Abstract
Non-peptidic delta opioid receptor agonists are being evaluated for a wide range of clinical applications; however, the clinical utility of piperazinyl benzamide delta agonists such as SNC80 may be limited by convulsant activity. The purpose of the present study was to evaluate the electroencephalographic and convulsant activity produced by a high dose of 10 mg/kg SNC80 IM in rhesus monkeys. EEG and behavioral activity were examined in four adult male rhesus monkeys after IM administration of SNC80. Monkeys were seated in a standard primate restraint chair, and EEG activity was recorded using an array of 16 needle electrodes implanted subcutaneously in the scalp in a bipolar (scalp-to scalp) montage in a longitudinal direction, with bilateral frontal, central, temporal, and occipital leads. Behavior was recorded using video monitoring equipment. Initially, all monkeys were tested with 10 mg/kg SNC80, which is a relatively high dose 3–10 fold greater than doses necessary to produce a variety of other behavioral effects. Behavioral convulsions and EEG seizures were observed in one of the four monkeys. In this monkey, neither behavioral convulsions nor EEG seizures were observed when a lower dose of 3.2 mg/kg was administered nine weeks later or when the same dose of 10 mg/kg SNC80 was administered one year later. These results suggest that IM administration of SNC80 is less potent in producing convulsant effects than in producing other, potentially useful behavioral effects (e.g. antinociception) in rhesus monkeys.
Keywords: delta opioid receptor, SNC80, convulsion, seizure, EEG, rhesus monkey
INTRODUCTION
Delta opioid receptors constitute one of the three main types of opioid receptors found in mammalian brain (i.e. delta, mu and kappa opioid receptors) (Martin et al., 1976; Lord et al., 1977; Kieffer, 1995). The recent development of systemically active, non-peptidic delta agonists such as BW373U86 and its O-methylated derivative SNC80 has greatly facilitated research on the physiological and behavioral consequences of delta receptor activation (Chang et al., 1993; Calderon et al., 1994). Preclinical studies conducted with these and other related drugs suggest that delta agonists may have clinical utility as analgesics, antidepressants, cardioprotective agents, and modulators of immune function (Chang et al., 1993; Calderon et al., 1994; Brandt et al., 2001a; Gross et al., 2004; Jutkiewicz and Woods, 2004; Ossipov et al., 2004; Weber and Gomez-Flores, 2004). However, it was recognized at an early stage that piperazinyl benzamide delta agonists such as BW373U86 and SNC80 also produced convulsions in mice and rats (Comer et al., 1993; Bilsky et al., 1995; Hong et al., 1998; Broom et al., 2002a; Broom et al., 2002b; Jutkiewicz et al., 2004). These convulsions in rodents are typically brief and non-lethal (but see (Hong et al., 1998), but they are also delta-receptor mediated and produced at doses similar to those that produce potentially useful effects such as antinociception. Consequently, the convulsant effects of delta agonists have emerged as one category of untoward effects that poses an impediment to further development of this class of drugs for clinical applications.
Delta agonist-induced convulsions may be less problematic if they occurred only in rodents and were not apparent in non-human primates or humans. In support of the possibility of species-dependent drug effects, there are significant species differences in opioid receptor distribution between rodents and primates (Mansour et al., 1987; Mansour et al., 1988; Hiller and Fan, 1996; Peckys and Landwehrmeyer, 1999; Mathieu-Kia et al., 2001; Mennicken et al., 2003). Delta receptors, for example, are diffusely expressed in the spinal cord of rats and mice, whereas their expression in non-human primates and humans is limited to the superficial laminae of the spinal dorsal horn (Mennicken et al., 2003). Moreover, behavioral studies have provided some evidence to suggest that there may be species differences in the convulsant effects of delta agonists. Although moderate doses of BW373U86 and some other piperazinyl benzamides produced convulsions in both squirrel monkeys and rhesus monkeys (Dykstra et al., 1993; Negus et al., 1994), SNC80 did not produce convulsions in rhesus monkeys across a range of doses that did produce other behavioral effects, including antinociceptive effects in a model of inflammatory pain (Negus et al., 1998; Brandt et al., 2001a). These results raised the possibility that some piperazinyl benzamide delta agonists, such as SNC80, might retain the ability to produce clinically useful effects in primates while displaying a reduced propensity to produce convulsions.
