Abstract
Urocortin 3 (Ucn 3), a member of the corticotropin-releasing factor (CRF) family of peptides, is strongly expressed in mammalian pancreatic β cells and has been shown to stimulate insulin secretion. Here we report the investigation of the hypothesis that endogenous Ucn 3 regulates insulin secretion, particularly in the presence of nutrient excess. Secretion of Ucn 3-like immunoreactivity from cultured β cells was stimulated by high glucose and insulin secretagogs such as GLP-1; furthermore, 5 pancreatic Ucn 3 mRNA levels in vivo were increased during the positive energy balance caused by high-fat diet and by the absence of leptin. Immunoneutralization of Ucn 3 or pharmacologic blockade of its receptor, the type 2 CRF receptor (CRFR2), attenuated high but not low glucose-induced insulin secretion from isolated islets in vitro. Cultured islets isolated from Ucn 3-null mice also secreted less insulin in response to high glucose concentrations. Consistently, peripheral injection of a selective CRFR2 antagonist before the administration of a glucose challenge significantly attenuated glucose-induced insulin secretion in vivo. Ucn 3-null mice were relatively protected from the hyperinsulinemia, hyperglycemia, glucose intolerance, hepatic steatosis, and hypertriglyceridemia induced by high-fat diet. Additionally, we found that aged Ucn 3-null mice maintained better glucose tolerance than age-matched wild-type littermates. These results suggest that endogenous Ucn 3 in the pancreas is induced under excessive caloric conditions and acts locally to augment insulin production, which in the long-term may contribute to reduced insulin sensitivity and harmful metabolic consequences.
Keywords: corticotropin-releasing factor, type 2 diabetes, Urocortin 3 knockout
Urocortin 3 (Ucn 3) is a new member of the corticotropin-releasing factor (CRF) family of peptides that is expressed in discrete brain areas including the hypothalamus and amygdala (1, 2). It binds the type 2 CRF receptor (CRFR2) with high affinity while displaying minimal binding to the type 1 CRFR (CRFR1) (1, 2). In the periphery, Ucn 3 is mainly expressed in pancreatic β cells (3), although it is not stored with insulin in the same secretory vesicles (3). Along with Ucn 3, both CRFR subtypes are found in pancreatic islets (4). The presence of both Ucn 3 and its receptor in pancreatic islets suggests that Ucn 3 may be a local factor regulating islet function such as hormone secretion. Consistent with this notion, we have reported (3) that exogenous Ucn 3, acting through CRFR2, stimulates insulin and glucagon secretion. Furthermore, the effect of Ucn 3 on insulin release is a result of direct activation of CRFR2 on β cells and is independent of the insulin-releasing effect of glucagon. It remains to be determined whether endogenous Ucn 3, acting through CRFR2 in the pancreas, plays an important role in modulating insulin secretion and overall energy homeostasis. In the present study, we first determined that expression of Ucn 3 in the pancreas is associated with hyperinsulinemia and hyperglycemia and that insulin-releasing agents including glucose stimulated Ucn 3 secretion from β cells. Furthermore, we determined that endogenous Ucn 3 plays a key role in high glucose-induced insulin secretion and that deficiency in Ucn 3 protects mice under two circumstances (high-fat diet and aging) associated with reduced insulin sensitivity and metabolic consequences.
