Matalon S. Am J Physiol. 1998;274:L369–L377. doi: 10.1152/ajplung.1998.274.3.L369. [DOI] [PubMed] [Google Scholar]
- 26.Emala C, Black C, Curry C, Levine MA, Hirshman CA. Am J Respir Cell Mol Biol. 1993;8:668–675. doi: 10.1165/ajrcmb/8.6.668. [DOI] [PubMed] [Google Scholar]
- 27.Hotta K, Emala CW, Hirshman CA. Am J Physiol. 1999;276:L405–L411. doi: 10.1152/ajplung.1999.276.3.L405. [DOI] [PubMed] [Google Scholar]
- 28.Saloman Y, Londos C, Rodbell M. Anal Biochem. 1974;58:541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- 29.Dumasius B, Sznajder JI, Azzam ZS, Boja J, Mutlu GM, Maron MB, Factor P. Circ Res. 2001;89:907–914. doi: 10.1161/hh2201.100204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.