Abstract
Eukaryotic translation initiation factor 5A (eIF5A), the only known protein containing the polyamine-derived amino acid hypusine, modulates protein synthesis. We show that neurotrophic and neuroprotective actions of nerve growth factor (NGF) are mediated by hypusinated eIF5A, which can account for the known roles of polyamines in cell growth and survival. NGF treatment of PC12 cells stimulates eIF5A formation. Moreover, prevention of hypusine formation by a selective inhibitor of deoxyhypusine synthase and by its depletion with RNA interference blocks the NGF-elicited augmentation of neurite outgrowth and cell survival of PC12 cells. In brain cultures, inhibition of hypusine formation also inhibits neuronal process extension.
Keywords: hypusine, spermidine, nerve growth factor, arginase, deoxyhypusine synthase
In all eukaryotic organisms, protein translation is regulated by a variety of translation initiation factors, which in the nervous system are responsible for neuronal survival and neurite extension (1, 2). One of these, eukaryotic translation initiation factor 5A (eIF5A), is the only known cellular protein to contain the unique polyamine-derived amino acid hypusine (3) (Fig. 1). The name hypusine reflects the composition of this amino acid, a combination of hydroxyputrescine and lysine (3). Hypusine is formed from spermidine by the sequential action of two enzymes. Deoxyhypusine synthase (DHS) transfers the 4-aminobutyl group of spermidine to the epsilon-amino group of a specific lysine in eIF5A with the resultant deoxyhypusine intermediate then hydroxylated by deoxyhypusine hydroxylase (DOHH) (3, 4). eIF5A, a 17-kDa acidic protein highly conserved throughout eukaryotes, associates with translation machinery (5, 6) and enhances methionyl-puromycin synthesis in a model assay for translation initiation (7), and its deletion in yeast is lethal (8). eIF5A is implicated in the regulation of p53 expression and thereby p53-dependent apoptosis through interactions with syntenin, independent of its influences on protein translation (9). Other proposed functions of eIF5A include serving as a cofactor of HIV-1 REV (10, 11) to regulate nuclear export (12) and RNA turnover (13–15) as well as maintaining cell wall integrity and actin polarity (16, 17).
Fig. 1.
Pathway of polyamine biosynthesis and hypusine modification in eIF5A. Arginase catalyzes the production of ornithine, which is subsequently converted into putrescine, spermidine, and spermine. Hypusine modification involves two sequential steps. DHS transfers the 4-aminobutyl moiety from spermidine to the ε-amino group of one specific lysine residue (Lys-50 in the human protein) in eIF5A generating eIF5A intermediate, which is then hydroxylated by deoxyhypusine hydroxylase to form the mature hypusinated eIF5A.
Arginine, a dietary precursor of polyamines, is converted by arginase to ornithine, which is subsequently transformed to putrescine, spermidine, and spermine (18–20) (Fig. 1). Polyamine biosynthesis and ornithine decarboxylase (ODC) are dramatically augmented in numerous forms of rapid tissue growth and many tumors (21, 22). ODC inhibitors and other agents interfering with polyamine synthesis prevent rapid tissue growth (20, 22). Arginine is also the direct precursor of nitric oxide (NO), as NO synthase (NOS) abstracts NO from the guanidino group of arginine giving rise to citrulline as a by-product (23). Both NO and polyamines have been implicated in neuronal growth and survival (24, 25), and differential movement of arginine into the polyamine and NO pathways influences neuronal survival (26). In the present study, we provide evidence that hypusinated eIF5A physiologically regulates nerve process extension and neuronal survival both in PC12 cells and brain neurons.
Results
Arginase-I is induced by neurotrophic stimuli in dorsal root ganglia neurons (27) and superior cervical ganglion (28). In PC12 cells stimulated with nerve growth factor (NGF) after serum starvation, we observe a 2-fold increase in arginase-I protein (Fig. 2 A and B). The increased protein levels of arginase-I are associated with a substantial augmentation of arginine transformation to its products with a 3-fold increase in urea generation (Fig. 2C). Additionally, the generation of CO2 from carboxyl-labeled [14C]arginine is increased 2- to 3-fold after NGF treatment (Fig. 2D). This increase is abolished after treatment with the arginase inhibitor S-(2-boronoethyl)-l-cysteine (BEC) or the ODC inhibitor difluoromethylornithine (DMFO) (Fig. 2D), consistent with findings that NGF induces expression of ODC in superior cervical ganglion (29) and PC12 cells (30).
Fig. 2.