Results
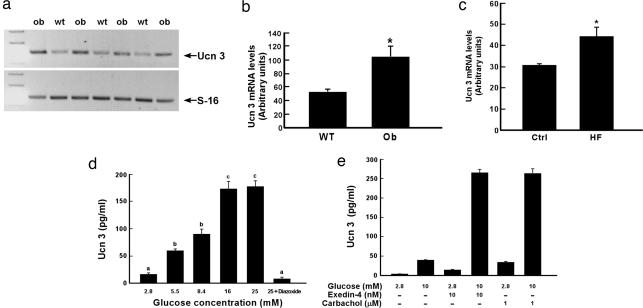
To probe the physiological role of Ucn 3 in the pancreas, we first examined the expression of Ucn 3 mRNA in the pancreas of two diabetic models: ob/ob obese mice and rats fed with high-fat diet (HFD). We found that Ucn 3 mRNA levels in the pancreas of ob/ob mice were significantly higher than the wild-type (WT) littermates (Fig. 1 a and b). Similarly, Ucn 3 mRNA levels were also elevated in the pancreas of rats kept on HFD compared with rats fed regular chow (Fig. 1c). We also studied Ucn 3 secretion from β cells by using a mouse clonal β cell line, MIN6, as a model. We found that glucose stimulated Ucn 3 secretion in a dose-dependent manner (Fig. 1d). The effect of glucose on Ucn 3 secretion plateaued at 16.8 mM. Pretreating the cells with a KATP channel blocker, diazoxide (100 μM), effectively blocked glucose-induced Ucn 3 secretion (Fig. 1d), indicating that the effect of glucose on Ucn 3 secretion is mediated by the KATP channel. Several agents including glucagon-like peptide-1 (GLP-1) and acetylcholine have been shown to stimulate insulin secretion in a glucose-dependent manner (5, 6). Treating MIN6 cells with exendin-4, a long-acting GLP-1 analogue, or carbachol, a cholinergic agonist, induced minimal or moderate Ucn 3 secretion in the presence of 2.8 mM glucose (Fig. 1e). On the other hand, both exendin-4 and carbachol significantly stimulated Ucn 3 release in the presence of 10 mM glucose (Fig. 1e). In summary, pancreatic Ucn 3 mRNA levels are up-regulated in obese and diabetic rodent models, and Ucn 3 is secreted in conditions that are also stimulatory to insulin secretion, suggesting that endogenous Ucn 3 may be involved in insulin secretion stimulated by nutrient excess.
Fig. 1.
Elevation of pancreatic Ucn 3 expression in diabetic rodent models and stimulation of Ucn 3 release from β cells by glucose. (a) Representative agarose gel electrophoresis showing Ucn 3 and S16 PCR products. (b) Summary of Ucn 3 expression in ob/ob obese mice and WT littermates. *, P < 0.05 vs. WT. (c) Ucn 3 mRNA levels in the pancreas of high-fat-fed rats (HF) and chow-fed controls (Ctrl). *, P < 0.05 vs. Ctrl. (d) Ucn 3 secretion from MIN6 cells in response to various glucose concentrations. (e) Ucn 3 release from MIN6 cells in response to glucose and/or insulin secretagogs. Values with different superscripts are significantly different with P < 0.01.
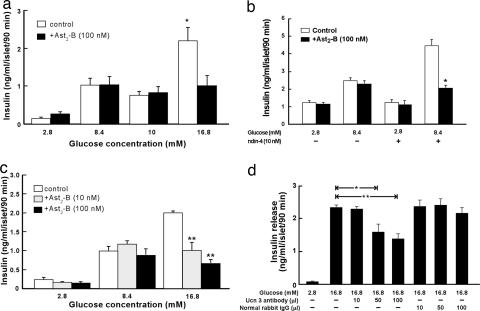
To investigate the possible involvement of Ucn 3 and its receptor, CRFR2, on glucose-induced insulin secretion, isolated rat islets were treated with the high-affinity CRFR2 selective peptide antagonist, Astressin2-B (Ast2-B) (7), before stimulation by varying concentrations of glucose. As shown in Fig. 2a, Ast2-B treatment did not affect insulin release induced by 8.4 or 10 mM glucose (Fig. 2a). In contrast, Ast2-B significantly attenuated insulin release induced by 16.8 mM glucose in a dose-dependent manner (Fig. 2 a and b). We also tested whether blocking CRF2 receptors modulated insulin secretion induced by GLP-1. As shown in Fig. 2c, exendin-4 significantly stimulated insulin release from isolated islets in the presence of 8.4 mM glucose and this effect was greatly attenuated by Ast2-B. To determine whether it was Ucn 3 or another ligand that activates CRFR2 under high glucose condition, isolated rat islets were treated with an affinity purified Ucn 3 antibody or normal rabbit IgG before glucose stimulation. As shown in Fig. 2d, anti-Ucn 3 IgG attenuated 16.8 mM glucose-induced insulin secretion in a dose-dependent manner. By contrast, normal rabbit IgG failed to alter glucose-induced insulin secretion. Together, these data indicate that endogenous Ucn 3 is released under high glucose condition in response to insulin augmenters such as GLP-1 ligands and in turn signals through CRFR2 to facilitate insulin secretion.