Observational measures of behavioral convulsions provide only a crude tool for evaluating potentially untoward effects of delta agonists on brain activity. One recent study addressed this issue in rodents by comparing electroencephalographic (EEG) activity with behavioral observations in rodents, and it was found that non-peptidic delta agonists including SNC80 produced EEG seizures and behavioral convulsions at similar doses (Jutkiewicz et al., 2006). The purpose of the present study was to compare EEG activity and behavioral measures of convulsions in non-human primates. Due to the potential for short-term and long-term negative health consequences associated with convulsant activity, the goal of the present study was not to fully evaluate the pharmacology of SNC80-induced convulsions and seizures. Rather, the goal was to assess the degree to which seizures/convulsions might be produced by a relatively high dose of SNC80 that has been shown previously to produce other behavioral effects in rhesus monkeys. Accordingly, rhesus monkeys were tested with a dose of 10 mg/kg SNC80 IM, which is at the upper end of the dose range for producing other behavioral effects such as antinociception, suppression of food-maintained responding, and discriminative stimulus effects (Negus et al., 1998; Brandt et al., 1999; Brandt et al., 2001a).
METHODS
Subjects
Four male rhesus monkeys (Macaca mulatta) were used. Subjects weighed 9.6–11 kg during the course of these studies. All monkeys had prior exposure to drugs (primarily opioid compounds) including SNC80, but they had not received SNC80 or other delta opioids for > 6 months. This period of no exposure to delta opioids was deemed to be important to minimize the possibility that subjects were to delta opioid effects (Comer et al., 1993; Brandt et al., 2001b). The subjects were individually housed, and water was freely available. Their diet consisted of PMI Feeds Jumbo monkey diet (PMI Feeds, Inc., St. Louis, MO). This diet was supplemented with fresh fruit twice daily. A 12 hr light/12 hr dark cycle was in effect (lights on from 7AM–7PM).
Animal maintenance and research were conducted in accordance with the guidelines provided by the NIH Committee on Laboratory Animal Resources. The facility was licensed by the United States Department of Agriculture, and protocols were approved by the Institutional Animal Care and Use Committee. The health of the monkeys was monitored daily by technical staff and periodically by a consulting veterinarian. Monkeys had visual, auditory and olfactory contact with other monkeys throughout the study. Monkeys also had access to puzzle feeders, mirrors and chew toys to provide environmental enrichment. Nature videotapes or music were played daily in all housing rooms.
Monitoring EEG activity and behavior
Monkeys were removed from their home cages, seated in restraint chairs equipped with a V-shaped headrest to minimize head movement, and transferred to a separate testing room. Once in the testing room, the top of each monkey’s head was shaved with electric barber shears. EMLA gel (2.5% Lidocaine+2.5% Prilocaine; Astra Zeneca, Wilmington, DE) was subsequently applied to the top of each monkey’s head to induce a local anesthetic effect. Five min later, stainless steel needle electrodes were placed on the scalp of each monkey. Specifically, a 16-electrode recording, using the 10–20 system, and with impedances below 5000 Ohms, was used to obtain the EEG recording. EEG activity was recorded with needle electrodes in a bipolar (scalp-to scalp) montage in a longitudinal direction, with bilateral frontal, central, temporal, and occipital leads. All electrodes were connected to a Voyageur TCP/IP Gateway EEG machine (Nicolet Biomedical Inc., Madison, WI). The EEG data were collected with the following settings: high filter at 70 Hz, low filter at 0.1 Hz, sensitivity 7 uV/mm, and paper speed 10 mm/sec. Video monitoring equipment (D-Link Systems, Inc., Fountain Valley, CA), consisting of a high-speed wireless router (DI-624), wireless PCI adapter (DWL-520) and wireless internet camera (DCS-900W), was set up to record each monkey’s behavior during the EEG session.
Baseline EEG activity was recorded for at least 15 min. In the initial study, monkeys were then injected IM with 10 mg/kg of the delta opioid agonist SNC80. This dose of SNC80 was selected for study because it is a relatively high dose more than 10-fold greater than the ED50 values for SNC80 to produce antinociceptive effects in an assay of prostaglandin-induced thermal hypersensitivity, rate-suppression in an assay of food-maintained operant responding, or discriminative stimulus effects in an assay of SNC80 discrimination (Negus et al., 1998; Brandt et al., 2001a). Electrographic activity was monitored for further analysis (see below). In addition, animals were videotaped for up to 2 hr after SNC80 injection to permit assessment of overt behavior (e.g. convulsions) that might be associated with SNC80-induced changes in EEG activity.