NGF stimulates arginine metabolism in primed PC12 cells. (A) Induction of arginase I expression in primed PC12 cells after NGF treatment. Western blot of lysates from PC12 cells treated with NGF (50 ng/ml) for 14 h is shown. (B) Quantification of arginase I expression (control, 1.15 ± 0.02; NGF, 1.92 ± 0.08). Data are presented as mean ± SEM from three experiments. ∗, P < 0.01. (C) Arginase activity. Primed PC12 cells were treated with NGF (50 ng/ml) for 14 h followed by addition of arginine labeled with 14C at the guanido group and incubated for 1 h. Radiolabeled urea was separated by TLC and quantified by scintillation counting (control, 2,061 ± 112; NGF, 6,976 ± 226; NGF plus BEC, 257 ± 29 in dpm). Results are presented as mean ± SEM from three independent experiments. ∗, P < 0.01. (D) ODC. Primed PC12 cells were treated with NGF (50 ng/ml) for 14 h followed by addition of arginine labeled with 14C at the carboxyl group and incubated for 1 h. CO2 was captured by 1 M KOH and measured by scintillation counting (control, 164 ± 7.5; NGF, 397 ± 33; NGF plus BEC, 110 ± 9.4; NGF ± DMFO, 94 ± 10 in dpm). Results are presented as mean ± SEM from three independent experiments. ∗, P < 0.01.
To monitor hypusinated eIF5A, we used ion exchange chromatography to analyze the formation of 3H-labeled hypusine (31). Treatment of PC12 cells with NGF elicits a 2- to 3-fold augmentation in hypusine (Fig. 3A) and 3H-labeled eIF5A (Fig. 3B), consistent with the increase in polyamine formation. Gel Coomassie staining does not display any major change in overall protein level, indicating that increased hypusine generation reflects augmented formation of hypusinated eIF5A (Fig. 3B).
Fig. 3.
NGF enhances hypusine modification of eIF5A. Primed PC12 cells were treated with NGF and labeled with 1,8-[3H]spermidine·HCl. (A) Formation of 3H-labeled hypusine was monitored by ion-exchange chromatography. (B) 3H-labeled hypusinated eIF5A was detected by a fluorogram (Right), and total proteins were revealed by Coomassie blue staining of an SDS/PAGE (Left).
We wondered whether the increase in hypusine synthesis and hypusinated eIF5A formation mediates the neurotrophic and prosurvival actions of NGF. First, we examined whether neurite outgrowth is influenced by inhibition of polyamine formation (Fig. 4). In PC12 cells treated with NGF, the arginase inhibitor BEC significantly reduces the number of neurites with long processes and triples cells with short processes. Similarly, the ODC inhibitor DMFO decreases the number of long processes and quintuples the number of cells with short processes.
Fig. 4.
Inhibition of arginase or ODC attenuates NGF-induced neurite outgrowth in primed PC12 cells. (A) Primed PC12 cells, which express GFP, were plated in serum-free medium or in medium with NGF (50 ng/ml), NGF plus 25 μM BEC, or NGF plus 2 mM DMFO as indicated. Cells were fixed 14 h after incubation and visualized with fluorescent microscopy. (B) Quantification of neurite outgrowth. Cells were divided into two groups with long vs. short processes as described in the experimental procedure. Approximately 40 cells were counted in each experiment (the number of cells with long processes in control, 4 ± 1.5; NGF, 37 ± 1; NGF plus BEC, 26.3 ± 1.7; NGF plus DMFO, 23.5 ± 1.6 vs. the number of cells with short processes in control, 36 ± 1.5; NGF, 3.8 ± 0.4; NGF plus BEC, 12.3 ± 1.3; and NGF plus DMFO, 17.3 ± 1.6). Data are presented as mean ± SEM from four experiments. ∗, P < 0.01.
To determine whether hypusinated eIF5A mediates the influences of polyamines on neurite outgrowth, we prevented eIF5A formation in two ways. First, we treated PC12 cells with the potent DHS inhibitor N-guanyl-1,7-diaminoheptane (GC-7) (IC50, 17 nM; refs. 32 and 33). GC-7 (1 μM) inhibits hypusine synthesis in PC12 cells (Fig. 5 A and B). The inhibition of hypusine synthesis by GC-7 is cell-density dependent so that with lower cell densities, GC-7 more potently inhibits hypusine formation (1 μM, ≈60% inhibition; 10 μM, 90% inhibition) (data not shown). In NGF-treated PC12 cells, GC7 substantially reduces the number of cells with long processes and triples the number with short processes (Fig. 5 E and F). These effects do not reflect any general effect on polyamine synthesis, because GC-7 does not alter polyamine pools in Chinese hamster ovary cells (32) or in PC12 cells (data not shown). We also depleted hypusine synthesis and hypusinated eIF5A with DHS RNA interference (RNAi) (Fig. 5 C and D), which reduces the number of cells with long processes by half and more than quintuples the number of cells with short processes (Fig. 5 E and F).