Fig. 2.
Blocking Ucn 3 or CRFR2 attenuates high glucose-induced insulin secretion. (a) Insulin secretion from isolated rat islets induced by various concentrations of glucose with (filled bars) or without (open bars) concurrent treatment of a CRFR2 selective antagonist, Ast2-B (100 nM). *, P < 0.05 vs. 16.8 mM glucose alone. (b) Insulin secretion induced by 16.8 mM glucose is attenuated by Ast2-B in a dose-dependent manner. **, P < 0.01 vs. 16.8 mM glucose alone. (c) Exendin-4 (10 nM) stimulated insulin release in the presence of 8.4 mM glucose, and the effect was attenuated by Ast2-B. *, P < 0.05 vs. exendin-4 + 8.4 mM glucose alone. (d) Anti-Ucn 3 antibody, but not normal rabbit IgG, dose-dependently attenuated 16.8 mM glucose-induced insulin secretion. *, P < 0.05; **, P < 0.01 vs. 16.8 mM glucose alone.
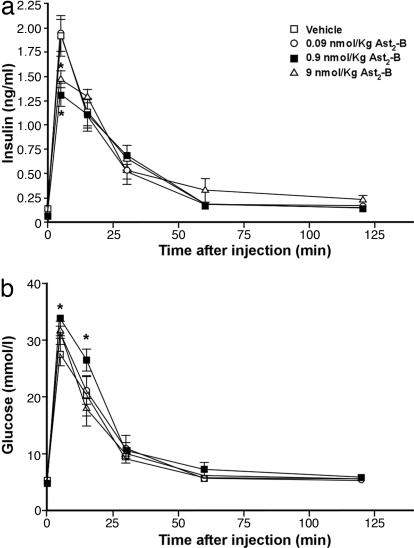
To determine whether blocking of CRFR2 function also modulates glucose-induced insulin release in vivo, various doses of Ast2-B (0.09–9 nmol/kg) or vehicle were injected intravenously into male rats through a jugular vein cannula 20 min before injection of a glucose (2.8 mmol/kg) bolus. As shown in Fig. 3a, Ast2-B injection attenuated glucose-induced insulin secretion in a dose-dependent manner, albeit the highest dose (9 nmol/kg) appears to be less effective compared with the dose of 0.9 nmol/kg. Plasma glucose levels also mirrored the results of insulin (Fig. 3b). This result extends our in vitro observations and suggests that endogenous CRFR2 in the periphery plays an important role in modulating insulin secretion. Although CRFR2 is also expressed in brain areas that are potentially involved in peripheral insulin secretion (8), the effect of Ast2-B on glucose-induced insulin secretion was probably because of a peripheral action as this peptide antagonist is unlikely to cross the blood brain barrier (9).
Fig. 3.
Blocking peripheral CRFR2 in vivo attenuates glucose-induced insulin secretion. Plasma insulin (a) and glucose (b) levels in male rats treated with vehicle or Ast2-B (0.09–9 nmol/kg) are shown. *, P < 0.05 vs. vehicle.
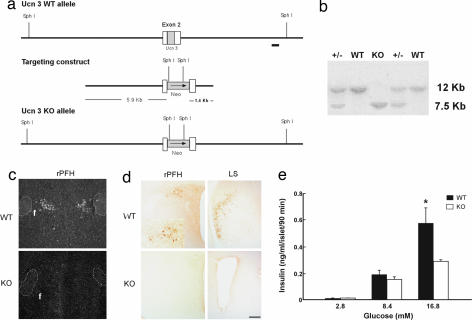
We generated Ucn 3-deficient mice (knockout; KO) through homologous recombination (Fig. 4) to further explore the role(s) of endogenous Ucn 3 in vivo. The mutant mice were viable and fertile and did not exhibit gross abnormalities. Under regular diet, the mutant mice showed similar responses to WT littermates to glucose and insulin tolerance tests (data not shown). Interestingly, the mutant mice had somewhat lower basal plasma insulin compared with the WT mice (data not shown). To test whether loss of Ucn 3 affected the response of β cells to glucose, we examined whether islets from Ucn 3 null mice had a perturbed insulin secretory response to glucose. As shown in Fig. 4e, low and medium glucose induced comparable levels of insulin release from both Ucn 3 WT and mutant islets, but insulin secretion induced by 16.8 mM glucose from Ucn 3 mutant islets was significantly lower than from the WT islets. These results thus confirm the proposed role of endogenous Ucn 3 in high glucose-induced insulin secretion.
