Abstract
The mammalian neocortex is organized into unique areas that serve functions such as sensory perception and modality-specific behaviors. The sizes of primary cortical areas vary across species, and also within a species, raising the question of whether area size dictates behavioral performance. We show that adult mice genetically engineered to overexpress the transcription factor EMX2 in embryonic cortical progenitor cells, resulting in reductions in sizes of somatosensory and motor areas, exhibit significant deficiencies at tactile and motor behaviors. Even increasing the size of sensorimotor areas by decreasing cortical EMX2 levels can lead to diminished sensorimotor behaviors. Genetic crosses that retain ectopic Emx2 transgene expression subcortically but restore cortical Emx2 expression to wild-type levels also restore cortical areas to wild-type sizes and in parallel restore tactile and motor behaviors to wild-type performance. These findings show that area size can dictate performance at modality-specific behaviors and suggest that areas have an optimal size, influenced by parameters of its neural system, for maximum behavioral performance. This study underscores the importance of establishing during embryonic development appropriate levels of regulatory proteins that determine area sizes, thereby influencing behavior later in life.
Keywords: cortex, EMX2, sensorimotor performance, cortical area patterning, somatosensory cortex
The mammalian neocortex is tangentially organized into areas that serve unique functions such as sensory perception and modality-specific behaviors. In humans, the primary areas, motor (M1), somatosensory (S1), and visual (V1), vary ≈2-fold in size in a smooth continuum (1–5). In mice, the sizes of V1 and the S1 barrelfield are very consistent within inbred strains of mice, but their mean sizes can differ between some inbred strains of mice (6).
These observations raise the question of whether the size of a cortical area influences behavioral performance. Normal humans exhibit significant differences in proficiency at visual psychophysical tasks (7), but these differences have not been correlated to differences in area size. Complementing these findings are studies of adult cortical plasticity. For example, repetitive use of a digit, or microstimulation of its cortical representation, results in an enlarged functional representation in M1 and S1 resulting in an increased overlap with functional representations of adjacent digits. These enlargements, which are evident physiologically but not anatomically, do not extend across area borders, do not increase area size, and have not been shown to affect systems-level behavior (8, 9).
Here we address whether differences in cortical area size influence modality-specific behaviors in adults by using genetic manipulations that act during embryonic development to alter cortical area size in a consistent, reproducible manner. Establishing a relationship between area size and behavioral performance could be predictive for behavioral differences between individuals and provide insight into the functional importance of cortical areas (2). These relationships could also provide insights into developmental cognitive disorders (10) and the potential for aberrant behavior due to polymorphisms that influence the expression of regulatory genes that control area patterning (11).
Area size is controlled in part by transcription factors, including the homeodomain protein EMX2 expressed by cortical progenitors, that specify during embryonic development the area identities of cortical progenitors and their neuronal progeny (12–14). Analyses of nestin-Emx2 transgenic (ne-Emx2) mice and heterozygous Emx2 knockout (Emx2+/−) mice, which have increased or decreased levels of Emx2 expression in cortical progenitors, respectively, show opposing changes in sizes of S1 and motor areas in mature mice (14). For example, in ne-Emx2 mice, increasing Emx2 expression in cortical progenitors by driving an Emx2 transgene under control of a neuron-specific nestin promoter results in a significant decrease in sizes of sensorimotor areas but has no effect on overall cortical size or lamination. Changes in EMX2 levels in cortical progenitors appear to result in a complete change in area identity of their neuronal progeny to match the properties appropriate for the area in which they reside (14) (A.L., S.-J.C., and D.D.M.O., unpublished work).
We used ne-Emx2 and Emx2+/− mice as models to test whether cortical area size influences behavioral performance. We focused on tests for behaviors that are modality-specific for sensorimotor areas because they are well established and better developed for mice than tests for cortically-mediated visual behavior (15). To validate our findings we carried out genetic “rescue” experiments by crossing ne-Emx2 and Emx2+/− mice to generate offspring that retain ectopic subcortical expression of the Emx2 transgene, as in ne-Emx2 mice, and have cortical Emx2 levels restored to wild-type (wt) levels.
Results
Mice Engineered to Have Smaller Sensorimotor Areas Have Diminished Performance on Tests of Tactile and Motor Behaviors.
To determine whether cortical area size correlates with behavioral performance, we initially tested, blind to genotype, adult male ne-Emx2 (n = 11) and matched wt mice (n = 11) of the same age range (10 to 15 weeks), experience and background. The sizes of S1 and motor areas in ne-Emx2 mice are reduced on average by 25% and 36%, respectively (14) (Fig. 1A). We first assessed general features that could affect performance on behavioral tests of sensorimotor skills, including body weight, mobility, and grip strength, and find ne-Emx2 mice to be similar to wt (see Experimental Procedures for details for all tests described in Results, and see the figure legends for data and statistics). In addition, performances of ne-Emx2 mice on auditory startle response and visual cliff tests are similar to wt, indicating that ne-Emx2 mice have functional audition and vision.
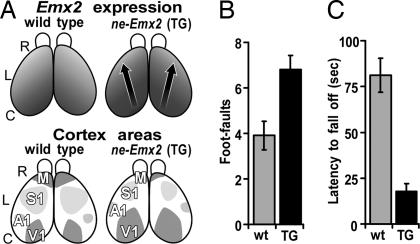
Fig. 1.