Fig. 5.
Inhibition of hypusine modification attenuates NGF-induced neurite outgrowth. (A) Primed PC12 cells were treated with GC-7 at concentrations of 0.1, 1, or 10 μM, respectively and labeled with 1,8-[3H]spermidine·HCl. Formation of [3H]hypusine was monitored by ion exchange chromatography. (B) Fluorogram for detecting [3H]hypusinated eIF5A (Right) and Coomassie blue staining for total protein (Left). (C) PC12 cells transfected with a control or a DHS RNAi plasmid were labeled with 1,8-[3H]spermidine·HCl. [3H]hypusine was monitored by ion-exchange chromatography. (D) Fluorogram of 3H-hypusinated eIF5A (Right) and Coomassie blue staining for total proteins (Left). (E) Primed PC12 cells were replated in serum-free medium or in medium with NGF (50 ng/ml), NGF plus 1 μM GC-7, or NGF plus DHS RNAi for 24 h, followed by fixation and imaging. (F) Quantification of neurite outgrowth (the number of cells with long processes in control, 5.3 ± 0.7; NGF, 40.8 ± 2.4; NGF plus GC-7, 27.3 ± 2.7; NGF plus DHS RNAi, 15.3 ± 1.1 vs. the number of cells with short processes in control, 34.5 ± 2.1; NGF, 4.3 ± 0.6; NGF plus GC-7, 13.5 ± 1.3; NGF plus DHS RNAi, 24.8 ± 2.5). Data are presented as mean ± SEM from four experiments. ∗, P < 0.01.
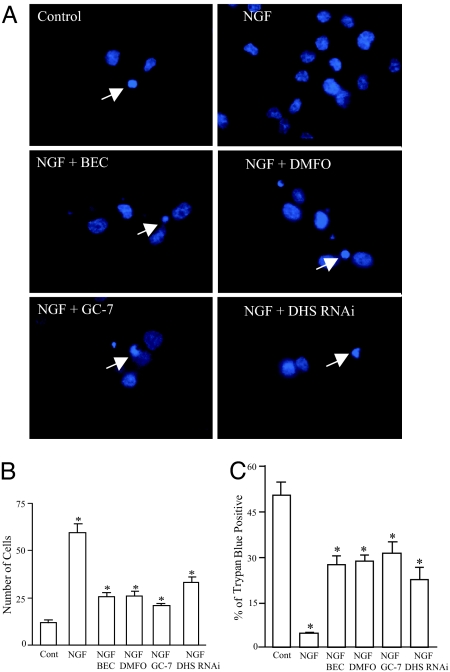
We explored whether hypusinated eIF5A mediates the ability of NGF to enhance neuronal survival by monitoring the influence of BEC, DMFO, GC-7, and DHS depletion by RNAi (Fig. 6). All four treatments reduce total cell number by 50% (Fig. 6B) and increase the number of dying, Trypan blue-positive cells by 5- to 6-fold (Fig. 6C).
Fig. 6.
Hypusine modification is required for NGF-dependent survival of primed PC12 cells. (A) Primed PC12 cells were cultured as described above for 24 h, followed by fixation and DAPI staining. Arrows indicate the condensed nuclei in dying cells. (B) Quantification of cells 24 h after plating (control, 12 ± 1.3; NGF, 59.5 ± 4.7; NGF plus BEC, 25.5 ± 2.4; NGF plus DMFO, 26 ± 2.6; NGF plus GC-7, 20.8 ± 1.3; NGF plus DHS RNAi, 33.3 ± 2.8). Data are presented as mean ± SEM from four experiments. ∗, P < 0.01. (C) Trypan blue staining for dying cells. Percentages of Trypan blue-positive cells are 50 ± 4.3 for control, 4.5 ± 0.3 for NGF, 27.3 ± 2.6 for NGF plus BEC, 28.3 ± 2.2 for NGF plus DMFO, 30.8 ± 3.8 for NGF plus GC-7, and 22.3 ± 4.1 for NGF plus DHS RNAi. Data are presented as mean ± SEM from four experiments. ∗, P < 0.01.
We wondered whether the increased number of dying cells elicited by BEC, DMFO, and GC-7 reflects nonspecific toxicity. All of the above experiments used primed PC12 cells. Accordingly, we examined the effects of these agents on PC12 cells that were not primed but had also been treated with NGF with similar augmentation of neurite extension and cell survival as in the primed cells. These preparations are much less sensitive to effects of the drugs on number of dying cells than the primed cells, indicating that drug actions in primed cells do not reflect nonspecific toxicity (data not shown). The inhibition of neurite extension and increase of cell death caused by depletion of DHS with RNAi also cannot be attributed to nonspecific toxicity, because a scrambled RNAi construct fails to elicit these effects.
