Abstract
Transmitters such as glutamate and ATP are released from brain astrocytes. Several pathways for their release have been proposed, including exocytosis. In the present study we sought to measure exocytosis from astrocytes with single vesicle imaging methods using synaptopHlourin (SpH) as an optical reporter. We imaged single SpH-laden vesicles with total internal reflection fluorescence (TIRF) microscopy. We observed spontaneous, as well as evoked, single-vesicle exocytosis events. Analysis of the kinetics and spatial spread associated with these events indicated two discernible forms of single vesicle exocytosis. One form, constituting ≈40% of the spontaneous events, was akin to kiss-and-run vesicle fusion and captured a mobile proton buffer from the extracellular medium. The other form seems to represent full vesicle fusion, constitutes ≈60% of the spontaneous events, and is associated with complete mixing of the vesicle and plasma membranes. Activation of calcium-mobilizing receptors on the astrocyte surface selected between the different forms of exocytosis. These data provide evidence for two forms of simultaneously occurring single-vesicle exocytosis events in astrocytes, and also suggest that SpH imaging and TIRF microscopy is useful to study the mechanisms of astrocyte transmitter release.
Keywords: imaging, calcium, ATP
Increasing evidence suggests that specialized glial cells, called astrocytes, participate in brain function (1, 2). Unlike neurons, astrocytes do not fire action potentials but manifest excitability in the form of intracellular Ca2+ concentration increases (3) ([Ca2+]i), which have been quite extensively studied in vitro (1, 2, 4, 5). Recent experiments with two-photon calcium imaging from the cortex show that astrocyte [Ca2+]i oscillations also occur in vivo and that they are regulated by alterations in neuronal activity (6). Astrocytes within the barrel cortex of rats showed [Ca2+]i transients upon whisker stimulation in vivo, proving that astrocytes are activated during sensory physiological stimuli (7). Astrocyte [Ca2+]i transients are known to be triggered by neuronal activity (8) and are known to occur independently of neuronal activity because of intracellular Ca2+ release (9, 10). [Ca2+]i increases, between a few nanomolar (11) and tens of micromolar (12) triggered by flash photolysis of caged Ca2+ or pharmacological means (13), trigger release of transmitters from astrocytes into the extracellular space, which may then activate receptors on other nearby cells, including neurons. For instance, recent experiments suggest that [Ca2+]i increases within single astrocytes can release glutamate, which activates extrasynaptic NMDA receptors on neurons (14, 15), leading to synchrony (14). In addition in the olfactory bulb, astrocytes release GABA to cause inhibition of mitral cells (15). Moreover, paroxysmal depolarizing shifts in neuronal membrane potential that accompany models of epilepsy may be triggered by release of glutamate from astrocytes (16). Additionally, astrocyte-derived transmitters such as ATP and adenosine affect synaptic plasticity in the hippocampus (17).
SynaptopHlourin (SpH) is a fusion protein between VAMP2 and a pH-sensitive GFP mutant (18). It is incorporated into vesicles, such that the GFP is quenched in the vesicle lumen, which has an acidic pH. Exocytosis increases the quantum yield of SpH, as deprotonation of the fluorophore occurs (18–20). We used SpH to determine whether we could image astrocyte exocytosis at the single-vesicle level. Our data suggest that SpH imaging can be used to record exocytosis from astrocytes with single-vesicle resolution. Additionally, the analysis revealed that astrocyte exocytosis was of two forms, and receptor agonists that elevate calcium within astrocytes differed in their capacity to trigger different forms of exocytosis.
Results and Discussion
Initial Observations.
Astrocytes contain vesicles that are between 30 and 300 nm in size (13, 21), and possess the vesicular machinery needed to package and release glutamate (13, 22–30). We transfected astrocytes with plasmids encoding SpH (18), in the expectation that SpH would become incorporated within astrocyte vesicles. Consistent with this expectation we observed vesicle like structures 1–2 days after transfection. Of these structures, 86 ± 4% colocalized with vGlut1, suggesting that most of the SpH laden vesicles were glutamatergic [supporting information (SI) Fig. 6D; n = 7]. We next imaged SpH expressing astrocytes with total internal reflection fluorescence (TIRF) microscopy. The area of the astrocytes closest to glass (the “footprint”) (31) was weakly fluorescent (SI Fig. 7A). We applied 25 mM NH4Cl to alkalanize intracellular compartments within astrocytes, and recorded a dramatic increase in the number of fluorescent vesicles (SI Fig. 7A), likely because intravesicular SpH was unquenched (20). Application of extracellular buffers at pH 5.5 resulted in reversible quenching of the diffuse footprint fluorescence (SI Fig. 7A), suggesting a pool of plasma membrane SpH. We determined the pKa of this surface SpH fraction to be 7.4 ± 0.02 (SI Fig. 7 B and C; n = 6), close to that previously reported (20).
Imaging Astrocyte Exocytosis with spH.
