Abstract
Pituitary adenomas are common neoplasms of the anterior pituitary gland. Germ-line mutations in the aryl hydrocarbon receptor-interacting protein (AIP) gene cause pituitary adenoma predisposition (PAP), a recent discovery based on genetic studies in Northern Finland. In this population, a founder mutation explained a significant proportion of all acromegaly cases. Typically, PAP patients were of a young age at diagnosis but did not display a strong family history of pituitary adenomas. To evaluate the role of AIP in pituitary adenoma susceptibility in other populations and to gain insight into patient selection for molecular screening of the condition, we investigated the possible contribution of AIP mutations in pituitary tumorigenesis in patients from Europe and the United States. A total of 460 patients were investigated by AIP sequencing: young acromegaly patients, unselected acromegaly patients, unselected pituitary adenoma patients, and endocrine neoplasia-predisposition patients who were negative for MEN1 mutations. Nine AIP mutations were identified. Because many of the patients displayed no family history of pituitary adenomas, detection of the condition appears challenging. Feasibility of AIP immunohistochemistry (IHC) as a prescreening tool was tested in 50 adenomas: 12 AIP mutation-positive versus 38 mutation-negative pituitary tumors. AIP IHC staining levels proved to be a useful predictor of AIP status, with 75% sensitivity and 95% specificity for germ-line mutations. AIP contributes to PAP in all studied populations. AIP IHC, followed by genetic counseling and possible AIP mutation analysis in IHC-negative cases, a procedure similar to the diagnostics of the Lynch syndrome, appears feasible in identification of PAP.
Keywords: immunohistochemistry, growth hormone/prolacting–secreting adenomas, acromegaly
Pituitary adenomas are common, benign, monoclonal neoplasms of the anterior pituitary gland. They account for ≈15% of intracranial tumors (1). Approximately two-thirds produce pituitary hormones in excess; among these, prolactin (PRL)- and growth hormone (GH)-oversecreting adenomas are the most common. GH-secreting adenomas cause acromegaly and gigantism. Less common are adrenocorticotropin hormone (ACTH)-secreting adenomas, causing Cushing's disease. The remaining one-third of pituitary adenomas is endocrinologically silent, known as nonfunctioning pituitary adenomas, and cause symptoms or signs due to tumor growth (1–3). Pituitary adenomas are components of rare, well established syndromes, such as multiple endocrine neoplasia type 1 (MEN1) and Carney complex (CNC) (4, 5). Recent data suggest that a genetic predisposition to pituitary tumors is less rare than thought and that genes other than those for MEN1 and CNC are also involved (5–7).
Recently, we showed that germ-line mutations of the aryl hydrocarbon receptor-interacting protein (AIP) gene cause pituitary adenoma predisposition (PAP) (8). A nonsense mutation, p.Q14X, was found in members of two Finnish families. The mutation segregated perfectly with the GH phenotype and was also present in three prolactinoma patients. In addition, a nonsense mutation, p.R304X, was found in two Italian siblings with GH-secreting adenomas. In a population-based series from Northern Finland, AIP mutations accounted for 16% of all patients diagnosed with pituitary adenomas secreting GH and for 40% of patients younger than 35 years of age. Typically, PAP patients were of a young age at disease onset and did not display a strong family history of pituitary adenomas. Loss of the normal allele was detected in eight of eight pituitary adenomas; AIP is likely to act as a tumor suppressor gene (8).
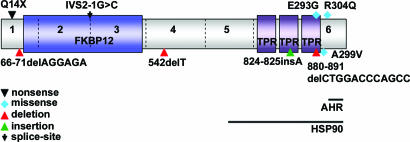
AIP encodes a protein of 330 aa. The protein contains an FKBP-homology domain, and three tetratricopeptide (TPR) repeats. AIP forms interactions with the aryl hydrocarbon receptor (AHR, also known as dioxin receptor), two HSP90 proteins, PDE4A5, PPARα, and survivin (9–12).
In our first study, for gene identification purposes, we focused on a defined, homogeneous population (8). To gain insight into clinical features and approaches to diagnose the condition, it was relevant to examine the contribution of germ-line AIP mutations in other patient materials as well. Here we sequenced the whole AIP coding region in a large, heterogeneous collection of 460 pituitary adenoma patients and patients from families with MEN1 features and who were derived from different populations in Europe and the United States. In addition, because the genetic evidence suggests that many AIP-associated pituitary adenomas are null for AIP protein, we tested whether negative staining in AIP immunohistochemistry (IHC) in pituitary adenomas would be a useful marker for PAP.
