Introduction
The specific phosphorylation of inositol 1,4,5-trisphosphate (Ins(1,4,5)P3) in the 3-position (EC 2.7.1.127) was discovered 20 yr ago (Batty et al., 1985; Irvine et al., 1986a, b). At that time it represented an unexpected complication in the metabolism of Ins(1,4,5)P3, whose second messenger function was just emerging (Streb et al., 1983; Berridge and Irvine, 1984). Moreover, given that we already knew that Ins(1,4,5)P3 can be inactivated by a specific 5-phosphatase (EC 3.1.3.56) (Downes et al., 1982), it was something of a puzzle as to why cells should also consume ATP to phosphorylate Ins(1,4,5)P3. This conundrum was further enhanced when we found that Ins(1,3,4,5)P4 did not mobilize Ca2+ (Irvine et al., 1986a, b) so, superficially, Ins(1,4,5)P3 3-kinase (IP33 K) could indeed be viewed as just a ‘redundant’ off-switch for Ins(1,4,5)P3.
In the ensuing time, we have learned an enormous amount about the three members of the mammalian IP33K family (for reviews of their overall molecular biology and properties see Communi et al. (1995); Irvine and Schell (2001); Pattni and Banting (2004)). We have also gone a long way towards an understanding of the ‘why’ question—why do cells bother to make Ins(1,3,4,5)P4, and consume an ATP molecule, when they have a perfectly good 5-phosphatase to inactivate Ins(1,4,5)P3? The answer to this question is a long and complicated one, and its principal components lie firstly in our knowledge of the evolution of the IP33Ks and, secondly, in what we know about their localization and regulation.
Evolution of Ins(1,4,5)P3 3-kinases
One of our major sources of enlightenment has stemmed from an understanding of where IP33Ks came from. We now know that IP33K is a member of a larger family of enzymes that began early in eukaryotic evolution (Irvine and Schell, 2001; Irvine, 2005), either as ‘InsP3 multi-kinases’ (EC 2.7.1.151, i.e., inositol phosphate kinases that can phosphorylate several substrates), or InsP6 kinases (EC 2.7.4.21). Together these enzymes form a major part of a route of synthesis of the pyro-phosphorylated inositol phosphates Ins(PP)P5 and Ins(PP)2P4 (more loosely known as InsP7 and InsP8, respectively). Discussion of these pathways and their functions can be found in, e.g., Irvine and Schell (2001); Shears (2001); Irvine (2005); York et al. (2005), and we return to them at the end of this review. Note that a particularly intriguing possible function for Ins(PP)P5 has emerged recently with the suggestion that it may directly (and non-enzymatically) phosphorylate some proteins, particularly proteins found in the nucleolus (Saiardi et al., 2004).
From this recent cloning activity we can see that the IP33Ks evolved out of an inositol phosphate kinase family late in eukaryotic evolution, probably about the time when metazoans emerged (Irvine and Schell, 2001). Moreover, from a comparison of the primary sequences of the family viewed in the context of how IP33KA binds its Ins(1,4,5)P3 substrate with such exquisite specificity (Gonzalez et al., 2004), we can see that the crucial evolutionary change was the insertion of three extra α-helices in the InsP-binding domain. This insertion holds the Ins(1,4,5)P3-binding residues in a precise orientation (see Fig. 1), and ensures an absolute specificity for the substrate, including not least ensuring that it will not phosphorylate PtdIns(4,5)P2 to generate the (already established when IP33Ks evolved) second messenger PtdIns(3,4,5)P3 (Gonzalez et al., 2004; Miller and Hurley, 2004); note the contrast (Fig. 1) with the related ‘InsP3 multikinase’, which lacks these three α-helices (Gonzalez et al., 2004), and can phosphorylate PtdIns(4,5)P2 to PtdIns(3,4,5)P3 (Resnick et al., 2005). So, as the first IP33K emerged in the form of a specific Ins(1,4,5)P3 3-kinase, why did evolution seize on it, keep it and use it extensively? The answer to this lies in the three possible functions of IP33Ks, and it is these functions, and the regulation of the IP33Ks’ location and activity, that are the subject of the rest of this paper.
Fig. 1.
Ins(1,4,5)P3 binding to IP3KA. This illustration is derived from the structure of IP3KA with Ins(1,4,5)P3 and ATP bound to it, as described by Gonzalez et al. (2004). On the left is the original structure, and on the right is exactly the same, but with the three-helices insert (IP lobe) that is unique to the Ins(1,4,5)P3 3-kinases (Gonzalez et al., 2004) removed. Thus, the structure on the right envisions how Ins(1,4,5)P3 might bind to an InsP3 multikinase (lacking these three helices) which could, therefore, enable a wider range of substrates binding, including PtdIns(4,5)P2 (Resnick et al., 2005).
