Abstract
During their development from haematopoietic progenitors, lymphocytes go through a series of progressive cell fate decisions. The underlying changes in gene expression programs are controlled in part by the assembly of transcription factor complexes on regulatory regions of their target genes. The propagation of gene expression programs through mitosis in a heritable manner – and hence cellular identity – is achieved by epigenetic processes that affect the accessibility of transcription factors to regulatory regions [1–4]. In mammals, epigenetic information is encoded by differences in the methylation of DNA on cytosines, composition of histone proteins, post-translational histone modifications, and higher-order chromatin structure. Here we discuss recent advances in the epigenetic control of T cell development and function.
Chromatin, histone modifications, and DNA methylation
In the nucleus DNA is contained in chromatin. The basic subunit of chromatin is the nucleosome, comprised of ~160 bp of DNA wrapped around an octamer consisting of two copies each of histones H2A, H2B, H3 and H4 [3]. Nucleosomes are dynamic structures that can be ‘remodeled’ to facilitate or impede transcription factor binding and transit of RNA polymerases [5]. Transcriptional accessibility is also influenced by post-translational histone modifications. For example, at the 5' ends of active genes H3 is typically acetylated on lysines 9 and 14 (H3K9/K14Ac) [6]. Lysine residues can also be methylated. For example, H3 trimethylated on lysine 4 (H3K4me3) co-localizes with H3K9/14Ac, while H3K4me2 is a mark of transcriptional competence present both within genes and at more distant regulatory regions [2,6]. Histone arginine methylation is also a characteristic of active genes. Conversely, H3K9me3 or H3K27me3 is associated with “closed” chromatin and silenced genes. Histones can also be modified by phosphorylation, ubiquitination, and ADP-ribosylation, but the effects of these modifications are less well characterized.
How histone modifications influence transcriptional competence is incompletely understood. Acetyl groups reduce the positive charge of histones, thereby reducing strength of binding to negatively-charged DNA, and also create binding sites for bromodomain-containing proteins, including histone acetyl transferases (HATs) [2]. Methylation of histones does not affect their charge, but can create binding sites for proteins that influence chromatin accessibility, e.g., heterochromatin protein 1 (HP1) binds to H3K9me3, the Polycomb protein/H3K27 methyltransferase EZH2 binds to H3K27me3, and WDR5-containing/H3K4 methyltransferase complexes bind to H3K4me2 [2]. These proteins in turn help to sustain the marks to which they bind, providing site-specific cellular memory of transcriptional competence. The multiple modifications possible for each histone molecule could potentially act in a combinatorial manner - a concept known as the “histone code”. Whether specific combinations of histone modifications actually encode discrete transcriptional states is controversial [7] but resolution may come from comprehensive genomic analyses of epigenetic modifications [6].
Histone acetylation is dynamic - HATs and histone deacetylases (HDACs) rapidly add or remove acetyl groups. Histone methylation was thought to be more stable until the recent discovery of enzymes that demethylate lysine (LSD1) and arginine (PADI4) [2]. LSD1 can demethylate mono- or dimethyl-H3K4 or -H3K9. H3K4me3 or H3K9me3 may be removed by H3 replacement [8]. A variant of H3 - H3.3 - can be incorporated into nucleosomes in a replication-independent manner and typically shows transcriptionally favorable histone modifications [9].
Methylation of cytosines in CpG dinucleotides is mediated by DNA methyltransferases (Dnmts) [4]. DNA methylation can repress gene expression through direct mechanisms, by blocking the binding of certain transcription factors, and by recruitment of methyl-CpG-binding proteins like MeCP2, MBD1 and MBD2. Methyl-CpG-binding proteins can exclude transcription factors and recruit HDACs and H3K9 methyltransferases, linking DNA methylation to of repressive histone modifications. The only established mechanism by which to demethylate cytosines is through its passive loss during DNA replication. While numerous reports suggest active demethylation, a molecular mechanism for enzymatic cytosine demethylation has not been defined in mammalian cells.
DNA methylation, histone modifications and higher order chromatin structure operate in concert to yield stable programs of gene expression. Higher order genome organisation and RNA interference-directed DNA and histone methylation may help to target these processes to specific sites.
Higher order genome organisation
The one-dimensional sequence of our genome - about 2 metres of DNA - is packed into a nucleus only a few microns across and brought to life by transcription factors, chromatin proteins, machines that remodel chromatin, transcribe DNA into RNA, process RNA transcripts or replicate DNA in preparation for cell division. This may sound chaotic, but a closer look reveals a considerable level of organisation. Important activities like transcription and DNA replication are centralised [10] and loci assume positions within the nucleus according to their expression status.
Nuclear positioning reflects gene activity and silencing
The localisation of chromosomes in the interphase nucleus reflects gene density, so that gene-rich chromosomes occupy more central positions [11]. Many active genes are located centrally, but others associate with nuclear pore proteins at the nuclear envelope [12]. Silent – but not active – genes often localise to heterochromatic areas around centromeres [13, 14]. In DP thymocytes, repositioning to centromeric heterochromatin precedes the stable silencing of the Tdt locus [14, 15] and the spreading of repressive histone modifications from the Tdt promoter to the surrounding chromatin [15]. Similarly, the CD4/CD8 lineage choice is reflected in locus repositioning prior to overt differentiation into CD4 or CD8 SP cells [16, 17].
Intrachromosomal interactions: control of gene expression by contacts between distant regulatory elements
Genes in higher eukaryotes are typically controlled by multiple regulatory elements, often separated by many basepairs from each other and the coding region [18]. Techniques like chromosome conformation capture, 3C [19], reveal that regulatory sites interact in 3-dimensional nuclear space [20–25]. Analysis of the DNA sequences [25] and the DNA binding proteins involved [26] points to a scenario where transcription factor binding - directly or indirectly - bridges distant regulatory elements to regulate gene expression. For example, GATA-1 and its cofactor FOG-1 are required for the interaction between the β-globin locus control region (LCR) and the β-major globin promoter [26].
