Abstract
Transient global ischemia is a neuronal insult that induces delayed, selective death of hippocampal CA1 pyramidal neurons. A mechanism underlying ischemia-induced cell death is activation of the gene silencing transcription factor REST (repressor element-1 silencing transcription factor)/NRSF (neuron-restrictive silencing factor) and REST-dependent suppression of the AMPA receptor subunit GluR2 in CA1 neurons destined to die. Here we show that REST regulates an additional gene target, OPRM1 (μ opioid receptor 1 or MOR-1). MORs are abundantly expressed by basket cells and other inhibitory interneurons of CA1. Global ischemia induces a marked decrease in MOR-1 mRNA and protein expression that is specific to the selectively vulnerable area CA1, as assessed by quantitative real-time RT-PCR, Western blotting, and ChIP. We further show that OPRM1 gene silencing is REST-dependent and occurs via epigenetic modifications. Ischemia promotes deacetylation of core histone proteins H3 and H4 and dimethylation of histone H3 at lysine-9 (H3-K9) over the MOR-1 promoter, an signature of epigenetic gene silencing. Acute knockdown of MOR-1 gene expression by administration of antisense oligodeoxynucleotides to hippocampal slices in vitro or injection of the MOR antagonist naloxone to rats in vivo affords protection against ischemia-induced death of CA1 pyramidal neurons. These findings implicate MORs in ischemia-induced death of CA1 pyramidal neurons and document epigenetic remodeling of expression of OPRM1 in CA1 inhibitory interneurons.
Keywords: epigenetic modification, gene silencing, ischemia, neuronal death
The transcriptional repressor REST silences a large network of genes essential for neuronal function including neurotransmitter receptors, ion channels, synaptic vesicle protein, and neuronal trophic factors (1–5) and restricts their expression to neurons in a variety of genetic contexts. During the late stages of neuronal differentiation, REST activation is important for the acquisition of the neural phenotype (5). Mice with targeted deletion of the REST gene die very early in development (6), and overexpression of REST in differentiating neurons disrupts neuronal gene expression and increases axon-guidance error (4).
REST is a Krüppel-type zinc-finger transcription factor that interacts with a 21-bp cis-element RE1 (repressor element 1, also called NRSE, neuron-restrictive silencing element) found in the regulatory regions of neuron-specific target genes (1, 2, 7, 8). REST silences target genes via association with distinct corepressor platforms involving mSin3A (9–11) and CoREST (5, 12), which recruit histone deacetylases (HDACs) 1 and 2 (5, 9, 10, 13–17). HDACs silence gene transcription by deacetylation of core histones and tightening of the core chromatin complex (18, 19). In addition, CoREST effects long-term gene silencing by association with methyl-CpG binding protein 2 (MeCP2), site-specific histone methyltransferase G9a (13, 20–22), which promotes dimethylation of histone 3 at lysine-9 (H3-K9) (23, 24), and the newly discovered histone demethylase LSD1, which removes methyl groups from lysine-4 of histone 3 (H3-K4) (25, 26). Whereas dimethylation of histone H3-K9 is an epigenetic marker of gene silencing, trimethylation of H3-K4 is a marker of active transcription (18, 19).
MORs are members of the large superfamily of G protein-coupled receptors and are activated by endogenous opioid peptides (27, 28). Upon activation, MORs inhibit adenylyl cyclase, reduce Ca2+ currents, stimulate inwardly rectifying K+ currents and activate mitogen-activated protein kinases via pertussis toxin-sensitive Gi/G0 proteins. Pharmacological studies indicate the existence of three MOR subtypes: μ1, μ2, and morphine-6β-glucuronide receptors (27, 28). Studies involving mice with targeted deletion of the OPRM1 gene implicate MORs in the euphoria and craving of exogenous opiates and other drugs of abuse (29). Within CA1, MORs are abundantly expressed on the terminals of basket cells, GABAergic inhibitory interneurons in the stratum pyramidale and strategically poised to influence synaptic efficacy in response to neuronal activity and/or insult (30–32). Upon activation, MORs inhibit release of GABA onto CA1 pyramidal cells, thereby increasing neuronal excitability (33) and epileptogenesis (34).
The present study was undertaken to determine the impact of global ischemia on MOR expression in CA1 and to examine a possible role for MORs in ischemia-induced death of CA1 pyramidal neurons. Here we show that REST binds and suppresses MOR promoter activity and gene expression specifically in the CA1 subfield where pyramidal cells die; presumably the suppression is restricted to the inhibitory interneurons, which actually survive the ischemic insult. Acute knockdown of MOR expression by application of antisense oligodeoxynucleotides (ODN) in vitro or pharmacological blockade of MORs by injection of the antagonist naloxone in vivo protects hippocampal neurons against ischemic damage. These observations implicate MORs in the selective, delayed death of CA1 neurons and identify MORs as a target for therapeutic intervention and amelioration of neuronal cell loss and cognitive deficits associated with global ischemia.
Results
Global Ischemia Suppresses MOR-1 mRNA and Protein in Vulnerable CA1 Neurons.