Using TIRF microscopy we recorded spontaneous increases in fluorescence intensity at discrete regions of interest (ROI; Fig. 1), herein called “SpH events.” On average, there were 0.26 ± 0.13 events per min/1,000 μm2 of astrocyte footprint (n = 27), equating to 1–2 events per min in whole astrocytes. Consistent with the hypothesis that the SpH events represent exocytosis, the Ca2+ ionophore ionomycin (10 μM) increased their frequency to 52 ± 14 per min/1,000 μm2, or ≈200–400 per astrocyte/min, and increased the intensity of the footprint by 93 ± 7% (n = 4) presumably as SpH diffused into the plasma membrane. To further test whether SpH events represented exocytosis we next compared their numbers from control astrocytes to those from cells transfected with plasmids encoding the light chain of botulinum toxin E. This neurotoxin specifically cleaves proteins associated with synaptic vesicle exocytosis (32). We used plasmids (32) rather than the toxin itself (33) because astrocytes do not readily take up botulinum toxin from the extracellular buffer. In control astrocytes we recorded the expected number of SpH events (0.18 ± 0.09 min/1,000 μm2 of footprint area; n = 7; see above section), but in astrocytes transfected with plasmids encoding botulinum toxin E we measured no SpH events (n = 9), although we could still observe mobile quenched SpH laden vesicles within astrocytes at high camera gain.
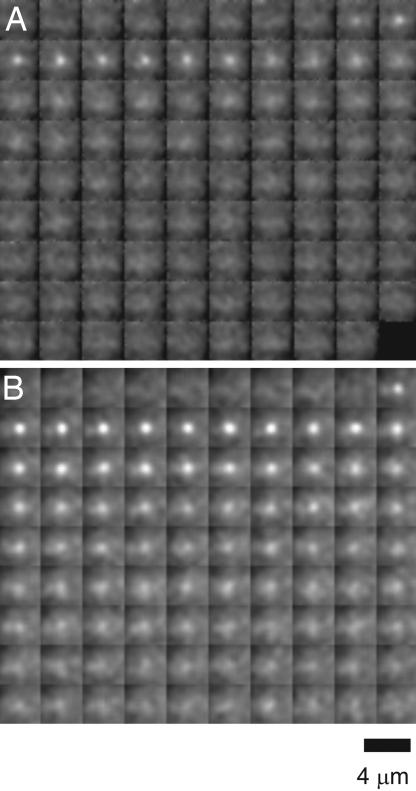
Fig. 1.
Representative image frames of spontaneous SpH events. (A) Fast confined SpH events (one frame every 83 ms). (B) Slow-spreading SpH events (one frame every 83 ms). The detailed analysis of images such as these is presented in Figs. 2–4.
Two Forms of Astrocyte Single-vesicle Exocytosis: Fast-Confined and Slow Spreading SpH Events.
Visual examination of the spontaneous SpH events suggested heterogeneity (Fig. 1); we briefly describe these differences now and then present the quantification (Figs. 2–4). We observed events that appeared rapidly as a point into a central ROI (within 1 frame), and were followed by no increase of fluorescence intensity in surrounding annuli (Figs. 1A and 2 A and B). For these events the intensity decayed in ≈1.5 s, and we call these fast-confined SpH events. In contrast other events also appeared rapidly, but were followed by a delayed increase in the fluorescence into a surrounding annulus (Figs. 1B and 2 A and C), and the event intensity decayed more slowly in ≈5 s. We call these slow-spreading SpH events.
Fig. 2.
Representative analysis of two types of spontaneous SpH events from astrocytes. (A) The schematic illustrates regions of interest (ROI) selected for subsequent image analysis. The center ROI was fixed on the events, and annuli expanding away from this center were selected. (B) Two examples for the time course of the fluorescence change for a central ROI, and five surrounding annuli (drawn in A). There was no fluorescence increase into any annulus. (Lower) Normalized traces for the center ROI and the 4th annulus; there was no clear peak in the 4th ROI. (C) Two examples for the time course of the fluorescence change for a central ROI, and five surrounding annuli (drawn in A). There were clear fluorescence increases into the annuli. (Lower) Normalized traces for the center ROI and the 4th annulus; there was a clear delayed peak in the 4th ROI in both cases.
Fig. 3.
Analysis of spontaneous SpH events. (A) Schematic of an SpH event illustrating the measured parameters. (B) SpH event peak ΔF/F histogram. (C) SpH event 4th annulus peak ΔF/F histogram; note it is bimodal. (D) SpH event half-width histogram; note it is bimodal.
Fig. 4.
Properties of spontaneous SpH events. (A and B) Properties of slow spreading events. (A) Event decay time (τd) distribution for the fluorescence reports from central ROIs. (B) Event decay time (τd) distribution for the fluorescence report from the 4th annulus. Note that they have near identical means, and overlapping widths. The central traces illustrate (for 1 event) the fluorescence report from a central ROI (black trace) and from the 4th surrounding annulus ROI (see Fig. 2A). (C and D) Properties of fast confined events. (C) Event kinetics (center ROIs) in different concentrations of Hepes in the extracellular buffer, as indicated. (D) Plot of the event decay time as a function of Hepes concentration. There was an approximately linear relationship.