From intra- to interchromosomal interactions: contacts between loci on different chromosomes
In addition to interactions in cis, sequences located on different chromosomes can come together in trans. Examples include the pairing of homologous chromosomes and the coalescence of centromeres in Drosophila and the formation of nucleoli by several hundred ribosomal (rDNA) genes on different chromosomes in mammalian cells [27]. Inactive genes on different chromosomes can colocalise in association with polycomb proteins [28] or in the vicinity of centromeric heterochromatin [13] and active genes from different chromosomes colocalise at shared sites of transcription [29]. Such sites had been predicted to exist as so-called ‘transcription factories’ [10]. A recent study found co-localisation even without transcription: in naive helper T cells, the IFNγ locus on mouse chromosome 10 apparently colocalises with the Th2 cytokine gene cluster (comprising IL-4, IL-15 and IL-13) on chromosome 11 [30]. Significantly, this interaction occurs in naive T cells, which have the potential to express low levels of both IFNγ and Th2 cytokines upon activation. In contrast, Th1 and Th2 cells express either IFNγ or Th2 cytokines (but not both) and interactions between IFNγ and the Th2 loci are lost.
Fluorescence in situ hybridisation (FISH) of interphase nuclei shows that individual chromosomes form distinct territories – as opposed to a random ‘spaghetti’ arrangement. This compartmentalisation of the nucleus appears to be at odds with the numerous cases where chromosomal loci appear to behave as individuals, for example the interchromosomal interactions discussed above [30] and the co-localisation of transcribed [29] or silenced loci [13, 14, 28]. One explanation for this paradox is that genes represent a relatively small proportion of our chromosomes and the probes used to define territories are therefore mainly directed at intergenic regions. The more genes are found on a stretch DNA (and the more ‘open’ its chromatin structure) the more often it is found outside its chromosome territory [11]. This results in a significant degree of overlap between territories (Branco and Pombo, personal communication).
Lymphoid tumours are characterised by recurrent chromosomal translocations, which typically bring genes that promote proliferation and/or survival under the control of active regulatory regions. Such translocations confer a selective advantage to cells in which they occur, and - even if they occur rarely - will often be seen in malignancies. In addition, genes involved in recurring translocations have a tendency to co-localise even in normal cells. In B lymphocytes, immunoglobulin genes are often close to Myc and Bcl-2 loci, perhaps foreshadowing Ig/Myc and Ig/Bcl-2 translocations B cell leukaemias [31, 32]. Analysis of the entire set of chromosomes correlates the probability of overlap between chromosome territories with the frequency of lymphocyte-specific translocations (Branco and Pombo, personal communication). These data link genome organisation to neoplastic transformation and suggest that translocations arise when double-strand breaks occur in areas of overlap between chromosome territories and are repaired by non-homologous end joining.
Transcriptional and posttranscriptional silencing by small RNAs
Double stranded RNA molecules are emerging as mediators of chromatin structure and gene expression at the transcriptional and post-transcriptional level by RNA interference (RNAi). Central to this pathway are the RNAse III enzymes Drosha and Dicer, which process double stranded RNAs, and Argonaute, the executioner of RNAi [33]. RNAi can block mRNA translation and initiate mRNA degradation, as widely exploited by researchers for siRNA-mediated post-transcriptional gene silencing [34, 35].
siRNA, transcriptional silencing and the structure of heterochromatin
In plants, the fission yeast S. Pombe and several other organisms, RNAi mediates the establishment of a repressive chromatin structure, the transcriptional silencing of constitutive heterochromation (such as pericentromeric repeats) and transposable elements in [36]. The discovery that transcriptional silencing by RNAi requires RNA polymerase II [37, 38] provides a mechanism for localising RNAi to the nucleus, and for selectively directing it to DNA sequences targeted by RNAi. The concept that siRNA-mediated transcriptional silencing requires transcription may seem paradoxical at first. One attractive explanation is that RNAi has evolved in response to transcriptionally active transposable elements and other invaders, who's silencing would have been advantageous to the host [39].
In organisms from yeast to mammals, defects in RNAi result in the accumulation of centromeric transcripts. Nuclear run-on assays showed that these transcripts also occur in wild type cells, but are rapidly processed into siRNAs [40]. Centromeric transcripts accumulate in Dicer deficient vertebrate cell lines [41, 42] but it is unclear whether this is due to increased transcription or to defective processing in the absence of Dicer. In several model organisms, the repressive chromatin structure of pericentromeric repeats clearly depends on the RNAi pathway: without it the structural integrity of centromeres is lost and chromosomes no longer segregate properly in mitosis [36]. However, the role of RNAi for the maintenance of a repressive chromatin structure at centromeres in vertebrate cells is less clear-cut [40, 41–45]. This may be due to the presence in vertebrates of redundant silencing mechanisms, including CpG methylation.
Is transcriptional silencing limited to repetitive DNA sequences or does it regulate protein-coding genes involved in cellular differentiation and function? Earlier reports in S. pombe have recently been called into question [46, 47]. While transfection of mammalian cells with siRNAs complementary to promoter regions can induce DNA methylation and transcriptional silencing [48, 49], most known endogenous double stranded RNAs target not promoters but 3’ untranslated regions (3’UTR) and affect gene expression at the post-transcriptional level.
miRNAs regulate translation and RNA stability
In contrast to siRNAs, micro RNAs (miRNAs) do not arise from centromeric repeats, RNA viruses or transposable elements but from specialised miRNA genes [33, 50]. Since each miRNA can target 3’ UTRs of many different transcripts - affecting translation and sometimes RNA stability [51, 52] - a sizeable proportion of protein coding transcripts is regulated by miRNAs [50, 52]. miRNAs expression is cell type- and developmental stage-specific and distinguishes normal from cancer cells, including lymphoid malignancies [53, 54]. Chromosomal translocations (see above) in leukaemias frequently involve miRNA genes, and miRNAs of the miR-17-92 cluster can cooperate with oncogenes to promote murine leukaemia [54] as well as restrict oncogene-driven proliferation by limiting E2F1 expression [55].