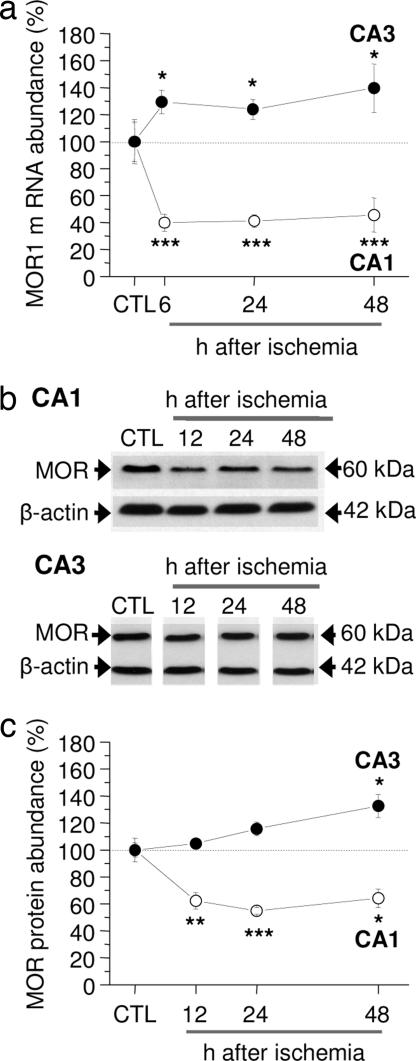
The role of MORs in ischemia-induced neuronal death is not well delineated. To address this issue, we subjected rats to global ischemia or sham operation. Transient global or forebrain ischemia in rats provides a well established model of neuronal insult in which there is a highly specific, delayed death of CA1 pyramidal neurons (35). Because ischemia activates the transcriptional repressor REST specifically in CA1 (14, 36), and MOR-1 is a known REST target (37, 38), we assessed the time course of MOR-1 mRNA expression in CA1 and CA3 by quantitative real-time PCR. In control CA1, MOR-1 mRNA expression was high (n = 15). Ischemia triggered a marked decrease in MOR-1 mRNA selectively in CA1 (decrease by: 6 h, 60 ± 6%, n = 6, P < 0.001; 24 h, 58 ± 4.8%, n = 5, P < 0.001; 48 h, 54 ± 13%, n = 5, P < 0.001 vs. control) (Fig. 1a). In contrast, MOR-1 mRNA was enhanced in CA3 (increase by: 6 h, 29 ± 8%, n = 4, P < 0.05; 24 h, 24 ± 7%, n = 4, P < 0.05; 48 h, 39 ± 18%, n = 4, P < 0.05) (Fig. 1a).
Fig. 1.
Global ischemia decreases MOR-1 mRNA and protein expression in CA1. (a) Real-time PCR of RNA samples from the CA1 and CA3 of control and experimental animals at 6, 24, and 48 h after ischemia. Bars represent relative mRNA quantity measured by the Ct values ± SEMs, where Ct is the number of cycles needed for detection of the fluorescence signal; significance was assessed by using the REST software with which transcript differences are tested for significance by a pairwise fixed reallocation–randomization test (55). ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001. (b and c) Representative Western blots probed with anti-MOR antibody (b) and relative MOR abundance in samples from the CA1 and CA3 at 48 h after sham operation (control) or at 12, 24, and 48 h after ischemia (c). Values are expressed as the ratio of band densities for experimental samples to band density of the corresponding control sample after correction for loading. Bars represent mean ± SEMs. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001 (Student's unpaired t test).
Because mRNA changes are not always followed by changes in protein abundance, we next examined the impact of ischemic insults on MOR protein in CA1 and CA3 by Western blot analysis. In control hippocampus, MOR protein expression, evident as a band of Mr = 60 kDa, was robust (Fig. 1b). Ischemia markedly decreased MOR protein abundance in CA1, evident as late as 48 h (decrease by: 12 h, 38 ± 6%, n = 7, P < 0.005 vs. control; 24 h, 46 ± 2%, n = 7, P < 0.0005; 48 h, 36 ± 7%, n = 5, P < 0.05 vs. control; shams, n = 8) (Fig. 1c). In contrast, ischemia did not significantly alter MOR protein abundance in CA3 at 12 h and 24 h and modestly increased MOR protein at 48 h (increase by 32 ± 8%, n = 5, P < 0.05) (Fig. 1c). Thus, ischemia-induced changes in MOR-1 mRNA and MOR protein are cell-specific and are consistent with the possibility that REST silences MOR-1 expression.
Ischemia Promotes Association of REST with the MOR-1 Promoter in CA1.
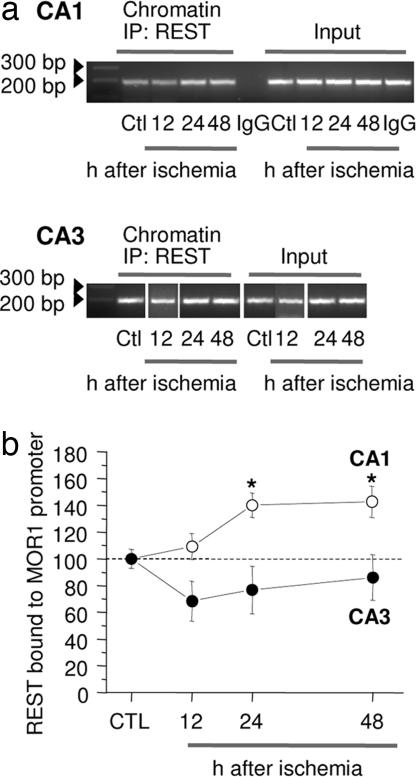
The finding, that global ischemia silences MOR-1 mRNA and protein expression in selectively vulnerable CA1 neurons, prompted us to analyze chromatin for association of REST with the promoter of the MOR-1 gene by ChIP assay. In control CA1, REST was bound to the RE1 region of the MOR-1 promoter at low, but significant levels (Fig. 2a, Ctl lanes). Ischemia enhanced association of REST with the MOR-1 promoter in the CA1, but not CA3, of experimental animals, evident at 24 and 48 h after ischemia (increase by: 24 h, 40 ± 9%, n = 4, P < 0.05 vs. control; 48 h, 42 ± 11%, n = 4, P < 0.05 vs. control) (Fig. 2a, center lanes). In contrast, ischemia did not significantly alter REST over the MOR-1 promoter in CA3 at times examined (Fig. 2b, center lanes). REST immunoreactivity was negligible in the control IgG immunoprecipitation. This finding validates the specificity of the ChIP assay. Thus, ischemia promotes association of REST with the MOR-1 promoter in vulnerable CA1.