In simple terms, there are three possible functions for IP33Ks: (a) to remove Ins(1,4,5)P3, thus terminating its second messenger function; (b) to synthesize Ins(1,3,4,5)P4, which acts as a second messenger in its own right; and (c) to serve as the first step in a route of synthesis of the higher inositol phosphates.
Functions (a)—removal of Ins(1,4,5)P3
As hinted at above, to avoid redundancy of IP33K with Ins(1,4,5)P3 5-phosphatase as a remover of Ins(1,4,5)P3, we would expect there to be clear differences between the two enzymes in terms of their physiology. Indirect evidence for this comes from the different phenotypes engendered in Caenorhabditis elegans when the IP33K (Clandinin et al., 1998) versus the Ins(1,4,5)P3 5-phosphatase (Bui and Sternberg, 2002) are knocked out. The first difference in actual properties between the two enzymes to be discovered was that IP33K has a higher affinity but lower Vmax than Ins(1,4,5)P3 5-phosphatase (Irvine et al., 1986a, b; Connolly et al., 1987), implying that perhaps IP33K is the principal route of Ins(1,4,5)P3 metabolism in cells, with the phosphatase being there to remove rapidly any excess Ins(1,4,5)P3 (a relationship reminiscent of that between the high-affinity, low-capacity Ca2+-ATPases, and the low-affinity, high-capacity Ca2+/Na+ exchangers). One might, therefore, expect that at low doses of agonists (and thus at low Ins(1,4,5)P3 levels) the route of Ins(1,4,5)P3 metabolism is mostly by the kinase, and some data on inositol phosphates generated support that suggestion indirectly. But interestingly, in mice with either IP33KA (Jun et al., 1998) or IP33KB (Pouillon et al., 2003) knocked out, the authors reported no difference in Ins(1,4,5)P3 levels in relevant tissues, whereas in cells with reduced Ins(1,4,5)P3 5-phosphatase, the authors documented increased Ins(1,4,5)P3 levels, leading to a change in cellular physiology (Speed et al., 1999). These issues may be complicated by the possible existence of multiple pools of Ins(1,4,5)P3 (see (Irvine and Schell, 2001 for discussion) and, overall, we still have no clear answer to the relative contributions of the two enzymes to Ins(1,4,5)P3 removal; it may be that it varies between tissues and organisms. More interesting and compelling evidence for a separation of the functions of the two enzymes lies in their very different regulation and localization.
At an early stage in its evolution, some time between the emergence of nematodes and arthropods (Irvine and Schell, 2001), IP33K became regulated by Ca2+/calmodulin (Communi et al., 1995). Later (in vertebrates), some IP33K isoforms became activatable by phosphorylation on a threonine residue that is close to the catalytic site (Gonzalez et al., 2004) by CaMKII (Communi et al., 1997, 1999). This is in marked contrast to the inhibition of Ins(1,4,5)P3 5-phosphatase caused by CaMKII phosphorylation (Communi et al., 2001). Thus, an activation of CaMKII will divert Ins(1,4,5)P3 specifically towards Ins(1,3,4,5)P4, which might be taken as indirect evidence for some specific function of the latter (see below).
Another factor that separates the kinase and phosphatase is their distinctly different localization. The Ins(1,4,5)P3 5-phosphatase is localized to the plasma membrane by being farnesylated (De Smedt et al., 1997). However, the A and B isoforms of IP33K are targeted either to the actin skeleton or to the endoplasmic reticulum (e.r.), and this targeting and its regulation represents a crucial aspect of their physiological function.
Localization of IP33Ks
The localization of the C isoform of IP33K is controversial in that one group have claimed a partially nuclear (and dynamic) localization (Nalaskowski et al., 2003), and the other a cytosolic localization (Dewaste et al., 2003). We will not discuss this issue further, but will instead focus on the localization of the A and B isoforms, which is more fully understood.
IP33KA is confined to the testis and to neurons, and in neurons its localization is dynamic and under tight regulation. IP33KA is expressed at highest levels in the cerebellum and the hippocampus, especially in the neuropil of the CA1 and dentate gyrus regions of the latter (Mailleux et al., 1991; Schell and Irvine, 2006). Moreover, this high level of enzyme is further concentrated by a clear localization to the post-dendritic spines of the neurons, a localization that is effected by a specific and novel F-actin binding domain consisting of the N-terminal 66 amino acids of IP33KA (Schell et al., 2001). In such a position, where it lies between the major site of Ins(1,4,5)P3 generation (by the post-synaptic density) and the site of its action (the e.r.), IP33KA is perfectly poised to act as a sort of ‘firewall’ (Schell et al., 2001), dictating by its activity the degree to which Ins(1,4,5)P3 can ‘escape’ from the spine and mobilize Ca2+ (Schell et al., 2001). The acute and long-term regulation of IP33KA by Ca2+ that is discussed above can then be viewed as a way in which stimulation of a spine to increase its Ca2+ will in turn dictate how it regulates the actions of Ins(1,4,5)P3.