Reduction in sizes of sensorimotor areas in ne-Emx2 mice correlates with diminished performance on tests of tactile and motor behaviors. (A) Dorsal views of mouse neocortex to show relative levels of graded Emx2 expression and area patterning in wt and ne-Emx2 transgenic (TG) mice. R, rostral; L, lateral; C, caudal. (Upper) Emx2 expression in embryonic cortex. Arrows indicate shifts in area patterning. Darker shading indicates higher Emx2 expression. (Lower) Size and position of primary sensory areas, somatosensory (S1), visual (V1), auditory (A1), and motor area (M), in adult cortex. Compared with wt, M and S1 are reduced in size in ne-Emx2 mice. Overall cortical size is the same as in wt. (B) Grid walk. Mice walk over a wire mesh grid, and performance is analyzed (16). Analysis was done by counting the number of errors in foot placement (foot-faults) per 20 steps. The wt made significantly fewer foot-faults (3.9 ± 0.6, n = 11) than ne-Emx2 (TG) mice (6.8 ± 0.6, n = 11) (Student's t test, P = 0.0034). In addition, ne-Emx2 mice often fall off the grid, a behavior never seen in wt (see SI Movie 1). (C) Rotarod. Mice were placed on a rotating rod that smoothly accelerated from 5 to 70 rpm over 3 min, and the latency to fall off was measured as described (17). ne-Emx2 (TG) mice show a significantly reduced performance (average fall-off latency = 17.8± 4.4 s, n = 11) compared with wt (average fall-off latency = 81.2 ± 9.2 s, n = 11; Student's t test P = 4.29E-06) (see SI Movie 2).
In contrast, ne-Emx2 mice are significantly deficient on sensorimotor behavioral tests compared with wt. These tests include performance on a grid walk [Fig. 1B and supporting information (SI) Movie 1] (16) and a rotarod (Fig. 1C and SI Movie 2), which evaluate in distinct ways forelimb and hindlimb motor coordination and balance (17). These diminished performances are not due to weaker strength or aberrant neuromuscular function of the hindlimbs and forelimbs in ne-Emx2 mice, as confirmed by performance on the grip strength test (see data in Experimental Procedures).
These findings indicate that diminished performance at sensorimotor behaviors in ne-Emx2 mice is due to the reduced sizes of sensorimotor cortical areas. An alternative interpretation is that the behavioral deficiencies are due to subcortical defects produced by transient ectopic subcortical expression of the Emx2 transgene. However, this is unlikely because an influence of the Emx2 transgene is expected to be limited to neural structures that normally express Emx2, primarily dorsal telencephalon (i.e., embryonic cerebral cortex) with limited exceptions (18). Further, behavioral deficiencies exhibited by ne-Emx2 mice, for example on the rotarod test, originally designed to test cerebellar defects, are comparable to those in mice with severe anatomical defects in the cerebellum: mice must lose 90% or more of their cerebellar Purkinje cells and associated cell types before they exhibit a statistically significant deficiency in rotarod performance (19, 20). However, our anatomical and marker analyses do not reveal defects in the cerebellum or other subcortical structures (ref. 14 and data not shown).
Genetic Rescue Studies.
Crossing ne-Emx2 mice with Emx2+/− mice restores cortical Emx2 to wt levels and cortical areas to wt sizes, and retains ectopic subcortical Emx2 transgene expression.
It is not feasible to analyze in ne-Emx2 mice all subcortical structures and circuits potentially involved in mediating sensorimotor behaviors. Therefore, to address whether the behavioral deficiencies in ne-Emx2 mice are due to subcortical defects produced by ectopic subcortical expression of the Emx2 transgene, we performed a genetic “rescue” by crossing ne-Emx2 mice with Emx2+/− mice. Our goal was to generate mice homozygous for the Emx2 transgene and heterozygous for the endogenous wt Emx2 allele (referred to as ne-Emx2; Emx2+/−), which we predicted should have total cortical Emx2 restored to a level that approximates that in wt cortex, which should restore area sizes to wt, but have ectopic subcortical expression of the Emx2 transgene comparable to that in ne-Emx2 mice (Fig. 2A).
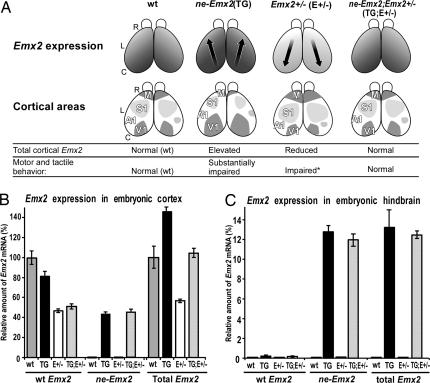
Fig. 2.