We assessed the importance of hypusinated eIF5A for neuronal disposition in the brain by treating primary hippocampal neuronal cultures with GC-7 (1 μM) for 48 h and monitoring neurite length (Fig. 7 A and B). GC-7 treatment reduces the number of neurons with the longest neurites by >50% and augments 2- to 3-fold those with the shortest neurites 2- to 3-fold (Fig. 7C). The cultures include pyramidal cells, granule cells, and interneurons, all of which are affected similarly by GC-7.
Fig. 7.
Inhibition of DHS attenuates neurite outgrowth of primary hippocampal neurons. (A) Primary hippocampal cultures at in vitro day 3 were treated with 1 μM GC-7, fixed 2 days after treatment, and visualized by F-actin staining. (B) Quantification of neurite outgrowth (the number of cells with long processes in control, 33.5 + 2.4 and GC-7, 13.5 + 1.5 vs. with short processes in control, 9.5 ± 1.7 and GC-7, 29 ± 2.8). Data are presented as mean ± SEM from four experiments. ∗, P < 0.01.
Discussion
The principal findings of our study are that NGF-dependent neurite outgrowth and survival of PC12 cells as well as mammalian brain neurons are critically dependent on hypusinated eIF5A. Inhibition of polyamine formation by agents that block arginase and ODC prevents neurite outgrowth and reduces cell viability. More importantly, highly selective inhibition of hypusine formation by the DHS inhibitor GC-7 and depletion of DHS by RNAi both block neurite outgrowth and markedly reduce cell survival.
The polyamines spermidine and spermine and their precursor putrescine have been studied extensively for many years as potential regulators of nucleic acid disposition, protein formation, and tissue growth and regeneration (19, 20). Because of their marked positive charges, polyamines bind nucleic acids and other negatively charged macromolecules. They have been implicated in gene transcription, mRNA translation (34), synaptic plasticity (35, 36), and tumorigenesis (20, 22). In neuronal systems, polyamines regulate glutamate receptors (35). Conceivably, incorporation of spermidine into hypusine alters its availability for regulation of glutamate receptors, which might affect neurotransmission and/or neuronal growth.
Despite abundant investigation of these multiple mechanisms, none have been definitively linked to the regulation by polyamines of tissue growth and cellular survival. Our study provides substantial evidence that the transformation of spermidine to hypusine in the formation of eIF5A mediates the effects of polyamines on neuronal process extension and survival. Other influences of polyamines on tissue growth may also involve hypusinated eIF5A. Thus, deletion of eIF5A or DHS in yeast reduces cell growth and is lethal (37–40). Moreover in C. elegans (41) and Drosophila (42), deletion of eIF5A, DHS, or DOHH is lethal.
The PC12 cells used in this study were primed with NGF, then stripped off the culture dish and replated, a procedure that models axotomized sympathetic neurons (43). Zigmond and associates (44) showed that spermidine enhances regrowth of neurites in these preparations. Treatment with polyamines also accelerates regeneration of damaged sympathetic nerves in rat superior cervical ganglia (45). In our experiments, inhibitors of polyamine biosynthesis and hypusine formation were substantially more effective in preventing influences of NGF on neurite outgrowth and survival in primed than in unprimed PC12 cells. Thus, polyamines and hypusinated eIF5A may be particularly important for tissue restoration after damage. Such actions may involve a wide range of tissues. For instance, Filbin and associates (27) recently showed that BDNF stimulates axonal growth of dorsal root ganglion (DRG) neurons through induction of arginase I with biosynthesis of polyamines and conceivably hypusinated eIF5A mediating these efforts.
How might eIF5A impact tissue growth and survival? eIF5A associates with translational machinery components (5, 6) and regulates the stability of RNA transcripts (13, 15), which may mediate cell growth and differentiation (46). Additionally, eIF5A regulates p53 and p53-dependent apoptosis (9). The importance of p53 for survival of many types of cells including neurons suggests that this pathway may mediate the neuronal alterations we have observed. Which of these proposed actions of eIF5A is critical for the tissue restorative influences is unclear. Regardless of the exact molecular mechanism, our findings indicate a requirement of hypusinated eIF5A for the growth and survival of neurons and presumably many other cell types. Agents selectively influencing its disposition may have therapeutic relevance in diverse conditions with altered cell growth and survival.
Materials and Methods
Cell Cultures.