We have explored this activation by Ca2+ in transfected cells (SML-B, JCHY, RFI and MJS, unpublished), and shown that even in a simple system like a HeLa cell, the influence of calmodulin and CaMKII is detectable in the ability of IP33KA to decrease Ins(1,4,5)P3-induced Ca2+ mobilization. Moreover, the localization to the actin skeleton also has a clear effect on the efficacy of IP33KA, again, even in such an unpolarized cell as a HeLa (SML-B, JCHY, RFI and MJS, unpublished), so when we consider such a highly polarized structure as a dendrite, the localization of IP33KA adds a new dimension to the inter-relationship between the various stimulatory inputs into postsynaptic spines (Schell et al., 2001).
Our more recent investigations of IP33KA have now added another crucial dimension to this complexity, the dimension of time. Firstly, by using IP33KA as a sort of real-time marker for F-actin, we have revealed that the F-actin in spines is in a highly dynamic state. As far as we can tell it is not turning over very rapidly, but rather the entire F-actin is in a state of dynamic equilibrium, whereby Ca2+ entry (especially through NMDA receptors) is driving it as a single entity out of the spines and into the main dendritic shaft (Fig. 2 and (Schell and Irvine, 2006)), carrying IP33KA with it. This is a physiological response in so far as resting synaptic activity has a significant effect on IP33KA localization (Schell and Irvine, 2006). Moreover, at high levels of stimulation with exogenous glutamate, which could be taken as a mimicking of massive stimulation such as follows ischemic shock, the exit of IP33KA is total and rapid (within seconds), and may form the first stage in the pronounced changes in spine structure that follow such events (Halpain et al., 1998). It is important to note, however, that provided the stimulation with glutamate is not too prolonged, it remains fully reversible within about 20 min (Fig. 2 and (Schell and Irvine, 2006)). It remains to be seen what the functional consequences of this movement of IP33KA are—as we have discussed elsewhere (Schell and Irvine, 2006); both removing IP33KA from the spines and placing it in the shafts will have profound consequences for Ins(1,4,5)P3-mediated Ca2+ signalling. Moreover, as the principal Ins(1,4,5)P3-generating receptors in this system (metabotropic and muscarinic) have a minimal influence on IP33KA movement, another consequence of these discoveries is to expand the complexity of the relationship between the different types of glutamate receptors.
Fig. 2.
Dynamic localization of Ins(1,4,5)P3 3-kinase A in postsynaptic spines. The figure shows IP3KA transfected into hippocampal neurones, and illustrates (A) before, (B) 2 min after, treatment with 100 μM glutamate, and (C) 20 min after removal of 100 μM glutamate and the addition of MK-801, an NMDA receptor antagonist. The reversible shift from the spines to the shaft induced by glutamate is plainly visible. See (Schell and Irvine, 2006) for all details.
Secondly, an extra layer of complexity in the dimension of time can be found in the spatial relationship between the e.r. and the actin skeleton. Our three-dimensional reconstruction of images (Schell and Irvine, 2006) has confirmed the close spatial relationship between these structures in those spines into which the e.r. penetrates (Capani et al., 2001). However, all these images depict motionless structures, and the picture changes when we think about how spines themselves are moving (Fischer et al., 1998), and that movement itself impinges on the Ca2+ kinetics within the spine (Majewska et al., 2000). Moreover, we have shown that the e.r. itself is a highly motile organelle whose movements are under acute regulation by Ca2+ in both ATP-dependent and independent mechanisms (Brough et al., 2005a, b). Taking this remarkable complexity concerning IP33KA and the actions of Ins(1,4,5)P3 in dendrites overall, we can begin to see that there is no functional redundancy with Ins(1,4,5)P3 5-phosphatase—in parallel experiments to those described above (Schell and Irvine, 2006), Ins(1,4,5)P3 5-phosphatase does not localize to dendritic spines, but is instead distributed all along the plasma membrane of the dendrite, and it does not move perceptibly when glutamate is added (MJS and RFI, unpublished). Thus, it makes every sense that a primary function of IP33KA could be to remove Ins(1,4,5)P3.