Epigenetic Regulation of T cell development
The influence of RNAi and other epigenetic mechanisms on T cell development is gene-, process-, and developmental stage-dependent. Enforced expression of one particular miRNA, miR-181, in haematopoietic progenitors biases lymphoid differentiation towards the B cell lineage at the expense of T cells [63]. And although the RNAi pathway is required for mouse development [64], its role in T cells is being assessed using conditional alleles and Cre transgenes driven by lineage- and developmental stage-specific promoters. Deletion of Dicer early in T cell development results in the depletion of miRNAs by the DP stage and a 10-fold reduction in αβ - but not γδ - thymocytes [44]. Surprisingly, the CD4/CD8 lineage choice appears largely unaffected: CD4- and CD8-lineage specific genes are expressed correctly and Tdt is appropriately silenced [44]. Dicer deletion later in development results in reduced numbers of peripheral T cells affecting the CD8 more than the CD4 lineage [45].
Similar to the effects of Dicer deletion, conditional deletion of Dnmt1 early in T cell development leads to progressive DNA demethylation and a profound reduction in numbers of TCRαβ but not TCRγδ lineage cells [56]. A similar phenotype is also seen in thymocytes lacking lymphocyte specific helicase [57], which remodels chromatin and facilitates DNA methylation, and in thymocytes lacking mSin3a [58], which recruits HDACs. Together, these studies indicate that RNAi, DNA methylation, histone acetylation and chromatin remodeling complexes [5, 59] are important for proper homeostasis of TCRαβ but not TCRγδ lineage progenitors. Perhaps surprisingly, V(D)J recombination does not appear to be affected in thymocytes with any of these defects.
Chromatin remodeling complexes, HDACs, and HP1 also contribute to permanent silencing of CD4 as DP thymocytes differentiate into CD8 SP cells [5,59]. Conversely, Mi-2β, which normally recruits HDACs, facilitates CD4 expression by recruiting the p300 HAT to the proximal CD4 enhancer [59]. Similarly, Runx proteins and BAF-containing chromatin remodeling complexes bind to enhancers E8I, E8II and E8V, recruit HATs to these regions and activate CD8 expression [60, 61]. While DNA methylation does not appear to play an important role in CD4 regulation, it does regulate CD8 expression in T cell progenitors: Aberrant CD8α and CD8β expression occurs in a large fraction of Dnmt1-deficient TCRγδ lineage cells [56]; variegated expression of CD8 in DP thymocytes from E8I plus E8II knockout mice is associated with increased CpG methylation within E8V and the CD8b promoter, and conditional deletion of Dnmt1 partially reverses variegated CD8 expression [62]. Nonetheless, mechanisms other than DNA methylation are sufficient to silence CD8 after commitment to the CD4 lineage, since CD4+CD8+ T cells are not found in the periphery of conditional Dnmt1 knockout mice [56].
Epigenetic Regulation of T cell cytokine production
IL-2 is produced abundantly by naïve T cells, so one would predict a fully accessible Il2 locus in these cells. Contrary to this prediction, the Il2 locus is contained in inactive chromatin in resting naïve T cells, but undergoes dynamic and reversible chromatin remodeling and histone acetylation in response to activation [65]. Moreover, Bruniquel et al showed that CpG cytosines in the proximal Il2 promoter are completely methylated in naïve CD4 T cells, but some of these cytosines are rapidly demethylated after T cell stimulation but before entry into S phase, suggesting active demethylation [66]. This finding is controversial, since Thomas et al found a much lower degree of cytosine methylation in naïve T cells (<33%) and only a modest 2-fold reduction following activation in vitro [65]. In any case, promoter demethylation appears to be required for optimal Il2 promoter activity in vitro [19] and activated Dnmt1-deficient T cells express ~5-fold more IL-2 than controls [67].
Dnmt1-deficient naïve T cells also express ~5-10-fold more IFN-γ following activation in vitro, although expression remains responsive to other cues – increasing in Th1 conditions and diminishing in Th2 conditions [67]. Consistent with these findings, naïve T cells lacking the methyl-CpG binding protein MBD2 produce increased amounts of IFN-γ and exhibit a Th1 bias in vivo [68]. As a consequence, MBD2-deficient mice on the susceptible BALB/c background show increased resistance to infection with Leishmania major, and on a B6/129 background have impaired Th2-dependent immunity against Trichuris muris. These findings suggest that the dominant effect of DNA methylation and MBD2 on T cell cytokine production in vivo is repression of IFN-γ.
Since CpGs in the murine Ifng promoter are constitutively demethylated in naïve T cells [69], it is likely that DNA methylation and MBD2 repress IFN-γ production either indirectly or through actions at other regulatory regions of the Ifng gene. Evolutionarily conserved nucleotide sequence elements (CNSs) extend >50kb upstream and downstream of Ifng [70–72]. Two of these CNSs, one located ~5kb upstream and the other ~18kb downstream from the ATG start codon, contain Th1 and CD8 effector T cell-specific DNase HS sites, demethylated DNA, acetylated H3 and H4 and methylated H3K4, and exhibit in vitro enhancer activity [72, 73]. The importance of these CNSs in Ifng regulation is also supported by the finding that the upstream CNS is approximated by chromatin looping to the Ifng promoter in naïve, Th1 and Th2 cells, whereas the downstream CNS is approximated to the promoter in Th1, but not naïve and Th2 CD4 T cells [30].