Rationale of genetic rescue experiments and quantification of Emx2 transcripts. (A) Schematic of rescue rationale. Description similar to Fig. 1A but includes two additional genotypes: Emx2 heterozygous mice (Emx2+/−) and ne-Emx2; Emx2+/− mice, homozygous for the ne-Emx2 transgene and heterozygous for the endogenous Emx2 allele. Relative levels of graded Emx2 expression are shown. In Emx2+/− mice, S1 and M areas are increased in size, whereas in ne-Emx2; Emx2+/− mice, the level of overall cortical Emx2 expression (endogenous and transgene) is similar to wt, and cortical area sizes revert toward wt. Motor and tactile behavioral performances are summarized. Emx2+/− mice show diminished performance in the grid walk and rotarod, albeit to a lesser degree than ne-Emx2 mice, but performance in the adhesive patch test is not statistically different from wt (indicated by an asterisk). (B and C) Emx2 expression level in embryonic day 12.5 embryos, an age of peak Emx2 expression, determined by quantitative real-time PCR. y-coordinate: amount of Emx2 mRNA, normalized to an endogenous reference (GABDH mRNA) and relative to total Emx2 mRNA in embryonic day 12.5 wt cortex. Sources of mRNA: wt, ne-Emx2 (TG), Emx2+/− (E+/−), and ne-Emx; Emx2+/− (TG;E+/−) mice. Levels were determined independently for the mRNA transcribed from the endogenous Emx2 gene (wt Emx2 transcript), from the ne-Emx2 transgene (ne-Emx2 transcript), and for the pooled mRNA derived from both genes (total Emx2 transcripts). (B) Embryonic cortex. Compared with wt, total Emx2 mRNA is increased by 43 ± 3.3% (P = 0.002) in ne-Emx2 mice and reduced to 45 ±1.5% in Emx2+/− mice (P = 0.00006). In ne-Emx2; Emx2+/− (TG;E+/−) mice, total Emx2 mRNA (104 ± 6.9%) is statistically indistinguishable from wt (P = 0.64). (C) In hindbrain [and other subcortical regions, e.g., spinal cord (data not shown)], total Emx2 mRNA in ne-Emx2; Emx2+/− mice (12.44 ± 0.35%) is indistinguishable (P = 0.69) from ne-Emx2 mice (13.21 ± 2.01%). Endogenous Emx2 mRNA is not detectable in hindbrain of wt and Emx2+/− mutant embryos. (Values from three independent experiments, error bars represent ± SEM, P values from unpaired two-tailed Student's t test.)
If the behavioral deficiencies exhibited by ne-Emx2 mice are due to subcortical defects, then the subcortical defects and the behavioral deficiencies would persist in ne-Emx2; Emx2+/− mice regardless of any affect on cortical area size because of the retained subcortical expression of the Emx2 transgene. However, if the behavioral deficiencies exhibited by ne-Emx2 mice are due to the reduction in sensorimotor areas, then if the cross restores cortical areas to their normal sizes, it should also restore the deficient sensorimotor performance to normal wt levels (Fig. 2A).
Quantification of Emx2 transcripts using real time PCR at embryonic day 12.5, near the peak of Emx2 expression, confirms our predictions for Emx2 expression (Fig. 2 B and C). Emx2 transcripts are significantly decreased in cortex of Emx2+/− mice whereas the combined level of endogenous and transgene Emx2 transcripts are significantly increased in cortex of ne-Emx2 mice compared with endogenous Emx2 transcripts in wt cortex. In contrast, the combined level of Emx2 transcripts in ne-Emx2; Emx2+/− cortex is statistically indistinguishable from wt. Subcortical expression of the Emx2 transgene in ne-Emx2; Emx2+/− mice is statistically indistinguishable from ne-Emx2 mice.
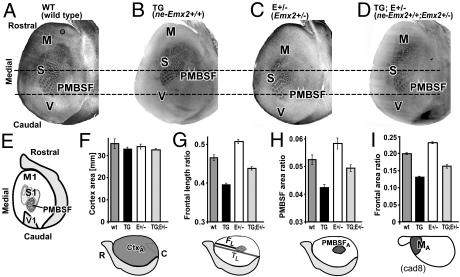
Measurements of area sizes were done on cytochrome oxidase (CO) and serotonin-stained tangential sections of 8–17 postnatal day 7 mice of the four genotypes obtained as littermates from the same set of crosses. Compared with wt, ne-Emx2 mice and Emx2+/− mice exhibit opposing changes in sizes of sensorimotor areas, although overall cortical size is similar to wt (Figs. 2A and 3). In contrast, ne-Emx2; Emx2+/− mice show a significant “rescue” of sizes of S1 and motor areas, which revert toward their wt sizes (Fig. 3).
Fig. 3.
Genetic crosses of ne-Emx2 transgenic and Emx2+/− heterozygous mice restore cortical Emx2 expression to wt levels and restore cortical area sizes toward wt sizes. (A–D) Tangential sections through layer 4 of flattened mouse cortices at postnatal day 7, stained for cytochrome oxidase to reveal area patterning landmarks. Sections are from four different genotypes that express different levels of EMX2 encoding transcripts in progenitor cells of the cortex during development (see Fig. 2B). (A) Wild type. (B) ne-Emx2+/+ transgenics. (C) Emx2+/− heterozygotes. (D) ne-Emx2+/+; Emx2+/− double mutants. CO staining patterns reveal differences in sizes and positions of cortical areas between genotypes. Horizontal lines across the sections support visualizing those changes. M, motor cortex; S, somatosensory cortex; PMBSF, posteromedial barrel subfield of somatosensory cortex; V, visual cortex. For a schematic of the area size changes, see Fig. 2A; for quantification, see F–I. (E) Schematic of flattened cortical hemisphere: indicated area landmarks, as revealed by CO and serotonin staining (serotonin sections not shown), were used for the area measurements and quantifications shown in F–I. Gray shaded area marks nonneocortical regions (olfactory bulb, rhinal cortex). (F–I) Histograms of cortical sizes, cortical area ratios, and cortical length ratios at postnatal day 7 of wt, TG (ne-Emx+/+), E+/− (Emx2+/−), and