We selected all events post hoc, as regions showing an abrupt increase in fluorescence intensity. We plotted the intensity profile of regions of interest over time, starting with a central ROI, and expanding into annuli of increasing size (Fig. 2A). In Fig. 2B, we present two examples of fast-confined SpH events, where the fluorescence did not spread into any annulus. In Fig. 2C, we present two examples of slow-spreading events where fluorescence did spread into annuli, progressively further from the central ROI with a time delay of 2.0 ± 0.2 s (n = 27) for the 4th annulus (SI Table 1).
We measured three parameters for the SpH events: the peak change in fluorescence (ΔF/F) for central ROIs, the peak changes in fluorescence for the 4th annuli and the half widths (in seconds; Figs. 2A and 3A and SI Table 1). For central ROIs the histogram of peak ΔF/F for all events was unimodal (Fig. 3B and SI Table 1), indicating that the vesicles underlying fast-confined and slow-spreading SpH events carry equal amounts of synaptopHlourin, as expected if the vesicles were of similar size. However, the histogram of ΔF/F for the 4th annulus was bimodal: there was one peak near ≈0, and one peak near ≈0.13 (Fig. 3C and SI Table 1), indicating that only in some cases did SpH spread significantly away from the site of release and into the 4th annulus. We next examined the half width histogram of the events, and found it too was bimodal (Fig. 3D). These data provide evidence for the existence of distinct events (Figs. 1–4). The events differed in their kinetics (Figs. 2 and 3D and SI Table 1) and in the extent to which fluorescence spreads into the 4th annulus (Figs. 2 and 3C and SI Table 1). The events with brief half widths at ≈1.5 s were the fast-confined SpH events, which did not spread significantly into the 4th annulus (SI Table 1; Fig. 3C). Whereas the events with half widths at ≈5 s were the slow-spreading events, that did spread into the 4th annulus (SI Table 1; Fig. 3C).
The finding that the event peak ΔF/F histogram for central ROIs was unimodal (Fig. 3B) suggests that the vesicles carry equal amounts of SpH, which would be expected if they were of equal size. To further explore this possibility we examined the ΔF/F of fast confined and slow-spreading SpH events separately: they were similar at 0.29 ± 0.03 (n = 37) and 0.30 ± 0.02 (n = 27), respectively. In both cases the change in fluorescence returned to baseline levels (>95% recovery) at the end of the analyzed period (Fig. 2).
The X-Y dimensions of the vesicles (measured as full-width half maxima; FWHM) were that of the point-spread-function (PSF) of the microscope at ≈500 nm. Based on electron microscopy astrocytes are known to possess vesicles that are ≈30 and ≈300 nm in diameter. Might the SpH laden vesicles in our experiments be 30 nm or 300 nm? This is a non trivial issue to address with light microscopy, because both vesicle sizes are below the resolution limit of our microscope, but to make inroads we exploited the fact that TIR illumination results in an evanescent wave with a penetration depth of ≈100 nm (31). During TIR objects smaller than 100 nm in diameter (e.g., a 43-nm bead and a 30-nm vesicle) are optimally excited, whereas objects larger (e.g., a larger bead or a 300 nm vesicle) are only partially excited. However, TIR is compromised if one adjusts the laser beam position (SI Fig. 8 A and B). In this case, oblique illumination occurs, resulting in a greater penetration depth approaching that of epifluorescence illumination. In this scenario, both the small (43 nm bead and 30 nm vesicle) and large objects (larger bead or 300 nm vesicle) are maximally excited. These considerations indicate that objects larger than the TIR penetration depth (≈100 nm) will increase in intensity upon switching to oblique illumination. Indeed, exactly this increase was observed for a 4000 nm bead and whole astrocyte footprints (3- to 4-fold increase in intensity; SI Fig. 8 C and D). In contrast objects smaller than the penetration depth are expected to decrease in intensity, because with oblique illumination they blur into the enhanced background fluorescence. Exactly this decrease was observed for 43-nm beads, and for the synaptopHlourin-laden vesicles (decreasing to half their intensity; SI Fig. 8 C and D). Thus the behaviors of the SpH laden vesicles (and 43 nm beads as controls) during TIR and oblique illumination suggest a size less ≤100 nm, which is consistent with the co localization with vGlut1 (SI Fig. 6). Our data do not rule out the possibility that astrocytes also express vesicles that are ≈300 nm in diameter (21). However, the data are most consistent with the view that the SpH laden vesicles we have imaged represent ≈30–50 nm glutamatergic vesicles.
Differences Between Slow-Spreading and Fast-Confined SpH Events.