Fig. 2.
Ischemia enhances REST binding to the MOR-1 promoter in CA1. (a) ChIP analysis of cross-linked chromatin from the CA1 and CA3 of control (Ctl) and experimental animals at 12, 24, and 48 h after ischemia immunoprecipitated with an anti-REST antibody. Immunoprecipitation with nonimmune IgG showed negligible PCR product, indicating specificity of the ChIP technique. Left lanes show ODN length standards. (b) Band densities for PCR products were normalized to that of the corresponding control input. Bars represent mean ± SEMs. ∗, P < 0.05 (Student's unpaired t test).
Ischemia Promotes Histone Deacetylation of H3 and H4 over the MOR-1 Promoter.
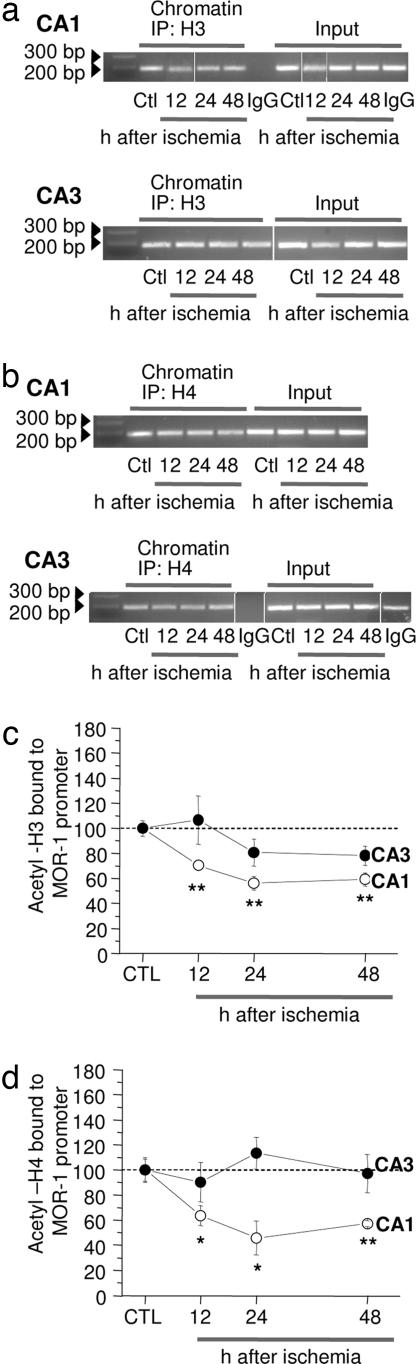
Upon binding to the RE1 element of target genes, REST recruits the corepressors, mSin3A and CoREST, which exist in stable complexes with HDAC. HDAC-mediated histone deacetylation and gene silencing is associated primarily with a dynamic mode of repression (1, 14, 15). We next examined the impact of ischemia on the acetylation status of core histones H3 and H4 physically associated with the MOR-1 promoter in the vulnerable CA1 and resistant CA3 by ChIP analysis. Ischemia enhanced deacetylation of core histone protein H3 over the MOR-1 promoter in CA1 (decrease by: 12 h, 29 ± 4%, n = 4, P < 0.01; 24 h, 43 ± 5%, n = 5, P < 0.002; 48 h, 40 ± 5%, n = 5, P < 0.002) (Fig. 3 a and b). Ischemia enhanced deacetylation of H4 in CA1, evident at 12, 24, and 48 h after ischemia (decrease by: 12 h, 36 ± 8%, n = 5, P < 0.05; 24 h, 54 ± 13% at 24 h, n = 5, P < 0.02; 48 h, 42 ± 5%, n = 5, P < 0.01) (Fig. 3 c and d). Ischemia did not significantly alter the abundance of acetylated H3 or H4 over the MOR-1 promoter in CA3 at any times examined (Fig. 3).
Fig. 3.
Ischemia promotes deacetylation of H3 and H4 over the MOR-1 promoter in CA1. ChIP analysis (a and b) of CA1 and CA3 from control and experimental animals 12, 24, and 48 h after ischemia immunoprecipitated with antibody to acetyl-H3 (a and c) or acetyl-H4 (b and d). The IgG immunoprecipitate showed negligible PCR product, indicating little or no immunoprecipitation in the absence of primary antibody. Band densities for PCR products were normalized as in Fig. 2. Left lanes show ODN length standards in a and b. Bars represent mean ± SEMs. ∗, P < 0.05; ∗∗, P < 0.01 (Student's unpaired t test).
REST Recruits Histone Methyltransferase G9a to the MOR-1 Promoter.
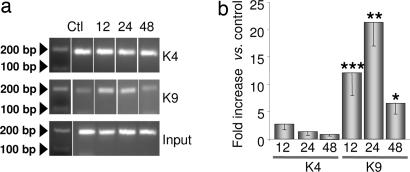
The CoREST corepressor platform is a multiprotein complex that contains the site-specific histone methyltransferase G9a, which promotes dimethylation of H3-K9 (18, 19). To examine whether ischemia promotes association of G9a with the RE1 site of the MOR-1 gene, we examined the methylation status of H3-K9 and K4. Toward this end, we performed ChIP analysis of cross-linked chromatin from the CA1 and CA3 with antibodies directed against dimethyl-K4 of H3 (diMeK4-H3), and dimethyl-K9 of H3 (diMeK9-H3), and assessed the amount of MOR-promoter associated with modified histones at times after ischemia by quantitative real-time PCR. In control CA1 (n = 6), dimethyl-K4-H3 and dimethyl-K9-H3 associated with the MOR-1 promoter was low, but detectable (Fig. 4). Ischemia dramatically increased dimethylation of H3-K9 (but not H3-K4) (increase to: 12 h, 12 ± 4 times control, n = 6, P < 0.001 vs. control; 24 h, 21 ± 4.3 times control, n = 5, P < 0.01 vs. control; 48 h, increase to 6.5 ± 2 times control, n = 5, P < 0.05 vs. control) (Fig. 4). In contrast, ischemia did not significantly alter the methylation status of residue K4 of histone H3. Immunoprecipitation with nonimmune IgG yielded very high Ct values, indicative of negligible precipitation of the MOR-1 promoter (data not illustrated). Together, these findings indicate that the REST corepressor complex is functionally active in postischemic CA1 neurons and document REST-mediated silencing of its target gene MOR-1 via histone deacetylation and methylation.