TG;E+/− (ne-Emx+/+; Emx2+/−) mice. Schematics below histograms relate to the schematic in E and indicate the specific measurements performed. (F) Overall surface area of neocortex in millimeters. Overall surface areas are not significantly different from wt (wt: 35.3 ± 2.1, n = 17; TG: 32.9 ± 0.9, n = 13; E+/−: 34.0 ± 1.1, n = 9; TG;E+/−: 32.6 ± 0.7, n = 13). All P values are >0.14 (unpaired Student's t test). (G) Frontal length ratio, defined as the ratio of FL (length between the rostral edge of PMBSF and the rostral pole of neocortex) to TL (total length of neocortex from rostral pole to occipital pole). Compared with wt (n = 13), the frontal ratio is significantly decreased in TG (−15 ± 0.8%∗∗, n = 12), significantly increased in E+/− (+9 ± 1.42%∗∗, n = 8), and reverts toward wt in TG;E+/− (−6 ± 1.17%∗, n= 11). (H) PMBSF area ratio, defined as the ratio of PA (area of PMBSF) to TA (area of the entire neocortex). Compared with wt (n + 8), the PMBSF ratio is significantly decreased in TG (−21 ± 2.3%∗∗, n = 6), significantly increased in E+/− (+11 ± 3.7%∗∗, n = 8), and reverts toward wt in TG;E+/− (−6 ± 2.6%, P = 0.0771; n = 8). (I) Frontal area ratio, defined as the ratio of MA [area covered by the rostral (motor) cad8 expression domain] to TA (area of entire cortex). Compared with wt (n = 6), the size of the rostral motor domain is significantly reduced in TG (−34 ± 1.7%∗∗, n = 9), significantly increased in E+/− (+15 ± 1.5%∗∗, n = 6), and reverts towards wt in TG;E+/− (−18 ± 2.7%∗∗, n = 9). Measurements were performed on whole mounts of cad8 in situ hybridization without flattening of the hemisphere. (Scale bar in A for A–D: 1 mm.) Bars in the histograms represent the means, and error bars represent SEM. Symbols used in this legend indicate the following: %, percent increase or decrease relative to wt; ±, SEM; n, number of cases: ∗, P < 0.05; ∗∗, P < 0.01 (unpaired Student's t tests).
We extended these findings by analyzing with whole-mount in situ hybridization in postnatal day 7 mice, the size of the rostral cortical expression domain of cadherin8 (cad8), a marker of the cortical motor field (21). Compared with wt (n = 6), the rostral cad8 motor domain is significantly decreased in ne-Emx2 mice (n = 9), is significantly increased in Emx2+/− mice (n = 6), and is restored in ne-Emx2; Emx2+/− mice (n = 9) to a size similar to wt (Fig. 3I).
In conclusion, the reduced sizes of sensorimotor areas in ne-Emx2 mice and their increased sizes in Emx2+/− mice are restored to wt sizes in ne-Emx2; Emx2+/− mice, which have levels of cortical Emx2 expression similar to wt, and ectopic subcortical expression of the Emx2 transgene similar to ne-Emx2 mice.
Deficiencies in behavioral performance in ne-Emx2 mice and Emx2+/− mice are rescued in ne-Emx2; Emx2+/− mice.
To test whether the rescue of area sizes results in a rescue of the behavioral deficiencies, even though ectopic subcortical Emx2 transgene expression is retained, forty 10- to 15-week-old male mice of the four different genotypes (ten of each genotype), obtained as littermates from the same set of crosses of ne-Emx2 mice with Emx2+/− mice, were behaviorally tested blind to genotype. Similar to the original group of ne-Emx2 mice tested (Fig. 1), the ne-Emx2 mice generated by these crosses have smaller sensorimotor areas (Fig. 3) and exhibit statistically significant deficiencies in the grid walk (Fig. 4A) and rotarod tests (Fig. 4B) compared with wt.
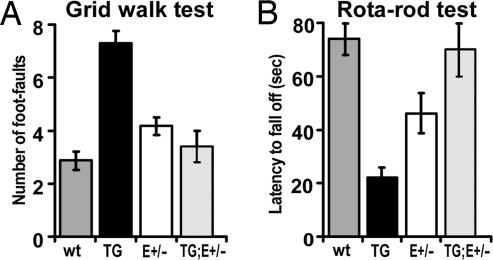
Fig. 4.
Sensorimotor behavioral deficiencies in ne-Emx2 mice are rescued by crosses that rescue area sizes but retain full level of subcortical Emx2 transgene expression. Forty adult male mice, ten of each of the four genotypes indicated and littermates from the same breeding group, were analyzed blind for behavioral performance at grid walk (A) and rotarod (B). Tests were done as described in Fig. 1. Mice are from a different breeding group than those tested in Fig. 1. Data were analyzed with unpaired Student's t test and one-way ANOVA followed by post hoc Dunnett's and Fisher (LSD) multiple comparison tests. Mean values with standard errors are presented. (A) Grid walk. The four genotypes performed significantly different (ANOVA, P = 1E-07, F = 26.10, Fcritical = 3.01). The ne-Emx2 mice (TG) (7.3 ± 0.4) made significantly more foot-faults than wt (2.86 ± 0.318) (t test and ANOVA: P < 0.0001). Emx2+/− mice (E+/−) (4.17 ± 0.3) performed worse than wt (t test, P = 0.0156; ANOVA, P = 0.0490). In contrast, ne-Emx2; Emx2+/− rescue mice (TG;E+/−) (3.4 ± 0.6) were indistinguishable from wt (t test, P = 0.46; ANOVA, P = 0.42) and performed significantly better than ne-Emx2 (TG) mice (t test, P = 0.0007; ANOVA, P < 0.0001). (B) Rotarod. Depending on the genotype, animals performed differently at this task (one-way ANOVA, P = 2.69E-05, F = 12.93, Fcritical = 2.99). The ne-Emx2 mice (TG) fell off the rod with an average latency of 21.6 ± 3.28 s, demonstrating a severe impairment in performance compared with wt (73.79 ± 5.38 s) (t test, P < 0.0001; ANOVA, P < 0.0001). Emx2+/− mice (E+/−) (46.25 ± 7.6 s) also performed significantly poorer than wt (t test, P = 0.0160; ANOVA, P = 0.0061). In contrast, ne-Emx2; Emx2+/− rescue mice (TG;E+/−) (69.6 ± 9.91 s) perform as well as wt (t test, P = 0.73; ANOVA, P = 0.66) and significantly better than ne-Emx2 mice (TG) (t test, P = 0.0073; ANOVA, P < 0.0001).