Results
Mutation Analysis.
Nine presumably pathogenic mutations were identified. These mutations, and the features of the respective patients, are depicted in Table 1 and Fig. 1. It is typically challenging to robustly evaluate the nature of missense changes in hereditary predisposition. Here, if a missense change was not seen in controls and was associated with a phenotype strongly suggestive of PAP, it was presumed pathogenic. Other changes were presumed to be neutral. It is clear that this subdivision is preliminary and should be interpreted with some caution. The results are reviewed and the details of the missense changes are depicted below.
Table 1.
AIP mutations identified in pituitary adenoma patients from the European and North American populations
| Patients | Mutation* | Fragment | No of patients with AIP mutation (%) | Clinical data | Normal AIP allele lost in tumor | Sex | Age at diagnosis, years | Family history of pituitary adenoma | Controls | |
|---|---|---|---|---|---|---|---|---|---|---|
| Young acromegaly | ||||||||||
| Germany | c.66-71delAGGAGA | Exon | 1 | 1 of 27 (3.7) | Acromegaly-GHoma | Yes | M | 20 | Yes (acromegaly) | 0 of 532 |
| c.878-879AG→GT (p.E293G) and c.880→891delCTGGACCCAGCC | Exon | 6 | 1of 27 (3.7) | Acromegaly-GHoma | Yes | F | 29† | NA | 0 of 255 | |
| Finland | c.40C→T (p.Q14X) | Exon | 1 | 2 of 36 (5.5) | Acromegaly-GHoma | NA | M | 36 | No | 0 of 532 |
| — | — | — | Acromegaly-GHoma | NA | F | 41 | No | 0 of 532 | ||
| Unselected acromegaly | ||||||||||
| Italy | — | — | 0 of 71 | — | — | — | — | — | — | |
| Unselected pituitary adenoma | ||||||||||
| U.S. | IVS2-1G→C | Intron 2 | 1 of 113 (0.9) | Acromegaly-GHoma | NA | M | 20 | No | 0 of 202 | |
| c.824-825insA | Exon 6 | 1 of 113 (0.9) | GHoma | Yes | M | 8 | No | 0 of 201 | ||
| Poland | c.911G→A (p.R304Q) | Exon 6 | 1 of 122 (0.8) | Cushing's disease–ACTHoma | NA | NA | 26 | No | 0 of 255 | |
| MEN1-negative | ||||||||||
| Spain | c.542delT | Exon | 4 | 1 of 55 (1.8) | Acromegaly–GHoma | NA | M | 18 | Yes (acromegaly) | 0 of 203 |
| The Netherlands | c.896C→T (p.A299V) | Exon | 6 | 1 of 36 (2.8) | Acromegaly–GHoma | NA | F | 16 | No | 0 of 255 |
ACTHoma, ACTH-secreting adenoma; F, female; GHoma, GH-secreting adenoma; M, male; NA, not available.
*Only putative pathogenic changes are depicted.
†Age at time of operation; age at time of diagnosis is not known.
Fig. 1.
Diagram of AIP displaying the presumably pathogenic mutations identified in this study. The locations of the FKBP-homology region and the three tetratricopeptide repeats (TPRs) are indicated by colored boxes. AHR and HSP90 interaction regions are indicated by black lines.
Young acromegaly patients.
In the German samples, two AIP mutations were identified (2 of 27, 7.4%; see Table 1). In addition, a heterozygous intronic change, IVS1-18C→T, was identified in one sample but was not predicted to have an effect on splicing as tested in silico. This same intronic change was also detected in 1 of 107 Centre d'Étude du Polymorphism Humain controls (1%) and 1 of 96 Caucasian U.K. controls (1%), suggesting that the variant is a polymorphism.
From the Helsinki University Central Hospital (HUCH) patient cohort, 36 Finnish patients were analyzed. The Finnish germ-line founder mutation was identified in two (5.5%; see Table 1) patients.
Unselected acromegaly patients.