In the initial study, SNC80 produced EEG seizures and a tonic-clonic convulsion in one of the four monkeys tested. Diazepam (1 mg/kg, i.m.) was administered to terminate the electrographic seizure activity as well as the behavioral convulsions. Two follow-up studies were then conducted in this monkey. First, the monkey was tested again after a period of approximately 9 weeks with a lower dose of 3.2 mg/kg SNC80. This study was conducted to assess the ability of a lower SNC80 dose to produce electrographic seizures and/or behavioral convulsions in this monkey. Second, the same monkey was tested again 1 year later with the original dose of 10 mg/kg SNC80. During this replicate test with 10 mg/kg SNC80, the monkey was observed for the presence of convulsant activity, but EEG measures were not recorded due to career transitions of key personnel.
Data Analysis
The EEG composition in primates is similar to that of humans, and consists in the awake relaxed adult animal of three major frequencies; theta (5–7 Hz), which is primarily seen over the temporal regions, alpha (9–11 Hz), which is most prominent occipitally, and beta (12–15 Hz), which can be seen most clearly over the frontal lobes. Records from all EEG channels were visually analyzed for interictal spike and slow wave discharges. The EEG composition, with regards to the amount of theta, alpha, and beta activity was analyzed with fast Fourier transformation to yield a power density vs. frequency (in Hz) graph. The centro-occipital and temporal-occipital derivations contained the least amount of movement and muscle artifacts and were selected for the final analysis. Twenty-five 30-second epochs were collected from the baseline EEG for all four monkeys and compared with corresponding epochs collected after drug administration. Fast Fourier analysis was performed on the mean values, and a two-way, repeated-measures ANOVA was used to determine statistical significance in the amount of theta, alpha and beta frequencies after drug administration.
Drugs
SNC80 (free base) was synthesized by Drs. Kenner C. Rice and John E. Folk (National Institutes of Health, Bethesda, MD), and was dissolved in 3% lactic acid to a final concentration of 50 mg/ml. Dilutions were made with sterile water. SNC80 was administered IM in the thigh.
RESULTS
Acute Effects of SNC80 (10 mg/kg)
Out of four monkeys tested, administration of SNC80 (10 mg/kg IM) caused a seizure in one monkey (monkey 95C271). EEG recording showed two short bursts of generalized spike and slow wave activity; the first at 2 minutes, and the second at 2 minutes 57 seconds after drug administration (see Figure 1). Neither the first nor the second burst of these electrographic seizure discharges was accompanied by behavioral convulsions. The EEG activity then changed character, and bursts of generalized sharp and slow waves started to occur at 3 minutes 16 second after the injection (see Figure 2). As with the initial discharges, the animal did not show any signs of behavioral convulsant activity at this time. At 4 minutes 16 seconds after drug administration, the EEG displayed generalized spike and slow wave activity (see Figure 3) that was accompanied by a behavioral convulsion. The animal initially stiffened and stopped ongoing activity. The animal then displayed brief tonic muscle activity with extension and pronation of all four extremities. This was followed by generalized clonic activity and loss of sphincter control, at which time diazepam (1 mg/kg IM) was administered to terminate seizures. In the postictal phase, the EEG showed characteristic low voltage slow activity for another 15 minutes. Clinically however, the monkey continued to be drowsy and less responsive for an additional 10 minutes after the episode.
Figure 1.
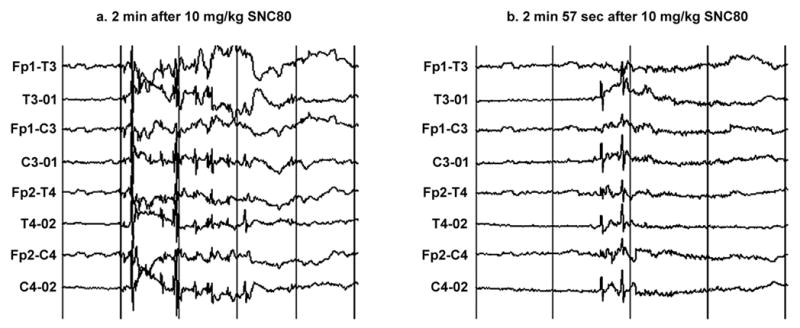
Generalized spike and slow wave discharges that started (a) 2 min and (b) 2 min 57 seconds after administration of 10 mg/kg SNC80 in monkey 95C271. No behavioral convulsions were noted in the animal during either episode of electrographic seizure discharge. Sensitivity is 30uV/mm on the vertical axis. Abbreviations on the vertical axis designate the location of bipolar leads associated with a given trace. Fp-frontoparietal; T-temporal; O-occipital; C-central. Odd and even numbers designate leads on the left and right side of the head, respectively. For example, Fp1-T3 marks a bipolar lead involving the left frontoparietal to left temporal regions of the head. The paper speed was 10 mm/second on the horizontal axis, which corresponds to 1 second between the vertical bars.