Surprisingly, the Emx2+/− mice, which have larger sensorimotor areas than wt (Fig. 3), also have statistically significant deficient behaviors in the grid walk and rotarod tests compared with wt (Fig. 4 A and B), albeit less severe than ne-Emx2 mice. Because expression of endogenous Emx2 is largely limited to the cerebral cortex (18), the behavioral deficiencies in Emx2+/− mice (Fig. 2B) almost certainly have a cortical locus and are not due to defects in subcortical sensorimotor structures (e.g., cerebellum that does not express Emx2).
In contrast to deficiencies of ne-Emx2 mice and Emx2+/− mice in tactile and motor behaviors, ne-Emx2; Emx2+/− mice exhibit performances at the grid walk (Fig. 4A) and rotarod tests (Fig. 4B) that are statistically indistinguishable from wt. Thus, the reduction in the sizes of sensorimotor areas and the diminished sensorimotor behavioral performances found in ne-Emx2 mice are rescued in parallel in ne-Emx2; Emx2+/− mice (Figs. 3 and 4). Because the Emx2 transgene is still expressed subcortically in ne-Emx2; Emx2+/− mice at levels that match those in ne-Emx2 mice (Fig. 2C), any potential defects caused by subcortical transgene expression would also be present in ne-Emx2; Emx2+/− mice. Therefore, the diminished tactile and motor behaviors in ne-Emx2 mice cannot be due to subcortical defects and must be related to the reduced sizes of S1 and motor areas.
The behavioral rescue in the performance of ne-Emx2; Emx2+/− mice on the grid walk and rotarod tests is particularly compelling because these mice are generated by a cross between ne-Emx2 mice and Emx2+/− mice, each of which exhibit significantly diminished performance on both of these tests (Fig. 4). The parallel rescue of behavioral deficiencies and area size in ne-Emx2; Emx2+/− mice also supports the conclusion that the deficient behavioral performances in Emx2+/− mice are due to increases in the sizes of sensorimotor areas compared with wt.
A Unique Sensorimotor Test Corroborates Major Findings from Grid Walk and Rotarod Tests.
To probe behaviors using a distinct type of sensorimotor test, we analyzed the performance of the same forty mice on the adhesive patch test, which provides a unique test of tactile perception as well as coordination between the hindlimbs, head and jaws (see Experimental Procedures and SI Movie 3). Compared with wt, ne-Emx2 mice are significantly less likely to initiate contact with the patch (Fig. 5A), suggesting a sensory perception deficit, and are significantly less likely to remove it, indicative of sensorimotor impairment (Fig. 5B). In addition, ne-Emx2 mice take significantly longer than wt to contact (Fig. 5C) and remove (Fig. 5D) the patch, again indicating a sensorimotor deficiency. The performance of Emx2+/− mice is not statistically different from wt, although they exhibit a trend toward poorer performance in each of the four measures.
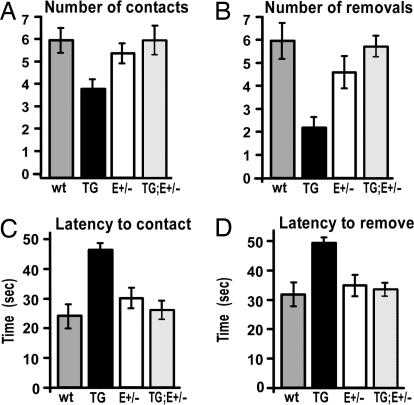
Fig. 5.
