Abstract
Silencing gene expression by RNAi is a powerful method for exploring gene function and validating drug targets and potentially for therapy. Lymphocytes and other primary blood cells are resistant to lipid-based transfection in vitro and are difficult to target in vivo. We show here that antibody-protamine fusion proteins targeting the human integrin lymphocyte function-associated antigen-1 (LFA-1) efficiently deliver siRNAs and specifically induce silencing in primary lymphocytes, monocytes, and dendritic cells. Moreover, a fusion protein constructed from an antibody that preferentially recognizes activation-dependent conformational changes in LFA-1 selectively targets activated leukocytes and can be used to suppress gene expression and cell proliferation only in activated lymphocytes. The siRNA-fusion protein complexes do not cause lymphocyte activation or induce IFN responses. K562 cells expressing latent WT or constitutively activated LFA-1 engrafted in the lungs of SCID mice are selectively targeted by intravenously injected fusion protein–siRNA complexes, demonstrating the potential in vivo applicability of LFA-1-directed siRNA delivery.
Keywords: affinity up-regulation, antiinflammation, cell adhesion molecule, drug delivery, AL-57 and TS1/22
RNAi can be activated by introducing synthetic short dsRNA fragments, termed siRNAs, into cells to silence genes bearing complementary sequences. RNAi is a powerful tool to evaluate the role of specific genes in cellular and disease processes with potential applications for therapy (1, 2). Using siRNAs to manipulate gene expression in lymphocytes would be of great interest not only for understanding lymphocyte biology but also for developing novel therapeutic approaches to dampen inflammation and harmful immune responses that occur during autoimmunity, transplant rejection, and graft vs. host disease, to suppress lymphotropic viral infections such as HIV, or to treat leukemia or lymphoma (1, 2). For some therapeutic applications, targeted siRNA delivery only to activated leukocytes would be an attractive approach to minimize potential immunosuppressive effects on bystander lymphocytes. However, primary lymphocytes are highly resistant to transfection using conventional transfection reagents (e.g., cationic lipids and polymers) (3–5). Although they can be transduced by electroporation in vitro (6), this method of introducing nucleic acids into cells cannot be used to deliver siRNAs into lymphocytes dispersed throughout the body in vivo and is complicated by significant cell death in vitro. Antibody fragment-protamine fusion proteins can deliver siRNAs by cell surface receptors to specific cell types and tissues efficiently and induce gene silencing in s.c. tumors when fusion protein–siRNA complexes are injected intravenously (7). As a proof of concept, a fusion protein, designed by using an Fab fragment of an anti-HIV envelope (env) antibody covalently linked to protamine, mixed with siRNAs was shown to silence gene expression in HIV-infected lymphocytes that express HIV env, but not in uninfected cells (7).
Lymphocytes dramatically alter their gene expression upon activation, acquiring helper or effector function critical for host defense (8, 9). Moreover, some key cell surface molecules already present on naïve and/or resting cells change their conformation following recognition of antigen or other signals of immune stimulation. In particular, the integrin lymphocyte function-associated antigen-1 (LFA-1) expressed on all leukocytes, which mediates heterotypic adhesion to intercellular adhesion molecules on other immune cells, undergoes dramatic conformational changes in response to stimulation (10, 11). LFA-1 is usually in the low-affinity nonadhesive form on naïve cells and is rapidly converted to the high-affinity (HA) adhesive form upon leukocyte activation (12). The LFA-1 ligand-binding domain, the inserted (I) domain, changes from a closed to an open form with a progressive 10,000-fold increase in affinity in the activated state (10). To study affinity up-regulation of LFA-1, we recently developed an engineered antibody, AL-57, that preferentially binds to the active conformation of LFA-1 (13, 14). Unlike most LFA-1 antibodies that bind equally to the HA and low-affinity forms (e.g., TS1/22), AL-57 binds to HA LFA-1 on activated lymphocytes but not to low-affinity LFA-1 on unstimulated cells (14). Thus, AL-57 could be used to target activated lymphocytes selectively.
The goal of this study is to determine whether LFA-1-directed antibody-protamine fusion proteins can target siRNAs to primary leukocytes and to probe whether the specificity of targeting can be used to silence gene expression only in activated leukocytes. Silencing gene expression only in activated cells during a disease could provide the possibility of interfering with unwanted pathogenic immune stimulation without global immunosuppressive effects on bystander immune cells that complicate most current immunosuppressive therapies (15). We designed an AL-57 single-chain variable region fragment (scFv)-protamine fragment fusion protein (AL-57-PF) and tested its ability to deliver siRNAs and silence gene expression in activated cells. Here we demonstrate selective gene silencing in activated lymphocytes by targeting the HA conformation of LFA-1.
Results
Expression of Anti-LFA-1 Fusion Proteins.
To incorporate an LFA-1-targeting moiety into an siRNA delivery reagent, the heavy- and light-chain variable genes of the LFA-1 IgG antibodies TS1/22 (16) and AL-57 (13, 14) were converted to scFvs, which were fused at their C termini in a bacterial expression plasmid with the sequence for a basic peptide from human protamine, corresponding to amino acids 8–29, as described (7). AL-57-PF and TS1/22-PF were expressed in bacteria with a His6 tag and purified to homogeneity from the periplasm by sequential Ni-NTA affinity and ion-exchange chromatography (data not shown).
AL-57-PF Delivers siRNA to Silence Gene Expression Selectively in HA LFA-1-Expressing Cells.