Fig. 4.
Generation of Ucn 3-null mice and insulin secretion in islets isolated from Ucn 3 WT and null (KO) mice. (a) Schematic representation of Ucn 3 WT and KO allele and targeting construct. The position of the 3′ probe used in genomic Southern blot analysis is shown by a black bar. (b) Southern blot analysis of SphI-digested genomic DNA from WT, +/−, and KO mice. (c and d) In situ hybridization (c) and immunohistochemistry (d) of adult male WT and KO mouse brain sections for Ucn 3 mRNA and peptide, respectively. (Scale bar: 200 μm.) (e) Insulin secretion of islets isolated from Ucn 3-null (KO) and WT mice in response to various concentrations of glucose. *, P < 0.05 vs. WT.
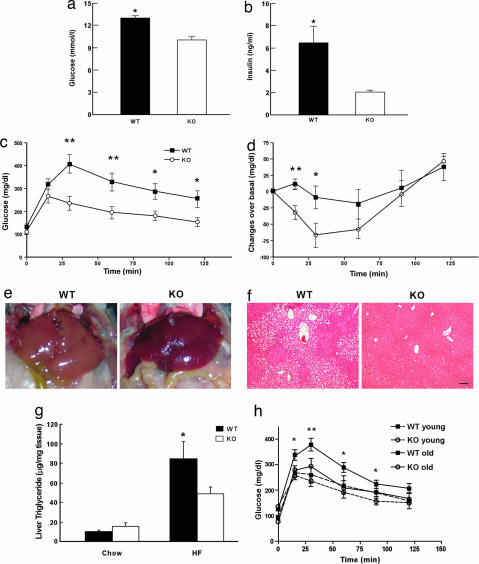
The involvement of Ucn 3 in the regulation of insulin secretion and glucose homeostasis in vivo under excess energy states was explored by feeding male Ucn 3-null mice and WT littermates HFD or regular chow for 16 weeks. Both Ucn 3-null mice and WT littermates gained significant weight under HFD compared with chow-fed controls (HFD: WT, 43.3 ± 1.6 g, and KO, 42.6 ± 0.7 g vs. Chow: WT, 31.5 ± 1.4 g, and KO, 30.23 ± 0.9 g). Mice fed with HFD also exhibited lower basal food intake compared with the chow-fed animals (HFD: WT, 3.53 ± 0.11 g, and KO, 3.57 ± 0.1 g vs. Chow: WT, 4.39 ± 0.07 g, and KO, 4.8 ± 0.15 g). This result may be due to an adaptation to the excess energy content of the HFD. Under HFD, basal plasma insulin and blood glucose concentrations of the KO mice were significantly lower than those of the WT mice (Fig. 5 a and b). Unexpectedly, we found that whereas the WT mice clearly displayed impaired glucose tolerance, the mutant mice did not develop significant glucose intolerance (Fig. 5c). Consistent with the glucose tolerance test, the insulin tolerance study revealed that the WT mice on HFD developed insulin resistance, but the mutant mice remained sensitive to insulin (Fig. 5d). Mice fed with HFD experienced an increase in fat mass and developed fatty liver and hepatic steatosis (10, 11). Gross examination of both genotypes showed that both WT and KO mice under HFD had increased fat mass (epididymal fat pad, HFD: WT, 1.53 ± 0.1 g, and KO, 1.42 ± 0.27 g vs. Chow: WT, 0.55 ± 0.18 g, and KO, 0.41 ± 0.05 g). On the other hand, WT, but not KO, mice developed severe liver steatosis under HFD (Fig. 5e). Histological examination showed that the liver of the WT mice had significantly more lipid accumulation than that of Ucn 3 KO (Fig. 5f). Biochemical analysis confirmed that triglyceride content in the WT livers were significantly higher than in the mutant mice (Fig. 5g). Thus, these results argue that under HFD, loss of Ucn 3 protects the animals from developing liver steatosis and insulin resistance.