Finally, a brief consideration of IP33KB supports this conclusion. Our understanding of the regulation of this isoform is not quite so advanced as for IP33KA but, nevertheless, we know that in addition to its dual stimulation by CaM and CaMKII (Communi et al., 1999), IP33KB also has an interesting localization as a part of its function. Originally, it was described as being either cytosolic or in the e.r. (Soriano et al., 1997), but subsequent cloning of the full length enzyme (the original cloning was missing some of the N-terminus) has led to the realization that it is partially localized to the cytoskeleton (Dewaste et al., 2003), because it too has an actin-binding domain (Brehm et al., 2004). The localization in the e.r. is due to a distinct part of the N-terminus (Pattni et al., 2003), and we have recently shown that removal of the first 150 amino acids from the N-terminus of IP33KB causes the enzyme to move from the actin skeleton to the e.r. (Yu et al., 2005). Moreover, this localization is subject to regulation by proteolytic cleavage (Pattni et al., 2003; Yu et al., 2005), an event that we have shown takes place under physiological conditions, at least with the transfected enzyme (Yu et al., 2005). Finally, as with the A-isoform (SML-B, JCHY, RFI and MJS, unpublished), the localization of the enzyme alters its ability to metabolize Ins(1,4,5)P3, as assayed by decreases in Ins(1,4,5)P3-induced Ca2+ signals (Yu et al., 2005).
Functions (b)—synthesis of Ins (1,3,4,5)P4
The possibility that Ins(1,3,4,5)P4 is a second messenger in its own right has been extensively discussed elsewhere (e.g. Irvine and Schell, 2001), and needs no expansion here. The only directly relevant recent information concerns our own attempts to gain evidence for the hypothesis (Irvine, 1989; Soriano and Banting, 1997; Irvine and Schell, 2001) that Ins(1,3,4,5)P4 acts to modify the structure of the e.r., thus regulating the mobilization of Ca2+ from the e.r. by Ins(1,4,5)P3. We have made concerted effort to seek evidence for such an effect on e.r. structure or movement using FRAP in several cell preparations, but have had to conclude either that the hypothesis is wrong, or that it cannot be detected by the technology we used (Brough et al., 2005a, b). There remain intriguing and strong data showing that Ins(1,3,4,5)P4 can exert profound influences on Ins(1,4,5)P3-regulated Ca2+ homeostasis (e.g. (Changya et al., 1989; Loomis-Husselbee et al., 1996), but the mechanism or universality of such responses remain unclear.
A very enticing indirect piece of evidence emerged recently from two independent reports that in the absence of the activity of IP33KB, mice cannot mature their T cells, leading to immunocompromized animals (Pouillon et al., 2003; Wen et al., 2004). T lymphocyte Ca2+ signalling appeared superficially normal in both studies, and Wen et al. (2004) presented evidence that MAP kinase signalling might be altered. Both groups raised the issue that GAP1IP4BP, a putative Ins(1,3,4,5)P4 receptor (Cullen et al., 1995, 1997), which is very highly expressed in circulating lymphocytes (Lockyer et al., 1999), might be the protein involved, thus directly implicating Ins(1,3,4,5)P4 as the important factor missing in these mice.
Subject to some caveats, these findings are arguably the most compelling evidence yet that Ins(1,3,4,5)P4 is a physiological second messenger. The two caveats to this interpretation are, firstly that, as has been shown for many other proteins (including, e.g., the yeast InsP3 multikinase (Dubois et al., 2000) and PI-PLCγ (Patterson et al., 2002)), IP33KB may play a structural role, independent of its catlytic activity, as an indispensible member of a multi-protein complex. The second caveat is that the important missing component may be InsP6 (or another higher inositol phosphate), rather than Ins(1,3,4,5)P4.
Functions (c)—the first step in the synthesis of higher inositol phosphates
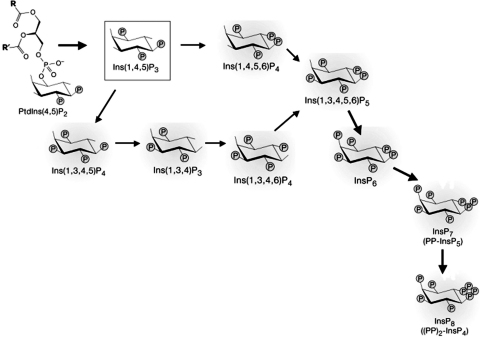
The suggested synthesis pathway in mammalian cells for the higher inositol phosphates (functionally the most interesting of these are currently: Ins(3,4,5,6)P4, Ins(1,3,4,5,6)P5, InsP6, InsP7 and InsP8 (see Irvine and Schell (2001); Shears (2001); Irvine (2005) for reviews)) was first pieced together largely by Shears and Balla and their co-workers in the 1980s (Balla et al., 1989; Shears, 1989). The cloning of inositol phosphate kinases in the following decades culminated in the identification of all the enzymes responsible for this pathway (Fig. 3), which goes as: Ins(1,4,5)P3 to Ins(1,3,4,5)P4 to Ins(1,3,4)P3 to Ins(1,3,4,6)P4 to Ins(1,3,4,5,6)P5 to InsP6 to InsP7 (InsP7 kinase has yet to be cloned).