Genetic rescue of sensorimotor area sizes also rescues deficiencies on a unique sensorimotor behavioral test. The same 40 adult male mice described in Fig. 4 were analyzed at the adhesive removal test (31). Each mouse received four trials; in each trial an adhesive patch was placed on both hind paws, for a total of eight possible contacts and eight possible removals (SI Movie 3). One-way unpaired Student's t test and one-way ANOVA followed by post hoc multiple comparison tests [Dunnett's and Fisher (LSD)] were performed to determine the significance of differences between genotypes. Mean values with standard errors are presented. (A) Number of contacts with the patch. ne-Emx2 mice (TG) are significantly less likely to contact the patch (3.90 ± 0.41 contacts) than wt mice (6.00 ± 0.53 contacts) (t test, P = 0.00920; ANOVA, P = 0.00276). However, ne-Emx2; Emx2+/− (TG;E+/−) rescue mice (6.00 ± 0.63 contacts) perform significantly better than ne-Emx2 mice (TG) (t test, P < 0.0001; ANOVA, P = 0.0082), at a level statistically indistinguishable from wt (t test, P = 1; ANOVA, P = 1). Emx2+/− mice (E+/−) (5.43 ± 0.43 contacts) do not perform significantly different from wt (t test, P = 0.42; ANOVA, P = 0.42). (B) Number of removals. ne-Emx2 mice (TG) are significantly less likely to remove the patch (1.80 ± 0.42 removals) than wt (5.00 ± 0.68 removals) (t test, P = 0.0018; ANOVA, P = 0.0001). However, ne-Emx2; Emx2+/− rescue mice (TG;E+/−) (4.80 ± 0.37 removals) are indistinguishable from wt (t test, P = 0.80; ANOVA, P = 0.82) but significantly different from ne-Emx2 mice (TG) (t test, P = 0.0002; ANOVA, P = 0.0012). Emx2+/− mice (E+/−) (3.86 ± 0.56 removals) did not differ significantly from wt (t test, P = 0.229; ANOVA, P = 0.155). (C) Latency to contact the patch. ne-Emx2 mice (TG) take significantly longer to contact the patch (46.25 ± 2.11 s) than wt (23.94 ± 3.95 s) (t test, P < 0.0001; ANOVA, P < 0.0001). In contrast, ne-Emx2; Emx2+/− rescue mice (TG;E+/−) (26.00 ± 2.93 s) are statistically indistinguishable from wt (t test, P = 0.68; ANOVA, P = 0.68), and significantly different from ne-Emx2 mice (TG) (t test, P = 0.0005; ANOVA, P < 0.0001). Emx2+/− mice (E+/−) (30.29 ± 3.40 s) did not perform significantly different from wt (t test, P = 0.23; ANOVA, P = 0.16). (D) Latency to remove the patch. ne-Emx2 mice (TG) take significantly longer to remove the patch (54.70 ± 1.70 s) than wt (35.25 ± 4.31 s) (t test, P = 0.0005; ANOVA, P < 0.0001), but the ne-Emx2; Emx2+/− rescue mice (TG;E+/−) (37.20 ± 2.60 s) performed identical to wt (t test, P = 0.70; ANOVA, P = 0.71) and significantly different from ne-Emx2 (TG) (t test, P < 0.0001; ANOVA, P = 0.0008). Emx2+/− animals (E+/−) (38.64 ± 4.04 s) were statistically indistinguishable from wt (t test, P = 0.57; ANOVA, P = 0.47).
In contrast to the deficient behaviors of ne-Emx2 mice, ne-Emx2; Emx2+/− mice exhibit a rescued behavior at the adhesive patch test, and their performance at each component of the test is statistically indistinguishable from wt (Fig. 5). These findings confirm our conclusions that the deficient performances observed in the grid walk and rotarod tests by ne-Emx2 mice are due to the reduction in the sizes of their sensorimotor areas, and not to the ectopic subcortical expression of the Emx2 transgene.
Discussion
We show that cortical area size determined during embryonic development can have profound effects on performance at modality-specific behaviors in adults. We used mice in which sizes of S1 and motor areas are decreased or increased by altering the expression of Emx2, a homeodomain protein that determines area size (12, 14, 22). We show that ne-Emx2 transgenic mice with reduced sensorimotor cortical areas have dramatically diminished performances at tests of tactile and motor behaviors, as well as balance and coordination, whereas Emx2+/− mice with larger sensorimotor areas have significantly reduced behavioral performance, albeit limited to more physically demanding tests of sensorimotor skills.
The behavioral deficiencies are restored to a wt level by genetic crosses that in parallel restore cortical levels of Emx2 and cortical area sizes to wt but retain ectopic Emx2 transgene expression subcortically (Fig. 2A). These rescue studies show that the changes in sizes of sensorimotor areas are a primary cause of the behavioral deficiencies. They also show that the behavioral deficiencies in ne-Emx2 mice are not a result of potential subcortical defects, including cerebellar, due to ectopic expression of the Emx2 transgene. Further, because expression of endogenous Emx2 is largely limited to the cerebral cortex (18), the behavioral deficiencies in Emx2+/− mice are unlikely due to subcortical defects [e.g., cerebellum does not express Emx2 and therefore cannot be directly affected by a partial Emx2 deficiency (ref. 18 and the present study)].
The differences between Emx2+/− mice and ne-Emx2 mice in behavioral performance could have several explanations, including that (i) reducing area size may have a more significant impact on behavioral performance than increasing area size relative to wt; (ii) compared with wt, the percentage increase in the size of sensorimotor areas in Emx2+/− mice is less than the percentage reduction of their size in ne-Emx2 mice; and (iii) physically more demanding tests (e.g., rotarod vs. adhesive patch removal) may more readily reveal behavioral deficiencies.
Our findings support the hypothesis that cortical areas have evolved an optimal size in the context of their neural systems to maximize behavioral performance (23). We show in mice that relatively modest changes in area size can have profound effects on behavioral performance, suggesting that in humans behavioral performance could differ substantially in relationship to the 2-fold size continuum reported for human cortical areas (1–5). We predict that a specific range of area size would result in optimal behavioral performance, and that this size range would be influenced by parameters of that area's neural system, some of which likely vary among individuals, particularly in out-bred populations. Our findings also have implications for human cognitive disorders. For example, recent findings suggest a link between the sizes of cortical areas located in the planum temporale of the cerebral cortex with the developmental cognitive disorder Williams syndrome (10).
Why can changes in area size result in diminished behavioral performance? The effects of area size changes could be modified, possibly amplified, by adaptive or secondary changes intracortically and subcortically. One possibility is a difference in convergence of thalamocortical inputs, which relay sensory input onto cortical neurons, and potential effects on intra- or inter-columnar circuitry that results in suboptimal cortical processing of sensory input. This suggestion is consistent with a correlation between cortical magnification in human V1 and Vernier acuity, which concludes that individuals with a larger V1 have lower overall Vernier acuity, i.e., a diminished resolution of a visual image (24). Other possibilities include decreased effectiveness of cortical output projections that modulate modality-specific behaviors, perhaps by altering the convergence of cortically-processed sensorimotor input onto corticospinal projection neurons that contribute to coordinated activation of limb muscles, motor coordination and balance.
