Abstract
Histological studies suggest that hippocampal subfields are differently affected by aging and Alzheimer’s disease (AD). The aims of this study were: (1) To test if hippocampal subfields can be identified and marked using anatomical landmarks on high resolution MR images obtained on a 4T magnet. (2) To test if age-specific volume changes of subfields can be detected. Forty-two healthy controls (21–85 years) and three AD subjects (76–86 years) were studied with a high resolution T2 weighted fast spin echo sequence. The entorhinal cortex (ERC), subiculum, CA1, CA2 and CA3/4 and dentate were marked. A significant correlation between age and CA1 (r = −0.51, p = 0.0002) which was most pronounced in the seventh decade of life was found in healthy controls. In AD subjects, CA1 and subiculum were smaller than in age-matched controls. These preliminary findings suggest that measurement of hippocampal subfields may be helpful to distinguish between normal aging and AD.
Keywords: Hippocampus, CA1, Entorhinal cortex, Subfield, High resolution imaging
1. Introduction
Aging can be associated with alterations of cognitive functions even in people spared from brain diseases commonly associated with older age and known to affect cognition, e.g., cerebrovascular disease or Alzheimer’s disease (AD) [23]. One of the most prominently affected cognitive domains is memory [2]. This suggests that the hippocampal formation, which is crucial for storage and retrieval of information, might be particularly vulnerable to the aging process. The reasons for the age-associated impairment of the hippocampus are not completely understood and are most probably complex, as a variety of biochemical and electrophysiological changes compromising neuronal excitability and plasticity in old age have been described [7,25]. Whether these functional changes are also associated with neuronal cell loss is a matter of debate. Some histological studies reported significant hippocampal neuronal cell loss with increasing age [22,38] while others found no significant cell loss despite the presence of corresponding behavioral changes [21,28,31]. Neuroimaging findings, i.e., MRI based hippocampal volumetry, are similarly ambiguous. Some studies have shown significant hippocampal volume loss with increasing age [18,29,34] and others found no hippocampal volume differences between aged and young subjects [12,40,41]. However, an accurate characterization of the typical age-related hippocampal changes in vivo is very important because AD in the early stages is also associated with hippocampal volume loss [15,16] and mild cognitive impairment, making the differentiation from normal aging difficult.
The hippocampus is not a homogeneous structure but divided into several subfields with distinctive histological characteristics: the subiculum (with the subdivisions pre-subiculum, para-subiculum and subiculum proper), the four cornu ammonis sectors (CA1–4) and the dentate gyrus. These subfields are functionally interconnected in two main intrahippocampal pathways: (1) The polysynaptic intrahippocampal pathway, which is composed of the entorhinal cortex, the dentate gyrus, CA3, 4 and CA1 and the subiculum. (2) The direct pathway, which is composed of the entorhinal cortex, CA1 and the subiculum [5]. Despite the tight functional interconnection, there is evidence of a functional specialization of the subfields, e.g., CA1 seems to be involved in temporal pattern association and intermediate term memory while CA3 is responsible for spatial pattern association, detection of novelty and short-term memory [20]. Furthermore, there is also evidence that different disease processes affect subfields differently and several histopathological studies suggest that this is also the case for aging and AD [9,32,47]. Therefore, measuring volume loss in hippocampal subfields might yield a better distinction between normal aging and early AD than measuring global hippocampal volume loss. However, this requires that the inner structure of the hippocampal formation can be depicted on MRI. On a clinical 1.5 T magnet the sensitivity of the MR signal is usually too low to obtain sufficient resolution to identify individual subfields without the application of sophisticated, often lengthy imaging protocols. Nonetheless, there have been several attempts to visualize age and AD related structural and perfusion changes in hippocampal subfields [1,39]. Recent advancements with high field MRI (3–4 T), achieving increased gray/white matter contrast due to the increased signal sensitivity at high fields, additional magnetization transfer effects and T1 weighting, have resulted in superb anatomical images of the brain at sub-millimeter resolution, that can be acquired within a few minutes [4,43]. Therefore, using a 4 T MRI system, the aims of this study were the following: (1) To test if hippocampal subfields, particularly, entorhinal cortex, subiculum, CA1, CA2 and CA3 and CA4, can be reliably identified and marked using anatomical landmarks on high resolution MR images. (2) To test if age-specific volume changes of subfields can be detected. Based on the findings of histological studies, we expected to find age-related changes in the subiculum [47,48] and CA1 [38] and the latter to be more pronounced in Alzheimer’s disease [47].