Similarly, there are multiple DNase HS sites within the Th2 cytokine locus, many – but not all – of which are within CNSs. A number of these HS sites and the Il4, Il13 and Il5 promoters are physically approximated in T and NK cells through intrachromosomal interactions forming a ‘hub’ that may facilitate their coordinate expression [24]. Two groups recently identified additional Th2-specific DNAse HS sites in the 3' region of the ubiquitously expressed Rad50 gene, which is located between the Il5 and Il13 genes [74, 75]. One of these sites (RHS7) is a Th2-specific enhancer/locus control region [25]. Deletion of RHS7 impairs intrachromsomal interactions within the Th2 locus in T cells, interchromosomal interactions between the Th2 locus and the Ifng locus, and the expression not only of Th2 cytokines, but also of IFN-γ[30□. Another HS site, HSIV, located downstream of the Il4 gene, silences inappropriate expression of IL-4 and IL-13 in naïve and Th1 T cells [76], and in these cells exhibits an unusual pattern of permissive (acetylated H3 and methylated H3K4) plus repressive (methylated H3K27) histone modifications; the permissive marks are retained but methylated H3K27 is selectively lost under Th2 culture conditions [77].
Like HSIV, DNA methylation and MBD2 act as non-redundant repressors of inappropriate Th2 cytokine expression. Dnmt1 is recruited to Il4/Il13 gene regulatory regions during S phase to maintain CpG methylation under non-polarizing or Th1 conditions, but is excluded from these regions in Th2 conditions [78]. Exclusion of Dnmt1 results in passive, slow DNA demethylation, which may help stabilize rather than initiate high-level Th2 cytokine expression. However, one specific CpG in RHS7 undergoes complete demethylation within 48 hr of activation in Th2 conditions in a manner suggesting active rather than passive DNA demethylation, and may thereby facilitate early Th2 cytokine production [74]. Consistent with this possibility, Dnmt1-deficient CD4 and CD8 T cells express ~10-fold and ~1000-fold more IL-4, IL-13, and IL-5, respectively, than control cells in the first 3 days after activation in non-polarizing conditions [20]. Dnmt1-deficient and MBD2-deficient T cells also express increased amounts of IL-4, even in Th1 conditions [67, 79]. Together with findings regarding IL-2 and IFN-γ, these results support a model in which DNA methylation, methyl-CpG binding proteins, and histone modifications collaborate to silence inappropriate Th1 and Th2 cytokine expression and to maintain Th1, Th2 and CD8 effector T cell lineage commitment.
Th1 and Th2 cells also have distinct patterns of miRNA expression [45, 80]. Repression of IFN-γ appears to be defective in Dicer-deficient T cells under Th2 culture conditions [45], but it is not clear if this is due to transcriptional silencing of the Ifng locus, post-transcriptional silencing of Ifng message, or indirect effects.
Conclusions
Although the past few years have provided a number of insights regarding chromatin and gene regulation in T cells, these insights have in turn generated new questions that require clarification: While a marked increase in IFN-γ expression is seen in Dicer-, MBD2-and Dnmt1-deficient T cells, does this mean that RNAi targets DNA methylation to the Ifng locus? To address this question and to understand the role of RNAi in T cell development and function, we need to mechanistically link the phenotypes observed in mice with T cell lineage-specific disruption of the RNAi pathway to the loss of specific double-stranded RNAs and to other epigenetic processes. It also remains to be shown whether miRNAs contribute to the control of cytokine RNA stability by co-stimulatory signals and RNA storage in memory T cells. We also need to learn more about how epigenetic modifications are targeted to or excluded from the regulatory regions of genes governing T cell development and function, whether T cell activation results in active DNA demethylation in cytokine gene regulatory regions, and if so, by what enzymatic mechanism. Work in the near future should illuminate the extent to which ongoing expression of transcription factors versus epigenetic processes determine lineage fidelity as T cell develop, differentiate and die.
Figure 1.
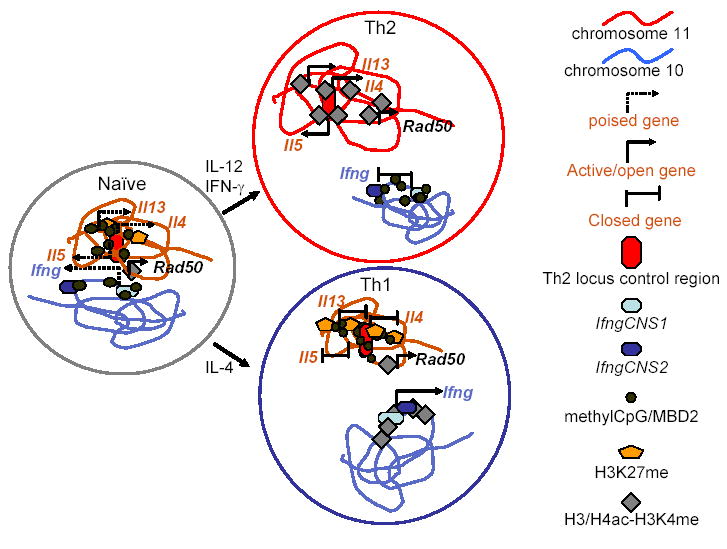
Schematic representation of chromatin modifications and intrachromosomal and interchromosomal interactions in the Th2 locus on murine chromosome 11 and in the Ifng locus on murine chromosome 10. In naïve CD4 T cells, these cytokine loci are in a poised configuration. Repressive histone modifications are limited (minimal, localized H3K27me at the Th2 locus; there are no data regarding H327me at the Ifng locus) or absent (H3K9me). DNA methylation is present at distal regulatory elements in these loci but is absent from the promoter region of the Ifng gene and is present in distal but not proximal regions of the Il4 promoter. The two chromosomes interact, so that the Il5 and Ifng promoters are approximated to each other and to the RHS6 DNase HS site of the Th2 locus control region (located in the 30 end of the Rad50 gene). In Th1 and Th2 cells, the interchromosomal interactions between the Ifng locus and Th2 locus are lost. In Th1 cells, the Ifng locus acquires transcriptionally favorable histone modifications (H3/H4ac and H3K4me), the IfngCNS1 and IfngCNS2 distal regulatory elements undergo progressive DNA demethylation and acquire DNase hypersensitivity sites, and IfngCNS2 is brought into proximity to the Ifng promoter. In the Th2 locus, H3K27me and DNA methylation increase and spread upstream and downstream from the Il4 gene. Conversely, in Th2 cells changes inverse to those seen in Th1 cells occur, although there are no published data regarding H3K27me at the Ifng locus. The Rad50 gene is constitutively active in each of these cell types.