The mechanisms that lead to variations in the size of an area among humans are not known but could be due to “expression level polymorphisms” (ELPs) characterized by differences between individuals in the expression level of genes (11). These differences due to ELPs approximate those genetically created for Emx2 in mice used in the present study. Therefore, modest differences in gene expression due to ELPs could underlie the differences in area size in humans if the affected genes control area patterning, which in turn may result in substantial differences in behavior.
Experimental Procedures
General.
All analyses were done blind to genotype. Data were analyzed by using Microsoft Excel and XLSTAT (Addinsoft, New York). Statistical analyses are described in the figure legends or below. For further details, see SI Experimental Procedures and SI Movies 1–3.
Animals.
Mice (wt, Emx2+/−, ne-Emx2+/+, and ne-Emx2+/+; Emx2+/−) were obtained by crosses of Emx2+/− knockout mice (25) and ne-Emx2+/+ transgenic mice (14), and genotyped by real-time PCR. F2 littermates were used for behavioral tests. No genetic background effects were detected either anatomically or behaviorally.
Gene Expression, Anatomy, and Statistics.
Gene expression levels were quantified by quantitative RT-PCR. Cytochrome oxidase and serotonin staining, cadherin8 whole-mount in situ hybridization, and area measurements were done as described (14, 26).
Behavioral Analyses.
Body weight.
Mean body weights were statistically indistinguishable between the genotypes behaviorally tested (wt: 29.6 ± 1.0 g, n = 8; ne-Emx2: 30.5 ± 0.6 g, n = 10; Emx2+/−: 30.6 ± 0.6 g, n = 7; ne-Emx2; Emx2+/−: 30.2 ± 1.4 g, n = 5).
Swimming/general mobility.
Injuries to sensorimotor areas disrupt normal swimming in rodents (27). We compared swimming between wt and ne-Emx2 mice in a water-filled Plexiglas box, recorded for 45 s. Normal mice stroke with hindlimbs; forelimbs were briefly used for directional change. No differences were observed between genotypes in any parameter, including swimming posture (e.g., torso position and ability to hold forelimbs immobile during swimming), swim pattern, swim path, or speed (wt: 18.24 ± 2.38 cm/s, n = 11; ne-Emx2: 17.48 ± 3.96 cm/s, n = 11; P = 0.59; Student's t test).
Grip strength.
The dual-sensor Grip Strength Meter (Columbus Instruments, Columbus, OH) was used to measure the peak amount of force applied by hindlimbs or forelimbs grasping pull bar assemblies (17). Forelimb strength exhibited no significant difference between wt and ne-Emx2 mice (peak tension sustained by wt: 80.41 ± 6.24 g; by ne-Emx2: 74.39 ± 4.35 g, P = 0.44; Student's t test), but ne-Emx2 mice showed stronger hindlimbs (54.99 ± 2.86 g) than wt (44.86 ± 1.70 g, P < 0.01).
Acoustic startle test.
Acoustic startle is a reflex in mice to a sudden, loud noise (28). Mice were left in their home cage in a quiet room, a sudden, brief 100-dB noise was produced above the cage, and the response was recorded (29). Ten of 11 wt mice and 11 of 11 ne-Emx2 mice showed a strong startle response.
Visual cliff test.
The visual cliff test (28, 30) was done by using a Plexiglas box designed to create the visual appearance of a “cliff” with a drop of 0.5 m but actually is a solid horizontal surface. A “safe” response is scored when mice step on a solid appearing checkered surface versus a clear surface that gives the appearance of a cliff. The wt and ne-Emx2 mice exhibited no significant difference (wt: 87.27 ± 1.94% safe responses; ne-Emx2 mice: 86.36 ± 2.78% safe responses; P = 0.8) and were similar to other sighted strains (e.g., A/J, Sm/J, C57BL/6, and C57BL/10), which show 80–90% safe responses, whereas a blind strain (C3H/HeJ) show a random choice (52% safe response) (30).
Grid walk, rotarod, and adhesive removal tests.
These tests evaluate balance and tactile and motor abilities of hindlimbs and forelimbs (refs. 16, 17, and 31, respectively).
Supplementary Material
Acknowledgments
We thank Geoff Boynton, Jonathan Horton, William Newsome, Chuck Stevens, and Brian Wandell for discussions; Mary Lynn Gage for editing; Berta Higgins and Haydee Gutierrez for technical assistance; and Matthias Kaeser for advice on genomic qPCR. This work was supported by National Institutes of Health Grant R37 NS31558 (to D.D.M.O.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0611723104/DC1.