Among the 71 Italian sporadic acromegaly patients, one heterozygous germ-line missense change was found: p.R16H (c.47G→A, resulting in the substitution of arginine at position 16 by histidine). Loss-of-heterozygosity analysis from this individual's pituitary tumor tissue was negative. p.R16H was absent in 181 Caucasian U.K., 52 Italian, and 209 Finnish controls. The change was found in 1 of 90 healthy German controls (1%), 1 unselected pituitary adenoma patient from the United States, and in 3 Polish unselected pituitary adenoma patients (see below), suggesting that this change may be a neutral polymorphism.
Unselected pituitary adenoma patients.
AIP mutation analysis performed in 113 unselected pituitary adenoma patients from the United States revealed two germ-line mutations (2 of 113, 1.8%; see Table 1) and two heterozygous missense changes that are likely to be polymorphisms. A heterozygous c.906G→A, resulting in the silent p.V302V change in exon 6, was found in three cases. When p.V302V was tested in silico, the prediction programs showed no significant effect on splicing. None of the three patients had a family history of pituitary adenomas. Loss-of-heterozygosity analysis was possible from tumor DNA samples of 2/3 individuals, and showed retention of the wild type allele. p.V302V was not detected in 109 Centre d'Étude du Polymorphism Humain, 94 Caucasian U.K., and 52 Italian controls. Finally, the previously seen missense change p.R16H in exon 1, was found in one individual: a 78 year-old woman of Polish descent. Tumor DNA sequence did not show loss of heterozygosity.
In 122 unselected pituitary adenoma patients from Poland, three different germ-line heterozygous missense changes were detected, of which one was considered disease associated (1 of 122, 0.8%; Table 1). In addition, the previously detected p.R16H change was seen also in three Polish individuals all diagnosed with Cushing's disease. A heterozygous c.696G→C, resulting in the silent p.P232P change in exon 5, was found in one patient with Cushing's disease. This change did not have any predicted effect on splicing as tested in silico. p.P232P was absent in 108 Centre d'Étude du Polymorphism Humain or 95 Caucasian U.K. controls.
Patients counseled and examined for MEN1 with negative genetic testing results.
In the 55 Spanish samples analyzed, one AIP mutation was identified (1 of 55, 1.8%; see Table 1). Likewise, of the 36 Dutch samples, an AIP germ-line mutation was identified in one specimen (1 of 36, 2.8%; see Table 1).
AIP Immunohistochemical Staining.
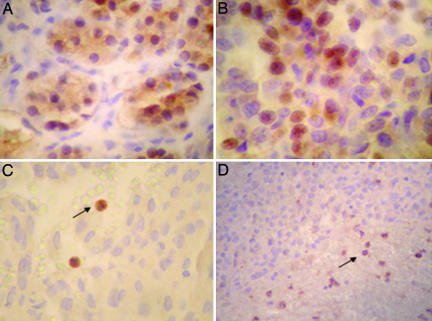
AIP immunoreaction was observed in both the cytoplasm and the nucleus in normal adenohypophysis (Fig. 2A). Most AIP mutation-negative adenomas (36 of 38) had preserved cytoplasmic and nuclear immunoreaction against AIP (Fig. 2B), whereas most AIP-deficient adenomas (9 of 12) lacked both cytoplasmic and nuclear immunoreactivity against AIP (Fisher's Exact test, P = 0.000004). In tumor tissues, leukocytes served as internal positive controls (Fig. 2 C and D). AIP IHC had 75% sensitivity and 95% specificity for truncating AIP germ-line mutations.
Fig. 2.
AIP IHC. (A) AIP immunoreaction is observed in both the cytoplasm and the nucleus in normal adenohypophysis. (B) AIP expression in AIP-proficient adenoma. (C and D) AIP-deficient adenomas from two acromegaly patients bearing the p.Q14X mutation. There is complete loss of immunoreaction in adenoma cells, whereas AIP heterozygous peripheral blood leucocytes display positive immunoreaction, indicated by black arrows.
Discussion
In our original study, we evaluated the contribution of AIP in a population-based material of acromegaly patients, diagnosed between 1980 and 1999 in the Oulu region of Northern Finland. In this isolated population, two germ-line AIP mutations (p.Q14X and IVS3-1G→A) accounted for 16% of all patients diagnosed with pituitary adenomas secreting GH and for 40% of patients that were younger than 35 years at the age of diagnosis (8). In the current study, we examined the role of AIP in more heterogeneous patient groups to provide clues to clinical and molecular identification of PAP.