For the slow-spreading SpH events there was a delay in the fluorescence intensity increase for an annulus of 2.0 ± 0.2 s (n = 27) in relation to an enclosed center ROI (see Fig. 2C for traces). This delay is indicative of a fluorescence report that spreads out from a point, and we interpret these events to represent complete vesicle fusion associated with significant mixing of SpH with the plasma membrane. If significant mixing were the case one would expect the fluorescence intensity profile of annulus ROIs to decay back to baseline levels as the SpH diffused progressively further away into the plasma membrane bulk. We quantified this spreading by measuring the decay time for the events from a central ROI and the 4th annulus (Fig. 4 A and B). From the histograms in Fig. 4 A and B it is apparent the fluorescence signal decays with equal kinetics for both central and annulus ROIs. We also investigated this issue by measuring a ratio of center ROI decay time/4th annulus decay time for each vesicle, which was 0.80 ± 0.12 (n = 37), and not significantly different to 1. The analysis is consistent with the suggestion that slow-spreading events represent exocytosis, with SpH diffusing away from a point source (vesicle) in to surrounding annuli, and subsequently out of these annuli into the plasma membrane bulk (Fig. 2).
Why do the fast-confined events (Fig. 2B) not spread markedly in to annuli? It is noteworthy that the fast confined event half-width was shorter than for slow-spreading events (Figs. 2B and 3D). Might this time course also represent reacidification of the vesicles after endocytosis? In this case, increasing the H+ buffering capacity of the extracellular medium is expected to increase the time it takes for the vesicle to be acidified (19). To test for this increase, we analyzed only those events that did not show any spread of fluorescence into an annulus (see Fig. 2). We then compared the decay time of these fast-confined events under four conditions of H+ buffering (2.5, 25, 60 and 100 mM Hepes). We found overall there was a near linear dependence of event decay time with the buffering capacity of the extracellular buffer (Fig. 4 C and D). These data support the hypothesis that fast confined events represent exocytosis followed by endocytosis and protonation of the lumen after the vesicles are recaptured.
Distinct Pharmacologically Evoked Calcium Transients in Astrocytes.
The experiments described above report on spontaneous SpH events. We next sought to evoke exocytosis. We used pharmacological means to elevate astrocyte [Ca2+]i levels because hippocampal astrocytes do not express voltage-gated Ca2+ channels (33). Previous work shows that ADPβS can elevate [Ca2+]i levels in astrocytes (33); we additionally used the PAR-1 receptor agonist thrombin (1–10 units/ml) to induce [Ca2+]i transients in astrocytes (33–35). ADPβS and thrombin elevated [Ca2+]i in a concentration-dependent manner (SI Fig. 9 A and B) with EC50 values of 1.3 μM (n = 8) and 0.14 units/ml (n = 10), respectively for ADPβS and thrombin. Both ADPβS and thrombin elevated [Ca2+]i by similar amounts because the peak change in Fluo-4 fluorescence was similar (SI Fig. 9 C and D). Interestingly, however ADPβS and thrombin-evoked [Ca2+]i transients differed in their kinetics. Thus the ADPβS evoked transients lasted for ≈5 s (mean 5 s with standard deviation at 1 s, n = 36; SI Fig. 9 E and G), whereas the thrombin evoked events lasted longer (mean 86 s with standard deviation at 22 s; n = 20; SI Fig. 9 F and G). We also estimated the [Ca2+]i changes evoked by ADPβS and thrombin with ratiometeric fura-2 fluorescence imaging, and estimate that the peak [Ca2+]i increases to ≈0.3–0.5 μM for both ADPβS and thrombin, i.e., to levels expected to affect transmitter release (11, 12, 30). In subsequent experiments we used ADPβS (100 μM) and thrombin (1–10 units/ml) to evoke astrocyte [Ca2+]i transients in astrocytes.
Distinct Forms of Pharmacologically Evoked Exocytosis.
Application of ADPβS evoked more SpH events (Fig. 5 A–E and E Inset), providing strong evidence that activation of P2Y1 receptors on astrocytes leads to exocytosis. Moreover, in cells expressing botulinum toxin E, ADPβS failed to evoke any events (n = 6). Interestingly, histograms of the ADPβS evoked event half widths, center ROI peak ΔF/F and 4th annulus peak ΔF/F were unimodal, with means of ≈1.5 s, 0.3 and 0, respectively (Fig. 5 C–E and SI Table 1). These data, along with the traces in Fig. 5B, indicate that ADPβS only evokes fast confined events. This result did not depend on the agonist concentration, because only these types of events were observed at EC100 (10 μM; Fig. 5C) and ≈EC50 concentrations of ADPβS (1 μM center ROI decay time was 1.5 ± 0.3 s; 5 events from 13 cells). Thus the ADPβS-evoked events resemble the fast-confined spontaneous events, associated with little mixing of SpH with the plasma membrane (SI Table 1).
Fig. 5.
Two types of evoked SpH events from astrocytes. (A–E) Experiments with ADPβS. (A) Representative images of 1 event over time. (B) A representative event profile for a center and 4th annulus. (C) ADPβS-evoked event half-width distribution. (D) ADPβS-evoked event ΔF/F histogram. (E) ADPβS-evoked event 4th annulus ΔF/F histogram. (Inset) Data from single footprints (open circles) and average data (filled circles) of event frequency before (ctrl) and during ADPβS. Event numbers are presented as event frequency per 1,000 μm2 of footprint to account for variations in the size of the footprints. (F–J) as in A–E but for thrombin-evoked events.