Acknowledgments
We thank David Fitzpatrick and other colleagues and researchers in this field for their contributions to the work described above and Miguel Branco, Ana Pombo and Wilfried Ellmeier for communication of unpublished results. Work in the authors' labs was supported in part by grants from the NIH (CBW, MM), March of Dimes (CBW) and the MRC (MM).
References
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Ansel KM, Lee DU, Rao A. An epigenetic view of helper T cell differentiation. Nat Immunol. 2003;4:616–623. doi: 10.1038/ni0703-616. [DOI] [PubMed] [Google Scholar]
- 2.Bannister AJ, Kouzarides T. Reversing histone methylation. Nature. 2005;436:1103–1106. doi: 10.1038/nature04048. [DOI] [PubMed] [Google Scholar]
- 3.Mellor J. The dynamics of chromatin remodeling at promoters. Mol Cell. 2005;19:147–157. doi: 10.1016/j.molcel.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 4.Wilson CB, Makar KW, Shnyreva M, Fitzpatrick DR. DNA methylation and the expanding epigenetics of T cell lineage commitment. Semin Immunol. 2005;17:105–119. doi: 10.1016/j.smim.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Chi T. A BAF-centred view of the immune system. Nat Rev Immunol. 2004;4:965–977. doi: 10.1038/nri1501. [DOI] [PubMed] [Google Scholar]
- 6.*.Bernstein BE, Kamal M, Lindblad-Toh K, Bekiranov S, Bailey DK, Huebert DJ, McMahon S, Karlsson EK, Kulbokas EJ, 3rd, Gingeras TR, et al. Genomic maps and comparative analysis of histone modifications in human and mouse. Cell. 2005;120:169–181. doi: 10.1016/j.cell.2005.01.001. Using chromatin immunoprecipitation in concert with genomic microarrays (Chip-on-chip), this study demonstrates patterns of global histone acetylation and methylation on human chromosome 21 and 22 and the Th2 cytokine locus. [DOI] [PubMed] [Google Scholar]
- 7.Liu CL, Kaplan T, Kim M, Buratowski S, Schreiber SL, Friedman N, Rando OJ. Single-nucleosome mapping of histone modifications in S. cerevisiae. PLoS Biol. 2005;3:e328. doi: 10.1371/journal.pbio.0030328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tagami H, Ray-Gallet D, Almouzni G, Nakatani Y. Histone H3.1 and H3.3 complexes mediate nucleosome assembly pathways dependent or independent of DNA synthesis. Cell. 2004;116:51–61. doi: 10.1016/s0092-8674(03)01064-x. [DOI] [PubMed] [Google Scholar]
- 9.McKittrick E, Gafken PR, Ahmad K, Henikoff S. Histone H3.3 is enriched in covalent modifications associated with active chromatin. Proc Natl Acad Sci U S A. 2004;101:1525–1530. doi: 10.1073/pnas.0308092100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cook PR. The organization of replication and transcription. Science. 1999;284:1790–1795. doi: 10.1126/science.284.5421.1790. [DOI] [PubMed] [Google Scholar]
- 11.Sproul D, Gilbert N, Bickmore WA. Nat Rev Genetics. 2005;6:775–781. doi: 10.1038/nrg1688. [DOI] [PubMed] [Google Scholar]
- 12.Casolari JM, Brown CR, Komili S, West J, Hieronymus H, Silver PA. Genome wide localization of the nuclear transport machinery couples transcriptional status and nuclear organization. Cell. 2004;117:427–439. doi: 10.1016/s0092-8674(04)00448-9. [DOI] [PubMed] [Google Scholar]
- 13.Brown KE, Guest SS, Smale ST, Hahm K, Merkenschlager M, Fisher AG. Association of transcriptionally silent genes with Ikaros complexes at centromeric heterochromatin. Cell. 1997;91:845–854. doi: 10.1016/s0092-8674(00)80472-9. [DOI] [PubMed] [Google Scholar]
- 14.Brown KE, Baxter J, Graf D, Merkenschlager M, Fisher AG. Dynamic repositioning of genes in the nucleus of lymphocytes preparing for cell division. Mol Cell. 1999;3:207–217. doi: 10.1016/s1097-2765(00)80311-1. [DOI] [PubMed] [Google Scholar]
- 15.Su RC, Brown KE, Saaber S, Fisher AG, Merkenschlager M, Smale ST. Dynamic assembly of silent chromatin during thymocyte maturation. Nat Genet. 2004;36:502–506. doi: 10.1038/ng1351. [DOI] [PubMed] [Google Scholar]
- 16.Delaire S, Huang YH, Chan SW, Robey EA. Dynamic repositioning of CD4 and CD8 genes during T cell development. J Exp Med. 2004;200:1427–1435. doi: 10.1084/jem.20041041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merkenschlager M, Amoils S, Roldan E, Rahemtulla A, O'connor E, Fisher AG, Brown KE. Centromeric repositioning of coreceptor loci predicts their stable silencing and the CD4/CD8 lineage choice. J Exp Med. 2004;200:1437–1444. doi: 10.1084/jem.20041127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.West AG, Fraser P. Remote control of gene transcription. Hum Mol Genet. 2005;14 Spec No 1:R101–11. doi: 10.1093/hmg/ddi104. [DOI] [PubMed] [Google Scholar]
- 19.Decker J, Rippe K, Dekker M, Kleckner N. Capturing chromosome conformation. Science. 1992;295:1306–1311. doi: 10.1126/science.1067799. [DOI] [PubMed] [Google Scholar]
- 20.Liu Z, Garrard WT. Long-range interactions between three transcriptional enhancers, active Vkappa gene promoters, and a 3' boundary sequence spanning 46 kilobases. Mol Cell Biol. 2005;25:3220–3231. doi: 10.1128/MCB.25.8.3220-3231.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tolhuis B, Palstra RJ, Splinter E, Grosveld F, de Laat W. Looping and interaction between hypersensitive sites in the active beta-globin locus. Mol Cell. 2002;10:1453–1465. doi: 10.1016/s1097-2765(02)00781-5. [DOI] [PubMed] [Google Scholar]