The analysis of 71 Italian acromegaly patients systematically collected from the Treviso region revealed no significant findings, but two siblings (2 of 73, 2.7%) belonging to this same sample collection were shown to display a truncating AIP mutation in our previous study (8).
In both of the two patient sets with acromegaly that presented at a young age, the Helsinki and Leipzig regions, two PAP patients were detected; altogether, 4 of 63 (6.3%) patients had PAP.
Two putative AIP mutations were found among unselected pituitary adenoma cases from the United States. IVS2-1G→C splice site mutation was detected in a patient diagnosed with acromegaly at the age of 20 years. c.824–825insA insertion, also causing a premature stop codon, was seen in a patient diagnosed with GH-secreting adenoma at the age of 8 years. These two cases account for 1.8% of the unselected pituitary adenoma cases in this series. The number of acromegaly patients in the sample set was only 13. Although the numbers are very small, it is noteworthy that the contribution of PAP in acromegaly in this consecutive U.S. series was similar to that seen in Northern Finland (2 of 13, 15%).
p.R304Q change was seen in one Polish unselected pituitary adenoma patient (1 of 122, 1%). If pathogenic, this AIP mutation would be previously unrecognized in Cushing's disease. We have detected this variant in an Italian patient with acromegaly (M.G., A.R., A.K., E.D.M., V.L., P.V., and L.A.A., unpublished data) but in none of the healthy controls, which strongly supports the notion that the change is pathogenic. Interestingly, p.R304Q locates on the AHR-binding region (13, 14) and possibly affects the interaction of AIP with AHR.
Approximately 10% of cases clinically suggestive of MEN1 do not seem to harbor germ-line MEN1 mutations (4, 15–17). Thus, we examined whether AIP is involved in such cases of unexplained endocrine neoplasia susceptibility. Two mutation-positive cases were found (2 of 91, 2%). The phenotypes of these two patients were in line with the above findings; both the Spanish and the Dutch patient were diagnosed with acromegaly at an early age: 18 and 16 years, respectively. The Spanish patient also had a positive family history, with two maternal uncles being diagnosed with acromegaly.
These data firmly confirm that AIP is directly implicated in the molecular pathogenesis of pituitary tumors, particularly of the GH/PRL lineage. In the future, it will be of interest to examine what the contribution of de novo mutations in PAP is. In the current work, the relevant additional samples were not available. The prevalence of AIP germ-line mutations varies in different clinical settings. In unselected sporadic pituitary tumors, the overall prevalence seems to be low: 2 of 113 and 1 of 122 from our U.S. and Poland cases, respectively. Also, none of the previously reported p.Q14X, p.R304X, and IVS3-1G→A mutations were found in a recently published U.S. series (18). On the contrary, AIP mutations are enriched in patients of a very young age at onset and/or a positive family history of acromegaly, although some of the PAP patients display neither of these features. Selection of patients for genetic counseling and possible genetic testing for PAP appeared challenging.
To help simplify selection, we tested AIP IHC in 50 pituitary adenomas as a tool for molecular screening for PAP. Because many mutations are truncating and because the condition is typically associated with loss of the wild-type allele in tumors, a strategy similar to that used to screen patients for hereditary nonpolyposis colorectal cancer or Lynch syndrome (19) appeared attractive: IHC screening of tumors for loss of the predisposition gene product, genetic counseling, and possible germ-line mutation testing in cases displaying negative IHC for AIP, followed by cascade screening in family members to identify individuals at risk. Because pituitary adenomas are examined immunohistochemically as a routine diagnostic practice, this screening method appears to be feasible. Indeed, we found that negative AIP IHC staining is a strong predictor of PAP. Two cases with a negative AIP mutation analysis but a negative IHC result may have had occult (such as deletion not detected by sequencing) germ-line mutations or somatic loss of AIP, although technical problems are also a possible explanation. The individuals had no family history of endocrine tumors but were 40 and 47 years of age, and, thus, PAP is possible. Similarly, in the three mutation-positive cases displaying positive AIP IHC, the unexpected IHC finding could have been due to technical difficulties such as unspecific staining or missense-type second hits enabling production of nonfunctional yet immunoreactive AIP protein. It is likely that, with greater experience with this approach, the ability to screen for AIP alterations by IHC will further improve. Although the cost of DNA sequencing will be reduced dramatically in the near future, IHC screening will remain useful, because direct DNA testing for AIP germ-line mutations would require prior genetic counseling. Genetic counseling demands resources and needs to be reserved for those pituitary adenoma cases that display features of hereditary susceptibility.