2. Methods
2.1. Subject population
Forty-two subjects were recruited from the community as controls for a dementia study by flyers and advertisements in local newspapers (cf. Table 1). Exclusion criteria included any poorly controlled medical illness (untreated diabetes, hypertension, thyroid disease) and/or use of medication or recreational drugs that could affect brain function, a history of brain trauma, brain surgery or evidence for ischemic events (lacunes, stroke) and skull defects on the MRI. Normal cognitive functioning was assessed by a battery of neuropsychological tests (mini mental state examination, California Verbal Learning Test (short form), Rey–Osterrieth complex figure, Verbal Fluency, Wechsler Adult Intelligence Score (digit symbol, digit span)); emotional state and functioning in daily living (only in subjects older than 50 years of age) were assessed with the Geriatric Depression Scale and the Functional Activities Questionnaire. In addition to this, three subjects diagnosed with Alzheimer’s disease according to the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association (NINCDS/ARDA) were recruited from local Memory Clinics. The study was approved by the committee of human research at the University of California, San Francisco (UCSF), and written informed consent was obtained from all subjects or their legal representatives according to the Declaration of Helsinki.
Table 1.
Demographic and neurosychological characteristics
| Standardized scores mean (S.D.)
|
|||||
|---|---|---|---|---|---|
| f/m | Digit symbol | Rey-delay | Short correct recall | Long correct recall | |
| Healthy subjects (total no. of subjects: 42 mean age 48.7 (range 21–85 years)) | |||||
| Third decade | 4/4 | 0.0 (0.8) | 0.0 (0.6) | 0.3 (1.2) | −0.1 (0.7) |
| Fourth decade | 0/5 | 1.2 (0.7) | 0.9 (0.4) | 1.0 (0.9) | 0.8 (0.3) |
| Fifth decade | 3/7 | 0.9 (1.6) | 0.4 (0.9) | 1.0 (1.0) | 0.9 (0.6) |
| Sixth decade | 4/5 | 0.4 (1.1) | 0.2 (1.2) | 0.6 (0.9) | 0.6 (0.8) |
| Seventh decade | 3/4 | 1.0 (0.8) | 0.1 (0.9) | 1.4 (1.1) | 0.9 (0.8) |
| Eighth plus decade | 2/5 | −0.3 (0.8) | −0.3 (0.4) | 0.1 (0.9) | −0.4 (0.9) |
| f/m | MMSE | ||||
| AD (total no. of subjects: 3 mean age 82.3 (range 77–86 years)) | |||||
| Male/female | 2/1 | 20.3 (range 16–26) | |||
2.2. MR imaging
All imaging was performed on a Bruker MedSpec 4T system controlled by a Siemens TrioTM console. Images were acquired using either a transmit/receive TEM resonator head coil (subjects 1–7, AD 1 and 2) or a USA instruments eight channel array coil that consisted of a separate transmit coil enclosing the eight receiver coils (subjects 8–42, AD 3). Age and gender distribution in the two groups were similar. The following sequences, which were part of a larger research imaging and spectroscopy protocol, were acquired: For the measurement of hippocampal subfields, a high resolution T2 weighted fast spin echo sequence TR/TE: 3500/19 ms, echo train length 15, 18.6 ms echo spacing, 160° flip angle, 100% oversampling in ky-direction, 0.4 mm × 0.5 mm in plane resolution, 2 mm slice thickness, 24 interleaved slices without gap, acquisition time 5:30 min (adapted from [4,41]), angulated perpendicular to the long axis of the hippocampal formation (cf. Fig. 1), and for the determination of the intracranial volume, a 3D T2 weighted turbospin echo sequence (TR/TE 3000/356 ms, echo train length 109, variable flip angle, 1 mm × 1 mm × 2 mm nominal resolution, 120 slices, acquisition time 3.40 min).
Fig. 1.