Fig. 4.
Ischemia enhances association of diMeK9-H3, but not diMeK4-H3, with the MOR-1 promoter in CA1. (a) ChlP analysis of cross-linked chromatin from the CA1 of control (Ctl) and experimental animals at 12, 24, and 48 h after ischemia immunoprecipitated with antibody to diMeK9-H3 (a Middle and b) or diMeK4-H3 (a Top and b) (Materials and Methods). (a) Representative ethidium bromide-stained agarose gel showing amplification of promoter fragments. Left lanes show ODN length standards. (b) Levels of diMeK9-H3 and diMeK4-H3 at MOR-1 promoter in CA1 were quantified by using real-time PCR. Ct values of immunoprecipitated samples were normalized to the corresponding value for input. Bars represent mean ± SEMs. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001 vs. control (Student's paired t test).
Acute Knockdown of MOR-1 Rescues Insulted CA1 Neurons.
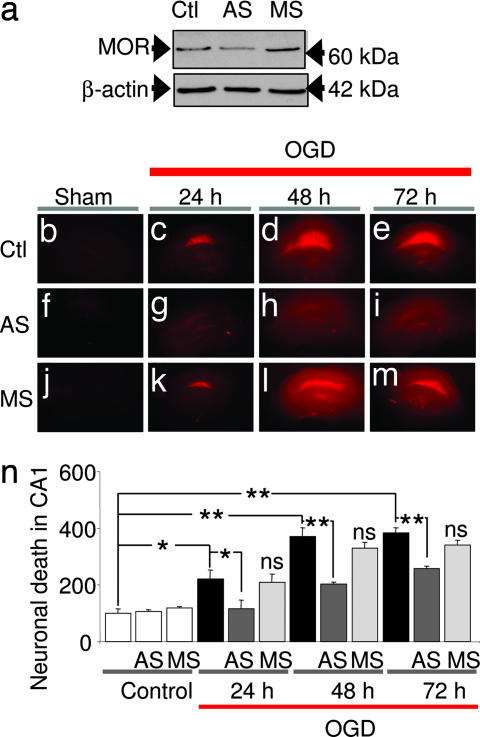
The results thus far document gene silencing of the MOR-1 gene via epigenetic modifications, but do not address whether MOR-1 expression is causally related to neuronal death. We next examined the impact of acute knockdown of MOR-1 expression on survival of insulted CA1 neurons. In CA1, MOR expression is localized primarily to inhibitory GABAergic interneurons (39) and MOR activation modulates synaptic plasticity in area CA1 of the rat hippocampus via inhibition of GABA release (40). Mice with targeted deletion of exon 1 of the MOR-1 gene exhibit enhanced survival of dentate gyrus neurons during development (41). We evaluated the effect of acute knockdown of MOR-1 expression by administration of antisense ODNs. Oxygen/glucose deprivation (OGD) of organotypically cultured hippocampal slice cultures provides an in vitro model of global ischemia and is ideal for administration of antisense ODNs (14). Neuronal death was assessed quantitatively by propidium iodide (PI) uptake. We used an antisense ODN directed against exon 1 of the MOR-1 gene, a sequence known to block morphine-mediated analgesia (42). Acute knockdown of MOR-1 by antisense technology is well established (42, 43). Because MORs have a turnover time of approximately three to four days in vivo (42), we applied antisense or missense ODNs (10 μM) to slices for 72 h before OGD. MOR-1 antisense induced a marked down-regulation of MOR protein, as assessed by Western analysis of whole slices (Fig. 5a). In contrast, missense did not significantly alter MOR protein (Fig. 5a).
Fig. 5.
MOR-1 antisense suppresses MOR protein expression and rescues CA1 neurons. (a) MOR antisense suppressed MOR protein expression, assessed by Western blot analysis of four hippocampal slices from different animals. (b–m) PI labeling of cultured hippocampal slices. (b) Control slice. (f) Control slice treated with MOR antisense. (j) Control slice treated with MOR missense. (c–e) Slices at 24, 48, and 72 h after OGD (30 min). The pyramidal cell layer of CA1 exhibited intense PI labeling, indicative of cell death, evident at 24 and 72 h. (g–i) MOR antisense (10 μM, 72 h before OGD) reduces OGD-induced neuronal death. (k–m) MOR-1 missense (10 μM, 72 h before OGD) was without effect. (n) Quantitation of cell death, assessed as the percentage of PI fluorescence per unit area over CA1 normalized to the control. ∗, P < 0.05; ∗∗, P < 0.01; n.s. vs. OGD (one-way ANOVA, followed by Dunnett's multiple comparison test). Ctl, control; AS, antisense; MS, missense.
OGD (30 min) induced selective, delayed death of CA1 pyramidal neurons, evident at 24 h and maximal at 72 h, as assessed by PI uptake (n = 8 per time point) (Fig. 5 c–e and n). MOR-1 antisense suppressed OGD-induced neuronal death at 24 h (reduction by 46 ± 9%, n = 5, P < 0.01 vs. OGD), 48 h (reduction by 62 ± 9%, n = 6, P < 0.05 vs. OGD), and 72 h (reduction by 63 ± 9%, n = 8, P < 0.01 vs. OGD) (Fig. 5 g–i and n). In contrast, MOR-1 missense (n = 4) (Fig. 5 k–n) did not detectably alter neuronal survival in slices subjected to OGD. Moreover, antisense and missense ODNs did not detectably alter cell survival in control slices subjected to normoxic conditions (n = 6 per treatment) (Fig. 5 f, j, and n). These findings demonstrate that MOR-1 antisense affords neuroprotection by suppressing MOR expression in CA1 and implicate MOR in ischemia-induced neuronal death.