Fig. 5.
Phenotypes of Ucn 3-null mice in glucose homeostasis. (a and b) Basal plasma glucose (a) and insulin (b) levels of Ucn 3 KO and WT mice after 16 weeks of HFD treatment. (c and d) Glucose (c) and insulin (d) tolerance tests in male Ucn 3 KO and WT mice (n = 8 per genotype). *, P < 0.05; **, P < 0.01 vs. WT. (e) Livers of male Ucn 3 KO and WT mice after HFD. (f) Histology of livers from Ucn 3 KO and WT mice fed HFD. (Scale bar: 40 μm.) (g) Liver triglyceride levels of Ucn 3 KO and WT mice. *, P < 0.05 vs. WT. (h) Glucose tolerance tests in Ucn 3 KO and WT mice at 10–12 weeks (young) and 12–13 months (old) of age. *, P < 0.05; **, P < 0.01 for old WT vs. old KO.
Finally, it is widely known that advancing age is associated with impaired glucose handling and insulin resistance (10, 11). The protective effect observed in Ucn 3 mutant mice in high-fat feeding prompted us to investigate whether Ucn 3 mutant mice could remain insulin-sensitive during old age. We compared glucose tolerance in Ucn 3 mutant and age-matched WT littermates at 10–12 weeks old and again at 12–13 months of age. No differences were found in responses to glucose tolerance between the mutant and WT controls at younger ages (Fig. 5h). In contrast, aged Ucn 3-null mice exhibited better glucose tolerance than the WT controls (Fig. 5h).
Discussion
The results presented here support the hypothesis that an endogenous peptide acting through the CRF2 receptor is an important local regulator of insulin secretion in response to high glucose concentrations. Although four different ligands could potentially stimulate the β cell CRF2 receptor, the attenuation of high glucose-mediated insulin secretion by a specific Ucn 3 antibody as well as studies on Ucn 3-null mice and their islets implicate Ucn 3 as being the ligand involved in this phenomenon. Presumably, at least under the conditions of these experiments, the local production of Ucn 3 by β cells under low and moderate levels of glucose is inadequate to facilitate insulin secretion. Ucn 3 joins the list of amplifiers of glucose-induced insulin release that includes neural amplifiers and enteric hormones such as GLP-1, which, interestingly, appear to be dependent, at least in part, on Ucn 3 signaling.
Based on results indicating that glucose-induced insulin secretion can be partially dependent on Ucn 3, we predicted that under excessive nutrient intake, such as when fed an HFD, mice without Ucn 3 would have reduced insulin secretion, which might lead to hyperglycemia and other symptoms of diabetes. Surprisingly, we found that the loss of Ucn 3 protected mice from developing severe hyperinsulinemia and liver steatosis under chronic HFD. Neither CRF1 nor CRF2 receptors have been found in the liver (12), and treatment of rat primary hepatocytes with Ucn 3 had no effect on the expression of several enzymes, including PEPCK, that are important in glucose metabolism (C.L., unpublished observations). Thus, the phenotype we observed is unlikely to be due to a loss of Ucn 3 signaling in the liver.
It is plausible that the relative protection against hyperglycemia in Ucn 3-null mice maybe related, at least in part, to a chronic limitation on the increased insulin secretion normally seen in animals on HFD. The excess energy available under HFD typically induces hyperinsulinemia, increases accumulation of excessive body fat, and facilitates the development of insulin resistance (13, 14). Loss of Ucn 3 under a high-nutrient state may mitigate excessive insulin release in the face of an excess of energy intake, which prevents animals from storing extra fuel and becoming insulin resistant. A recent parallel study by Steneberg et al. (15) showed that GPR40, a G protein-coupled receptor, is expressed in β cells and mediates the stimulatory effects of free fatty acids (FFAs) on insulin secretion. Importantly, GPR40-deficient β cells secrete less insulin in response to FFAs, and loss of GPR40 protects mice from obesity-induced hyperinsulinemia, hepatic steatosis, and glucose intolerance (15). This study is consistent with our hypothesis that limiting insulin secretion in response to excessive nutrients can protect animals from developing glucose intolerance and insulin resistance.