Fig. 3.
Pathways of InsP6 synthesis. The figure depicts the two likely pathways for InsP6 synthesis in mammlian cells. One route, the upper pathway (Odom et al., 2000), is found in yeast, may exist in mammals and does not involve any Ins(1,4,5)P3 3-kinase activity. The lower route (Balla et al., 1989; Shears, 1989) has IP33K as an essential first step.
If this is indeed the major pathway by which animals make InsP6, then acting as the first step on it is another potentially very important function for IP33Ks. Elsewhere, the arguments for and against whether other pathways of InsP6 synthesis are equally or more important are discussed (Irvine, 2005), and these would not be repeated here. In this context, the crucial issue is whether the ‘missing factor’ in IP33KB knock-out mice (Pouillon et al., 2003; Wen et al., 2004) is Ins(1,3,4,5)P4 itself, or another component of the above pathway. As mice lacking ‘InsP3 multikinase’ (Frederick et al., 2005) or InsP5 2-kinase (Verbsky et al., 2005) die at birth (compare the less severe phenotypes of IP33K knock-out mice, above), superficially, it seems likely that IP33K is not essential to higher inositol phosphate synthesis. We have recently set up a highly specific and sensitive mass assay for InsP6 (RF I, AJL and MJS, unpublished), and by assessing the levels of InsP6 in the above knock-out mice with this assay we hope to shed light on this issue.
Summary
It is 20 yr since the Ins(1,4,5)P3 3-kinase reaction was discovered, and this review summarizes and discusses some of the advances we have made in our understanding of the physiological significance of this reaction. The three major potential functions of Ins(1,4,5)P3 3-kinases are discussed in the context of their localization and regulation, the possible functions of their product, Ins(1,3,4,5)P4 and their potential contribution to the synthesis of higher inositol polyphosphates.
Acknowledgements
Our work is supported by the Royal Society (RFI, MJS), the Wellcome Trust (RFI, AJL), the Croucher Foundation (JCHY) and Merck Sharp and Doehme (SML-B).
References
- Balla T., Baukal A.J., Hunyady L., Catt K.J. Agonist-induced regulation of inositol tetrakisphosphate isomers and inositol pentakisphosphate in adrenal glomerulosa cells. J Biol Chem. 1989;264:13605–13611. [PubMed] [Google Scholar]
- Batty I.R., Nahorski S.R., Irvine R.F. Rapid formation of inositol 1,3,4,5-tetrakisphosphate following muscarinic receptor stimulation of rat cortical slices. Biochem J. 1985;232:211–215. doi: 10.1042/bj2320211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M.J., Irvine R.F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984;312:315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Brehm M.A., Schreiber I., Bertsch U., Wegner A., Mayr G.W. Identification of the actin-binding domain of Ins(1,4,5)P3 3-kinase isoform B (IP3K-B) Biochem J. 2004;382:353–362. doi: 10.1042/BJ20031751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brough D., Sim Y., Thorn P., Irvine R.F. The structural integrity of the endoplasmic reticulum, and its possible regulation by inositol 1,3,4,5-tetrakisphosphate. Cell Calcium. 2005;38:153–159. doi: 10.1016/j.ceca.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Brough D., Schell M.J., Irvine R.F. Agonist-induced regulation of mitochondrial and endoplasmic reticulum motility. Biochem J. 2005;392:291–297. doi: 10.1042/BJ20050738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui Y.K., Sternberg P.W. Caenorhabditis elegans inositol 5-phosphatase homolog negatively regulates inositol 1,4,5-triphosphate signaling in ovulation. Mol Biol Cell. 2002;13:1641–1651. doi: 10.1091/mbc.02-01-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capani F., Martone M.E., Deerinck T.J., Ellisman M.H. Selective localization of high concentrations of F-actin in subpopulations of dendritic spines in rat central nervous system: a three-dimensional electron microscopic study. J Comput Neurol. 2001;435:156–170. doi: 10.1002/cne.1199. [DOI] [PubMed] [Google Scholar]