Thrombin also evoked a significant increase in the frequency of events (Fig. 5 F–J and J Inset), a response that was totally absent in cells expressing botulinum toxin E (n = 9). The half-width (Fig. 5H), the center ROI peak ΔF/F (Fig. 5I) and the 4th annulus peak ΔF/F (Fig. 5J) histograms of these thrombin-evoked events were unimodal with means at ≈5 s, 0.3 (ΔF/F) and 0.13 (ΔF/F; Fig. 5 H–J), i.e., distinct from the ADPβS-evoked events in two key parameters. These data, along with the traces in Fig. 5G showing spreading into an annulus, indicate that thrombin evokes slow-spreading spontaneous events, associated with significant mixing of SpH with the plasma membrane. The time delay between fluorescence appearing in the central ROI and then spreading into a surrounding annulus was 2.4 ± 0.3 s (n = 25), and not significantly different from spontaneous slow-spreading events (Fig. 2; 2.0 ± 0.2 s). The ability of thrombin to evoke only slow spreading events did not seem to depend on the agonist concentration, because only these types of events were observed at EC100 (10 units/ml; Fig. 5H) and ≈EC30 concentrations (0.1 units/ml; center ROI decay time was 6.5 ± 0.4 s; 6 events from 11 cells). Thus the thrombin-evoked events resembled slow-spreading events. The ADPβS and thrombin-evoked events were identical in their peak amplitudes (ΔF/F; Fig. 5 D and I and SI Table 1), indicating that the vesicles underlying both carried approximately equal amounts of synaptopHlourin. Because ADPβS evoked only fast confined events and thrombin evoked only slow-spreading spH events these data suggest that astrocytes may select for distinct forms of exocytosis depending on how they are activated.
Summary.
The main findings of the present study are that (1) SpH TIRF imaging can be used to record exocytosis from astrocytes with single-vesicle resolution, (2) exocytosis seems to be of two forms, and (3) receptor agonists that elevate calcium within astrocytes differ in their capacity to trigger different forms of exocytosis. Our data are based on single-vesicle imaging with TIRF microscopy. This method allows one to image near membrane events with good resolution, but a limitation is that only the membrane surface within ≈100 nm of the glass coverslip is sampled (31). It remains unknown to what extent this experimental arrangement favored the dominance of slow-spreading SpH events over the fast-confined ones (60% versus 40%), but this unknown does not detract from the main finding that two forms of exocytosis were readily observed with TIRF.
Interestingly, the number of exocytotic events measured with SpH in this study was similar to the number of NMDA receptor mediated slow inward currents recorded from pyramidal neurons and thalamic neurons (5, 14, 36), which are all thought to result from astrocyte exocytosis. Additionally, the quanta like single-vesicle events are consistent with the brief pulsatile events associated with astrocyte transmitter release onto neurons (5, 14, 36). However, the measures of exocytosis reported in this study are smaller than the number of exocytotic events measured with acridine orange to mark vesicles in astrocytes (13). It is likely that these differences arise because acridine orange labels multiple acid compartments in the astrocytes, only some of which are transmitter-containing vesicles (37). Previously membrane capacitance measurements (30), acridine orange imaging (13) and amperometry (21) have been used to study astrocyte exocytosis. Our experiments extend those studies and indicate that SpH imaging with TIRF microscopy is useful to study astrocyte exocytosis, with the unexpected finding that two forms of exocytosis can be imaged simultaneously from the same astrocytes.
The present imaging data indicate that astrocytes release transmitter through two forms of single-vesicle exocytosis. The fast-confined SpH events are similar to kiss and run exocytosis, whereas the slow-spreading events likely represent full vesicle fusion. Recent amperometry experiments have provided evidence for kiss and run exocytosis from astrocytes (21). Do the distinct events described in the present study correspond to two different pools of vesicles or different modes of release of a common vesicle? Presumably the vesicles could differ based on morphological and/or functional criteria. If the vesicles are indeed from different pools, then they do not seem to differ substantially in SpH content, and therefore we presume size. Also their behaviors on switching between TIR and oblique illumination suggest that the vesicles are ≤100 nm, but our experiments fall short of accurately measuring the vesicle dimensions. A definitive answer to this issue must await advances in light microscopy that allow imaging below the diffraction limit in live cells. Recent electron microscopy studies have indeed shown ≈30 nm glutamatergic vesicles in astrocytes (13), which is consistent with our suggestion that SpH predominantly labels glutamatergic vesicles ≤100 nm in size.