Figure 2.
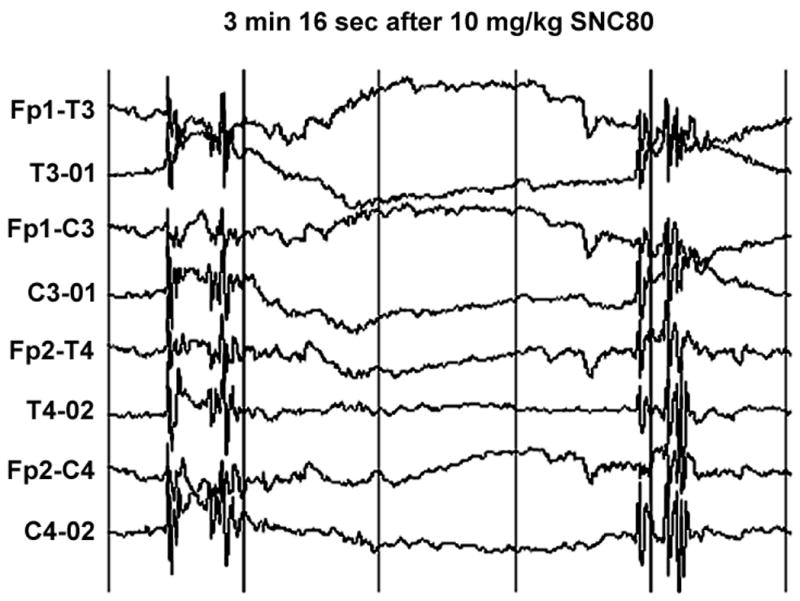
Repeated episodes of generalized spike and slow wave discharges that occurred at 3 minutes 16 seconds after administration of SNC80 in monkey 95C271. The monkey did not show behavioral convulsions at this time. See Fig. 1 for other details.
Figure 3.
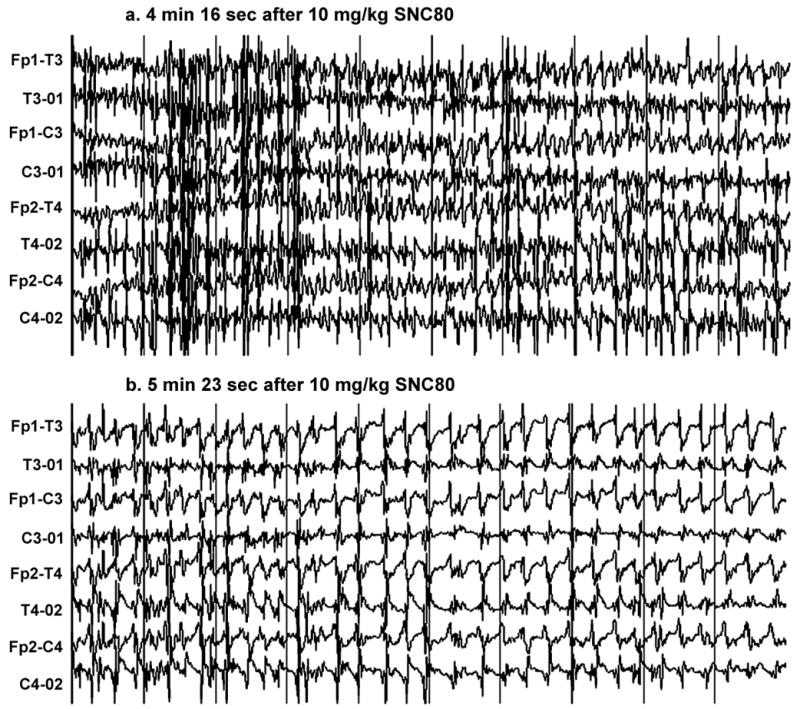
Generalized spike and wave discharges that started (a) 4 minutes 16 seconds and (b) 5 min 23 sec after administration of SNC80 in monkey 95C271. In 3a, the frequency of this primarily generalized discharge was 7–8 cycles per second. These discharges were accompanied by a behavioral convulsion. In 3b, the behavioral and electrographic seizure continued, and the frequency decreased. Diazepam was administered shortly after this trace. Sensitivity is 50 uv/millimeter. See Fig. 1 for other details
Administration of the same dose of SNC80 in the other three monkeys did not produce any epileptiform activity. Specifically, no discharges were seen in the EEG recording, nor were there any clinical correlates indicative of behavioral seizures in these monkeys. As shown in Figure 4, Fast Fourier analysis revealed no significant differences in the EEG composition in respect to amounts of theta, alpha and beta frequencies before and after administration of 10 mg/kg SCN80 in these monkeys (F1,48= 0.525, p= 0.544 and F1,48= 1.136, p= 0.398 for centro-occipital and temporal-occipital EEG compositions, respectively.)