References
- 1.Brodmann K. Allg Z Psychiatr (Berlin) 1918;74:564–568. [Google Scholar]
- 2.Dougherty RF, Koch VM, Brewer AA, Fischer B, Modersitzki J, Wandell BA. J Vis. 2003;3:586–598. doi: 10.1167/3.10.1. [DOI] [PubMed] [Google Scholar]
- 3.Stensaas SS, Eddington DK, Dobelle WH. J Neurosurg. 1974;40:747–755. doi: 10.3171/jns.1974.40.6.0747. [DOI] [PubMed] [Google Scholar]
- 4.White LE, Andrews TJ, Hulette C, Richards A, Groelle M, Paydarfar J, Purves D. Cereb Cortex. 1997;7:31–47. doi: 10.1093/cercor/7.1.31. [DOI] [PubMed] [Google Scholar]
- 5.White LE, Andrews TJ, Hulette C, Richards A, Groelle M, Paydarfar J, Purves D. Cereb Cortex. 1997;7:18–30. doi: 10.1093/cercor/7.1.18. [DOI] [PubMed] [Google Scholar]
- 6.Airey DC, Robbins AI, Enzinger KM, Wu F, Collins CE. BMC Neurosci. 2005;6:18. doi: 10.1186/1471-2202-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halpern SD, Andrews TJ, Purves D. J Cognit Neurosci. 1999;11:521–534. doi: 10.1162/089892999563580. [DOI] [PubMed] [Google Scholar]
- 8.Recanzone GH, Merzenich MM, Jenkins WM, Grajski KA, Dinse HR. J Neurophysiol. 1992;67:1031–1056. doi: 10.1152/jn.1992.67.5.1031. [DOI] [PubMed] [Google Scholar]
- 9.Hlustik P, Solodkin A, Noll DC, Small SL. J Clin Neurophysiol. 2004;21:180–191. doi: 10.1097/00004691-200405000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Eckert MA, Galaburda AM, Karchemskiy A, Liang A, Thompson P, Dutton RA, Lee AD, Bellugi U, Korenberg JR, Mills D, et al. NeuroImage. 2006;33:39–45. doi: 10.1016/j.neuroimage.2006.05.062. [DOI] [PubMed] [Google Scholar]
- 11.Cowles CR, Hirschhorn JN, Altshuler D, Lander ES. Nat Genet. 2002;32:432–437. doi: 10.1038/ng992. [DOI] [PubMed] [Google Scholar]
- 12.Bishop KM, Goudreau G, O'Leary DDM. Science. 2000;288:344–349. doi: 10.1126/science.288.5464.344. [DOI] [PubMed] [Google Scholar]
- 13.Mallamaci A, Muzio L, Chan CH, Parnavelas J, Boncinelli E. Nat Neurosci. 2000;3:679–686. doi: 10.1038/76630. [DOI] [PubMed] [Google Scholar]
- 14.Hamasaki T, Leingartner A, Ringstedt T, O'Leary DDM. Neuron. 2004;43:359–372. doi: 10.1016/j.neuron.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 15.Douglas RM, Alam NM, Silver BD, McGill TJ, Tschetter WW, Prusky GT. Vis Neurosci. 2005;22:677–684. doi: 10.1017/S0952523805225166. [DOI] [PubMed] [Google Scholar]
- 16.Z'Graggen WJ, Metz GA, Kartje GL, Thallmair M, Schwab ME. J Neurosci. 1998;18:4744–4757. doi: 10.1523/JNEUROSCI.18-12-04744.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaspar BK, Llado J, Sherkat N, Rothstein JD, Gage FH. Science. 2003;301:839–842. doi: 10.1126/science.1086137. [DOI] [PubMed] [Google Scholar]
- 18.Cecchi C. Gene. 2002;291:1–9. doi: 10.1016/s0378-1119(02)00623-6. [DOI] [PubMed] [Google Scholar]
- 19.Martin LA, Goldowitz D, Mittleman G. Eur J Neurosci. 2003;18:2002–2010. doi: 10.1046/j.1460-9568.2003.02921.x. [DOI] [PubMed] [Google Scholar]
- 20.Martin LA, Escher T, Goldowitz D, Mittleman G. Genes Brain Behav. 2004;3:158–166. doi: 10.1111/j.1601-183x.2004.00067.x. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki SC, Inoue T, Kimura Y, Tanaka T, Takeichi M. Mol Cell Neurosci. 1997;9:433–447. doi: 10.1006/mcne.1997.0626. [DOI] [PubMed] [Google Scholar]
- 22.Bishop KM, Rubenstein JLR, O'Leary DDM. J Neurosci. 2002;22:7627–7638. doi: 10.1523/JNEUROSCI.22-17-07627.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krubitzer L, Kaas J. Curr Opin Neurobiol. 2005;15:444–453. doi: 10.1016/j.conb.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Duncan RO, Boynton GM. Neuron. 2003;38:659–671. doi: 10.1016/s0896-6273(03)00265-4. [DOI] [PubMed] [Google Scholar]
- 25.Pellegrini M, Mansouri A, Simeone A, Boncinelli E, Gruss P. Development (Cambridge, UK) 1996;122:3893–3898. doi: 10.1242/dev.122.12.3893. [DOI] [PubMed] [Google Scholar]
- 26.Nakagawa Y, Johnson JE, O'Leary DDM. J Neurosci. 1999;19:10877–10885. doi: 10.1523/JNEUROSCI.19-24-10877.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stoltz S, Humm JL, Schallert T. Behav Brain Res. 1999;106:127–132. doi: 10.1016/s0166-4328(99)00100-x. [DOI] [PubMed] [Google Scholar]
- 28.Crawley JN. What's Wrong with My Mouse? New York: Wiley–Liss; 2000. [Google Scholar]
- 29.Logue SF, Owen EH, Rasmussen DL, Wehner JM. Neuroscience. 1997;80:1075–1086. doi: 10.1016/s0306-4522(97)00164-4. [DOI] [PubMed] [Google Scholar]
- 30.Fox MW. Anim Behav. 1965;13:232–233. doi: 10.1016/0003-3472(65)90040-0. [DOI] [PubMed] [Google Scholar]
- 31.Schallert T, Upchurch M, Wilcox RE, Vaughn DM. Pharmacol Biochem Behav. 1983;18:753–759. doi: 10.1016/0091-3057(83)90019-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.