- Changya L., Gallacher D.V., Irvine R.F., Potter B.V., Petersen O.H. Inositol 1,3,4,5-tetrakisphosphate is essential for sustained activation of the Ca2+-dependent K+ current in single internally perfused mouse lacrimal acinar cells. J Membr Biol. 1989;109:85–93. doi: 10.1007/BF01870793. [DOI] [PubMed] [Google Scholar]
- Clandinin T.R., DeModena J.A., Sternberg P.W. Inositol trisphosphate mediates a RAS-independent response to LET-23 receptor tyrosine kinase activation in C. elegans. Cell. 1998;92:523–533. doi: 10.1016/s0092-8674(00)80945-9. [DOI] [PubMed] [Google Scholar]
- Communi D., Vanweyenberg V., Erneux C. Molecular study and regulation of d-myo-inositol 1,4,5-trisphosphate 3-kinase. Cell Signal. 1995;7:643–650. doi: 10.1016/0898-6568(95)00035-n. [DOI] [PubMed] [Google Scholar]
- Communi D., Vanweyenberg V., Erneux C. d-myo-inositol 1,4,5-trisphosphate 3-kinase A is activated by receptor activation through a calcium:calmodulin-dependent protein kinase II phosphorylation mechanism. EMBO J. 1997;16:1943–1952. doi: 10.1093/emboj/16.8.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Communi D., Dewaste V., Erneux C. Calcium–calmodulin-dependent protein kinase II and protein kinase C-mediated phosphorylation and activation of d-myo-inositol 1,4, 5-trisphosphate 3-kinase B in astrocytes. J Biol Chem. 1999;274:14734–14742. doi: 10.1074/jbc.274.21.14734. [DOI] [PubMed] [Google Scholar]
- Communi D., Gevaert K., Demol H., Vandekerckhove J., Erneux C. A novel receptor-mediated regulation mechanism of type I inositol polyphosphate 5-phosphatase by calcium/calmodulin-dependent protein kinase II phosphorylation. J Biol Chem. 2001;276:38738–38747. doi: 10.1074/jbc.M105640200. [DOI] [PubMed] [Google Scholar]
- Connolly T.M., Bansal V.S., Bross T.E., Irvine R.F., Majerus P.W. The metabolism of tris- and tetraphosphates of inositol by 5-phosphomonoesterase and 3-kinase enzymes. J Biol Chem. 1987;262:2146–2149. [PubMed] [Google Scholar]
- Cullen P.J., Hsuan J.J., Truong O., Letcher A.J., Jackson T.R., Dawson A.P. Identification of a specific Ins(1,3,4,5)P4-binding protein as a member of the GAP1 family. Nature. 1995;376:527–530. doi: 10.1038/376527a0. [DOI] [PubMed] [Google Scholar]
- Cullen P.J., Loomis-Husselbee J.W., Dawson A.P., Irvine R.F. Inositol 1,3,4,5-tetrakisphosphate and Ca2+ homeostasis—the role of GAP1IP4BP. Biochem Soc Trans. 1997;25:991–996. doi: 10.1042/bst0250991. [DOI] [PubMed] [Google Scholar]
- De Smedt F., Missiaen L., Parys J.B., Vanweyenberg V., De Smedt H., Erneux C. Isoprenylated human brain type I inositol 1,4,5-trisphosphate 5-phosphatase controls Ca2+ oscillations induced by ATP in Chinese hamster ovary cells. J Biol Chem. 1997;272:17367–17375. doi: 10.1074/jbc.272.28.17367. [DOI] [PubMed] [Google Scholar]
- Dewaste V., Moreau C., De Smedt F., Bex F., De Smedt H., Wuytack F. The three isoenzymes of human inositol-1,4,5-trisphosphate 3-kinase show specific intracellular localization but comparable Ca2+ responses on transfection in COS-7 cells. Biochem J. 2003;374:41–49. doi: 10.1042/BJ20021963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C.P., Mussat M.C., Michell R.H. The inositol trisphosphate phosphomonoesterase of the human erythrocyte membrane. Biochem J. 1982;203:169–177. doi: 10.1042/bj2030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois E., Dewaste V., Erneux C., Messenguy F. Inositol polyphosphate kinase activity of Arg82/ArgRIII is not required for the regulation of the arginine metabolism in yeast. FEBS Lett. 2000;486:300–304. doi: 10.1016/s0014-5793(00)02318-8. [DOI] [PubMed] [Google Scholar]
- Fischer M., Kaech S., Knutti D., Matus A. Rapid actin-based plasticity in dendritic spines. Neuron. 1998;20:847–854. doi: 10.1016/s0896-6273(00)80467-5. [DOI] [PubMed] [Google Scholar]