Naloxone Protects Against Ischemia-Induced Death of CA1 Neurons in Vivo.
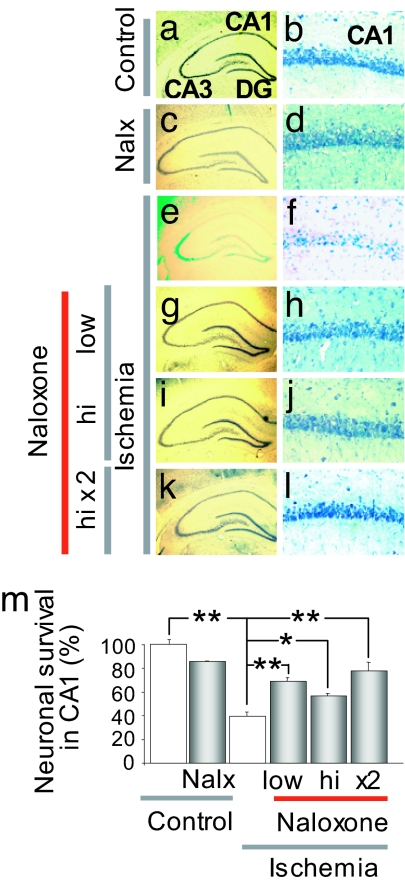
To examine whether MOR activity is causally related to ischemia-induced cell death in a clinically relevant animal model, we examined whether naloxone, a μ opioid antagonist, protects CA1 neurons when administered close to the time of ischemia. The role of opioid receptors in neuronal survival/cell death is not well delineated. To address this issue directly, we administered naloxone before or before and after ischemia. Ischemia induced extensive death of pyramidal cells in the CA1, assessed at 7 days after ischemia (reduction to 40 ± 3.6% of control, n = 12, P < 0.01 vs. control) (Fig. 6 e and f; summary data in Fig. 6m); the few remaining pyramidal neurons were severely damaged and appeared pyknotic (Fig. 6, compare e and f with a and b). Neuronal death was specific in that little or no cell loss occurred in the nearby CA2 or transition zone, CA3 or dentate gyrus (Fig. 6e and not illustrated). Administration of naloxone directly into the right cerebral ventricle at 1 h before ischemia at 0.1 mg/kg (low dose) or 1.0 mg/kg (high dose) or at 1 h before and 1 h after ischemia at 1 mg/kg did not significantly alter neuronal survival in the hippocampus of control (sham-operated) animals (Fig. 6 c and d) but afforded robust protection of CA1 neurons in animals subjected to global ischemia. The lower dose given before ischemia increased the number of surviving neurons to 69 ± 3% of control (n = 6, P < 0.01 vs. ischemia) (Fig. 6 g, h, and m); the higher dose given before ischemia increased the number of surviving neurons to 56 ± 2% of control (n = 5, P < 0.05 vs. ischemia) (Fig. 6 i, j, and m); the higher dose given before and after ischemia increased the number of surviving neurons to 74 ± 7% (n = 5, P < 0.01 vs. ischemia) (Fig. 6 k and l; summary data in Fig. 6m, no significant difference between doses). These data demonstrate that block of MOR receptors by the competitive antagonist naloxone affords protection of CA1 neurons against ischemia-induced neuronal death and are consistent with a role for MOR silencing in neuronal survival.
Fig. 6.
Naloxone protects CA1 neurons from postischemic neurodegeneration. (a–l) Representative toluidine blue-stained coronal brain sections at the level of the dorsal hippocampus from control and experimental animals subjected to saline (a and b), naloxone (1 mg/kg; c and d), ischemia and saline (e and f), ischemia and naloxone (0.1 mg/kg or 1 mg/kg, 1 h before ischemia; g–j), or ischemia and naloxone [1 mg/kg twice (1 h before and 1 h after ischemia); k and l). Animals were killed 7 days after reperfusion. (m) Summary data for a–l. Ischemia elicited selective, delayed death of CA1 pyramidal neurons. Naloxone at doses and times examined significantly protected CA1 neurons. ∗, P < 0.05; ∗∗, P < 0.01. Low- and high-magnification panel widths are 2.5 and 0.25 mm, respectively.
Discussion
Global ischemia is a neuronal insult that induces delayed, selective death of CA1 pyramidal neurons. A mechanism underlying ischemia-induced cell death is activation of the transcriptional repressor REST and REST-dependent silencing of the AMPAR subunit GluR2 in neurons destined to die (14). Here we show that global ischemia silences an additional REST target, MOR-1. Ischemia induces a marked down-regulation in MOR-1 mRNA and protein expression in selectively vulnerable CA1 (but not CA3). These findings document REST induction and silencing of MOR-1 expression in postischemic CA1, presumably in inhibitory interneurons. Ischemia promotes association of REST with the MOR-1 promoter in CA1 (but not CA3), consistent with a casual relation between REST induction and MOR-1 silencing in CA1. We further show that ischemia promotes deacetylation of histones H3 and H4 and dimethylation of H3-K9 (but not H3-K4) over the MOR-1 promoter in CA1 neurons destined to die, a signature of epigenetic gene silencing. To our knowledge, these findings document the first example of insult-induced histone methylation in neurons and implicate histone modification in the pathogenesis of neurodegeneration after global ischemia. We further show that MOR-1 expression is causally related to ischemia-induced neuronal death. Acute knockdown of MOR-1 gene expression by administration of antisense ODNs to hippocampal slices in vitro or intracerebroventricular injection of the MOR antagonist naloxone to rats in vivo affords protection against ischemia-induced death of CA1 neurons. These observations identify MORs as a novel target for therapeutic intervention and amelioration of the deleterious consequences associated with global ischemia. They also extend observations that the MOR agonist fentanyl exacerbates neurodegeneration after global ischemia (44).