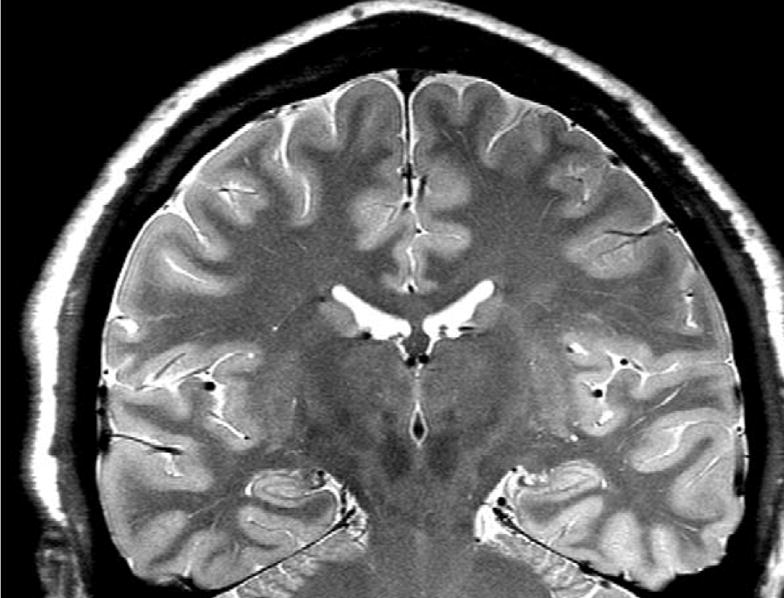
A typical high resolution T2 weighted image of the hippocampus. A hypointense line representing the leptomeningeal tissue in the vestigial hippocampal sulcus is clearly visible [5]. This line was used as landmark for the subdivision of the hippocampal formation.
2.3. Post-processing and measurement of the hippocampal subfields
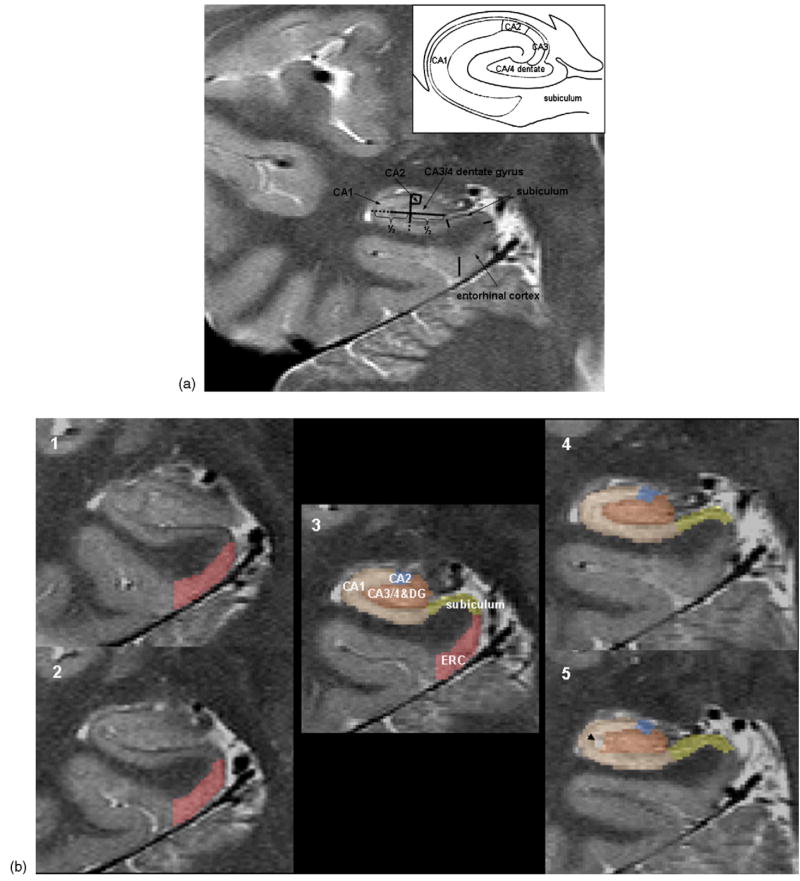
The images were transferred to an off line work station for post-processing. Using an in house developed display software, the angulation of each hippocampus on the high resolution T2 sequence was precisely determined and the images resliced using 3D sinc interpolation to create a left and a right hippocampal image on which the coronal axis was perpendicular to the long axis of the corresponding hippocampus. The measurement started on the first slice on which the head of the hippocampus was no longer visible. On this slice, the hippocampal subfields, subiculum and entorhinal cortex were marked manually. In addition, the entorhinal cortex was marked on the two slices anterior to the starting slice and the subiculum and the hippocampal subfields were marked on the two slices posterior to the starting slice. The marking scheme is depicted in detail in Fig. 2a and b. The most medial point of the temporal cortex was chosen as medial border of the entorhinal cortex (ERC) and the end of the collateral sulcus was chosen as its lateral border. In contrast to most other markings schemes for the ERC [17,30], we chose not to mark the part of the ERC within the collateral sulcus because the depth of this sulcus shows considerably within and between subject variability. The CA1/subiculum border was determined by drawing a line perpendicular to the edge of the subiculum touching the medial border of the hippocampus. This border was chosen because it could be easily and reliably identified although we were aware that by doing so a part of the prosubiculum and subiculum proper were counted towards the CA1 sector (cf. Fig. 2a). The CA1/CA2 border was determined by dividing the line along the longest diameter of the hippocampus by two and drawing a line perpendicular to this line. CA2 was marked as a square whose sides were determined by the thickness of CA1 at the CA1/CA2 border. The remainder of the hippocampal formation consisting of CA3, CA4 and dentate gyrus was marked as one region because there were no reliable marks to distinguish between these structures. The thickness of the individual subfields was determined by the distance between the hypointense line representing the leptomeningeal tissue in the vestigial hippocampal sulcus [5] and the outer edge of the hippocampus. The alveus and the fimbria were not included in the markings. Ten subjects were marked twice by two raters to assess the reliability of this marking scheme within and between raters. The volumes of the subfields were determined by multiplying the number of pixels within the markings by pixel size and slice thickness and then by adding the volumes on all marked slices. The volume of the total hippocampus was determined using a commercially available high dimensional brain tool (Medtronic Surgical Navigation Technologies, Louisville, CO) [13]. To adjust for differences in head size, all volumes were normalized to total intracranial volume (ICV) by using ICV as a covariate in the regression analysis. The ICV was determined from the 3D T2 weighted image using the BET program (FMRIB Image Analysis Group, Oxford University, http://www.fmrib.ox.ac.uk/fsl).