The preparation for primary hippocampal neurons is as described (47). Briefly, primary neurons were prepared from rat embryonic day 18–19 brains. Neurons were plated in 35-mm Petri dishes with glass coverslips, which were coated with poly-d-lysine (40 μg/ml), at a density of ≈2–4 × 105 per well in neurobasal medium with B27 supplement (Invitrogen, Carlsbad, CA). PC12 cells (ATCC, Manassas, VA) were maintained in DMEM with 10% heat-inactivated horse serum, 10% FBS, and penicillin–streptomycin (100 units/ml and 100 μg/ml, respectively) (Invitrogen). The primed PC12 cells were prepared as described (43). Briefly, PC12 cells were plated on collagen IV (Roche, Indianapolis, IN)-coated 10-cm dishes and treated with 50 ng/ml NGF (NGF-7S; Sigma, St. Louis, MO) for 5 days at 37°C in a humidified atmosphere with 5% CO2. Half of the medium was replaced with the same fresh medium every 2 days with a constant NGF concentration. The NGF-primed cells were washed four times with regular PC12 culture medium and dissociated from the culture dishes with forceful trituration in culture medium. Cells were spun down at 500 × g for 5 min and resuspended in fresh medium containing 10% DMSO and stored in liquid nitrogen. An aliquot of NGF-primed cells was washed once in serum-free DMEM, resuspended in serum-free medium, and plated on collagen IV-coated six-well plates or 35-mm dishes with coverslips (MatTek Corp., Ashland, MA). NGF (50 ng/ml) (Sigma), 25 μM BEC (Calbiochem, San Diego, CA), 2 mM DMFO (Sigma), and 1 μM GC-7 were used in all experiments.
DHS RNAi Construct.
Double-stranded oligos containing RNAi sequence derived from rat DHS gene (GCCCAUAAGAACCACAUAC) were subcloned into pSUPER neo + GFP vector (OligoEngine, Seattle, WA) linearized with HindIII and BglII. The expression cassette for short hairpin RNA is driven by the H1 promoter, and EGFP expression cassette is under control of a CMV promoter. PC12 cells were transfected with plasmids by using Lipofectamine 2000 (Invitrogen).
Immunoblots.
Cells were lysed in 1× sample buffer (Invitrogen) with 5% 2-mercaptoethanol, EDTA-free protease inhibitors (Roche), 1.5 mM Na3VO4, and 10 mM NaF. The primary GAPDH (Sigma) and arginase I (BD Bioscience, San Jose, CA) antibodies were diluted in blocking buffer (3% BSA/0.05% Tween 20 in PBS). The blots were developed by using supersignal west pico chemiluminescent substrate (Pierce, Rockford, IL).
Quantification of Neurite Growth.
Primed PC12 cells were resuspended in serum-free DMEM and plated onto collagen-coated 35-mm dishes with coverslips at the bottom at a low-density of 1 × 105 per well. Cells were treated with 50 ng/ml NGF alone or plus other agents (25 μM BEC, 2 mM DMFO, or 1 μM GC-7, respectively). Approximately 14–18 h later, cells were fixed in 4% paraformaldehyde in PBS at room temperature. Neuronal processes were visualized by expression of GFP with a plasmid (pSUPER) transiently transfected into PC12 cells during priming. In the DHS RNAi experiments, the RNAi plasmid was transiently transfected into PC12 cells 24 h before harvesting the primed cells. Individual images were acquired with a Meta 510 confocal microscope. PC12 cells were divided into two groups: one group of cells having processes longer than the diameter of their cell body and the other group of cells having shorter or no processes. Approximately 200 cells were counted for each experiment. Primary hippocampal neurons in vitro day two were treated with 1 μM GC-7 for 2–3 days. Neurons were then fixed in 4% paraformaldehyde in PBS at room temperature. Neuronal processes were visualized by phalloidin-FITC staining for F-actin.
Trypan Blue Staining and Cell Counting.
PC12 cells primed as described above were resuspended in serum-free DMEM and then plated onto collagen-coated 35-mm dishes at a low density of 1 × 105 per well. Cells were incubated either with 50 ng/ml NGF alone or plus other agents (25 μM BEC, 2 mM DMFO, or 1 μM GC-7, respectively). Twenty-four hours after treatment, cells were gently rinsed once with PBS and detached from the dish by trypsin treatment. Cells were pelleted by a brief spin, resuspended in 0.5 ml of culture medium, and manually counted by using a hemocytometer. Dying cells were counted by Trypan blue staining as follows. Culture dishes were sealed with parafilm and placed in a shaker at 37°C at 30 rpm for 30 min. Detached cells were collected by a brief spin, and the total number of cells and Trypan blue-positive cells were counted. Because 99% of cells still attached to the dishes after shaking were viable (Trypan blue-negative), these cells were dissociated from the dishes by trypsin treatment and counted. In the DHS RNAi experiment, ≈70–90% of primed PC12 cells were GFP-positive cells before plating. After a 24 h treatment, GFP-positive cells were counted by using a fluorescent microscope. Among the cells that remained attached to the dishes after shaking, 60% were GFP-positive, whereas 96% of cells that floated in medium were GFP-positive. This indicates that the Trypan blue-positive cells are largely transfected with DHS RNAi, whereas untransfected cells contribute little, if any, to the Trypan blue-positive staining.