- Frederick J.P., Mattiske D., Wofford J.A., Megosh L.C., Drake L.Y., Chiou S.T. An essential role for an inositol polyphosphate multikinase, Ipk2, in mouse embryogenesis and second messenger production. Proc Natl Acad Sci USA. 2005;102:8454–8459. doi: 10.1073/pnas.0503706102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez B., Schell M.J., Letcher A.J., Veprintsev D.B., Irvine R.F., Williams R.L. Structure of a human inositol 1,4,5-trisphosphate 3-kinase: substrate binding reveals why it is not a phosphoinositide 3-kinase. Mol Cell. 2004;15:689–701. doi: 10.1016/j.molcel.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Halpain S., Hipolito A., Saffer L. Regulation of F-actin stability in dendritic spines by glutamate receptors and calcineurin. J Neurosci. 1998;18:9835–9844. doi: 10.1523/JNEUROSCI.18-23-09835.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R.F. How do inositol 1,4,5-trisphosphate and inositol 1,3,4,5-tetrakisphosphate regulate intracellular Ca2+? Biochem Soc Trans. 1989;17:6–9. doi: 10.1042/bst0170006. [DOI] [PubMed] [Google Scholar]
- Irvine R.F. Inositide evolution—towards turtle domination? J Physiol. 2005;566:295–300. doi: 10.1113/jphysiol.2005.087387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R.F., Schell M.J. Back in the water: the return of the inositol phosphates. Nat Rev Mol Cell Biol. 2001;2:327–338. doi: 10.1038/35073015. [DOI] [PubMed] [Google Scholar]
- Irvine R.F., Letcher A.J., Heslop J.P., Berridge M.J. The inositol tris/tetrakisphosphate pathway—demonstration of Ins(1,4,5)P3 3-kinase activity in animal tissues. Nature. 1986;320:631–634. doi: 10.1038/320631a0. [DOI] [PubMed] [Google Scholar]
- Irvine R.F., Letcher A.J., Lander D.J., Berridge M.J. Specificity of inositol phosphate-stimulated Ca2+ mobilization from Swiss-mouse 3T3 cells. Biochem J. 1986;240:301–304. doi: 10.1042/bj2400301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun K., Choi G., Yang S.G., Choi K.Y., Kim H., Chan G.C. Enhanced hippocampal CA1 LTP but normal spatial learning in inositol 1,4,5-trisphosphate 3-kinase(A)-deficient mice. Learn Mem. 1998;5:317–330. [PMC free article] [PubMed] [Google Scholar]
- Lockyer P.J., Vanlingen S., Reynolds J.S., McNulty T.J., Irvine R.F., Parys J.B. Tissue-specific expression and endogenous subcellular distribution of the inositol 1,3,4,5-tetrakisphosphate-binding proteins GAP1(IP4BP) and GAP1(m) Biochem Biophys Res Comm. 1999;255:421–426. doi: 10.1006/bbrc.1999.0217. [DOI] [PubMed] [Google Scholar]
- Loomis-Husselbee J.W., Cullen P.J., Dreikausen U.E., Irvine R.F., Dawson A.P. Synergistic effects of inositol 1,3,4,5-tetrakisphosphate on inositol 2,4,5-triphosphate-stimulated Ca2+ release do not involve direct interaction of inositol 1,3,4,5-tetrakisphosphate with inositol triphosphate-binding sites. Biochem J. 1996;314:811–816. doi: 10.1042/bj3140811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailleux P., Takazawa K., Erneux C., Vanderhaeghen J.J. Inositol 1,4,5-trisphosphate 3-kinase distribution in the rat brain. High levels in the hippocampal CA1 pyramidal and cerebellar Purkinje cells suggest its involvement in some memory processes. Brain Res. 1991;539:203–210. doi: 10.1016/0006-8993(91)91622-8. [DOI] [PubMed] [Google Scholar]
- Majewska A., Tashiro A., Yuste R. Regulation of spine calcium dynamics by rapid spine motility. J Neurosci. 2000;20:8262–8268. doi: 10.1523/JNEUROSCI.20-22-08262.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G.J., Hurley J.H. Crystal structure of the catalytic core of inositol 1,4,5-trisphosphate 3-kinase. Mol Cell. 2004;15:703–711. doi: 10.1016/j.molcel.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Nalaskowski M.M., Bertsch U., Fanick W., Stockebrand M.C., Schmale H., Mayr G.W. Rat inositol 1,4,5-trisphosphate 3-kinase C is enzymatically specialized for basal cellular inositol trisphosphate phosphorylation and shuttles actively between nucleus and cytoplasm. J Biol Chem. 2003;278:19765–19776. doi: 10.1074/jbc.M211059200. [DOI] [PubMed] [Google Scholar]
- Odom A.R., Stahlberg A., Wente S.R., York J.D. A role for nuclear inositol 1,4,5-trisphosphate kinase in transcriptional control. Science. 2000;287:2026–2029. doi: 10.1126/science.287.5460.2026. [DOI] [PubMed] [Google Scholar]
- Patterson R.L., van Rossum D.B., Ford D.L., Hurt K.J., Bae S.S., Suh P.G. Phospholipase C-gamma is required for agonist-induced Ca2+ entry. Cell. 2002;111:529–541. doi: 10.1016/s0092-8674(02)01045-0. [DOI] [PubMed] [Google Scholar]