Fig. 2.
(a) The marking scheme used in the study. The inset shows a diagram of the hippocampal subfield derived from a histological specimen (adapted from Ref. [5]). As it is not possible to identify individual hippocampal layers at this field strength, the scheme was based on reliably recognizable anatomical landmarks even though this resulted in a part of the prosubiculum and subiculum proper being counted towards the CA1 sector. (b) A typical example of hippocampal subfield markings. No. 1 is the most anterior slice, No. 5 is the most posterior slice. Slice 3 is the referred in the text as “starting” slice. The arrow on slice 5 indicates a cyst in the vestigial hippocampal sulcus which was excluded from the measurement.
2.4. Statistical analysis
Reliability of subfield measurements was assessed by comparing volumes generated by marking subfields in a randomly selected subgroup of 10 subjects twice by the same rater (intra-rater reliability) and by two raters independently (inter-rater reliability). Raters were blind to identity and clinical status of each subject. The intraclass correlation coefficient (ICC) was calculated based on a two-way analysis of variance (ANOVA) with random effects since we were interested in how these raters were representative of a larger spectrum of raters. ICC values near unity indicate that measurements yielded very consistent results while values near 0.5 indicate unreliable measurements. Multiple linear regression analysis was used to test the influence of age and gender on each hippocampal subfield (sum of left and right side). To test if subfield volumes change disproportionately across the age range, subjects were grouped into five decades (third decade: 18–29 years; fourth decade: 30–39 years; fifth decade: 40–49 years; sixth decade: 50–59 years and seventh plus decade: more than 60 years of age). Differences between age groups were compared using a one-way ANOVA; corrections for multiple comparisons for post hoc analyses were performed with Duncan’s test. The level of significance was set at p < 0.05.
3. Results
3.1. Reliability
Table 2 displays the ICC for the two raters indicating generally high consistency within and between raters (ICC > 0.75). However, the markings of the right subiculum were unreliable (ICC < 0.5). This was mostly likely due to artifacts from the posterior cerebral artery causing geometrical distortions in the region of the subiculum which made its consistent marking difficult.
Table 2.
Intra- and inter-rater reliability for subfield markings in nine subjects
| ICC
|
|||
|---|---|---|---|
| Rater 1 | Rater 2 | Between-rater | |
| L ERC | 0.83 | 0.67 | 0.61 |
| R ERC | 1 | 0.98 | 0.88 |
| L Subiculum | 0.99 | 0.91 | 0.78 |
| R Subiculum | 0.99 | 0.66 | 0.39 |
| L CA1 | 0.99 | 0.98 | 0.93 |
| R CA1 | 0.99 | 0.87 | 0.91 |
| L CA2 | 0.96 | 0.81 | 0.86 |
| R CA2 | 0.77 | 0.78 | 0.68 |
| L CA3/4 and dentate | 0.98 | 0.86 | 0.88 |
| R CA3/4 and dentate | 0.99 | 0.96 | 0.89 |
ICC, intraclass correlation coefficient; ERC, entorhinal cortex, L, left, R, right.