The annual incidence of newly diagnosed cases of acromegaly ranges from 2.8 to >4 per million, with prevalences ranging from 34 to 120 cases per million (20). Although these observations suggest that ≈1,000 new cases will be diagnosed annually in the United States, the insidious nature of acromegaly and the frequent delays in diagnosis in this disease have led to estimates that GH-secreting pituitary adenomas are present but undiagnosed in as many as 20,000 persons in the Unites States alone (21). Because the majority of patients present with a macroadenoma, and younger patients frequently have larger, more invasive tumors with poorer outcomes, the potential for prolonged biochemical remission with any single modality is diminished (22, 23). In patients with more advanced disease, successful therapy eliminates or resolves all manifestations of the disease in a minority, and diminished quality-of-life persists in the majority (24). Overall morbidity and mortality, related primarily to chronic cardiovascular disease, is a function of biochemical control: risk of mortality may be as high as 3.5-fold greater in patients with persistent disease compared with those in remission (22, 23, 25–28). Although molecular diagnosis of PAP in unselected pituitary adenomas would be first performed in a research setting, thousands of paraffin-embedded somatotropinoma samples in the United States alone are available for prescreening of PAP by AIP IHC, pending consent from the patients. Although current management of patients with pituitary adenomas does not seem to be influenced by diagnosed AIP mutation positivity, offering genetic counseling and predictive testing to family members provides a powerful tool for prevention of morbidity in at-risk individuals. The great majority of AIP-related tumors are GH- and/or PRL-secreting adenomas, and the clinical diagnosis of acromegaly at onset is difficult. Therefore, we suggest that asymptomatic relatives testing positive for an AIP mutation should undergo annual PRL and IGF1 monitoring (29), as suggested for MEN1 carriers (30). Finally, we highlight the particular importance of AIP analyses in patients with a positive family history for acromegaly or with early onset of the tumor.
Materials and Methods
Study Subjects.
The study was approved by the appropriate ethics review committees. Appropriate informed consent was obtained from all subjects.
Young acromegaly patients.
DNA extracted from paraffin-embedded tumor tissue was available from 27 patients with acromegaly from the German pituitary tumor register, Institute of Pathology, Marienkrankenhaus Hamburg. The search was conducted for entries during the last 3 years for patients younger than 40 years old at the time of surgery.
DNA derived from blood was available from 36 Finnish acromegaly patients who were <45 years old and originally diagnosed and treated at the Department of Endocrinology, Helsinki University Central Hospital (HUCH). This cohort represented 57.1% of all young (<45 years) acromegaly patients diagnosed at HUCH between the years 1980–2005.
Unselected acromegaly patients.
Blood-extracted DNA samples from 71 unselected Italian acromegaly patients who were referred to Treviso General Hospital were available. Age at diagnosis ranged between 23 and 90 years, with a mean age of 45 years.
Unselected pituitary adenoma patients.
Altogether, 113 samples collected consecutively from patients undergoing resection of a pituitary tumor at the Cleveland Clinic were analyzed. DNA was isolated from either blood or tumor tissue. Age at diagnosis ranged between 8 and 87 years, with a mean age of 52 years. Of these 113 patients, all underwent biochemical and immunohistochemically confirmed diagnoses: 13 with Cushing's disease, 13 with acromegaly due to GH-secreting adenomas, 11 with hyperprolactinemia due to PRL-secreting adenomas. The remaining 76 patients had a nonfunctioning pituitary adenoma. None of the patients had a family history of pituitary tumors.
Blood-extracted DNA samples from 122 unselected Polish pituitary adenoma patients were collected at the International Hereditary Cancer Center, Pomeranian Medical University, in Szczecin, Poland. Of these, 74 patients were diagnosed with Cushing's disease, 30 with acromegaly, and 18 with pituitary adenomas of various types. Age of onset ranged between 8 and 67 years, with a mean age of 39 years. Age of onset was not known for 21 cases.