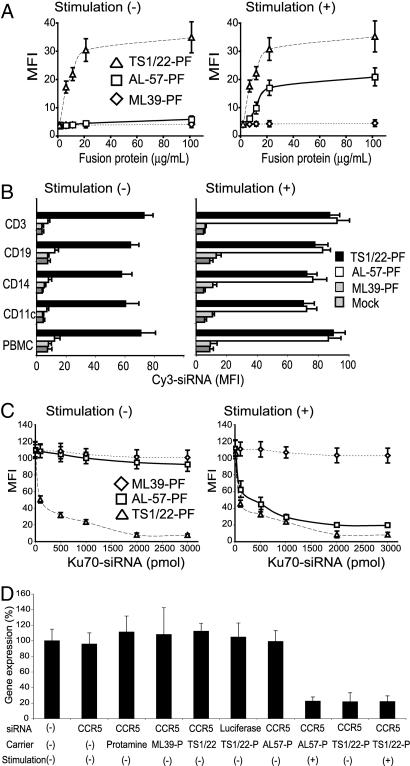
TS1/22 binds nonselectively to both low- and HA LFA-1 (17), whereas IgG AL-57 binds selectively to HA LFA-1 (13, 14). To verify that their binding specificities were preserved after conversion to scFv-PF, we used flow cytometry to assess binding to human peripheral blood mononuclear cells (PBMC) of Alexa-488-labeled AL-57-PF, TS1/22-PF, and ML39-PF [a control fusion protein that recognizes human ErbB2 (7)]. On circulating blood cells, LFA-1 is predominantly in the low-affinity form but can be converted to the HA form by stimulation in the presence of Mg2+ and EGTA with an activating antibody CBRLFA-1/2 (18). Stimulation with the activating antibody did not affect LFA-1 expression on any subset [supporting information (SI) Fig. 6A]. TS1/22-PF bound to PBMC independently of stimulation, but AL-57-PF bound only to stimulated PBMC (Fig. 1A and SI Fig. 6B). Because the fusion proteins bound to cells with the specificity of their respective antibodies, we next tested whether AL-57-PF specifically delivered fluorescently labeled siRNAs only into stimulated PBMC. Cy3-siRNA on its own or complexed with ML39-PF did not get into any subset of PBMC. TS1/22-PF efficiently delivered Cy3-siRNA to both unstimulated and stimulated PBMC of each subtype, CD3+ T and CD19+ B lymphocytes, CD14+ monocytes, and CD11c+ dendritic cells (Fig. 1B). In contrast, AL-57-PF delivered Cy3-siRNA only to a small subset of unstimulated T and B lymphocytes (≈1–2%) in PBMC. These were present as a distinct Cy3+ peak on flow cytometry that was not present in the control samples treated with Cy3-siRNA mixed with medium, irrelevant antibody, or protamine (Fig. 1B and data not shown). These small subpopulations likely represent the small numbers of circulating activated lymphocytes in healthy donors. However, AL-57-PF potently delivered Cy3-siRNA to all subsets of stimulated PBMC (Fig. 1B). These results demonstrate the selective siRNA delivery by AL-57-PF only to activated leukocytes.
Fig. 1.
Selective targeting of siRNAs to PBMC expressing HA LFA-1 by AL-57-PF. PBMC were either unstimulated (1 mM MgCl2, 1 mM CaCl2) or stimulated with 5 mM MgCl2, 1 mM EGTA, and 10 μg/ml CBRLFA-1/2 to activate LFA-1. (A) Activation-independent binding of TS1/22-PF and activation-dependent binding of AL-57-PF. (B) Selective delivery of Cy3-siRNA (1 nmol) to stimulated or unstimulated PBMC, measured 6 h after treatment. The LFA-1 antibody fusion proteins selectively delivered siRNAs to T lymphocytes (stained with CD3), B lymphocytes (CD19), monocytes (CD14), and dendritic cells (CD11c). (C) Silencing of Ku70 in PBMC. Ku70 expression was measured 3 d after treatment with Ku70-siRNA, delivered as indicated. (D) Silencing of CCR5 in T lymphocytes. Memory T lymphocytes were treated for 3 d in the presence or absence of LFA-1-activating antibody with 1 nmol of CCR5-siRNA, delivered as indicated. Expression of CCR5 mRNA relative to β-actin mRNA was measured by quantitative RT-PCR.
We next asked whether AL-57-PF-delivered siRNAs could induce silencing of the ubiquitously expressed Ku70 gene (7) selectively to HA LFA-1-expressing cells. Stimulated or unstimulated PBMC were analyzed 48 h after treatment with Ku70-siRNA delivered by polyethyleneimine (PEI), oligofectamine or scFv-PFs. siRNA complexed with PEI or oligofectamine did not significantly reduce Ku70 expression (SI Fig. 7), confirming that PBMC are resistant to conventional transfection reagents. Ku70-siRNA delivered by TS1/22-PF induced potent silencing independently of stimulation, whereas Ku70-siRNA delivered by AL-57-PF induced silencing only in stimulated cells (Fig. 1C and SI Fig. 7A). Silencing was readily detectable with 100 pmol of siRNA and plateaued at ≈2,000 pmol (Fig. 1C). Similar results were obtained when CD4–siRNA was delivered to primary stimulated and unstimulated lymphocytes to silence CD4 expression (SI Fig. 7B).
AL-57-PF Silences Chemokine Receptor CCR5 Selectively in HA LFA-1-Expressing Cells.