3.2. Age effect
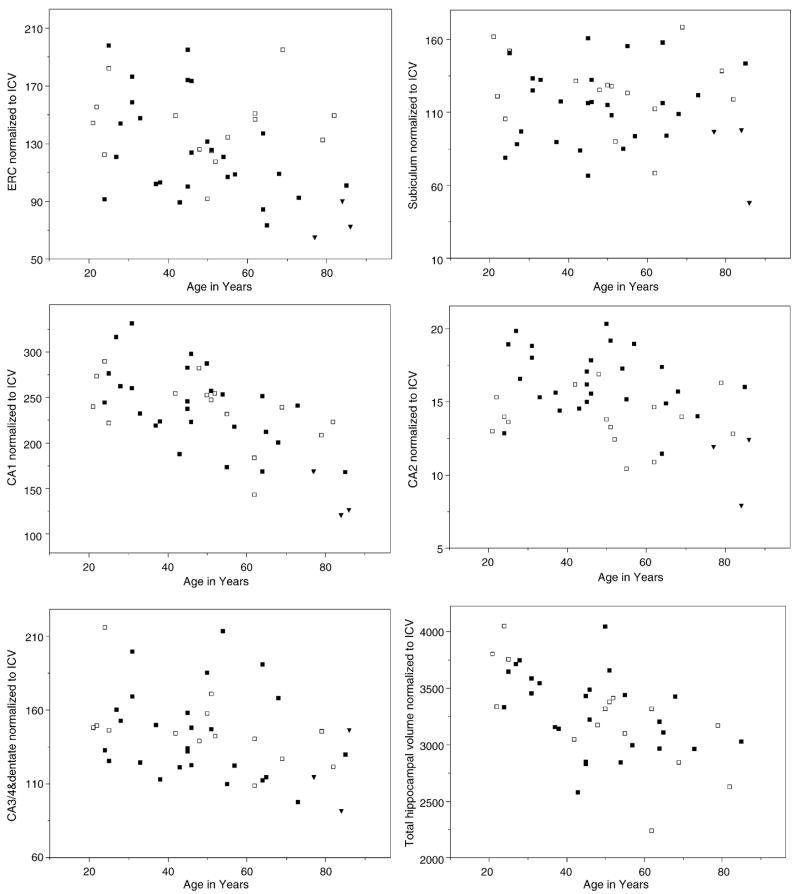
Regression analysis showed a strong negative effect of age (p = 0.0001) and ICV (p = 0.001) but not of gender on total hippocampal volume (F = 13.9; d.f. = 3, 38; p < 0.0001). Of the subfields, only CA1 showed an age effect (p = 0.0002) (cf. Fig. 3). As there were no effects of gender or ICV, age alone explained its variations (F = 7.55; d.f. = 3, 38; p = 0.0004). The scatterplot (cf. Fig. 3) suggested an increased volume loss after the age of 60. Therefore, age was also fitted as a quadratic term but this did not result in a better overall fit of the regression. However, when we compared the CA1 volumes between the different decades, we found a significant effect for age group (F = 4.15; d.f. = 4, 37; p = 0.007). Post hoc analyses showed that the seventh plus decade age group had significantly smaller CA1 than every other age group. There was no significant difference of CA1 between the other age groups. None of the other subfields showed a significant correlation with age or ICV but CA2 showed an effect of gender (p = 0.00003) with men having a larger CA2 volumes than women (F = 13.44; d.f. = 3, 38; p < 0.00001).
Fig. 3.
Scatterplots of subfield and total hippocampal volume measurements against age. All measurements were corrected for differences in head size using the following formula: normalized volume = raw volume in mm3 × 1000/ICV in mm3 (ICV = intracranial volume). Women are indicated with open squares, men with filled squares. The measurements of three AD patients (filled triangles) are shown for comparison: 77 years old AD subject, MMSE 26; 84 years old AD subject, MMSE 16; 86 years old AD subject, MMSE 19.
4. Discussion
There were two major findings of this study: (1) It is possible to reliably depict details of the internal structure of the hippocampal formation on high resolution T2 weighted MRIs at 4 T. These internal structures can be used to measure several hippocampal subfields in vivo. (2) Using this method, we found an age related volume loss in CA1 which was most pronounced in the seventh decade of life. This suggests that the age related loss of total hippocampal volume is mainly driven by a volume loss in a very circumscribed region of the hippocampus.