Epigenetic Remodeling of Insulted CA1 Neurons.
Posttranslational modification of histone amino-terminal tails is an important mechanism that dynamically regulates chromatin structure and gene transcription. A fundamental mechanism by which REST silences target genes is by recruitment of the corepressors mSin3A and CoREST, which in turn recruit HDACs to the promoters of target genes (13, 21, 22). HDACs are implicated in dynamic gene repression and silence target genes by removal of acetyl groups from the N-terminal tails of histone proteins and tightening of the core chromatin complex. Our finding that ischemia promotes REST-dependent deacetylation of core histones H3 and H4 over the MOR-1 promoter is consistent with dynamic, REST-dependent gene silencing. The REST/CoREST complex additionally contains the site-specific histone methyltransferase G9a (20). Our finding that ischemia promotes dimethylation of H3-K9 (but not H3-K4) over the MOR-1 promoter is consistent with long-term, REST-dependent gene silencing via G9a-dependent histone methylation. Together, the results document epigenetic remodeling of MOR-1 gene expression in insulted CA1 neurons.
MOR-1 and Neuronal Survival.
Our findings that MOR-1 gene silencing and pharmacological blockade promote cell survival are consistent with findings of others that pharmacological blockade of MOR is protective in in vivo and in vitro models of hypoxia-ischemia (45–48) and that mice with targeted deletion of exon-1 of the MOR-1 gene exhibit enhanced survival of dentate gyrus neurons (41) in development. Whereas the protective effects of naloxone have been ascribed not to its binding to classical opioid receptors, but rather to its anti-inflammatory actions, the observation that (−) naloxone, but not its inert stereoisomer (+) naloxone, is protective indicates a direct opioid receptor-mediated action (47). Our finding that MOR-1 silencing or blockade affords neuroprotection is consistent with findings that MOR blockade reduces the abundance of Fas, FasL and proapoptotic BCL-2 family members (Bad and Bax) in the mouse neocortex (49). Under physiological conditions, activation of MOR inhibits GABA release from inhibitory interneurons that selectively project onto the somata of pyramidal neurons, thereby enhancing the excitability and output of pyramidal neurons (50). A possible scenario is that ischemia-induced gene silencing of MOR disinhibits GABA release from inhibitory interneurons and attenuates excitability and activity of CA1 pyramidal neurons and reduces excitotoxicity of endogenous glutamate (51). In this light, MOR-1 silencing would represent a failed attempt of postischemic inhibitory interneurons to promote survival of CA1 pyramidal neurons.
Significance of Epigenetic Remodeling of Insulted Neurons.
Our findings that ischemia triggers REST-dependent histone modifications and gene silencing of MOR-1 (the present study) and AMPA receptor GluR2 gene expression (14) implicate REST as a compelling candidate for orchestrating epigenetic modifications in postischemic neurons. Our findings that ischemia triggers REST expression in neurons destined to die (ref. 14 and the present study) and that REST expression is causally related to neuronal death (14) implicate REST-activated epigenetic modifications and gene silencing in neuronal injury. The topic of REST-dependent epigenetic modifications and reprogramming of mature neurons is a new and emerging area of investigation. Dysregulation of REST and its target genes is implicated in pathogenesis of Down's syndrome (55), Alzheimer's disease (56), and status epilepticus (14, 36, 57). The present study adds neurodegeneration after global ischemia to the growing list of neurological disorders and diseases in which REST-mediated epigenetic modifications and gene silencing are dysregulated.
Materials and Methods
Global Ischemia and Naloxone Administration.
Male Sprague–Dawley rats (150–200 g) were subjected to transient global ischemia or sham operation by four-vessel occlusion as described (14, 36). At indicated times, animals were anesthetized and injected intracerebroventricularly with naloxone or saline [for details, see supporting information (SI)Materials and Methods ].
Real-Time PCR.
To assess MOR-1 mRNA abundance, animals were killed at times after surgery and CA1 and CA3 were removed. RNA isolation, reverse transcription, and DNA amplification were performed according to the manufacturer's protocol (Applied Biosystems, Foster City, CA). Primers are as described in SI Materials and Methods.
Western Blot Analysis.
Protein extracts were prepared from CA1 and CA3 of control and experimental animals and subjected to Western blot analysis (14). Blots were probed with an anti-MOR antibody (SI Materials and Methods).
ChIP Assays.
ChIP assays were performed according to the protocol from an Upstate Biotechnology ChIP kit as described (14). The following antibodies were used: anti-REST, anti-acetylated H3, anti-acetylated H4, anti-methylated H3 raised against dimethyl K4 H3, anti-methylated H3 raised against dimethyl K9 H3, and nonimmune rabbit IgG (SI Materials and Methods).
Slice Cultures and Antisense ODN Administration.
Hippocampal slice cultures were prepared and subjected to OGD as described (14, 36). For probes, see SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Lakshmi Devi (Mount Sinai School of Medicine, New York, NY) for providing the μ receptor antibody and Ms. Adrianna Latuszek-Barrantes for technical assistance. This work was supported by National Institutes of Health Grants NS46742 and NS45693 (to R.S.Z.) and NS45287 (to M.V.L.B.) and a generous grant from the F. M. Kirby Program in Neural Repair and Neuroprotection. M.V.L.B. is the Sylvia and Robert S. Olnick Professor of Neuroscience and Distinguished Professor at the Albert Einstein College of Medicine.