Figure 4.

Fast Fourier analysis of the EEG composition as a function of EEG power and frequency (in Hz) in the centro-occipital (F1,48= 0.525, p= 0.544) and temporal-occipital (F1,48= 1.136, p= 0.398) activities before and after administration of 10 mg/kg SNC80 in three monkeys that did not develop seizures after SNC80 administration.
Follow-up studies in monkey 95C271
The monkey that initially showed both electrographic seizures and behavioral convulsions was tested with a lower dose of 3.2 mg/kg SNC80 IM nine weeks after the initial study and with the original dose of 10 mg/kg SNC80 IM one year after the initial study. Treatment with 3.2 mg/kg SNC80 did not produce any spike and slow wave discharges in the EEG or behavioral convulsions. EEG was not recorded during the second treatment with 10 mg/kg SNC80, but behavioral convulsions were not observed.
DISCUSSION
This is the first study to evaluate the EEG effects of delta opioid agonists in non-human primates. In one monkey during one test, a high dose of 10 mg/kg SNC80 produced both EEG seizures and behavioral convulsions. These results confirm and extend other studies to suggest that SNC80 and other piperazinyl benzamides (e.g. BW373U86) may produce behavioral convulsions in mice (Comer et al., 1993; Bilsky et al., 1995; Hong et al., 1998; Broom et al., 2002b), rats (Broom et al., 2002a; Jutkiewicz et al., 2004; Jutkiewicz et al., 2006), squirrel monkeys (Dykstra et al., 1993; Allen et al., 2002) and rhesus monkeys (Negus et al., 1994). However, the convulsant effects of SNC80 were not replicated in this monkey during a second test with 10 mg/kg SNC80 one year later, and a lower dose of 3.2 mg/kg SNC80 also failed to produce either EEG seizures or behavioral convulsions in this monkey. Moreover, three other monkeys tested with 10 mg/kg SNC80 showed neither EEG seizures nor behavioral convulsions. Finally, SNC80 doses up to 32 mg/kg have failed to produce behavioral convulsions in our previous studies (Negus et al., 1998; Brandt et al., 2001a; Brandt et al., 2001b). Taken together, these results suggest that 10 mg/kg is at best a threshold dose for production of seizures and convulsions in rhesus monkeys.
In the one monkey in which SNC80-induced EEG seizures were observed, isolated spike and slow wave discharges without accompanying behavioral convulsions or other overt behavioral correlates were present within 3 minutes after SNC80 administration. Within the next minute, the seizure activity intensified as reflected by generalized discharges in the EEG and by the emergence of behavioral convulsions. The pattern of the electrographic seizures and behavioral convulsions produced by SNC80 resembled that of generalized clonic-tonic seizures (Casaubon et al., 2003; Moraes et al., 2005). Seizures were quickly terminated by diazepam, and the EEG showed typical postictal slowing and amplitude depression with EEG frequencies ranging between 4 to 5 Hz and an amplitude below 50 uV. There was also a moderate increase of high frequency beta activity noted approximately 15 minutes after the seizure. This increase was most likely caused by the administration of diazepam.