How do our experiments on astrocytes compare with recent work on hippocampal neurons? In making these comparisons it is important to consider that an absolute correspondence may be surprising, because astrocytes are quite distinct from neurons (see ref. 38 for a review of exocytosis). Overall we found that most single-vesicle events (≈60%) are consistent with full vesicle fusion, diffusion of SpH away from the release site, and complete mixing of SpH with the plasma membrane. Additionally, we found that 40% of events were not consistent with full fusion. In these cases the SpH did not diffuse, and at least in terms of SpH, the vesicle maintained its identity (19, 38). For these fast confined events the fluorescence signal returned to baseline levels over ≈1.5 s: endocytosis occurs quickly at the site of fusion, i.e., akin to kiss-and-run, and is followed by vesicle reacidification. In the case of hippocampal neurons kiss-and-run exocytosis has been observed by some (19, 39), but not by others (40) and factors that may constrain its prevalence have been considered (41, 42). Nonetheless, our experiments show that kinetically distinct forms of exocytosis can be readily detected in hippocampal astrocytes with single-vesicle imaging methods. Capacitance measurements that have single-vesicle resolution also provide support for kiss-and-run exocytosis from neurons (43, 44).
For the fast-confined events we measured a vesicle re acidification time course of ≈1.5 s, which is faster than that measured for hippocampal neurons at ≈4–5 s (40, 42). Interestingly, recent experiments show that an average brain synaptic vesicle contains ≈1.4 V-ATPase molecules (45), implying that variability between vesicles could give rise to distinct acidification rates for neurons and astrocytes. The number of V-ATPase molecules in the vesicles we have imaged is unknown. Moreover, vesicle reacidification may also depend on V-ATPase reassembly kinetics and the single molecule H+ transport rate, both of which are unknown. Taken together, these unknowns vitiate any precise interpretations about reacidification rates per se, or the similarities/differences between neurons and astrocytes.
Both the evoked and spontaneous single-vesicle events reported in this study were abolished by botulinum toxin E. This result suggests an important role of SNAP-25 in the release process (46), and extends previous work on astrocytes (23). In comparison spontaneous miniature synaptic currents persisted, whereas evoked synaptic current were abolished, in hippocampal neurons from mice with genetic deletion of SNAP-25 (47). From this perspective, both evoked and spontaneous astrocyte exocytosis may be SNAP-25 dependent, whereas evoked and spontaneous exocytosis from hippocampal neurons may differ in its dependence on SNAP-25 (47). The preceding three paragraphs present several similarities and differences between hippocampal neurons and astrocytes, and thus opportunities to fully understand the mechanisms of astrocyte exocytosis.
Astrocyte [Ca2+]i transients previously recorded in vivo differed in time course (6). Fortuitously we found that ADPβS and thrombin application evoked [Ca2+]i transients that also differed in time course. Based on SpH intensity as a measure of exocytosis (18–20) our data suggest that ADPβS and thrombin result in distinct modes of exocytosis. By analogy to studies with neurons ADPβS evoked exocytosis followed by endocytosis (fast confined events). Whereas thrombin evoked release consistent with full vesicle fusion (slow spreading events), and presumably compensatory endocytosis at other sites within the astrocyte. The simplest explanation for these data are that the distinct ADPβS and thrombin evoked [Ca2+]i transients within astrocytes result in different forms of exocytosis. However, other possibilities may also exist. For example ADPβS and thrombin may trigger [Ca2+]i transients and signaling events in distinct plasma membrane locales. We found no evidence for differing [Ca2+]i transient locales in the present work, but the possible existence of signaling locales and their impact on exocytosis will be the subject of future in depth studies. Astrocytes are known to express many distinct types of neurotransmitter receptors, many of which mobilize intracellular calcium levels. Our data thus imply that distinct receptor pathways may differ in their ability to select between modes of exocytosis, as exemplified by our findings with ADPβS and thrombin. From this perspective in future work it will be interesting to determine whether spontaneous [Ca2+]i transients also result in distinct modes of exocytosis, with separable neuronal consequences.
Materials and Methods
Ca2+ Imaging.
Mixed hippocampal neuron-astrocyte cultures were made and maintained as described (40, 48). Physiological saline comprised the following: 125 mM NaCl/5 mM KCl/1 mM MgCl2/10 mM d-glucose/2 mM CaCl2/25 mM Hepes. Cells were imaged and loaded with 5 μM Fluo-3/AM or fura-2/AM (Molecular Probes, Invitrogen, Paisley, U.K.) as described (33) (49). Calibration of the Ca2+ signal was achieved by permeabilizing the cells, determining the 340/380 ratio minimum (Rmin) and maximum (Rmax), and calibrating by using algorithms (50) assuming a Kd of 224 nM where [Ca2+]i = Kd. (R − Rmin)/(Rmax − R), and R is the 340/380 ratio.
Exocytosis Imaging.
Mixed hippocampal neuron-astrocyte cultures were transfected with 1–2 μg of synaptopHluorin (18, 19) cDNA by using Effectene (Qiagen). Twenty-four hours later, the cells were washed with buffer and imaged with evanescent wave microscopy as described (31, 49). The camera gain (Princeton Instruments cooled I-PentaMAX camera; Roper Scientific, Trenton, NJ) was adjusted for each astrocyte to provide the best signal to noise images, and so comparisons between astrocytes can only be reported as ΔF/F.
Chemicals.