Patients counseled and examined for MEN1 with negative genetic testing results.
Blood-extracted DNA from 36 Dutch patients was analyzed. Patients had been referred to the DNA Diagnostics Laboratory (Department of Medical Genetics, University Medical Centre Utrecht, The Netherlands) for MEN1 molecular diagnostics, during the period of 2004–2006. Patients suspected for MEN1 were defined as those with at least three of the following five lesions: hyperparathyroidism/parathyroid tumors, pancreatic endocrine tumors, pituitary adenomas, adrenal gland tumors, and/or neuroendocrine carcinoid tumors. The patients fulfilled the following criteria: young age at onset (<35 years) of any of the five MEN1-related lesions and/or multiple MEN1-related lesions in a single organ or two distinct organs, and at least one first-degree relative in whom at least one target organ was affected. Age at tumor diagnosis ranged between 15 and 81 years, with a mean age of 50 years.
Another set consisted of individuals suspected for MEN1 and referred to the Spanish National Cancer Center during the period of 1997–2006. Blood-extracted DNA samples from 55 unselected and consecutive MEN1-negative patients were available for AIP mutation analysis. Age at diagnosis ranged between 12 and 78 years, with a mean age of 50 years. Information was not available for two patients.
Control Samples.
DNA from unrelated, anonymous, individuals was used as control samples: 110 Caucasian Centre d'Étude du Polymorphism Humain individuals, 288 Caucasians from the U.K. (Human Random Control DNA Panels, Porton Down, Salisbury, Wiltshire, U.K.), 209 Finns, 90 Germans, and 52 Italians.
IHC Samples.
AIP protein expression was analyzed in 50 pituitary adenomas. Twelve tumors were from AIP mutation-positive individuals (nine cases with p.Q14X, and one case with IVS2-1G→C, c.824–825insA, and IVS3-1G→A, respectively) including 10 somatotropinomas and two prolactinomas. Thirty-eight mutation-negative adenomas included 32 somatotropinomas, five prolactinomas, and one GH- and PRL-negative adenoma.
Mutation Analysis.
Mutation analysis was performed by direct sequencing of genomic DNA. The whole coding region of AIP was sequenced, as well as flanking intronic sequences and 5′ and 3′ untranslated regions. PCR protocols and primer sequences have been described by Vierimaa et al. (8) and are available on request. DNA sequencing was performed using Big Dye 3.1 termination chemistry on an ABI3730 DNA sequencer (Applied Biosystems, Foster City, CA).
In Silico Analysis.
The potential effects on splicing of the detected intronic and silent changes were predicted in silico by computational methods by using NetGene2, Alternative Splice Site Predictor (ASSP), and SpliceScan programs (31–34).
AIP IHC.
Five-micrometer-thick sections were cut from the paraffin blocks. After deparaffinization and rehydration, sections were pretreated in either 0.01 M citrate (pH 6.0) buffer in a microwave oven at 800 W for 2 min and at 300 W for 10 min or in 0.01 M Tris-EDTA (pH 6.0) buffer in a microwave oven at 800 W for 2 min and at 300 W for 15 min. AIP was detected in tumors by using AIP antibody (AIP SP5213P; Acris Antibodies, Hiddenhausen, Germany) at a 1:4,000 dilution for 30 min. Positive antibody reaction was detected with diaminobenzidine (DAKO, Copenhagen, Denmark) with hematoxylin counterstain.
Acknowledgments
We thank all patients for their valuable help. We are grateful to R. Lehtonen for help with the in silico analysis; S. Marttinen, I. L. Svedberg, I. Vuoristo, P. Hannuksela, M. Aho, and R. Vuento for their excellent technical assistance; P. Ellonen for providing sequencing facilities and service; and D. K. Luedecke (University of Hamburg, Germany) for providing material and clinical data. This study was supported by Academy of Finland Grants 213183 (to V.L.) and 212901 (to P.V.), the Center of Excellence in Translational Genome-Scale Biology, the Sigrid Jusélius Foundation, the Cancer Society of Finland, Association for International Cancer Research Grant 05-001 (to A.K.), a Jalmari and Rauha Ahokas Foundation research grant (to M.G.), a Bodossaki Foundation postgraduate scholarship (to M.G.), the Melvin Burkhardt Chair in Neurosurgical Oncology, and the Karen Colina Wilson Research Endowment within the Brain Tumor Institute at the Cleveland Clinic Foundation.