The first major finding was that the increased resolution and improved signal to noise ratio of a high field magnet allowed marking of the hippocampal subfields in vivo in a group of healthy controls spanning an age range of 60 years and in a small group of subjects suffering from AD. Previously, Adachi et al. [1] used a multishot diffusion weighted imaging sequence on a 1.5 T magnet that delineates details of the internal structure of the hippocampal formation due to the different diffusion properties of water in different tissue types. Their study compared AD subjects with age-matched healthy controls in which they measured the width of different subfields at specified locations on one slice. In contrast, we used the increased gray/white matter contrast due to higher signal to noise at 4 T and additional magnetization transfer effects and T1 weightening effects of an optimized imaging sequence to delineate the inner structure of the hippocampus. Furthermore, we derived subfield volumes by measuring subfield areas on three continuous slices. Volumetric measurements are less influenced by shape variations or other anatomical irregularities, e.g. cysts in the vestigial hippocampal sulcus (cf. Fig. 2b) and thus more robust than thickness measurements. Previous MR studies [14,29] suggested that the hippocampal head is particularly susceptible to age related changes. Compared to the body and the tail, the anatomy of the internal structures of the head is rather complicated which makes a reliable identification of subfields without resolution of the different histological layers difficult. However, even at high field strength this is not possible in vivo without prohibitively long acquisition time [6]. Therefore, we used the vestigial hippocampal sulcus to determine the width of the subfields and arbitrary boundaries based on easily recognizable anatomical landmarks to subdivide the hippocampus into sectors corresponding approximately to the different subfields. The resolution of the leptomeningeal tissue in the vestigial hippocampal sulcus was good along the entire length of the hippocampus. However, because manual marking of the complete structure would have been very time consuming without necessarily providing additional information, we decided to restrict the measurements to the anterior portion of the hippocampal body. This subfield marking scheme, although lacking accurate histological definition, was highly reproducible between and within two raters.
Using this marking scheme, we found a significant negative correlation between CA1 volumes and age in cognitively normal subjects spanning an age range of six decades. The volume loss of CA1 was most pronounced in the seventh plus decade group. None of the other subfields showed a significant correlation with age. The findings that age effects on the hippocampal formation seem to be restricted rather than diffuse might explain why MR studies using whole hippocampal measurements yielded inconsistent results. Volume loss in gray matter structures in neuroimaging is generally equaled with neuron loss. This seems not necessarily to be true for the aging hippocampus as in the last years, several studies using quantitative unbiased stereology procedures for neuron counting suggested that the number of hippocampal pyramidal cells remains quite stable across the lifespan [26,47]. However, other age related changes, e.g., the loss of GABA-ergic interneurons [36], reductions of neuron sizes due to a decrease of synaptic density [3,10,37], astrocytosis [26,33], etc. could also result in hippocampal volume loss. There are several reasons why CA1 might be particularly vulnerable to age effects, e.g. a high concentration of glutamatergic N-methyl-D-aspartate receptors [19], an age-associated increase of L-type Ca2+ channel activity and thus elevated intracellular Ca2+ [42], a high intrinsic production of reactive oxygen species [45], reduced expression of protective factors like heat shock cognate protein or brain derived neurotrophic factor [11,44], lower levels of estrogen receptor levels and thus estrogen mediated neuroprotection [24,27], etc. Age related changes and age related vulnerabilities have also been described for other hippocampal subfields, particularly for the subiculum [47], CA3 [8,19] and the dentate gyrus/hilus [35,39,46,47]. We did not find age effects for these subfields but this might be due to limitations in the marking scheme. For example, it is possible that the way how the border between the subiculum and CA1 was defined (see Section 2) and the fact that the subiculum was in some cases distorted by artifacts from the posterior cerebral artery prevented us from detecting an age effect in this region. We also cannot exclude that by combining the CA3 and CA4 we might have missed subtle age effects in one of the two.
In contrast to aging, it is generally acknowledged that significant hippocampal neuronal cell loss, particularly in CA1 but also in the subiculum, is a typical finding in AD [47,48]. The number of AD subjects in this study is very small, but CA1 and the subiculum of the AD subjects were markedly smaller than those of the age-matched controls while the other subfields were in the normal range for this age group. This is also consistent with the findings of Adachi et al. [1]. Taken together, our results suggest that measuring subfields on high resolution images might eventually help to distinguish between normal aging and AD in vivo. However, a larger population of healthy controls and AD subjects needs to be studied to confirm these preliminary findings.