All chemicals used were from Tocris (Bristol, U.K.), Sigma (Poole, U.K.), Invitrogen (Paisley, U.K.), or Molecular Probes Invitrogen. The names of the chemicals are abbreviated as adenosine 5′-β-thio diphosphate (ADPβS).
Analysis.
Data were analyzed by using Origin 6.1 (Origin Lab Corp), and Graphpad Instat 3.06 for Windows (San Diego, CA). Images were analyzed by using Image J, Metamorph, and CorelDraw12. Data in the text and graphs are shown as mean ± SEM from n determinations as indicated.
Supplementary Material
Acknowledgments
We thank Dr. Aude Derevier for help with cell culture, Dr. G. Miesenböck (Yale University, New Haven, CT) for the synaptopHlourin cDNA, Drs. Michel Goedert and Ronald Kaback for comments on an earlier version of this manuscript, and Dr. B Davletov (Medical Research Council, Cambridge, U.K.) for plasmids encoding the light chain of botulinum toxin E. This work was supported by the Medical Research Council.
Abbreviations
- SpH
synaptopHlourin
- [Ca2+]i
intracellular calcium transient
- ROI
region of interest
- TIRF
total internal reflection fluorescence.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0607625104/DC1.
References
- 1.Haydon PG. Nat Rev Neurosci. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- 2.Fields RD. Sci Am. 2004;290(4):54–61. doi: 10.1038/scientificamerican0404-54. [DOI] [PubMed] [Google Scholar]
- 3.Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Science. 1990;247:470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- 4.Newman EA. Trends Neurosci. 2003;26:536–542. doi: 10.1016/S0166-2236(03)00237-6. [DOI] [PubMed] [Google Scholar]
- 5.Parri HR, Gould TM, Crunelli V. Nat Neurosci. 2001;4:773–774. doi: 10.1038/90507. [DOI] [PubMed] [Google Scholar]
- 6.Hirase H, Qian L, Bartho P, Buzsaki G. PLoS Biol. 2004;2:E96. doi: 10.1371/journal.pbio.0020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X, Lou N, Xu Q, Tian GF, Peng WG, Han X, Kang J, Takano T, Nedergaard M. Nat Neurosci. 2006;9:816–823. doi: 10.1038/nn1703. [DOI] [PubMed] [Google Scholar]
- 8.Dani JW, Chernjavsky A, Smith SJ. Neuron. 1992;8:429–440. doi: 10.1016/0896-6273(92)90271-e. [DOI] [PubMed] [Google Scholar]
- 9.Parri HR, Crunelli V. Neuroscience. 2003;120:979–992. doi: 10.1016/s0306-4522(03)00379-8. [DOI] [PubMed] [Google Scholar]
- 10.Newman EA. J Neurosci. 2003;23:1659–1666. doi: 10.1523/JNEUROSCI.23-05-01659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parpura V, Haydon PG. Proc Natl Acad Sci USA. 2000;97:8629–8634. doi: 10.1073/pnas.97.15.8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kreft M, Stenovec M, Rupnik M, Grilc S, Krzan M, Potokar M, Pangrsic T, Haydon PG, Zorec R. Glia. 2004;46:437–445. doi: 10.1002/glia.20018. [DOI] [PubMed] [Google Scholar]
- 13.Bezzi P, Gundersen V, Galbete JL, Seifert G, Steinhauser C, Pilati E, Volterra A. Nat Neurosci. 2004;7:613–620. doi: 10.1038/nn1246. [DOI] [PubMed] [Google Scholar]
- 14.Fellin T, Carmignoto G. J Physiol. 2004;559:3–15. doi: 10.1113/jphysiol.2004.063214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kozlov AS, Angulo MC, Audinat E, Charpak S. Proc Natl Acad Sci USA. 2006;103:10058–10063. doi: 10.1073/pnas.0603741103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian GF, Azmi H, Takano T, Xu Q, Peng W, Lin J, Oberheim N, Lou N, Wang X, Zielke HR, Kang J, Nedergaard M. Nat Med. 2005;11:973–981. doi: 10.1038/nm1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- 18.Miesenböck G, De Angelis DA, Rothman JE. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- 19.Gandhi SP, Stevens CF. Nature. 2003;423:607–613. doi: 10.1038/nature01677. [DOI] [PubMed] [Google Scholar]
- 20.Sankaranarayanan S, De Angelis D, Rothman JE, Ryan TA. Biophys J. 2000;79:2199–2208. doi: 10.1016/S0006-3495(00)76468-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen X, Wang L, Zhou Y, Zheng LH, Zhou Z. J Neurosci. 2005;25:9236–9243. doi: 10.1523/JNEUROSCI.1640-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pasti L, Zonta M, Pozzan T, Vicini S, Carmignoto G. J Neurosci. 2001;21:477–484. doi: 10.1523/JNEUROSCI.21-02-00477.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Araque A, Li N, Doyle RT, Haydon PG. J Neurosci. 2000;20:666–673. doi: 10.1523/JNEUROSCI.20-02-00666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parpura V, Fang Y, Basarsky T, Jahn R, Haydon PG. FEBS Lett. 1995;377:489–492. doi: 10.1016/0014-5793(95)01401-2. [DOI] [PubMed] [Google Scholar]