Abbreviations
- ODN
oligodeoxynucleotide
- HDAC
histone deacetylase
- MOR
μ opioid receptor
- PI
propidium iodide
- OGD
oxygen/glucose deprivation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0611704104/DC1
References
- 1.Ballas N, Mandel G. Curr Opin Neurobiol. 2005;15:500–506. doi: 10.1016/j.conb.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 2.Roopra A, Huang Y, Dingledine R. Mol Interv. 2001;1:219–228. [PubMed] [Google Scholar]
- 3.Bruce AW, Donaldson IJ, Wood IC, Yerbury SA, Sadowski MI, Chapman M, Gottgens B, Buckley NJ. Proc Natl Acad Sci USA. 2004;101:10458–10463. doi: 10.1073/pnas.0401827101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paquette AJ, Perez SE, Anderson DJ. Proc Natl Acad Sci USA. 2000;97:12318–12323. doi: 10.1073/pnas.97.22.12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ballas N, Battaglioli E, Atouf F, Andres ME, Chenoweth J, Anderson ME, Burger C, Moniwa M, Davie JR, Bowers WJ, et al. Neuron. 2001;31:353–365. doi: 10.1016/s0896-6273(01)00371-3. [DOI] [PubMed] [Google Scholar]
- 6.Chen ZF, Paquette AJ, Anderson DJ. Nat Genet. 1998;20:136–142. doi: 10.1038/2431. [DOI] [PubMed] [Google Scholar]
- 7.Schoenherr CJ, Anderson DJ. Science. 1995;267:1360–1363. doi: 10.1126/science.7871435. [DOI] [PubMed] [Google Scholar]
- 8.Chong JA, Tapia-Ramirez J, Kim S, Toledo-Aral JJ, Zheng Y, Boutros MC, Altshuller YM, Frohman MA, Kraner SD, Mandel G. Cell. 1995;80:949–957. doi: 10.1016/0092-8674(95)90298-8. [DOI] [PubMed] [Google Scholar]
- 9.Grimes JA, Nielsen SJ, Battaglioli E, Miska EA, Speh JC, Berry DL, Atouf F, Holdener BC, Mandel G, Kouzarides T. J Biol Chem. 2000;275:9461–9467. doi: 10.1074/jbc.275.13.9461. [DOI] [PubMed] [Google Scholar]
- 10.Roopra A, Sharling L, Wood IC, Briggs T, Bachfischer U, Paquette AJ, Buckley NJ. Mol Cell Biol. 2000;20:2147–2157. doi: 10.1128/mcb.20.6.2147-2157.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naruse Y, Aoki T, Kojima T, Mori N. Proc Natl Acad Sci USA. 1999;96:13691–13696. doi: 10.1073/pnas.96.24.13691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andres ME, Burger C, Peral-Rubio MJ, Battaglioli E, Anderson ME, Grimes J, Dallman J, Ballas N, Mandel G. Proc Natl Acad Sci USA. 1999;96:9873–9878. doi: 10.1073/pnas.96.17.9873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lunyak VV, Burgess R, Prefontaine GG, Nelson C, Sze SH, Chenoweth J, Schwartz P, Pevzner PA, Glass C, Mandel G, et al. Science. 2002;298:1747–1752. doi: 10.1126/science.1076469. [DOI] [PubMed] [Google Scholar]
- 14.Calderone A, Jover T, Noh KM, Tanaka H, Yokota H, Lin Y, Grooms SY, Regis R, Bennett MV, Zukin RS. J Neurosci. 2003;23:2112–2121. doi: 10.1523/JNEUROSCI.23-06-02112.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Y, Myers SJ, Dingledine R. Nat Neurosci. 1999;2:867–872. doi: 10.1038/13165. [DOI] [PubMed] [Google Scholar]
- 16.Humphrey GW, Wang Y, Russanova VR, Hirai T, Qin J, Nakatani Y, Howard BH. J Biol Chem. 2001;276:6817–6824. doi: 10.1074/jbc.M007372200. [DOI] [PubMed] [Google Scholar]
- 17.You A, Tong JK, Grozinger CM, Schreiber SL. Proc Natl Acad Sci USA. 2001;98:1454–1458. doi: 10.1073/pnas.98.4.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin C, Zhang Y. Nat Rev Mol Cell Biol. 2005;6:838–849. doi: 10.1038/nrm1761. [DOI] [PubMed] [Google Scholar]
- 19.Marmorstein R. Nat Rev Mol Cell Biol. 2001;2:422–432. doi: 10.1038/35073047. [DOI] [PubMed] [Google Scholar]
- 20.Ballas N, Grunseich C, Lu DD, Speh JC, Mandel G. Cell. 2005;121:645–657. doi: 10.1016/j.cell.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 21.Roopra A, Qazi R, Schoenike B, Daley TJ, Morrison JF. Mol Cell. 2004;14:727–738. doi: 10.1016/j.molcel.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 22.Shi Y, Sawada J, Sui G, Affar EB, Whetstine JR, Lan F, Ogawa H, Luke MP, Nakatani Y, Shi Y. Nature. 2003;422:735–738. doi: 10.1038/nature01550. [DOI] [PubMed] [Google Scholar]
- 23.Tachibana M, Sugimoto K, Fukushima T, Shinkai Y. J Biol Chem. 2001;276:25309–25317. doi: 10.1074/jbc.M101914200. [DOI] [PubMed] [Google Scholar]
- 24.Xiao B, Jing C, Wilson JR, Walker PA, Vasisht N, Kelly G, Howell S, Taylor IA, Blackburn GM, Gamblin SJ. Nature. 2003;421:652–656. doi: 10.1038/nature01378. [DOI] [PubMed] [Google Scholar]
- 25.Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, Casero RA, Shi Y. Cell. 2004;119:941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 26.Lee MG, Wynder C, Cooch N, Shiekhattar R. Nature. 2005;437:432–435. doi: 10.1038/nature04021. [DOI] [PubMed] [Google Scholar]