CCR5 is a chemokine receptor that plays a critical role in Th1 type immunity to pathogens (19) and is a coreceptor for HIV infection (20). Aberrant up-regulation of CCR5 in T lymphocytes is implicated in the induction of Th1-type responses in rheumatoid arthritis and transplant rejection (21). Therefore, selective attenuation of CCR5 expression in activated lymphocytes might be a novel approach to treat autoimmune disease or HIV infection. To investigate the feasibility of this approach in vitro, we tested delivery of CCR5-siRNA by LFA-1 antibody fusion proteins. Memory T cells express CCR5 and low-affinity LFA-1 that converts to the HA conformation after stimulation with CBRLFA-1/2 (14). Unstimulated or stimulated memory T cells were treated with CCR5–siRNA or control luciferase–siRNA mixed with the fusion proteins or their constituent components and analyzed by quantitative RT-PCR for CCR5 expression (Fig. 1D). Stimulation with CBRLFA-1/2 on its own did not alter CCR5 mRNA expression (not shown). As expected, CCR5–siRNA delivered by TS1/22-PF greatly reduced mRNA expression independently of stimulation, whereas CCR5 was reduced by CCR5–siRNA delivered by AL-57-PF only in stimulated lymphocytes. These results demonstrate potent and selective gene silencing only in activated PBMC and T lymphocytes after siRNA delivery with AL-57-PF and activation-independent gene silencing by siRNA delivered by TS1/22-PF in resting and activated mononuclear cells that are normally resistant to transfection.
AL-57-PF Selectively Targets HA LFA-1-Expressing Cells in Heterogeneous Populations.
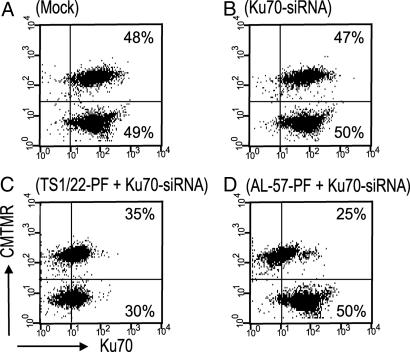
To demonstrate further the selective delivery of siRNAs to activated cells in heterogeneous populations of cells expressing both high- and low-affinity LFA-1, we delivered Ku70-siRNAs to mixed populations of K562 cells that were stably transfected to express LFA-1 (22) and then either exposed to the stimulating antibody CBRLFA-1/2 or the nonactivating control LFA-1 antibody TS2/4. The stimulated cells were labeled with CMTMR (CellTracker, Invitrogen, Carlsbad, CA) to identify them in the mixed population. Ku70-siRNA delivered by TS1/22-PF-reduced Ku70 protein expression in both CBRLFA-1/2- and TS2/4-treated cells, showing gene silencing independent of LFA-1 activation (Fig. 2 and SI Fig. 8). In contrast, Ku70-siRNA delivered by AL-57-PF selectively attenuated Ku70 expression in CBRLFA-1/2-treated cells, while leaving Ku70 expression in TS2/4-treated cells unchanged (Fig. 2). These results demonstrate siRNA delivery by AL-57-PF selectively targets HA LFA-1 expressing cells in heterogeneous populations.
Fig. 2.
Selective silencing of Ku70 in mixed populations of K562 cells transfected to express LFA-1. CMTMR-labeled CBRLFA-1/2-activated cells, expressing HA LFA-1, were cocultured with unlabeled cells treated with an LFA-1 nonactivating antibody that express low-affinity LFA-1. Three days after treatment with 1 nmol of Ku70-siRNA delivered as indicated, the cocultures were analyzed for Ku70 silencing. AL57-PF-delivered siRNAs silence only the labeled activated cells (D), whereas TS-1/22-PF-delivered siRNAs silence Ku70 in both populations (C).
AL-57-PF Delivers siRNA to Silence Gene Expression in Lymphocytes Activated by T Cell Receptor (TCR) or Chemokine Stimulation.
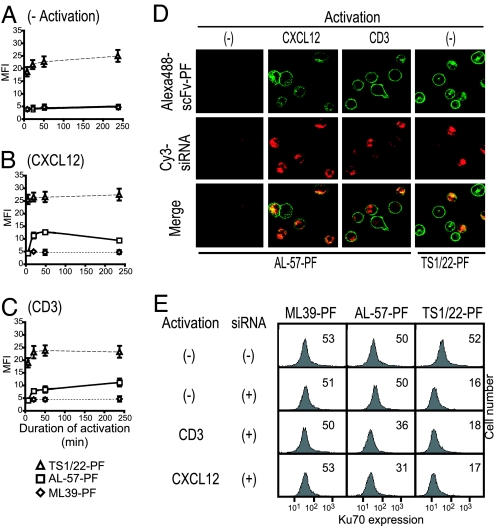
Activation of lymphocytes by engagement of the TCR or chemokine receptors elicits intracellular signaling cascades that lead to transient up-regulation of HA LFA-1 (12). During chronic inflammation, the HA conformation of LFA-1 persists in aberrantly activated lymphocytes (23, 24). To investigate whether AL-57-PF delivers siRNAs and silences gene expression in lymphocytes activated by physiologically relevant stimuli that would model chronic inflammation in vitro, T lymphocytes were exposed to immobilized CD3 antibody or immobilized CXCL12 chemokine, which elicit persistent activation of LFA-1. Binding of TS1/22-PF and AL-57-PF was used to verify the effects of these stimuli on LFA-1 conformation. As determined by binding of activation-insensitive TS1/22-PF, LFA-1 expression barely changed during activation (Fig. 3 A–C). Unstimulated normal donor T lymphocytes did not bind AL-57-PF in the absence of stimulation (Fig. 3A), but binding persisted for at least 4 h after exposure to the immobilized stimuli (Fig. 3 B and C).
Fig. 3.