An earlier study from our group (16) reported that mice deficient in CRFR2 fed with HFD exhibited lower basal blood glucose and insulin levels compared with WT mice and were protected from HFD-induced glucose intolerance. The present study raises the possibility that loss of Ucn 3 signaling in the pancreas may contribute to the phenotype of CRFR2 mutant mice under HFD. However, the phenotype of the Ucn 3 mutant mice differed from that of the CRFR2 mutants. Under HFD, CRFR2 mutants had less body fat and more lean mass compared with the WT controls, and the mutant mice ate significantly more than the WT mice (16) and exhibited increased thermogenesis and metabolic rate (17). On the other hand, Ucn 3 mutant mice fed with HFD did not have this phenotype. These additional abnormalities may be mediated by the other CRFR2 ligands, Ucn 1 or Ucn 2. Ucn 2 is expressed predominately in peripheral tissues including skeletal muscle, where CRFR2 is also expressed (18). Recently, we gained understanding of the role of Ucn 2 in glucose homeostasis by developing and analyzing Ucn 2-null mice (19). Ucn 2-null mice had less fat mass and more lean body mass compared with the WT littermates; furthermore, loss of Ucn 2 protected animals from HFD-induced insulin resistance. Additional studies revealed that Ucn 2, through CRFR2, attenuates insulin-induced signaling and glucose uptake in skeletal muscle (19). Thus, Ucn 2 acting locally in skeletal muscle is likely to be involved in a different aspect of CRFR2 function in glucose homeostasis and may contribute to other abnormalities observed in CRFR2 mutant mice.
We also observed that aged Ucn 3 mutant mice remained insulin sensitive and handled glucose loads better than the age-matched WT littermates, in keeping with the notion that the long-term limitation of insulin secretion may be beneficial in maintaining insulin sensitivity and glucose homeostasis.
In fact, it has been shown that restriction of insulin action, either by diet restriction (lower insulin levels) or by reducing insulin signaling, can be beneficial to the health of the animals and extends their life span (20–23). This is consistent with our findings that loss of Ucn 3 lowers the animal's ability to release insulin proportionally in response to excess energy intake and subsequently prevents the animals from developing overt diabetes under high-fat feeding or age-related accumulation of fuel storage.
In conclusion, our results support a role of Ucn 3 in the pancreas in the regulation of glucose-induced insulin secretion. Abrogation of Ucn 3 expression resulted in lowering insulin secretion in response to elevated glucose concentrations both in vitro and in vivo. In addition, our results are consistent with the current insight that limiting excess insulin secretion may be beneficial in maintaining long-term healthy glucose homeostasis.
Materials and Methods
Semiquantitative RT-PCR Analysis.
Total RNA was extracted from pancreas, and the cDNA products were used as templates for semiquantitative RT-PCR analysis by using specific primers for Ucn 3 and the ribosomal protein S16. Oligonucleotide primer sequences and PCR conditions are provided in supporting information (SI) Materials and Methods.
Cell Culture and Ucn 3 Secretion.
MIN6 cells (24) were cultured in DMEM supplemented with 10% FBS and 114 μM 2-mercaptoethanol. For Ucn 3 secretion, the cells were seeded in six-well plates and allowed to recover for 48 h. The cells were washed twice with Hepes-balanced Krebs–Ringer bicarbonate buffer (HKRB) containing 5 mM glucose and 0.2% BSA and were incubated in HKRB with various testing reagents for 2 h at 37°C. The incubation buffer was collected and vacuum-dried for measurement of Ucn 3 by RIA as described (3). Exendin-4 was purchased from Phoenix Pharmaceuticals (Burlingame, CA), and diazoxide and carbachol were purchased from Sigma (St. Louis, MO).
Animal Studies.