This study has several limitations. (1) The sample size is relatively small because the study was primarily designed as a feasibility study. (2) The study was cross-sectional in design and thus baseline volumes of each subject are not known. Therefore, biological variations of subfield sizes between the age groups could mimic age-related changes, e.g. subjects born 70 years ago might have been exposed to environmental influences associated with a reduced size of CA1. Furthermore, we cannot exclude that some of the individuals in the oldest group might have been suffering from pre-clinical AD, which could have contributed to the fact that we found the strongest age-related effect in this group. (3) Hippocampal subfields were only marked in a relatively small region of the anterior hippocampus. It cannot be ruled out that aging or other disease processes, e.g. vascular disease, affect hippocampal subfields in different parts of the hippocampus differently and thus will be missed by marking only a part of the whole structure. (4) We changed RF coils during the study. However, age and gender distribution of the subjects were similar (TEM coil: f/m: 3/6 mean age: 52.13 ± 18.4, 8-channel coil: f/m: 13/23, mean age: 46.3 ± 21.6) in both groups and the findings remained when each group was analysed separately.
In conclusion, these preliminary results suggest that hippocampal subfields can be reliably identified on high resolution MRI. The major finding was a significant negative correlation between age and CA1 but not other subfields, suggesting a selective vulnerability of CA1 to age effects. Further studies are needed to confirm this finding and determine the value of these measurements for the differentiation between normal aging and early AD or AD and other diseases which affect hippocampal subfields, e.g. stress, vascular disease, etc.
Acknowledgments
The authors wish to thank Ms. Crystal Paul and Ms. Sabrina Fox for expert technical assistance, Ms. Jennifer Hlavin and Jessica Black for subject recruitment and Dr. John Kornak for statistical advice. The study was supported by grant RO1 AG010897 to Dr. M.W. Weiner. The study was approved by the committee of human research at the University of California, San Francisco (UCSF), and written informed consent was obtained from all participating subjects or their legal representatives according to the Declaration of Helsinki.
References
- 1.Adachi M, Kawakatsu S, Hosoya T, Otani K, Honma T, Shibata A, et al. Morphology of the inner structure of the hippocampal formation in Alzheimer’s disease. Am J Neuroradiol. 2003;24:1575–81. [PMC free article] [PubMed] [Google Scholar]
- 2.Christensen H. What cognitive changes can be expected with normal aging? Aust NZ J Psychiatry. 2001;35:768–75. doi: 10.1046/j.1440-1614.2001.00966.x. [DOI] [PubMed] [Google Scholar]
- 3.Davies HA, Kelly A, Dhanrajan TM, Lynch MA, Rodriquez JJ, Stewart MG. Synaptophysin immunogold labeling of synapses decreases in dentate gyrus of the hippocampus of aged rats. Brain Res. 2003;986:191–5. doi: 10.1016/s0006-8993(03)03251-7. [DOI] [PubMed] [Google Scholar]
- 4.De Vita E, Thomas DL, Roberts S, Parkes HG, Turner R, Kinchesh P, et al. High resolution MRI of the brain at 4.7 Tesla using fast spin echo imaging. Br J Radiol. 2003;76:631–7. doi: 10.1259/bjr/69317841. [DOI] [PubMed] [Google Scholar]
- 5.Duvernoy HM. The human hippocampus. Functional anatomy, vascularization and serial sections with MRI. 3rd ed. Berlin: Springer Verlag; 2005. [Google Scholar]
- 6.Fatterpekar GM, Naidich TP, Delman BN, Aguiraldo JG, Gultekin SH, Sherwood CC, et al. Cytoarchitecture of the human cerebral cortex: MR microscopy of excised specimens at 9.4. Tesla Am J Neuroradiol. 2002;23:1313–21. [PMC free article] [PubMed] [Google Scholar]
- 7.Foster TC. Involvement of hippocampal synaptic plasticity in age-related memory decline. Brain Res Rev. 1999;30:216–49. doi: 10.1016/s0165-0173(99)00017-x. [DOI] [PubMed] [Google Scholar]
- 8.Fuchs E, Flügge G, Ohl F, Lucassen P, Vollmann-Honsdorf GK, Michaelis T. Psychosocial stress, glucocorticoids and structural alterations in the tree shrew hippocampus. Physiol Behav. 2001;73:285–91. doi: 10.1016/s0031-9384(01)00497-8. [DOI] [PubMed] [Google Scholar]