Arginase Activity Assay.
Primed PC12 cells were plated onto six-well plates in serum-free DMEM (Invitrogen) or in medium with 50 ng/ml NGF and then treated with 25 μM BEC, 2 mM DMFO, or 1 μM GC-7 for 14 h. Before the arginase activity assay, PC12 cells cultured in six-well plates were gently rinsed twice in 2 ml of washing buffer (124 mM NaCl/3 mM KCl/1.25 mM Na2HPO4/1.6 mM CaCl2/1.8 mM MgSO4/10 mM d-glucose/10 mM Hepes, pH 7.4) and then incubated for 45 min at 37°C in 0.5 ml of washing buffer with 5 μM unlabeled l-arginine plus 0.25 μM l-[guanido-14C]-arginine (5 μCi/ml; NEN, Boston, MA). After incubation, 1.5 ml of ice-cold methanol was directly added into wells. The plates were incubated on ice for 30 min with gentle shaking. Samples were transferred to Eppendorf tubes. Cell debris was removed by a brief spin. The supernatants were collected and concentrated by speed vacuum. Then, 10 μl of the concentrated sample was spotted onto TLC plates (Merck, Darmstadt, Germany), dried for 30 min at room temperature, and developed in a solvent system composed of chloroform/methanol/ammonium hydroxide/water in a ratio of 0.5/4.5/2.0/1.0 (vol/vol). 14C-labeled urea was used as a standard. TLC plates were dried and exposed to a film. Urea spots were cut from TLC plates and analyzed by scintillation counting.
ODC Activity Assay.
After treatment, PC12 cells cultured in six-well plates were gently rinsed twice in 2 ml of washing buffer (124 mM NaCl/3 mM KCl/1.25 mM Na2HPO4/1.6 mM CaCl2/1.8 mM MgSO4/10 mM d-glucose/10 mM Hepes, pH 7.4). One milliliter of washing buffer with 5 μM unlabeled l-arginine plus 0.25 μM l-14C(U)-arginine (5 μCi/ml; NEN) was added into each well. Whatman 3 MM paper (≈0.5 × 0.5 cm in size) was soaked with 100 μl of 1 M KOH and put in an Eppendorf tube cap, and then carefully placed into six-well plates. Each well was sealed with a rubber stopper. Forty-five minutes after incubation, the 3 MM paper was removed from the plates and immediately placed into a vial for scintillation counting.
Measurement of Hypusine by Ion-Exchange Chromatography and Detection of Hypusinated eIF5A by Fluorogram.
3H-hypusine was monitored as described (31). Briefly, primed PC12 cells were plated on 6- to 10-cm dishes in serum-free DMEM for 2 h. NGF (50 ng/ml), GC-7 (0.1, 1, and 10 μM, respectively), and 1,8-[3H]spermidine·HCl (30–40 μCi) (20–36 Ci/mmol; PerkinElmer Life Sciences, Boston, MA) were applied afterward. After incubation at 37°C for 14–18 h, PC12 cells were scraped and pelleted by a brief spin. Protein was precipitated with 10% trichloroacetic acid plus 1 mM polyamines and washed in the same buffer 3–4 times. The precipitated proteins were hydrolyzed in 6 M HCl at 110°C for 18 h. Samples were dried down by a speed vacuum and resuspended in 100 μl of water. The content of [3H]hypusine was determined after ion-exchange chromatographic separation. For DHS RNAi experiments, PC12 cells were transfected with a control or a DHS RNAi plasmid for 24 h and then labeled with [3H]spermidine (60 μCi) in serum-free medium with 50 ng/ml NGF for 18 h. The hypusinated eIF5A was also monitored by a fluorogram. Briefly, proteins from trichloroacetic acid precipitation were separated by SDS/PAGE. Gels were treated with sodium salicylate (1 M) for 30 min, dried down, and exposed to a film at −70°C for 4 days.
Acknowledgments
This work was supported by U.S. Public Health Service Grant MH 18501 and Research Scientist Award DA-00074 (to S.H.S.).