Male Sprague–Dawley rats (250–300 g) were used for the in vivo studies. Varying doses of the CRFR2 antagonist Ast2-B or vehicle were administered i.v., and blood samples (300 μl) were drawn through preimplanted i.v. cannulae at specified times. Blood samples were immediately centrifuged to separate plasma from blood cells and stored at −20°C until assay. Plasma insulin and glucose levels were determined by using commercial kits. All animal procedures were approved by the Salk Institute Institutional Animal Care and Use Committee.
Islet Culture Studies.
Islets from male Sprague–Dawley rats (300–350 g) or mice were isolated by collagenase digestion (3) and incubated overnight in M199 medium supplemented with 10% FBS. Islets were washed twice with Krebs–Ringer-buffered saline (KRB) and preincubated in KRB with 2.8 mM glucose for 60 min, followed by incubation in testing agents diluted in KRB for another 60 min. Testing buffer was then removed and stored at −20°C until RIA for insulin.
Generation of Ucn 3 Mutant Mice.
A targeting cassette consisting of neomycin-resistant gene was used to replace the entire Ucn 3 coding domain. A 465-bp 3′ fragment near the SphI site was used to screen for ES cell recombinants (Fig. 4). Ucn 3 deficiency was confirmed by in situ hybridization and immunohistochemistry as described in SI Materials and Methods. Mice were fed ad libitum with a standard mouse chow (Purina, St. Louis, MO) and kept under a light-dark cycle of 12 h. For HFD study, 6-week-old male Ucn 3-null mice and WT littermates were put on HFD (45 kcal % fat, D12451; Research Diets, New Brunswick, NJ) or regular chow for 16 weeks. Basal glucose levels were determined by glucometer, and basal plasma insulin was determined by RIA (Linco, St. Louis, MO).
Glucose and Insulin Tolerance Tests and Hepatic Triglyceride Measurements.
Glucose tolerance test was performed in overnight-fasted mice after i.p. injection of 2 g/kg glucose. Insulin tolerance test was conducted after 2 h of fasting with i.p. injection of 0.75 units/kg insulin. Blood glucose levels were monitored by using a glucometer. Hepatic triglyceride was extracted as described (25), and triglyceride content was determined enzymatically by using commercial kits (Sigma and Wako, Osaka, Japan). Values were calculated as micrograms per milligram of wet tissue.
Supplementary Material
Acknowledgments
We thank Dr. J. Rivier (The Salk Institute) for providing the peptides used in the study, Dr. T. Sung for advice in generating Ucn 3-null mice, and C. Chen, C. Yau, and R. Fernandez for technical assistance. This work was supported by National Institutes of Health (NIH) Grants HD034534 and DK-26741, The Alder Foundation, The Kleberg Foundation, and The Clayton Medical Research Foundation, Inc. W.V. is a Senior Investigator for the Clayton Medical Research Foundation. C.L. is supported by NIH Career Development Award DK-064745.
Abbreviations
- CRF
corticotropin-releasing factor
- Ucn 3
Urocortin 3
- CRFR1
CRF receptor type 1
- Ast2-B
Astressin2-B
- GLP-1
glucagon-like peptide 1
- HFD
high-fat diet
- KATP
ATP-sensitive potassium channel.
Footnotes
Conflict of interest statement: W.V. is a cofounder, consultant, equity holder, and member of the Board of Directors of Neurocrine Biosciences and Acceleron Pharma. The following have been licensed by the Salk Institute and/or Clayton Foundation: CRF to Ferring Pharmaceuticals and CRF1 receptor to Neurocrine Biosciences.
This article contains supporting information online at www.pnas.org/cgi/content/full/0611641104/DC1.