Persistent physiological stimulation of memory T cells activates sustained AL-57-PF binding and siRNA delivery. (A–C) Kinetics of affinity up-regulation of LFA-1 after activation of T cells. Cells stimulated for the indicated times with immobilized CXCL12 or anti-CD3 were analyzed for binding of Alexa-488-labeled fusion proteins. (D) Cy3-siRNA and Alexa-488-TS1/22-PF were taken up by unstimulated T cells, whereas uptake of Alexa- 488-AL-57-PF required T cell activation. (E) Activation-dependent silencing of Ku70 in T cells measured 3 d after treatment with 1 nmol of Ku70-siRNA delivered by scFv-PF. Mean fluorescence intensities (MFI) of representative histograms are shown.
We next used confocal microscopy to investigate the ability of Alexa-488-labeled AL-57-PF to bind and deliver Cy3-siRNA selectively to activated lymphocytes. Four hours after exposure of activated lymphocytes to the fluorescently labeled fusion protein–siRNA complexes, Alexa-488-AL-57-PF was distributed to both the plasma membrane and internal punctate structures, whereas Cy3-siRNA was predominantly intracellular, colocalizing with the fusion protein (Fig. 3D). The conformation-sensitive fusion protein AL-57-PF did not transduce unactivated lymphocytes. As expected, T cells treated with Alexa-488-TS1/22-PF internalized Cy3-siRNA with a similar staining pattern, but uptake was independent of cell activation (Fig. 3D and data not shown).
Using Ku70-siRNA, we next investigated the ability of AL-57-PF to silence genes selectively in lymphocytes activated by physiologic stimuli. Activation by immobilized CD3 mAb or CXCL12 did not affect Ku70 protein expression assessed by flow cytometry (data not shown). Exposure to AL-57-PF–siRNA complexes reduced Ku70 selectively in activated lymphocytes, whereas TS1/22-PF–siRNAs reduced Ku70 levels even in unstimulated lymphocytes (Fig. 3E and data not shown). These results demonstrate that AL-57-PF enables the manipulation of gene expression selectively in lymphocytes activated by physiologically relevant stimuli.
AL-57-PF-Mediated Knockdown of Cyclin D1 Suppresses Proliferation Selectively in Activated Lymphocytes.
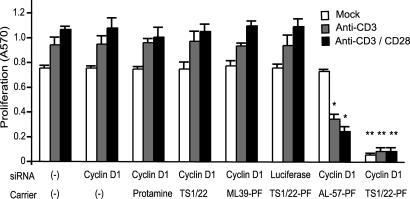
Proliferation of aberrantly activated lymphocytes has been implicated in the pathogenesis of autoimmune diseases. Cyclins and other cell-cycle regulating proteins represent a potential therapeutic target for autoimmune diseases and other diseases caused by overly exuberant immune activation (25). We therefore investigated whether we could selectively suppress cellular proliferation in activated lymphocytes using cyclin D1–siRNA. Basal proliferation of memory T cells, prepared by in vitro exposure of PBMC to IL-15, was enhanced by activation with immobilized CD3-mAb alone or together with CD28-mAb (Fig. 4). Proliferation measured by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay was not altered by exposure to cyclin D1-siRNA alone or mixed with protamine, TS1/22-scFv, ML39-PF. Luciferase-siRNA delivered by TS1/22-PF also had no effect on lymphocyte proliferation. However, cyclin D1-siRNA delivered by TS1/22-PF potently inhibited basal proliferation of memory T cells as well as the elevated proliferation of activated lymphocytes. Cyclin D1-siRNA delivered by AL-57-PF did not affect proliferation of unactivated memory T cells but significantly suppressed proliferation in activated lymphocytes (Fig. 4). Moreover, suppression was somewhat more effective in cells that were more fully stimulated by both antibodies. Experiments with CD3 and CD28 mAbs immobilized at 1 and 5 μg/ml produced similar results (Fig. 4 and data not shown). Proliferation measured by [3H] thymidine incorporation showed similar results (not shown). Suppression of proliferation correlated with levels of cyclin D1 knockdown (SI Fig. 9).
Fig. 4.
Selective inhibition of proliferation by AL-57-PF-delivered cyclin D1-siRNA to activated T cells. Proliferation was assayed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) incorporation 3 d after treatment with or without immobilized activating antibodies, combined with cyclin D1 or control siRNA complexed with scFv-PF fusion proteins, TS1/22 scFv, protamine, or medium. Silencing cyclin D1 using TS1/22-PF stopped proliferation of all T cells, whereas inhibition of proliferation using AL-57-PF required cell activation. ∗, P < 0.03; ∗∗, P < 0.01.
The Fusion Proteins Targeting LFA-1 Deliver siRNA in Vivo.
We next investigated whether LFA-1-targeted fusion proteins could deliver siRNA in vivo. Because AL-57 and TS1/22 antibodies do not recognize murine LFA-1, we used SCID mice engrafted with K562 cells stably transfected to express human WT LFA-1 (K562-WT LFA-1) or HA LFA-1 (K562-HA LFA-1) (22, 26). Five days after i.v. injection of K562 cells, when they formed numerous small nodules in the lung (not shown), we injected 1.2 nmol (40 μg) of fusion protein complexed with 6 nmol (100 μg) of Cy3-siRNA into the tail vein.