The one monkey in which a convulsion and EEG seizure were observed may have been unusually sensitive to the convulsant effects of SNC80. However, the same dose of SNC80 failed to produce a convulsion in this monkey during a subsequent test one year later that involved only observation without EEG monitoring. The reason for this inconsistency in convulsant effects is not known; however, it suggests that aspects of the EEG testing procedure (e.g. physical handling to shave the head and implant the scalp electrodes, presence of additional staff members to monitor EEG records) may have increased susceptibility to the convulsant effects of SNC80 in this monkey.
It is possible that higher SNC80 doses would have produced more reliable convulsant activity within and between monkeys in the present study. However, higher doses were not tested because 10 mg/kg is 3- to 10-fold greater than the ED50 for SNC80 to produce a range of other behavioral effects in rhesus monkeys (Negus et al., 1998; Brandt et al., 1999; Brandt et al., 2001a). In particular, even the most sensitive monkey in the present study did not display EEG seizures or behavioral convulsions after administration of 3.2 mg/kg SNC80. However, this dose is sufficient to produce maximal antinociceptive effects in an assay of thermal hypersensitivity induced by either prostaglandin-E2 or Freund’s Complete Adjuvant and approximately 60% maximal antinociception when thermal hypersensitivity is induced by capsaicin (Brandt et al., 2001a). Consequently, these results suggest that SNC80 in rhesus monkeys is more potent in producing potentially useful antinociceptive effects in models of inflammatory pain than in producing convulsant effects. It should be noted that doses of SNC80 up to 10 mg/kg are only weakly active or inactive in standard models of acute thermal pain (Negus et al., 1998); however, such models may not be optimal for detecting drugs (e.g. nonsteroidal antiinflammatory drugs) that have excellent value as analgesics against inflammatory pain (Negus et al., 2006). The higher potency of intramuscular SNC80 to produce potentially valuable antinociceptive effects as opposed to convulsions in rhesus monkeys parallels the finding that subcutaneous administration of SNC80 was also more potent in producing locomotor and antidepressant effects than convulsions in rats (Jutkiewicz et al., 2004).
As noted above, SNC80 doses up to 32 mg/kg did not produce behavioral convulsions in previous studies, and indeed, the single convulsion and EEG seizure observed in the present study marks the only time an SNC80-induced convulsion has been observed in non-human primates in this laboratory (Negus et al., 1998; Brandt et al., 2001a; Brandt et al., 2001b). One factor that may have contributed to the absence of convulsions in our previous studies was the use of a cumulative dosing procedure. It is well-established that tolerance can develop rapidly to the convulsant effects and some other behavioral effects of delta opioids (Comer et al., 1993; Brandt et al., 2001b), and as a result, it is possible that administration of low doses early in a cumulative dosing sessions attenuated any convulsant effects that might have been produced by subsequent administration of higher doses. However, other studies did use acute dosing at doses up to 10 mg/kg without producing convulsions (Brandt et al., 2001a), and in the present study, additional care was taken to minimize the possibility of tolerance by inserting a long hiatus (>6 months) in exposure to delta opioids prior to testing. Thus, it is unlikely that tolerance is sufficient to explain the apparently low relative potency of SNC80 to produce convulsions in rhesus monkeys.
In summary, the present study confirmed that SNC80 can produce both EEG seizures and behavioral convulsions in rhesus monkeys. However, SNC80 appears to be less potent in producing convulsant effects than in producing other, potentially useful behavioral effects. These results provide some hope that delta agonists could be developed that retain clinically useful effects such as a reduction of inflammatory pain but that do not produce convulsant effects.