References
- 1.Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, Vaughan J, Reyes TM, Gulyas J, Fischer W, et al. Proc Natl Acad Sci USA. 2001;98:7570–7575. doi: 10.1073/pnas.121165198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsu SY, Hsueh AJ. Nat Med. 2001;7:605–611. doi: 10.1038/87936. [DOI] [PubMed] [Google Scholar]
- 3.Li C, Chen P, Vaughan J, Blount A, Chen A, Jamieson PM, Rivier J, Smith MS, Vale W. Endocrinology. 2003;144:3216–3224. doi: 10.1210/en.2002-0087. [DOI] [PubMed] [Google Scholar]
- 4.Kanno T, Suga S, Nakano K, Kamimura N, Wakui M. Diabetes. 1999;48:1741–1746. doi: 10.2337/diabetes.48.9.1741. [DOI] [PubMed] [Google Scholar]
- 5.Drucker DJ. Cell Metab. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Gilon P, Henquin JC. Endocr Rev. 2001;22:565–604. doi: 10.1210/edrv.22.5.0440. [DOI] [PubMed] [Google Scholar]
- 7.Rivier J, Gulyas J, Kirby D, Low L, Perrin MH, Kunitake K, DiGruccio M, Vaughan J, Reubi JC, Waser B, et al. J Med Chem. 2002;45:4737–4747. doi: 10.1021/jm0202122. [DOI] [PubMed] [Google Scholar]
- 8.Chalmers DT, Lovenberg TW, De Souza EB. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rivier CL, Grigoriadis DE, Rivier JE. Endocrinology. 2003;144:2396–2403. doi: 10.1210/en.2002-0117. [DOI] [PubMed] [Google Scholar]
- 10.Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI. Science. 2003;300:1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbieri M, Rizzo MR, Manzella D, Paolisso G. Diabetes Metab Res Rev. 2001;17:19–26. doi: 10.1002/dmrr.178. [DOI] [PubMed] [Google Scholar]
- 12.Lovenberg TW, Chalmers DT, Liu C, De Souza EB. Endocrinology. 1995;136:4139–4142. doi: 10.1210/endo.136.9.7544278. [DOI] [PubMed] [Google Scholar]
- 13.Storlien LH, James DE, Burleigh KM, Chisholm DJ, Kraegen EW. Am J Physiol. 1986;251:E576–E583. doi: 10.1152/ajpendo.1986.251.5.E576. [DOI] [PubMed] [Google Scholar]
- 14.Storlien LH, Pan DA, Kriketos AD, Baur LA. Ann NY Acad Sci. 1993;683:82–90. doi: 10.1111/j.1749-6632.1993.tb35694.x. [DOI] [PubMed] [Google Scholar]
- 15.Steneberg P, Rubins N, Bartoov-Shifman R, Walker MD, Edlund H. Cell Metab. 2005;1:245–258. doi: 10.1016/j.cmet.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Bale TL, Anderson KR, Roberts AJ, Lee KF, Nagy TR, Vale WW. Endocrinology. 2003;144:2580–2587. doi: 10.1210/en.2002-0091. [DOI] [PubMed] [Google Scholar]
- 17.Carlin KM, Vale WW, Bale TL. Proc Natl Acad Sci USA. 2006;103:3462–3467. doi: 10.1073/pnas.0511320103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen A, Blount A, Vaughan J, Brar B, Vale W. Endocrinology. 2004;145:2445–2457. doi: 10.1210/en.2003-1570. [DOI] [PubMed] [Google Scholar]
- 19.Chen A, Brar B, Choi CS, Rousso D, Vaughan J, Kuperman Y, Kim SN, Donaldson C, Smith SM, Jamieson P, et al. Proc Natl Acad Sci USA. 2006;103:16580–16585. doi: 10.1073/pnas.0607337103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roth GS, Ingram DK, Lane MA. Ann N Y Acad Sci. 2001;928:305–315. doi: 10.1111/j.1749-6632.2001.tb05660.x. [DOI] [PubMed] [Google Scholar]
- 21.Bluher M, Kahn BB, Kahn CR. Science. 2003;299:572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- 22.Barbieri M, Bonafe M, Franceschi C, Paolisso G. Am J Physiol. 2003;285:E1064–E1071. doi: 10.1152/ajpendo.00296.2003. [DOI] [PubMed] [Google Scholar]
- 23.Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, et al. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyazaki J, Araki K, Yamato E, Ikegami H, Asano T, Shibasaki Y, Oka Y, Yamamura K. Endocrinology. 1990;127:126–132. doi: 10.1210/endo-127-1-126. [DOI] [PubMed] [Google Scholar]
- 25.Lee Y, Wang MY, Kakuma T, Wang ZW, Babcock E, McCorkle K, Higa M, Zhou YT, Unger RH. J Biol Chem. 2001;276:5629–5635. doi: 10.1074/jbc.M008553200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.