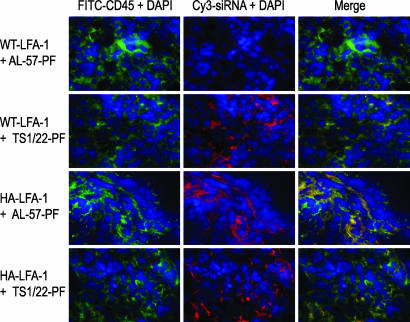
Four hours later, lung tissues were harvested and examined by immunohistochemistry (Fig. 5), and single-cell suspensions were analyzed by flow cytometry (SI Table 1) for uptake of fluorescent siRNA. Cy3-siRNA complexed with a control fusion protein (ML39-PF) was not taken up above background (SI Table 1 and data not shown). TS1/22-PF delivered Cy3-siRNA equally well to K562-WT LFA-1 and K562-HA LFA-1 but not to mouse lung cells (Fig. 5 and data not shown). AL-57-PF delivered Cy3-siRNA to K562-HA LFA-1 as well as TS1/22-PF, but siRNA delivery to K562-WT LFA-1 was much less efficient than delivery by TS1/22-PF. Neither protein induced significant uptake of Cy3-siRNA by parent K562 cells that do not express LFA-1 (not shown). These results demonstrate in vivo proof of principle for the effective systemic siRNA delivery by TS1/22-PF to LFA-1-expressing cells and the selective delivery by AL-57-PF to HA LFA-1-expressing cells.
Fig. 5.
Specific in vivo siRNA delivery by anti-LFA-1 fusion proteins to K562 cells expressing human WT LFA-1 or human HA LFA-1. Four hours after injection of Cy3-siRNA complexed with AL-57- or TS1/22-PF, siRNA delivery to K562 cells in the lungs of SCID mice was examined by fluorescence microscopy. Anti-human CD45 labeled K562 cells. TS1/22-PF delivered siRNA equally well to cells expressing WT and HA-LFA-1. By contrast, AL-57-PF preferentially delivered to K562-HA LFA-1. Mouse lung cells did not take up the siRNA.
Exposure to siRNA-Fusion Protein Complex Does Not Cause Lymphocyte Activation.
The engagement of LFA-1 by its natural ligand intercellular adhesion molecule-1 leads to lymphocyte activation (27). The LFA-1 targeting fusion proteins might have limited usefulness if they activated the cells they targeted. To determine whether TS1/22-PF or AL-57-PF complexes cause lymphocyte activation, we measured the induction of the early activation markers CD25 and CD69 on PBMC cultured for 48 h with the fusion proteins mixed with luciferase–siRNA. Neither fusion protein–siRNA complex induced expression of CD69 and CD25, whereas activation with phytohemagglutinin induced expression of both markers (SI Fig. 10 A and B). Therefore, transducing lymphocytes with the anti-LFA-1 scFv fusion proteins does not activate them.
LFA-1-Targeted siRNAs Do Not Elicit IFN Responses.
Another possible unwanted off-target effect of fusion protein-delivered siRNA would be activation of IFN-responsive genes (IRG) by activating cytosolic dsRNA-activated protein kinase PKR or by binding to Toll-like receptors 3, 7, and 8 that recognize RNA on the cell surface or in endosomes (28, 29). To examine whether AL-57-PF- or TS1/22-PF-siRNA complexes activate an IFN response, we used quantitative RT-PCR to measure mRNA expression of IFN-β, and two key IRG, 2′,5′-oligoadenylate synthetase and Stat-1 (7) in PBMC stimulated with Mg/EGTA plus CBRLFA-1/2 and then treated with the fusion protein-siRNA complexes (SI Fig. 10C). The IRG were not induced by the LFA-1 antibody but were induced by treatment with the known IFN inducers, LPS and poly(I:C). Treatment with as much as 1 μM luciferase-siRNA delivered by TS1/22-PF or AL-57-PF did not induce an IFN response. Therefore, even in highly sensitive primary cells, siRNA complexed with the scFv-protamine fragment fusion proteins does not trigger nonspecific IFN responses.
Discussion
Here we report LFA-1-targeted scFv-protamine fusion proteins as a nonviral delivery approach to induce RNAi in primary leukocytes. Primary lymphocytes are highly resistant to nonviral siRNA delivery with cationic lipid and polymer reagents (3–5), as confirmed in the present study. By fusing LFA-1-specific scFv antibodies (AL-57 and TS1/22) to a protamine fragment that condenses siRNA through charge interactions, we have developed an LFA-1-specific delivery method for efficient gene silencing. The conformation-insensitive TS1/22-PF enables potent gene silencing in all leukocytes independently of activation status. These include primary lymphocytes and dendritic cells, which are resistant to conventional transfection techniques. Furthermore, the HA LFA-1-specific AL-57-PF enables gene silencing selectively in activated leukocytes. Moreover, IFN responses were not triggered in primary cell PBMC mixtures containing highly sensitive antigen-presenting cells. Finally, using SCID mice engrafted with K562 cells expressing WT or HA LFA-1, we demonstrated the in vivo feasibility of activation-independent delivery by TS1/22-PF to LFA-1-bearing cells and activation-dependent delivery by AL-57-PF.
These results support the general applicability and high degree of specificity possible with antibody-protamine fusion proteins for targeted siRNA delivery to primary cells. Moreover, the targeting fusion proteins do not activate lymphocytes, even though they engage a cell surface signaling molecule. This may be because the targeting reagent is monomeric, because it is designed from a scFv and is not expected to cross-link the receptor.