Abbreviations
- AHR
aryl hydrocarbon receptor
- AIP
aryl hydrocarbon receptor-interacting protein
- GH
growth hormone
- IHC
immunohistochemistry
- MEN1
multiple endocrine neoplasia type 1
- PAP
pituitary adenoma predisposition
- PRL
prolactin.
Footnotes
References
- 1.Heaney AP, Melmed S. Nat Rev Cancer. 2004;4:285–295. doi: 10.1038/nrc1320. [DOI] [PubMed] [Google Scholar]
- 2.Melmed S. J Clin Invest. 2003;112:1603–1618. doi: 10.1172/JCI20401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arafah BM, Nasrallah MP. Endocr Relat Cancer. 2001;8:287–305. doi: 10.1677/erc.0.0080287. [DOI] [PubMed] [Google Scholar]
- 4.Daly AF, Jaffrain-Rea ML, Beckers A. Horm Metab Res. 2005;37:347–354. doi: 10.1055/s-2005-870135. [DOI] [PubMed] [Google Scholar]
- 5.Daly AF, Jaffrain-Rea ML, Ciccarelli A, Valdes-Socin H, Rohmer V, Tamburrano G, Borson-Chazot C, Estour B, Ciccarelli E, Brue T, et al. J Clin Endocrinol Metab. 2006;91:3316–3323. doi: 10.1210/jc.2005-2671. [DOI] [PubMed] [Google Scholar]
- 6.Frohman LA, Eguchi K. Growth Horm IGF Res. 2004;14(Suppl A):90–96. doi: 10.1016/j.ghir.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 7.Pellegata NS, Quintanilla-Martinez L, Siggelkow H, Samson E, Bink K, Hofler H, Fend F, Graw J, Atkinson MJ. Proc Natl Acad Sci USA. 2006;103:15558–15563. doi: 10.1073/pnas.0603877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vierimaa O, Georgitsi M, Lehtonen R, Vahteristo P, Kokko A, Raitila A, Tuppurainen K, Ebeling TM, Salmela PI, Paschke R, et al. Science. 2006;312:1228–1230. doi: 10.1126/science.1126100. [DOI] [PubMed] [Google Scholar]
- 9.Carver LA, Bradfield CA. J Biol Chem. 1997;272:11452–11456. doi: 10.1074/jbc.272.17.11452. [DOI] [PubMed] [Google Scholar]
- 10.Bolger GB, Peden AH, Steele MR, MacKenzie C, McEwan DG, Wallace DA, Huston E, Baillie GS, Houslay MD. J Biol Chem. 2003;278:33351–33363. doi: 10.1074/jbc.M303269200. [DOI] [PubMed] [Google Scholar]
- 11.Sumanasekera WK, Tien ES, Turpey R, Vanden Heuvel JP, Perdew GH. J Biol Chem. 2003;278:4467–4473. doi: 10.1074/jbc.M211261200. [DOI] [PubMed] [Google Scholar]
- 12.Kang BH, Altieri DC. J Biol Chem. 2006;281:24721–24727. doi: 10.1074/jbc.M603175200. [DOI] [PubMed] [Google Scholar]
- 13.Bell DR, Poland A. J Biol Chem. 2000;275:36407–36414. doi: 10.1074/jbc.M004236200. [DOI] [PubMed] [Google Scholar]
- 14.Petrulis JR, Perdew GH. Chem Biol Interact. 2002;141:25–40. doi: 10.1016/s0009-2797(02)00064-9. [DOI] [PubMed] [Google Scholar]
- 15.Hai N, Aoki N, Shimatsu A, Mori T, Kosugi S. Clin Endocrinol (Oxford) 2000;52:509–518. doi: 10.1046/j.1365-2265.2000.00966.x. [DOI] [PubMed] [Google Scholar]
- 16.Marx SJ. Nat Rev Cancer. 2005;5:367–375. doi: 10.1038/nrc1610. [DOI] [PubMed] [Google Scholar]
- 17.Cebrian A, Ruiz-Llorente S, Cascon A, Pollan M, Diez JJ, Pico A, Telleria D, Benitez J, Robledo M. J Med Genet. 2003;40:e72. doi: 10.1136/jmg.40.5.e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu R, Bonert V, Saporta I, Raffel LJ, Melmed S. J Clin Endocrinol Metab. 2006;91:5126–5129. doi: 10.1210/jc.2006-1731. [DOI] [PubMed] [Google Scholar]