Abbreviations
- eIF5A
eukaryotic initiation factor 5A
- DHS
deoxyhypusine synthase
- NGF
nerve growth factor
- GC-7
N-guanyl-1,7-diaminoheptane
- BEC
S-(2-boronoethyl)-l-cysteine
- DMFO
difluoromethylornithine
- ODC
ornithine decarboxylase.
Footnotes
The authors declare no conflict of interest.
References
- 1.Willis DE, Twiss JL. Curr Opin Neurobiol. 2006;16:111–118. doi: 10.1016/j.conb.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Degracia DJ, Kumar R, Owen CR, Krause GS, White BC. J Cereb Blood Flow Metab. 2002;22:127–141. doi: 10.1097/00004647-200202000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Park MH. J Biochem (Tokyo) 2006;139:161–169. doi: 10.1093/jb/mvj034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park JH, Aravind L, Wolff EC, Kaevel J, Kim YS, Park MH. Proc Natl Acad Sci USA. 2006;103:51–56. doi: 10.1073/pnas.0509348102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jao DL, Chen KY. J Cell Biochem. 2006;97:583–598. doi: 10.1002/jcb.20658. [DOI] [PubMed] [Google Scholar]
- 6.Zanelli CF, Maragno AL, Gregio AP, Komili S, Pandolfi JR, Mestriner CA, Lustri WR, Valentini SR. Biochem Biophys Res Commun. 2006;348:1358–1366. doi: 10.1016/j.bbrc.2006.07.195. [DOI] [PubMed] [Google Scholar]
- 7.Benne R, Hershey JW. J Biol Chem. 1978;253:3078–3087. [PubMed] [Google Scholar]
- 8.Kang HA, Hershey JW. J Biol Chem. 1994;269:3934–3940. [PubMed] [Google Scholar]
- 9.Li AL, Li HY, Jin BF, Ye QN, Zhou T, Yu XD, Pan X, Man JH, He K, Yu M, et al. J Biol Chem. 2004;279:49251–49258. doi: 10.1074/jbc.M407165200. [DOI] [PubMed] [Google Scholar]
- 10.Ruhl M, Himmelspach M, Bahr GM, Hammerschmid F, Jaksche H, Wolff B, Aschauer H, Farrington GK, Probst H, Bevec D, et al. J Cell Biol. 1993;123:1309–1320. doi: 10.1083/jcb.123.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hauber I, Bevec D, Heukeshoven J, Kratzer F, Horn F, Choidas A, Harrer T, Hauber J. J Clin Invest. 2005;115:76–85. doi: 10.1172/JCI21949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosorius O, Reichart B, Kratzer F, Heger P, Dabauvalle MC, Hauber J. J Cell Sci. 1999;112:2369–2380. doi: 10.1242/jcs.112.14.2369. [DOI] [PubMed] [Google Scholar]
- 13.Zuk D, Jacobson A. EMBO J. 1998;17:2914–2925. doi: 10.1093/emboj/17.10.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valentini SR, Casolari JM, Oliveira CC, Silver PA, McBride AE. Genetics. 2002;160:393–405. doi: 10.1093/genetics/160.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schrader R, Young C, Kozian D, Hoffmann R, Lottspeich F. J Biol Chem. 2006;281:35336–35346. doi: 10.1074/jbc.M601460200. [DOI] [PubMed] [Google Scholar]
- 16.Zanelli CF, Valentini SR. Genetics. 2005;171:1571–1581. doi: 10.1534/genetics.105.048082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chatterjee I, Gross SR, Kinzy TG, Chen KY. Mol Genet Genomics. 2006;275:264–276. doi: 10.1007/s00438-005-0086-4. [DOI] [PubMed] [Google Scholar]
- 18.Morris SM., Jr Am J Clin Nutr. 2006;83:508S–512S. doi: 10.1093/ajcn/83.2.508S. [DOI] [PubMed] [Google Scholar]
- 19.Tabor CW, Tabor H. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- 20.Gerner EW, Meyskens FL., Jr Nat Rev Cancer. 2004;4:781–792. doi: 10.1038/nrc1454. [DOI] [PubMed] [Google Scholar]
- 21.Russell D, Snyder SH. Proc Natl Acad Sci USA. 1968;60:1420–1427. doi: 10.1073/pnas.60.4.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pegg AE. J Biol Chem. 2006;281:14529–14532. doi: 10.1074/jbc.R500031200. [DOI] [PubMed] [Google Scholar]
- 23.Bredt DS, Snyder SH. Annu Rev Biochem. 1994;63:175–195. doi: 10.1146/annurev.bi.63.070194.001135. [DOI] [PubMed] [Google Scholar]