AL-57 is a ligand mimetic antibody that binds selectively to the HA conformation of LFA-1 (14). LFA-1 activation by a single encounter with an activating stimulus is transient; stimulation of lymphocytes with soluble anti-CD3 antibody (30) and soluble chemokine CXCL12 (31) increases LFA-1 adhesiveness only for 5–20 min. In contrast, as we found here using immobilized stimuli that constitutively engage TCR or CXCR4, sustained receptor engagement leads to persistent affinity up-regulation of LFA-1. Constitutive lymphocyte activation might mimic aberrant activation in chronic inflammation. To examine the potential therapeutic feasibility of AL-57-PF-directed siRNA delivery, we investigated whether we could suppress lymphocyte proliferation selectively in persistently stimulated populations. Cyclin D1-siRNA delivered by AL-57-PF suppressed lymphocyte proliferation only when cells were stimulated with CD3 or CD3/CD28. By contrast, TS1/22-PF suppressed lymphocyte proliferation independently of the state of lymphocyte activation. There are several potential therapeutic advantages of selective gene silencing. Selective targeting of activated lymphocytes would likely be sufficient to suppress inflammatory tissue injury. By leaving resting and naïve cells untouched, selective targeting would reduce iatrogenic immunodeficiency, a major problem associated with current immunosuppressive drugs (15). Moreover, the siRNA dose required to target a small subset of disease-causing cells is likely to be substantially less than that needed for indiscriminate targeting.
Other activation makers, such as CD69, CD25, CD40L, or OX40, could also be used for selective targeting of activated lymphocytes (15, 32, 33). The expression profiles of cell surface molecules after activation vary greatly depending on timing and the character and strength of the activating stimulus (32). Fusion proteins, based on antibodies or ligands to different activation markers, might allow targeting of overlapping but distinct phases of lymphocyte activation. Determining which targeting strategy would be most appropriate for different pathological conditions will require in vivo studies.
This study suggests that LFA-1-directed siRNA delivery reagents might be useful for targeting leukocytes in vivo for research to understand disease pathogenesis or discover useful drug targets or for RNAi-based therapy. LFA-1 is expressed on the surface of all leukocytes. Although methods have recently been described for efficient systemic siRNA delivery to the liver (34–36), so far there are no clinically relevant in vivo examples of systemic siRNA delivery to other organs or to moving targets, such as hematopoietic cells. Moreover, the ability to transduce only activated subsets of immune cells by taking advantage of the conformational change of LFA-1 on activated cells provides the potential for highly targeted research or therapeutic intervention. Although this study was done with human reagents that do not recognize mouse LFA-1, and we are currently engineering the murine analogs for in vivo testing, the feasibility study using SCID mice engrafted with K562 cells expressing human LFA-1 strongly supports the applicability of LFA-1 antibody fusion proteins for in vivo siRNA delivery. In SCID mice, whereas the selectivity of AL-57-PF to deliver siRNA to HA LFA-1 over WT LFA-1 was well maintained, K562-WT LFA-1, which rarely takes up siRNA in vitro (Fig. 2D and data not shown), showed some uptake of siRNA delivered by AL-57-PF (SI Table 1). This result suggests that WT LFA-1 in K562 transfectants may be activated in vivo by binding to intercellular adhesion molecule-1 in homotypic cell aggregates (37) and/or by the innate inflammatory responses elicited by xenogeneic reactions to K562 cells.
Targeting LFA-1 using siRNA-fusion protein complexes might have enhanced efficacy at suppressing immune activation and inflammation compared with other ways of delivering siRNA. Many LFA-1 antibodies, including AL-57, block leukocyte adhesion (13, 38), and LFA-1 blocking mAbs are effective in attenuating inflammatory disease in mouse models and in treating psoriasis patients (39, 40). Targeted siRNA delivery using blocking LFA-1 antibodies might produce additive or synergistic effects by both silencing proinflammatory molecules and inhibiting LFA-1-mediated cell adhesion. Because blocking LFA-1 by itself is insufficient to suppress inflammation in certain disease models (41), combining LFA-1-blocking antibodies with gene silencing might be a more powerful therapeutic approach.
Methods
siRNA Delivery and Gene Silencing.
siRNAs mixed with fusion proteins (in a 5:1 molar ratio), appropriate controls (i.e., scFv, protamine), or vehicles in 50 μl of PBS were preincubated for 30 min at room temperature and added to 2 × 105 PBMC or lymphocytes in 150 μl of RPMI medium 1640/10% FCS in the presence of 1 mM MgCl2/CaCl2 or 5 mM MgCl2/1 mM EGTA plus 10 μg/ml mAb CBRLFA-1/2. Cells were cultured for 6–72 h at 37°C, 5% CO2 and subjected to flow cytometry and/or RT-PCR analyses.
T Lymphocyte Activation Through CXCR4 and TCR.
Microtiter plates were coated for 1 h at 37°C with CXCL12 (5 μg/ml), anti-human CD3 mAb (5 μg/ml; clone 1304; Immunotech, Marseille, France), and/or anti-human CD28 mAb (5 μg/ml; clone 1373; Immunotech), washed, and blocked with RPMI medium 1640 containing 10% FCS for 1 h at 37°C. T lymphocytes (1 × 105 cells per well in 100 μl) were stimulated for the indicated times at 37°C, 5% CO2. To study the kinetics of fusion protein binding, Alexa-488-labeled fusion proteins (20 μg/ml) were added 15 min before the end of stimulation. Cells were fixed in cold 2% formaldehyde in Hanks' balanced salt solution (HBSS), washed three times with HBSS containing 2% glucose and 2% BSA, resuspended in HBSS, and analyzed by flow cytometry. To study siRNA delivery, cells were treated for 4 h with Cy3-siRNA on its own or delivered by fusion proteins and analyzed with fluorescent microscopy. To study silencing, cells were cultured for 3 days in the presence or absence of Ku70-siRNA alone or complexed with fusion proteins and analyzed with flow cytometry.
Mixed-Population Transduction Experiment.