- 25.Wilhelm A, Volknandt W, Langer D, Nolte C, Kettenmann H, Zimmermann H. Neurosci Res. 2004;48:249–257. doi: 10.1016/j.neures.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Maienschein V, Marxen M, Volknandt W, Zimmermann H. Glia. 1999;26:233–244. doi: 10.1002/(sici)1098-1136(199905)26:3<233::aid-glia5>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Q, Fukuda M, Van Bockstaele E, Pascual O, Haydon PG. Proc Natl Acad Sci USA. 2004;101:9441–9446. doi: 10.1073/pnas.0401960101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krzan M, Stenovec M, Kreft M, Pangrsic T, Grilc S, Haydon PG, Zorec R. J Neurosci. 2003;23:1580–1583. doi: 10.1523/JNEUROSCI.23-05-01580.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anlauf E, Derouiche A. Glia. 2005;49:96–106. doi: 10.1002/glia.20094. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Q, Pangrsic T, Kreft M, Krzan M, Li N, Sul JY, Halassa M, Van Bockstaele E, Zorec R, Haydon PG. J Biol Chem. 2004;279:12724–12733. doi: 10.1074/jbc.M312845200. [DOI] [PubMed] [Google Scholar]
- 31.Steyer JA, Almers W. Nat Rev Mol Cell Biol. 2001;2:268–275. doi: 10.1038/35067069. [DOI] [PubMed] [Google Scholar]
- 32.Bajohrs M, Rickman C, Binz T, Davletov B. EMBO Rep. 2004;5:1090–1095. doi: 10.1038/sj.embor.7400278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bowser DN, Khakh BS. J Neurosci. 2004;24:8606–8620. doi: 10.1523/JNEUROSCI.2660-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dery O, Corvera CU, Steinhoff M, Bunnett NW. Am J Physiol. 1998;274:C1429–C1452. doi: 10.1152/ajpcell.1998.274.6.C1429. [DOI] [PubMed] [Google Scholar]
- 35.Junge CE, Lee CJ, Hubbard KB, Zhang Z, Olson JJ, Hepler JR, Brat DJ, Traynelis SF. Exp Neurol. 2004;188:94–103. doi: 10.1016/j.expneurol.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 36.Perea G, Araque A. J Neurosci. 2005;25:2192–2203. doi: 10.1523/JNEUROSCI.3965-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brunk UT, Dalen H, Roberg K, Hellquist HB. Free Radical Biol Med. 1997;23:616–626. doi: 10.1016/s0891-5849(97)00007-5. [DOI] [PubMed] [Google Scholar]
- 38.Harata NC, Aravanis AM, Tsien RW. J Neurochem. 2006;97:1546–1570. doi: 10.1111/j.1471-4159.2006.03987.x. [DOI] [PubMed] [Google Scholar]
- 39.Harata NC, Choi S, Pyle JL, Aravanis AM, Tsien RW. Neuron. 2006;49:243–256. doi: 10.1016/j.neuron.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 40.Granseth B, Odermatt B, Royle SJ, Lagnado L. Neuron. 2006;51:773–786. doi: 10.1016/j.neuron.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 41.Fernandez-Alfonso T, Kwan R, Ryan TA. Neuron. 2006;51:179–186. doi: 10.1016/j.neuron.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 42.Atluri PP, Ryan TA. J Neurosci. 2006;26:2313–2320. doi: 10.1523/JNEUROSCI.4425-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klyachko VA, Jackson MB. Nature. 2002;418:89–92. doi: 10.1038/nature00852. [DOI] [PubMed] [Google Scholar]
- 44.He L, Wu XS, Mohan R, Wu LG. Nature. 2006;444:102–105. doi: 10.1038/nature05250. [DOI] [PubMed] [Google Scholar]
- 45.Takamori S, Holt M, Stenius K, Lemke EA, Gronborg M, Riedel D, Urlaub H, Schenck S, Brugger B, Ringler P. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 46.Davletov B, Bajohrs M, Binz T. Trends Neurosci. 2005;28:446–452. doi: 10.1016/j.tins.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 47.Washbourne P, Thompson PM, Carta M, Costa ET, Mathews JR, Lopez-Bendito G, Molnar Z, Becher MW, Valenzuela CF, Partridge LD, Wilson MC. Nat Neurosci. 2002;5:19–26. doi: 10.1038/nn783. [DOI] [PubMed] [Google Scholar]
- 48.Khakh BS, Gittermann D, Cockayne DA, Jones A. J Neurosci. 2003;23:7426–7437. doi: 10.1523/JNEUROSCI.23-19-07426.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fisher JA, Girdler G, Khakh BS. J Neurosci. 2004;24:10475–10487. doi: 10.1523/JNEUROSCI.3250-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grynkiewicz G, Poenie M, Tsien RY. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.