- 24.Dawson VL, Dawson TM, London ED, Bredt DS, Snyder SH. Proc Natl Acad Sci USA. 1991;88:6368–6371. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilad GM, Gilad VH. Brain Res. 1983;273:191–194. doi: 10.1016/0006-8993(83)91113-7. [DOI] [PubMed] [Google Scholar]
- 26.Estevez AG, Sahawneh MA, Lange PS, Bae N, Egea M, Ratan RR. J Neurosci. 2006;26:8512–8516. doi: 10.1523/JNEUROSCI.0728-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai D, Deng K, Mellado W, Lee J, Ratan RR, Filbin MT. Neuron. 2002;35:711–719. doi: 10.1016/s0896-6273(02)00826-7. [DOI] [PubMed] [Google Scholar]
- 28.Boeshore KL, Schreiber RC, Vaccariello SA, Sachs HH, Salazar R, Lee J, Ratan RR, Leahy P, Zigmond RE. J Neurobiol. 2004;59:216–235. doi: 10.1002/neu.10308. [DOI] [PubMed] [Google Scholar]
- 29.MacDonnell PC, Nagaiah K, Lakshmanan J, Guroff G. Proc Natl Acad Sci USA. 1977;74:4681–4684. doi: 10.1073/pnas.74.10.4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Volonte C, Greene LA. J Biol Chem. 1990;265:11050–11055. [PubMed] [Google Scholar]
- 31.Park MH, Liberato DJ, Yergey AL, Folk JE. J Biol Chem. 1984;259:12123–12127. [PubMed] [Google Scholar]
- 32.Park MH, Wolff EC, Lee YB, Folk JE. J Biol Chem. 1994;269:27827–27832. [PubMed] [Google Scholar]
- 33.Jakus J, Wolff EC, Park MH, Folk JE. J Biol Chem. 1993;268:13151–13159. [PubMed] [Google Scholar]
- 34.Childs AC, Mehta DJ, Gerner EW. Cell Mol Life Sci. 2003;60:1394–1406. doi: 10.1007/s00018-003-2332-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mott DD, Washburn MS, Zhang S, Dingledine RJ. J Neurosci. 2003;23:1179–1188. doi: 10.1523/JNEUROSCI.23-04-01179.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aizenman CD, Munoz-Elias G, Cline HT. Neuron. 2002;34:623–634. doi: 10.1016/s0896-6273(02)00674-8. [DOI] [PubMed] [Google Scholar]
- 37.Schnier J, Schwelberger HG, Smit-McBride Z, Kang HA, Hershey JW. Mol Cell Biol. 1991;11:3105–3114. doi: 10.1128/mcb.11.6.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wohl T, Klier H, Ammer H, Lottspeich F, Magdolen V. Mol Gen Genet. 1993;241:305–311. doi: 10.1007/BF00284682. [DOI] [PubMed] [Google Scholar]
- 39.Sasaki K, Abid MR, Miyazaki M. FEBS Lett. 1996;384:151–154. doi: 10.1016/0014-5793(96)00310-9. [DOI] [PubMed] [Google Scholar]
- 40.Park MH, Joe YA, Kang KR. J Biol Chem. 1998;273:1677–1683. doi: 10.1074/jbc.273.3.1677. [DOI] [PubMed] [Google Scholar]
- 41.Sugimoto A. Differentiation. 2004;72:81–91. doi: 10.1111/j.1432-0436.2004.07202004.x. [DOI] [PubMed] [Google Scholar]
- 42.Spradling AC, Stern D, Beaton A, Rhem EJ, Laverty T, Mozden N, Misra S, Rubin GM. Genetics. 1999;153:135–177. doi: 10.1093/genetics/153.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rukenstein A, Green LA. Brain Res. 1983;263:177–180. doi: 10.1016/0006-8993(83)91218-0. [DOI] [PubMed] [Google Scholar]
- 44.Schreiber RC, Boeshore KL, Laube G, Veh RW, Zigmond RE. Neuroscience. 2004;128:741–749. doi: 10.1016/j.neuroscience.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 45.Dornay M, Gilad VH, Shiler I, Gilad GM. Exp Neurol. 1986;92:665–674. doi: 10.1016/0014-4886(86)90307-9. [DOI] [PubMed] [Google Scholar]
- 46.Chen ZP, Yan YP, Ding QJ, Knapp S, Potenza JA, Schugar HJ, Chen KY. Cancer Lett. 1996;105:233–239. doi: 10.1016/0304-3835(96)04287-5. [DOI] [PubMed] [Google Scholar]
- 47.Huang Y, Man HY, Sekine-Aizawa Y, Han Y, Juluri K, Luo H, Cheah J, Lowenstein C, Huganir RL, Snyder SH. Neuron. 2005;46:533–540. doi: 10.1016/j.neuron.2005.03.028. [DOI] [PubMed] [Google Scholar]