K562 cells transfected to express LFA-1 were either treated for 30 min at 37°C with the activating antibody CBRLFA-1/2 (10 μg/ml) and labeled with 4 μM CMTMR (CellTracker, Invitrogen) or treated with the nonactivating LFA-1 antibody TS2/4 and mock-labeled. The two populations were washed and mixed in equal numbers and then cocultured for 48 h at 37°C, 5% CO2, in RPMI medium 1640/10% FCS in the presence of 1 nmol of Ku70-siRNA or luciferase-siRNA, alone or complexed with protamine or an indicated antibody-protamine fusion protein, before measuring intracellular Ku70 expression by flow cytometry.
In Vivo Delivery.
SCID mice on a CB17 background (5–7 weeks old) from Charles River Breeding Laboratory (Wilmington, MA) were injected by tail vein with 6 × 106 K562 cells transfected to express WT or HA LFA-1. Five days later, Cy3-siRNA (6 nmol) complexed with fusion protein in a 5:1 molar ratio in 100 μl of PBS was injected by tail vein. Mice were killed 4 h later, and the lungs were harvested. The right lung was placed in optimal cutting temperature compound (Tissue-Tek, Hatfield, PA) snap-frozen in liquid nitrogen, and used for immunohistochemistry. Single-cell suspensions, prepared by mechanical disruption of the left lung, were analyzed by flow cytometry. All animal procedures were approved by the Animal Care and Use Committee of the CBR Institute for Biomedical Research.
See SI Methods for additional methods.
Supplementary Material
Acknowledgments
We thank Dr. Timothy A. Springer (CBR Institute for Biomedical Research) for antibodies and hybridomas and Drs. Peter Sage, Simone Piva, Moonsoo Jin, Dipanjan Chowdhury, and Yichao Wu for technical assistance and advice. D.P. thanks the Dorot Foundation for a postdoctoral fellowship and Pfizer, Inc., for partial support. This work was supported by the Arthritis Foundation (C.V.C), the American Society of Hematology (M.S.), and National Institutes of Health Grants AI63421 (to M.S.) and AI45587 and AI58695 (to J.L.).
Abbreviations
- LFA-1
lymphocyte function-associated antigen-1
- scFv
single-chain variable region fragment
- PBMC
peripheral blood mononuclear cell
- HA
high-affinity
- TCR
T cell receptor.
Footnotes
Author contributions: D.P. and P.Z. contributed equally to this work; D.P., P.Z., C.V.C., J.L., and M.S. designed research; D.P., P.Z., and C.V.C. performed research; C.V.C., J.L., and M.S. contributed new reagents/analytic tools; D.P., P.Z., C.V.C., J.L., and M.S. analyzed data; and D.P., J.L., and M.S. wrote the paper. D.P., P.Z., C.V.C., and M.S. declare no conflict of interest. J.L. declares a financial interest. A prorizional patent on the antibody fusion protein method for siRNA delivery has been licensed.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0608491104/DC1.
References
- 1.Behlke MA. Mol Ther. 2006;13:644–670. doi: 10.1016/j.ymthe.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dykxhoorn DM, Lieberman J. Annu Rev Med. 2005;56:401–423. doi: 10.1146/annurev.med.56.082103.104606. [DOI] [PubMed] [Google Scholar]
- 3.Goffinet C, Keppler OT. FASEB J. 2006;20:500–502. doi: 10.1096/fj.05-4651fje. [DOI] [PubMed] [Google Scholar]
- 4.Marodon G, Mouly E, Blair EJ, Frisen C, Lemoine FM, Klatzmann D. Blood. 2003;101:3416–3423. doi: 10.1182/blood-2002-02-0578. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Lu H, LiWang P, Sili U, Templeton NS. Mol Ther. 2003;8:629–636. doi: 10.1016/s1525-0016(03)00231-4. [DOI] [PubMed] [Google Scholar]
- 6.Lai W, Chang CH, Farber DL. J Immunol Methods. 2003;282:93–102. doi: 10.1016/j.jim.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 7.Song E, Zhu P, Lee SK, Chowdhury D, Kussman S, Dykxhoorn DM, Feng Y, Palliser D, Weiner DB, Shankar P, et al. Nat Biotechnol. 2005;23:709–717. doi: 10.1038/nbt1101. [DOI] [PubMed] [Google Scholar]
- 8.Hutton JJ, Jegga AG, Kong S, Gupta A, Ebert C, Williams S, Katz JD, Aronow BJ. BMC Genomics. 2004;5:82. doi: 10.1186/1471-2164-5-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Staudt LM, Brown PO. Annu Rev Immunol. 2000;18:829–859. doi: 10.1146/annurev.immunol.18.1.829. [DOI] [PubMed] [Google Scholar]
- 10.Shimaoka M, Xiao T, Liu J-H, Yang Y, Dong Y, Jun C-D, McCormack A, Zhang R, Joachimiak A, Takagi J, et al. Cell. 2003;112:99–111. doi: 10.1016/s0092-8674(02)01257-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim M, Carman CV, Springer TA. Science. 2003;301:1720–1725. doi: 10.1126/science.1084174. [DOI] [PubMed] [Google Scholar]