- 27.Fields H. Nat Rev Neurosci. 2004;5:565–575. doi: 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
- 28.Kieffer BL, Gaveriaux-Ruff C. Prog Neurobiol. 2002;66:285–306. doi: 10.1016/s0301-0082(02)00008-4. [DOI] [PubMed] [Google Scholar]
- 29.Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le MM, Dolle P, et al. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- 30.Mansour A, Fox CA, Burke S, Akil H, Watson SJ. J Chem Neuroanat. 1995;8:283–305. doi: 10.1016/0891-0618(95)00055-c. [DOI] [PubMed] [Google Scholar]
- 31.Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, Watson SJ. J Comp Neurol. 1994;350:412–438. doi: 10.1002/cne.903500307. [DOI] [PubMed] [Google Scholar]
- 32.Tempel A, Zukin RS. Proc Natl Acad Sci USA. 1987;84:4308–4312. doi: 10.1073/pnas.84.12.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McQuiston AR, Saggau P. J Neurophysiol. 2003;90:1936–1948. doi: 10.1152/jn.01150.2002. [DOI] [PubMed] [Google Scholar]
- 34.Lee PH, Obie J, Hong JS. J Neurosci. 1989;9:692–697. doi: 10.1523/JNEUROSCI.09-02-00692.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zukin RS, Jover T, Yokota H, Calderone A, Simionescu M, Lau CG. In: Stroke: Pathophysiology, Diagnosis, and Management. Mohr JP, Choi DW, Grotta JC, Weir B, Wolf PA, editors. Philadelphia: Churchill Livingstone; 2004. pp. 829–854. [Google Scholar]
- 36.Noh KM, Yokota H, Mashiko T, Castillo PE, Zukin RS, Bennett MV. Proc Natl Acad Sci USA. 2005;102:12230–12235. doi: 10.1073/pnas.0505408102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim CS, Hwang CK, Choi HS, Song KY, Law PY, Wei LN, Loh HH. J Biol Chem. 2004;279:46464–46473. doi: 10.1074/jbc.M403633200. [DOI] [PubMed] [Google Scholar]
- 38.Andria ML, Simon EJ. Brain Res Mol Brain Res. 2001;91:73–80. doi: 10.1016/s0169-328x(01)00124-3. [DOI] [PubMed] [Google Scholar]
- 39.Drake CT, Milner TA. Hippocampus. 2002;12:119–136. doi: 10.1002/hipo.1107. [DOI] [PubMed] [Google Scholar]
- 40.Wagner JJ, Etemad LR, Thompson AM. J Pharmacol Exp Ther. 2001;296:776–781. [PubMed] [Google Scholar]
- 41.Harburg GC, Hall FS, Harrist AV, Sora I, Uhl GR, Eisch AJ. Neuroscience. 2006;144:77–87. doi: 10.1016/j.neuroscience.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pasternak GW, Standifer KM. Trends Pharmacol Sci. 1995;16:344–350. doi: 10.1016/s0165-6147(00)89068-9. [DOI] [PubMed] [Google Scholar]
- 43.Silva RM, Grossman HC, Hadjimarkou MM, Rossi GC, Pasternak GW, Bodnar RJ. J Pharmacol Exp Ther. 2002;301:513–518. doi: 10.1124/jpet.301.2.513. [DOI] [PubMed] [Google Scholar]
- 44.Kofke WA, Garman RH, Garman R, Rose ME. Brain Res. 1999;818:326–334. doi: 10.1016/s0006-8993(98)01228-1. [DOI] [PubMed] [Google Scholar]
- 45.Benzel EC, Musgrove CC, Kesterson L. South Med J. 1989;82:555–557. doi: 10.1097/00007611-198905000-00005. [DOI] [PubMed] [Google Scholar]
- 46.Zabramski JM, Spetzler RF, Selman WR, Roessmann UR, Hershey LA, Crumrine RC, Macko R. Stroke. 1984;15:621–627. doi: 10.1161/01.str.15.4.621. [DOI] [PubMed] [Google Scholar]
- 47.Liao SL, Chen WY, Raung SL, Chen CJ. Neurosci Lett. 2003;345:169–172. doi: 10.1016/s0304-3940(03)00540-8. [DOI] [PubMed] [Google Scholar]
- 48.McIntosh TK, Fernyak S, Hayes RL, Faden AI. J Neurotrauma. 1993;10:373–384. doi: 10.1089/neu.1993.10.373. [DOI] [PubMed] [Google Scholar]
- 49.San-Emeterio EP, Hurle MA. Neurosci Lett. 2006;403:276–279. doi: 10.1016/j.neulet.2006.04.053. [DOI] [PubMed] [Google Scholar]
- 50.Svoboda KR, Adams CE, Lupica CR. J Neurosci. 1999;19:85–95. doi: 10.1523/JNEUROSCI.19-01-00085.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwartz RD, Yu X, Katzman MR, Hayden-Hixson DM, Perry JM. J Neurosci. 1995;15:529–539. doi: 10.1523/JNEUROSCI.15-01-00529.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bahn S, Mimmack M, Ryan M, Caldwell MA, Jauniaux E, Starkey M, Svendsen CN, Emson P. Lancet. 2002;359:310–315. doi: 10.1016/S0140-6736(02)07497-4. [DOI] [PubMed] [Google Scholar]
- 53.Okazaki T, Wang H, Masliah E, Cao M, Johnson SA, Sundsmo M, Saitoh T, Mori N. Neurobiol Aging. 1995;16:883–894. doi: 10.1016/0197-4580(95)02001-2. [DOI] [PubMed] [Google Scholar]
- 54.Palm K, Belluardo N, Metsis M, Timmusk T. J Neurosci. 1998;18:1280–1296. doi: 10.1523/JNEUROSCI.18-04-01280.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pfaffl MW, Horgan GW, Dempfle L. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.