Acknowledgments
Supported by RO1-DA11460 from NIDA, NIH and by the Intramural Research Programs of NINDS and NIDDK, NIH. The authors would like to thank Sam McWilliams, Melissa Timm and Kevin Costa for technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen RM, Granger AL, Dykstra LA. Dextromethorphan potentiates the antinociceptive effects of morphine and the delta-opioid agonist SNC80 in squirrel monkeys. J Pharmacol Exp Ther. 2002;300:435–441. doi: 10.1124/jpet.300.2.435. [DOI] [PubMed] [Google Scholar]
- Bilsky EJ, Calderon SN, Wang T, Bernstein RN, Davis P, Hruby VJ, McNutt RW, Rothman RB, Rice KC, Porreca F. SNC 80, a selective, nonpeptidic and systemically active opioid delta agonist. J Pharmacol Exp Ther. 1995;273:359–366. [PubMed] [Google Scholar]
- Brandt MR, Furness MS, Mello NK, Rice KC, Negus SS. Antinociceptive effects of delta-opioid agonists in Rhesus monkeys: effects on chemically induced thermal hypersensitivity. J Pharmacol Exp Ther. 2001a;296:939–946. [PubMed] [Google Scholar]
- Brandt MR, Furness MS, Rice KC, Fischer BD, Negus SS. Studies of tolerance and dependence with the delta-opioid agonist SNC80 in rhesus monkeys responding under a schedule of food presentation. J Pharmacol Exp Ther. 2001b;299:629–637. [PubMed] [Google Scholar]
- Brandt MR, Negus SS, Mello NK, Furness MS, Zhang X, Rice KC. Discriminative stimulus effects of the nonpeptidic delta-opioid agonist SNC80 in rhesus monkeys. J Pharmacol Exp Ther. 1999;290:1157–1164. [PubMed] [Google Scholar]
- Broom DC, Jutkiewicz EM, Folk JE, Traynor JR, Rice KC, Woods JH. Convulsant activity of a non-peptidic delta-opioid receptor agonist is not required for its antidepressant-like effects in Sprague-Dawley rats. Psychopharmacology (Berl) 2002a;164:42–48. doi: 10.1007/s00213-002-1179-y. [DOI] [PubMed] [Google Scholar]
- Broom DC, Nitsche JF, Pintar JE, Rice KC, Woods JH, Traynor JR. Comparison of receptor mechanisms and efficacy requirements for delta-agonist-induced convulsive activity and antinociception in mice. J Pharmacol Exp Ther. 2002b;303:723–729. doi: 10.1124/jpet.102.036525. [DOI] [PubMed] [Google Scholar]
- Calderon SN, Rothman RB, Porreca F, Flippen-Anderson JL, McNutt RW, Xu H, Smith LE, Bilsky EJ, Davis P, Rice KC. Probes for narcotic receptor mediated phenomena. 19. Synthesis of (+)-4-[(alpha R)-alpha-((2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl)-3- methoxybenzyl]-N,N-diethylbenzamide (SNC 80): a highly selective, nonpeptide delta opioid receptor agonist. J Med Chem. 1994;37:2125–2128. doi: 10.1021/jm00040a002. [DOI] [PubMed] [Google Scholar]
- Casaubon L, Pohlmann-Eden B, Khosravani H, Carlen PL, Wennberg R. Video-EEG evidence of lateralized clinical features in primary generalized epilepsy with tonic-clonic seizures. Epileptic Disord. 2003;5:149–156. [PubMed] [Google Scholar]
- Chang KJ, Rigdon GC, Howard JL, McNutt RW. A novel, potent and selective nonpeptidic delta opioid receptor agonist BW373U86. J Pharmacol Exp Ther. 1993;267:852–857. [PubMed] [Google Scholar]
- Comer SD, Hoenicke EM, Sable AI, McNutt RW, Chang KJ, De Costa BR, Mosberg HI, Woods JH. Convulsive effects of systemic administration of the delta opioid agonist BW373U86 in mice. J Pharmacol Exp Ther. 1993;267:888–895. [PubMed] [Google Scholar]
- Dykstra LA, Schoenbaum GM, Yarbrough J, McNutt R, Chang KJ. A novel delta opioid agonist, BW373U86, in squirrel monkeys responding under a schedule of shock titration. J Pharmacol Exp Ther. 1993;267:875–882. [PubMed] [Google Scholar]
- Gross GJ, Fryer RM, Patel HH, Schultz JEJ. Cardioprotection and delta opioid receptors. In: Chang KJ, Porreca F, Woods JH, editors. The Delta Receptor. New York: Marcel Dekker; 2004. pp. 451–466. [Google Scholar]
- Hiller JM, Fan LQ. Laminar distribution of the multiple opioid receptors in the human cerebral cortex. Neurochem Res. 1996;21:1333–1345. doi: 10.1007/BF02532374. [DOI] [PubMed] [Google Scholar]