- 22.Carter D, Chakalova L, Osborne CS, Dai YF, Fraser P. Long-range chromatin regulatory interactions in vivo. Nat Genet. 2002;32:623–626. doi: 10.1038/ng1051. [DOI] [PubMed] [Google Scholar]
- 23.Murrell A, Heeson S, Reik W. Interaction between differentially methylated regions partitions the imprinted genes Igf2 and H19 into parent-specific chromatin loops. Nat Genet. 2004;36:889–893. doi: 10.1038/ng1402. [DOI] [PubMed] [Google Scholar]
- 24.Spilianakis CG, Flavell RA. Long-range intrachromosomal interactions in the T helper type 2 cytokine locus. Nat Immunol. 2004;5:1017–1027. doi: 10.1038/ni1115. [DOI] [PubMed] [Google Scholar]
- 25.*.Lee GR, Spilianakis CG, Flavell RA. Hypersensitive site 7 of the TH2 locus control region is essential for expressing TH2 cytokine genes and for long-range intrachromosomal interactions. Nat Immunol. 2005;6:42–48. doi: 10.1038/ni1148. Deletion of a DNase HS site (RHS7) in the 3' end of Rad50 reduces Th2 cytokine expression and reduces long-range intrachromosomal interactions between the locus control region and Th2 cytokine promoters. [DOI] [PubMed] [Google Scholar]
- 26.Vakoc CR, Letting DL, Gheldof N, Sawado T, Bender MA, Groudine M, Weiss MJ, Dekker J, Blobel GA. Proximity among distant regulatory elements at the beta-globin locus requires GATA-1 and FOG-1. Mol Cell. 2005;17:453–462. doi: 10.1016/j.molcel.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 27.Leung AK, Lamond AI. The dynamics of the nucleolus. Crit Rev Eukaryot Gene Expr. 2003;13:39–54. doi: 10.1615/critreveukaryotgeneexpr.v13.i1.40. [DOI] [PubMed] [Google Scholar]
- 28.Bantignies F, Grimaud C, Lavrov S, Gabut M, Cavalli G. Inheritance of Polycomb-dependent chromosomal interactions in Drosophila. Genes Dev. 2003;17:2406–2420. doi: 10.1101/gad.269503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.*.Osborne CS, Chakalova L, Brown KE, Carter D, Horton A, Debrand E, Goyenechea B, Mitchell JA, Lopes S, Reik W, Fraser P. Active genes dynamically colocalize to shared sites of ongoing transcription. Nat Genet. 2004;36:1065–1071. doi: 10.1038/ng1423. This study documents that genes with different chromosomal addresses can colocalise at sites of active transcription. [DOI] [PubMed] [Google Scholar]
- 30.**.Spilianakis CG, Lalioti MD, Town T, Lee GR, Flavell RA. Interchromosomal associations between alternatively expressed loci. Nature. 2005;435:637–645. doi: 10.1038/nature03574. Interchromosomal interactions between the Th2 locus and the Ifng promoter in naïve CD4 T cells are also identifed, and these interactions are substantially reduced when the RHS7 DNase HS site in the Th2 locus control region is deleted in naïve CD4 T cells or when naïve CD4 T cells differentiate into Th1 or Th2 cells. This and the following paper demonstrate long-range intrachromosomal interactions through chromatin looping between the Th2 locus control region in the 3′ end of the Rad50 gene and the Il4, Il5 and Il13 promoters on murine chromosome 10, and between the Ifng promoter and two regulatory elements located upstream and downstream of the Ifng gene on murine chromosome. [DOI] [PubMed] [Google Scholar]
- 31.Roix JJ, McQueen PG, Munson PJ, Parada LA, Misteli T. Spatial proximity of translocation-prone gene loci in human lymphomas. Nat Genetics. 2003;34:287–291. doi: 10.1038/ng1177. [DOI] [PubMed] [Google Scholar]
- 32.Parada LA, McQueen PG, Misteli T. Tissue-specific spatial organization of genomes. Genome Biol. 2004;5:R44. doi: 10.1186/gb-2004-5-7-r44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 34.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 35.Silva JM, Li MZ, Chang K, Ge W, Golding MC, Rickles RJ, Siolas D, Hu G, Paddison PJ, Schlabach MR, Sheth N, Bradshaw J, Burchard J, Kulkarni A, Cavet G, Sachidanandam R, McCombie WR, Cleary MA, Elledge SJ, Hannon GJ. Second-generation shRNA libraries covering the mouse and human genomes. Nat Genet. 2005;37:1281–1288. doi: 10.1038/ng1650. [DOI] [PubMed] [Google Scholar]
- 36.Bernstein E, Allis CD. RNA meets chromatin. Genes Dev. 2005;19:1635–1355. doi: 10.1101/gad.1324305. [DOI] [PubMed] [Google Scholar]
- 37.Kato H, Goto DB, Martienssen RA, Urano T, Furukawa K, Murakami Y. RNA polymerase II is required for RNAi-dependent heterochromatin assembly. Science. 2005;309:467–469. doi: 10.1126/science.1114955. [DOI] [PubMed] [Google Scholar]
- 38.Schramke V, Sheedy DM, Denli AM, Bonila C, Ekwall K, Hannon GJ, Allshire RC. RNA-interference-directed chromatin modification coupled to RNA polymerase II transcription. Nature. 2005;435:1275–1279. doi: 10.1038/nature03652. [DOI] [PubMed] [Google Scholar]
- 39.Martienssen R, Lippman Z, May B, Ronemus M, Vaughn M. Transposons, tandem repeats, and the silencing of imprinted genes. Cold Spring Harb Symp Quant Biol. 2004;69:371–9.40. doi: 10.1101/sqb.2004.69.371. [DOI] [PubMed] [Google Scholar]
- 40.Volpe TA, Kidner C, Hall IM, Teng G, Grewal SI, Martienssen RA. Regulation of heterochromatic silencing and histone H3 lysine-9 methylation by RNAi. Science. 2002;297:1833–1837. doi: 10.1126/science.1074973. [DOI] [PubMed] [Google Scholar]