- 12.Carman CV, Springer TA. Curr Opin Cell Biol. 2003;15:547–556. doi: 10.1016/j.ceb.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Huang L, Shimaoka M, Rondon IJ, Roy I, Chang Q, Po M, Dransfield DT, Ladner RC, Edge AS, Salas A, et al. J Leukocyte Biol. 2006;80:905–914. doi: 10.1189/jlb.1105649.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimaoka M, Kim M, Cohen E, Yang W, Astrof N, Peer D, Salas A, Ferrand A, Springer T. Proc Natl Acad Sci USA. 2006;103:13991–13996. doi: 10.1073/pnas.0605716103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolfraim LA. Arch Immunol Ther Exp (Warsz) 2006;54:1–13. doi: 10.1007/s00005-006-0001-7. [DOI] [PubMed] [Google Scholar]
- 16.Haskard D, Cavender D, Beatty P, Springer TA, Ziff M. J Immunol. 1986;137:2901–2906. [PubMed] [Google Scholar]
- 17.Ma Q, Shimaoka M, Lu C, Jing H, Carman CV, Springer TA. J Biol Chem. 2002;277:10638–10641. doi: 10.1074/jbc.M112417200. [DOI] [PubMed] [Google Scholar]
- 18.Petruzzelli L, Maduzia L, Springer TA. J Immunol. 1995;155:854–866. [PubMed] [Google Scholar]
- 19.Ma B, Liu W, Homer RJ, Lee PJ, Coyle AJ, Lora JM, Lee CG, Elias JA. J Immunol. 2006;176:4968–4978. doi: 10.4049/jimmunol.176.8.4968. [DOI] [PubMed] [Google Scholar]
- 20.Berger EA, Murphy PM, Farber JM. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 21.Ribeiro S, Horuk R. Pharmacol Ther. 2005;107:44–58. doi: 10.1016/j.pharmthera.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Lu C, Springer TA. J Immunol. 1997;159:268–278. [PubMed] [Google Scholar]
- 23.Arao T, Morimoto I, Kakinuma A, Ishida O, Zeki K, Tanaka Y, Ishikawa N, Ito K, Eto S. J Clin Endocrinol Metab. 2000;85:382–389. doi: 10.1210/jcem.85.1.6320. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka Y, Mine S, Figdor CG, Wake A, Hirano H, Tsukada J, Aso M, Fujii K, Saito K, van Kooyk Y, Eto S. Blood. 1998;91:3909–3919. [PubMed] [Google Scholar]
- 25.Goulvestre C, Chereau C, Nicco C, Mouthon L, Weill B, Batteux F. J Immunol. 2005;175:6959–6967. doi: 10.4049/jimmunol.175.10.6959. [DOI] [PubMed] [Google Scholar]
- 26.Shimaoka M, Lu C, Palframan R, von Andrian UH, McCormack A, Takagi J, Springer TA. Proc Natl Acad Sci USA. 2001;98:6009–6014. doi: 10.1073/pnas.101130498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dustin ML, Bivona TG, Philips MR. Nat Immunol. 2004;5:363–372. doi: 10.1038/ni1057. [DOI] [PubMed] [Google Scholar]
- 28.Hornung V, Guenthner-Biller M, Bourquin C, Ablasser A, Schlee M, Uematsu S, Noronha A, Manoharan M, Akira S, de Fougerolles A, et al. Nat Med. 2005;11:263–270. doi: 10.1038/nm1191. [DOI] [PubMed] [Google Scholar]
- 29.Sioud M. J Mol Biol. 2005;348:1079–1090. doi: 10.1016/j.jmb.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 30.Dustin ML, Springer TA. Nature. 1989;341:619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- 31.Constantin G, Majeed M, Giagulli C, Piccio L, Kim JY, Butcher EC, Laudanna C. Immunity. 2000;13:759–769. doi: 10.1016/s1074-7613(00)00074-1. [DOI] [PubMed] [Google Scholar]
- 32.Hargreaves RE, Monk NJ, Jurcevic S. Trends Mol Med. 2004;10:130–135. doi: 10.1016/j.molmed.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 33.Sugamura K, Ishii N, Weinberg AD. Nat Rev Immunol. 2004;4:420–431. doi: 10.1038/nri1371. [DOI] [PubMed] [Google Scholar]
- 34.Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M, Elbashir S, Geick A, Hadwiger P, Harborth J, et al. Nature. 2004;432:173–178. doi: 10.1038/nature03121. [DOI] [PubMed] [Google Scholar]
- 35.Morrissey DV, Lockridge JA, Shaw L, Blanchard K, Jensen K, Breen W, Hartsough K, Machemer L, Radka S, Jadhav V, et al. Nat Biotechnol. 2005;23:1002–1007. doi: 10.1038/nbt1122. [DOI] [PubMed] [Google Scholar]
- 36.Zimmermann TS, Lee AC, Akinc A, Bramlage B, Bumcrot D, Fedoruk MN, Harborth J, Heyes JA, Jeffs LB, John M, et al. Nature. 2006;441:111–114. doi: 10.1038/nature04688. [DOI] [PubMed] [Google Scholar]
- 37.Kim M, Carman CV, Yang W, Salas A, Springer TA. J Cell Biol. 2004;167:1241–1253. doi: 10.1083/jcb.200404160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu C, Shimaoka M, Salas A, Springer TA. J Immunol. 2004;173:3972–3978. doi: 10.4049/jimmunol.173.6.3972. [DOI] [PubMed] [Google Scholar]
- 39.Harlan JM, Winn RK. Crit Care Med. 2002;30:S214–S219. doi: 10.1097/00003246-200205001-00007. [DOI] [PubMed] [Google Scholar]
- 40.Lebwohl M, Tyring SK, Hamilton TK, Toth D, Glazer S, Tawfik NH, Walicke P, Dummer W, Wang X, Garovoy MR, et al. N Engl J Med. 2003;349:2004–2013. doi: 10.1056/NEJMoa030002. [DOI] [PubMed] [Google Scholar]
- 41.de Fougerolles AR. In: I Domains in Integrins. Gullberg D, editor. Georgetown, TX: Plenum; 2003. pp. 165–177. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.