- Hong EJ, Rice KC, Calderon SN, Woods JH, Traynor JR. Convulsive behavior of nonpeptide ∂-opioid ligands: Comparison of SNC80 and BW373U86 in mice. Analgesia. 1998;3:269–276. [Google Scholar]
- Jutkiewicz EM, Baladi MG, Folk JE, Rice KC, Woods JH. The convulsive and electroencephalographic changes produced by nonpeptidic delta-opioid agonists in rats: comparison with pentylenetetrazol. J Pharmacol Exp Ther. 2006 doi: 10.1124/jpet.105.095810. [DOI] [PubMed] [Google Scholar]
- Jutkiewicz EM, Eller EB, Folk JE, Rice KC, Traynor JR, Woods JH. Delta-opioid agonists: differential efficacy and potency of SNC80, its 3-OH (SNC86) and 3-desoxy (SNC162) derivatives in Sprague-Dawley rats. J Pharmacol Exp Ther. 2004;309:173–181. doi: 10.1124/jpet.103.061242. [DOI] [PubMed] [Google Scholar]
- Jutkiewicz EM, Woods JH. Antidepressant-like effects of delta opioid receptor agonists. In: Chang KJ, Porreca F, Woods JH, editors. The Delta Receptor. New York: Marcel Dekker; 2004. pp. 355–371. [Google Scholar]
- Kieffer BL. Recent advances in molecular recognition and signal transduction of active peptides: receptors for opioid peptides. Cell Mol Neurobiol. 1995;15:615–635. doi: 10.1007/BF02071128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord JA, Waterfield AA, Hughes J, Kosterlitz HW. Endogenous opioid peptides: multiple agonists and receptors. Nature. 1977;267:495–499. doi: 10.1038/267495a0. [DOI] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Autoradiographic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. J Neurosci. 1987;7:2445–2464. [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Anatomy of CNS opioid receptors. Trends Neurosci. 1988;11:308–314. doi: 10.1016/0166-2236(88)90093-8. [DOI] [PubMed] [Google Scholar]
- Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976;197:517–532. [PubMed] [Google Scholar]
- Mathieu-Kia AM, Fan LQ, Kreek MJ, Simon EJ, Hiller JM. Mu-, delta- and kappa-opioid receptor populations are differentially altered in distinct areas of postmortem brains of Alzheimer’s disease patients. Brain Res. 2001;893:121–134. doi: 10.1016/s0006-8993(00)03302-3. [DOI] [PubMed] [Google Scholar]
- Mennicken F, Zhang J, Hoffert C, Ahmad S, Beaudet A, O’Donnell D. Phylogenetic changes in the expression of delta opioid receptors in spinal cord and dorsal root ganglia. J Comp Neurol. 2003;465:349–360. doi: 10.1002/cne.10839. [DOI] [PubMed] [Google Scholar]
- Moraes MF, Chavali M, Mishra PK, Jobe PC, Garcia-Cairasco N. A comprehensive electrographic and behavioral analysis of generalized tonic-clonic seizures of GEPR-9s. Brain Res. 2005;1033:1–12. doi: 10.1016/j.brainres.2004.10.066. [DOI] [PubMed] [Google Scholar]
- Negus SS, Butelman ER, Chang KJ, DeCosta B, Winger G, Woods JH. Behavioral effects of the systemically active delta opioid agonist BW373U86 in rhesus monkeys. J Pharmacol Exp Ther. 1994;270:1025–1034. [PubMed] [Google Scholar]
- Negus SS, Gatch MB, Mello NK, Zhang X, Rice K. Behavioral effects of the delta-selective opioid agonist SNC80 and related compounds in rhesus monkeys. J Pharmacol Exp Ther. 1998;286:362–375. [PubMed] [Google Scholar]
- Negus SS, Vanderah TW, Brandt MR, Bilsky EJ, Becerra L, Borsook D. Preclinical assessment of candidate analgesic drugs: recent advances and future challenges. J Pharmacol Exp Ther. 2006 doi: 10.1124/jpet.106.106377. in press. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Lai J, Vanderah T, Porecca F. The Delta Opioid Receptor Subtypes and Pain Modulation. In: Chang KJ, Porreca F, Woods JH, editors. The Delta Receptor. New York: Marcel Dekker; 2004. pp. 297–329. [Google Scholar]
- Peckys D, Landwehrmeyer GB. Expression of mu, kappa, and delta opioid receptor messenger RNA in the human CNS: a 33P in situ hybridization study. Neuroscience. 1999;88:1093–1135. doi: 10.1016/s0306-4522(98)00251-6. [DOI] [PubMed] [Google Scholar]
- Weber RJ, Gomez-Flores R. Delta opioids and immune function. In: Chang KJ, Porreca F, Woods JH, editors. The Delta Receptor. New York: Marcel Dekker; 2004. pp. 383–399. [Google Scholar]