- 41.Fukagawa T, Nogami M, Yoshikawa M, Ikeno M, Okazaki T, Takami Y, Nakayama T, Oshimura M. Dicer is essential for formation of the heterochromatin structure in vertebrate cells. Nat Cell Biol. 2004;6:784–791. doi: 10.1038/ncb1155. [DOI] [PubMed] [Google Scholar]
- 42.Kanellopoulou C, Muljo SA, Kung AL, Ganesan S, Drapkin R, Jenuwein T, Livingston DM, Rajewsky K. Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes Dev. 2005;19:489–501. doi: 10.1101/gad.1248505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murchison EP, Partridge JF, Tam OH, Cheloufi S, Hannon GJ. Characterization of Dicer-deficient murine embryonic stem cells. Proc Natl Acad Sci U S A. 2005;102:12135–12140. doi: 10.1073/pnas.0505479102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.*.Cobb BS, Nesterova TB, Thompson E, Hertweck A, O'Connor E, Godwin J, Wilson CB, Brockdorff N, Fisher AG, Smale ST, Merkenschlager M. T cell lineage choice and differentiation in the absence of the RNase III enzyme Dicer. J Exp Med. 2005;201:1367–1373. doi: 10.1084/jem.20050572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.*.Muljo SA, Ansel KM, Kanellopoulou C, Livingston DM, Rao A, Rajewsky K. Aberrant T cell differentiation in the absence of Dicer. J Exp Med. 2005;202:261–269. doi: 10.1084/jem.20050678. Together, ref 44 and 45 provide the first insight into the role of the RNAse III enzyme Dicer in lymphocyte development and function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schramke V, Sheedy DM, Denli AM, Bonila C, Ekwall K, Hannon GJ, Allshire R. Retraction: RNA-interference-directed chromatin modification coupled to RNA polymerase II transcription. Nature. 2005 Oct 13;437(7061):1057. doi: 10.1038/nature04181. [DOI] [PubMed] [Google Scholar]
- 47.Allshire R. Retraction. Hairpin RNAs and retrotransposon LTRs effect RNAi and chromatin-based gene silencing. Science. 2005 Oct 7;310(5745):49. doi: 10.1126/science.310.5745.49b. [DOI] [PubMed] [Google Scholar]
- 48.Kawasaki H, Taira K. Induction of DNA methylation and gene silencing by short interfering RNAs in human cells. Nature. 2004;431:211–217. doi: 10.1038/nature02889. [DOI] [PubMed] [Google Scholar]
- 49.Morris KV, Chan SW, Jacobsen SE, Looney DJ. Small interfering RNA-induced transcriptional gene silencing in human cells. Science. 2004;305:1289–1292. doi: 10.1126/science.1101372. [DOI] [PubMed] [Google Scholar]
- 50.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 51.Jing Q, Huang S, Guth S, Zarubin T, Motoyama A, Chen J, Di Padova F, Lin SC, Gram H, Han J. Involvement of microRNA in AU-rich element-mediated mRNA instability. Cell. 2005;120: 623–634. doi: 10.1016/j.cell.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 52.Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 53.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR. MicroRNA expression profiles classify human cancers. Nature. 2005 Jun 9;435(7043):834–8. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 54.He L, Thomson JM, Hemann MT, et al. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828–833. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O'Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc-regulated microRNAs modulate E2F1 expression. Nature. 2005;435:839–843. doi: 10.1038/nature03677. [DOI] [PubMed] [Google Scholar]
- 56.Lee PP, Fitzpatrick DR, Beard C, Jessup HK, Lehar SM, Makar KW, Perez-Melgosa M, Sweetser MT, Schlissel MS, Nguyen S, et al. A critical role for Dnmt1 and DNA methylation in T cell development, function and survival. Immunity. 2001;15:763–774. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- 57.Yan Q, Cho E, Lockett S, Muegge K. Association of Lsh, a regulator of DNA methylation, with pericentromeric heterochromatin is dependent on intact heterochromatin. Mol Cell Biol. 2003;23:8416–8428. doi: 10.1128/MCB.23.23.8416-8428.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cowley SM, Iritani BM, Mendrysa SM, Xu T, Cheng PF, Yada J, Liggitt HD, Eisenman RN. The mSin3A chromatin-modifying complex is essential for embryogenesis and T-cell development. Mol Cell Biol. 2005;25:6990–7004. doi: 10.1128/MCB.25.16.6990-7004.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.*.Williams CJ, Naito T, Arco PG, Seavitt JR, Cashman SM, De Souza B, Qi X, Keables P, Von Andrian UH, Georgopoulos K. The chromatin remodeler Mi-2beta is required for CD4 expression and T cell development. Immunity. 2004;20:719–733. doi: 10.1016/j.immuni.2004.05.005. This study shows that Mi-2b, which typically recruits histone deacetylases to regulatory regions thereby repressing gene expression, recruits p300 histone acetyltransferase to the CD4 enhancer to activate CD4 expression in T cell progenitors. [DOI] [PubMed] [Google Scholar]
- 60.Kioussis D, Ellmeier W. Chromatin and CD4, CD8A and CD8B gene expression during thymic differentiation. Nat Rev Immunol. 2002;2:909–919. doi: 10.1038/nri952. [DOI] [PubMed] [Google Scholar]