- Pattni K., Banting G. Ins(1,4,5)P3 metabolism and the family of IP3-3 kinases. Cell Signal. 2004;16:643–654. doi: 10.1016/j.cellsig.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Pattni K., Millard T.H., Banting G. Calpain cleavage of the B isoform of Ins(1,4,5)P3 3-kinase separates the catalytic domain from the membrane anchoring domain. Biochem J. 2003;375:643–651. doi: 10.1042/BJ20030505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouillon V., Hascakova-Bartova R., Pajak B., Adam E., Bex F., Dewaste V. Inositol 1,3,4,5-tetrakisphosphate is essential for T lymphocyte development. Nat Immunol. 2003;4:1136–1143. doi: 10.1038/ni980. [DOI] [PubMed] [Google Scholar]
- Resnick A.C., Snowman A.M., Kang B.N., Hurt K.J., Snyder S.H., Saiardi A. Inositol polyphosphate multikinase is a nuclear PI3-kinase with transcriptional regulatory activity. Proc Nat Acad Sci USA. 2005;102:12783–12788. doi: 10.1073/pnas.0506184102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiardi A., Bhandari R., Resnick A.C., Snowman A.M., Snyder S.H. Phosphorylation of proteins by inositol pyrophosphates. Science. 2004;306:2101–2105. doi: 10.1126/science.1103344. [DOI] [PubMed] [Google Scholar]
- Schell MJ, Irvine RF. Calcium-triggered exit of F-actin and IP3 3-kinase A from dendritic spines is rapid and reversible. Eur J Neurosci, 2006, in press. [DOI] [PubMed]
- Schell M.J., Erneux C., Irvine R.F. Inositol 1,4,5-trisphosphate 3-kinase A associates with F-actin and dendritic spines via its N terminus. J Biol Chem. 2001;276:37537–37546. doi: 10.1074/jbc.M104101200. [DOI] [PubMed] [Google Scholar]
- Shears S.B. The pathway of myo-inositol 1,3,4-trisphosphate phosphorylation in liver. Identification of myo-inositol 1,3,4-trisphosphate 6-kinase, myo-inositol 1,3,4-trisphosphate 5-kinase, and myo-inositol 1,3,4,6-tetrakisphosphate 5-kinase. J Biol Chem. 1989;264:19879–19886. [PubMed] [Google Scholar]
- Shears S.B. Assessing the functional omnipotence of inositol hexakisphophosphate. Cell Signal. 2001;13:151–158. doi: 10.1016/s0898-6568(01)00129-2. [DOI] [PubMed] [Google Scholar]
- Soriano S., Banting G. Possible roles of inositol 1,4,5-trisphosphate 3-kinase B in calcium homeostasis. FEBS Lett. 1997;403:1–4. doi: 10.1016/s0014-5793(96)01516-5. [DOI] [PubMed] [Google Scholar]
- Soriano S., Thomas S., High S., Griffiths G., D’Santos C., Cullen P. Membrane association, localization and topology of rat inositol 1,4,5-trisphosphate 3-kinase B: implications for membrane traffic and Ca2+ homoeostasis. Biochem J. 1997;324:579–589. doi: 10.1042/bj3240579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speed C.J., Neylon C.B., Little P.J., Mitchell C.A. Underexpression of the 43 kDa inositol polyphosphate 5-phosphatase is associated with spontaneous calcium oscillations and enhanced calcium responses following endothelin-1 stimulation. J Cell Sci. 1999;112:669–679. doi: 10.1242/jcs.112.5.669. [DOI] [PubMed] [Google Scholar]
- Streb H., Irvine R.F., Berridge M.J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983;306:67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Verbsky J., Lavine K., Majerus P.W. Disruption of the mouse inositol 1,3,4,5,6-pentakisphosphate 2-kinase gene, associated lethality, and tissue distribution of 2-kinase expression. Proc Natl Acad Sci USA. 2005;102:8448–8453. doi: 10.1073/pnas.0503656102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen B.G., Pletcher M.T., Warashina M., Choe S.H., Ziaee N., Wiltshire T. Inositol (1,4,5) trisphosphate 3 kinase B controls positive selection of T cells and modulates Erk activity. Proc Natl Acad Sci USA. 2004;101:5604–5609. doi: 10.1073/pnas.0306907101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York S.J., Armbruster B.N., Greenwell P., Petes T.D., York J.D. Inositol diphosphate signaling regulates telomere length. J Biol Chem. 2005;280:4264–4269. doi: 10.1074/jbc.M412070200. [DOI] [PubMed] [Google Scholar]
- Yu C.H.Y., Lloyd-Burton S.M., Irvine R.F., Schell M.J. Regulation of the localisation and activity of inositol 1,4,5-trisphosphate 3-kinase B in intact cells by proteolysis. Biochem J. 2005;392:435–441. doi: 10.1042/BJ20050829. [DOI] [PMC free article] [PubMed] [Google Scholar]