- 19.Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, Nakagawa H, Sotamaa K, Prior TW, Westman J, et al. N Engl J Med. 2005;352:1851–1860. doi: 10.1056/NEJMoa043146. [DOI] [PubMed] [Google Scholar]
- 20.Stewart PM. Eur J Endocrinol. 2004;151:431–432. doi: 10.1530/eje.0.1510431. [DOI] [PubMed] [Google Scholar]
- 21.Melmed S. In: Endocrinology. DeGroot LJ, Jameson JL, editors. Philadelphia: Elsevier Saunders; 2006. pp. 411–428. [Google Scholar]
- 22.Drange MR, Fram NR, Herman-Bonert V, Melmed S. J Clin Endocrinol Metab. 2000;85:168–174. doi: 10.1210/jcem.85.1.6309. [DOI] [PubMed] [Google Scholar]
- 23.Besser GM, Burman P, Daly AF. Eur J Endocrinol. 2005;153:187–193. doi: 10.1530/eje.1.01968. [DOI] [PubMed] [Google Scholar]
- 24.Biermasz NR, Pereira AM, Smit JW, Romijn JA, Roelfsema F. J Clin Endocrinol Metab. 2005;90:2731–2739. doi: 10.1210/jc.2004-2297. [DOI] [PubMed] [Google Scholar]
- 25.Holdaway IM, Rajasoorya RC, Gamble GD. J Clin Endocrinol Metab. 2004;89:667–674. doi: 10.1210/jc.2003-031199. [DOI] [PubMed] [Google Scholar]
- 26.Colao A, Ferone D, Marzullo P, Lombardi G. Endocr Rev. 2004;25:102–152. doi: 10.1210/er.2002-0022. [DOI] [PubMed] [Google Scholar]
- 27.Kauppinen-Makelin R, Sane T, Reunanen A, Valimaki MJ, Niskanen L, Markkanen H, Loyttyniemi E, Ebeling T, Jaatinen P, Laine H, et al. J Clin Endocrinol Metab. 2005;90:4081–4086. doi: 10.1210/jc.2004-1381. [DOI] [PubMed] [Google Scholar]
- 28.Swearingen B, Barker FG, II, Katznelson L, Biller BM, Grinspoon S, Klibanski A, Moayeri N, Black PM, Zervas NT. J Clin Endocrinol Metab. 1998;83:3419–3426. doi: 10.1210/jcem.83.10.5222. [DOI] [PubMed] [Google Scholar]
- 29.Giustina A, Barkan A, Casanueva FF, Cavagnini F, Frohman L, Ho K, Veldhuis J, Wass J, Von Werder K, Melmed S. J Clin Endocrinol Metab. 2000;85:526–529. doi: 10.1210/jcem.85.2.6363. [DOI] [PubMed] [Google Scholar]
- 30.Brandi ML, Gagel RF, Angeli A, Bilezikian JP, Beck-Peccoz P, Bordi C, Conte-Devolx B, Falchetti A, Gheri RG, Libroia A, et al. J Clin Endocrinol Metab. 2001;86:5658–5671. doi: 10.1210/jcem.86.12.8070. [DOI] [PubMed] [Google Scholar]
- 31.Brunak S, Engelbrecht J, Knudsen S. J Mol Biol. 1991;220:49–65. doi: 10.1016/0022-2836(91)90380-o. [DOI] [PubMed] [Google Scholar]
- 32.Hebsgaard SM, Korning PG, Tolstrup N, Engelbrecht J, Rouze P, Brunak S. Nucleic Acids Res. 1996;24:3439–3452. doi: 10.1093/nar/24.17.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang M, Marin A. Gene. 2006;366:219–227. doi: 10.1016/j.gene.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 34.Tchourbanov A, Ali HH, Deogun J. Proceedings of the 2004 IEEE Computational Systems Bioinformatics Conference; Los Alamitos, CA: IEEE Comput Soc; 2004. pp. 672–673. [Google Scholar]