- 61.Sato T, Ohno S, Hayashi T, Sato C, Kohu K, Satake M, Habu S. Dual functions of Runx proteins for reactivating CD8 and silencing CD4 at the commitment process into CD8 thymocytes. Immunity. 2005;22:317–328. doi: 10.1016/j.immuni.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 62.*.Bilic I, Kösters C, Unger B, Sekimata M, Hertweck A, Maschek R, Wilson CB, Ellmeier W. Negative regulation of CD8 expression via CD8 enhancer-mediated recruitment of the zinc finger protein MAZR. Nat Immunol. 2006 doi: 10.1038/ni1311. submitted. This study demonstrates that variegated expression of CD8 in DP thymocytes in which CD8 enhancers E8I and E8II have been deleted is associated with reduced H3 and H4 acetylation and H3K4 methylation and persistent DNA methylation in several regulatory regions of the CD8ab locus. CD8 expression is partially restored by deletion of DNA methyltransferase 1, indicating that these enhancers facilitate CD8 expression in part by creating an epigenetically accessible locus in DP thymocytes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303:83–86. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- 64.Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, Li MZ, Mills AA, Elledge SJ, Anderson KV, Hannon GJ. Dicer is essential for mouse development. Nat Genet. 2003;35:215–217. doi: 10.1038/ng1253. [DOI] [PubMed] [Google Scholar]
- 65.Thomas RM, Gao L, Wells AD. Signals from CD28 induce stable epigenetic modification of the IL-2 promoter. J Immunol. 2005;174:4639–4646. doi: 10.4049/jimmunol.174.8.4639. [DOI] [PubMed] [Google Scholar]
- 66.Bruniquel D, Schwartz RH. Selective, stable demethylation of the interleukin-2 gene enhances transcription by an active process. Nat Immunol. 2003;4:235–240. doi: 10.1038/ni887. [DOI] [PubMed] [Google Scholar]
- 67.Makar KW, Wilson CB. DNA methylation is a non-redundant repressor of the Th2 effector program. J Immunol. 2004;173:4402–4406. doi: 10.4049/jimmunol.173.7.4402. [DOI] [PubMed] [Google Scholar]
- 68.Hutchins AS, Artis D, Hendrich BD, Bird AP, Scott P, Reiner SL. Cutting edge: a critical role for gene silencing in preventing excessive type 1 immunity. J Immunol. 2005;175:5606–5610. doi: 10.4049/jimmunol.175.9.5606. [DOI] [PubMed] [Google Scholar]
- 69.Winders BR, Schwartz RH, Bruniquel D. A distinct region of the murine IFN-gamma promoter is hypomethylated from early T cell development through mature naive and Th1 cell differentiation, but is hypermethylated in Th2 cells. J Immunol. 2004;173:7377–7384. doi: 10.4049/jimmunol.173.12.7377. [DOI] [PubMed] [Google Scholar]
- 70.Chang S, Aune TM. Histone hyperacetylated domains across the Ifng gene region in natural killer cells and T cells. Proc Natl Acad Sci U S A. 2005;102:17095–17100. doi: 10.1073/pnas.0502129102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee DU, Avni O, Chen L, Rao A. A distal enhancer in the interferon-gamma (IFN-gamma) locus revealed by genome sequence comparison. J Biol Chem. 2004;279:4802–4810. doi: 10.1074/jbc.M307904200. [DOI] [PubMed] [Google Scholar]
- 73.Shnyreva M, Weaver WM, Blanchette M, Taylor SL, Tompa M, Fitzpatrick DR, Wilson CB. Evolutionarily conserved sequence elements that positively regulate IFN-gamma expression in T cells. Proc Natl Acad Sci U S A. 2004;101:12622–12627. doi: 10.1073/pnas.0400849101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fields PE, Lee GR, Kim ST, Bartsevich VV, Flavell RA. Th2-specific chromatin remodeling and enhancer activity in the Th2 cytokine locus control region. Immunity. 2004;21:865–876. doi: 10.1016/j.immuni.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 75.Lee DU, Rao A. Molecular analysis of a locus control region in the T helper 2 cytokine gene cluster: a target for STAT6 but not GATA3. Proc Natl Acad Sci U S A. 2004;101:16010–16015. doi: 10.1073/pnas.0407031101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ansel KM, Greenwald RJ, Agarwal S, Bassing CH, Monticelli S, Interlandi J, Djuretic IM, Lee DU, Sharpe AH, Alt FW, et al. Deletion of a conserved Il4 silencer impairs T helper type 1-mediated immunity. Nat Immunol. 2004;5:1251–1259. doi: 10.1038/ni1135. [DOI] [PubMed] [Google Scholar]
- 77.Koyanagi M, Baguet A, Martens J, Margueron R, Jenuwein T, Bix M. EZH2 and histone 3 trimethyl lysine 27 associated with Il4 and Il13 gene silencing in Th1 cells. J Biol Chem. 2005;280:31470–31477. doi: 10.1074/jbc.M504766200. [DOI] [PubMed] [Google Scholar]
- 78.Makar KW, Perez-Melgosa M, Shnyreva M, Weaver WM, Fitzpatrick DR, Wilson CB. Active recruitment of DNA methyltransferases regulates interleukin 4 in thymocytes and T cells. Nat Immunol. 2003;4:1183–1190. doi: 10.1038/ni1004. [DOI] [PubMed] [Google Scholar]
- 79.Hutchins AS, Mullen AC, Lee HW, Sykes KJ, High FA, Hendrich BD, Bird AP, Reiner SL. Gene silencing quantitatively controls the function of a developmental trans-activator. Mol Cell. 2002;10:81–91. doi: 10.1016/s1097-2765(02)00564-6. [DOI] [PubMed] [Google Scholar]
- 80.Monticelli S, Ansel KM, Xiao C, Socci ND, Krichevsky AM, Thai TH, Rajewsky N, Marks DS, Sander C, Rajewsky K, Rao A, Kosik KS. MicroRNA profiling of the murine hematopoietic system. Genome Biol. 2005;6:R71. doi: 10.1186/gb-2005-6-8-r71. [DOI] [PMC free article] [PubMed] [Google Scholar]