- 9.Fukutani Y, Kobayashi K, Nakamura I, Watanabe K, Isaki K, Cairns NJ. Neurons, intracellular and extracellular neurofibrillary tangles in subdivisions of the hippocampal cortex in normal ageing and Alzheimer’s disease. Neurosci Lett. 1995;200:57–60. doi: 10.1016/0304-3940(95)12083-g. [DOI] [PubMed] [Google Scholar]
- 10.Geinisman Y, De Toledo-Morrell L, Morrell F, Persina IS, Rossi M. Age-related loss of axospinous synapses formed by two afferent systems in the rat dentate gyrus as revealed by the unbiased stereological dissector technique. Hippocampus. 1992;2:437–44. doi: 10.1002/hipo.450020411. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi M, Mitsunaga F, Ohira K, Shimizu K. Changes in BDNF-immunuoreactive structures in the hippocampal formation of aged macaque monkey. Brain Res. 2001;918:191–6. doi: 10.1016/s0006-8993(01)03002-5. [DOI] [PubMed] [Google Scholar]
- 12.Head D, Snyder AZ, Girton LE, Morris JC, Buckner RL. Fronto-hippocampal double dissociation between normal aging and Alzheimer’s disease. Cerebral Cortex. 2005;15:732–9. doi: 10.1093/cercor/bhh174. [DOI] [PubMed] [Google Scholar]
- 13.Hsu YY, Schuff N, Du AT, Mark K, Zhu X, Hardin D, et al. Comparison of automated and manual MRI volumetry of hippocampus in normal aging and dementia. J Magn Reson Imaging. 2002;16:305–10. doi: 10.1002/jmri.10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jack CR, Petersen RC, Xu YC, Waring SC, O’Brien PC, Tangalos EG, et al. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer’s disease. Neurology. 1997;786:794. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jack CR, Petersen RC, Xu YC, O’Brien PC, Smith GE, Ivnik RJ, et al. Prediction of AD with MRI based-hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jack CR, Dickson DW, Parisi JE, Xu YC, Cha RH, O’Brien PC, et al. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology. 2002;58:750–7. doi: 10.1212/wnl.58.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Insausti R, Juottonen K, Soininen H, Insausti AM, Partanen K, Vainio P, et al. MR volumetric analysis of the human entorhinal, perirhinal and temporopolar cortices. Am J Neuroradiol. 1998;19:659–71. [PMC free article] [PubMed] [Google Scholar]
- 18.Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, et al. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging. 2001;11:581–94. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- 19.Kadar T, Dachir S, Shukitt-Hale B, Levy A. Sub-regional hippocampal vulnerability in various animal models leading to cognitive dysfunction. J Neural Transm. 1998;105:987–1004. doi: 10.1007/s007020050107. [DOI] [PubMed] [Google Scholar]
- 20.Kesner RP, Lee I, Gilbert P. A behavioral assessment of hippocampal function based on a subregional analysis. Rev Neurosci. 2004;15:333–51. doi: 10.1515/revneuro.2004.15.5.333. [DOI] [PubMed] [Google Scholar]
- 21.Keuker JIH, De Birrun G, Luiten PGM, Fuchs E. Preservation of hippocampal neuron numbers and hippocampal subfield volumes in behaviorally characterized ages tree shrews. J Comp Neurol. 2004;468:509–17. doi: 10.1002/cne.10996. [DOI] [PubMed] [Google Scholar]
- 22.Landfield PW, Braun ID, Pitler TA, Lindsay JD, Lynch G. Hippocampal aging in rats: a morphometric study of multiple variables in semithin sections. Neurobiol Aging. 1981;4:265–75. doi: 10.1016/0197-4580(81)90034-8. [DOI] [PubMed] [Google Scholar]
- 23.Lindeboom J, Weinstein H. Neuropsychology of cognitive ageing, minimal cognitive impairment, Alzheimer’s disease and vascular cognitive impairment. Eur J Pharmacol. 2004;490:83–6. doi: 10.1016/j.ejphar.2004.02.046. [DOI] [PubMed] [Google Scholar]
- 24.Mehra RD, Sharma K, Nyakas C, Vij U. Estrogen receptor alpha and beta immunoreactive neurons in normal adult and aged female rat hippocampus: a qualitative and quantitative study. Brain Res. 2005;1056:22–35. doi: 10.1016/j.brainres.2005.06.073. [DOI] [PubMed] [Google Scholar]
- 25.Miller DB, O’Callaghan JP. Effects of aging and stress on hippocampal structure and function. Metabolism. 2003;52(10 Suppl 2):17–21. doi: 10.1016/s0026-0495(03)00296-8. [DOI] [PubMed] [Google Scholar]
- 26.Miller DB, O’Callaghan JP. Aging, stress and the hippocampus. Age Res Rev. 2005;4:123–40. doi: 10.1016/j.arr.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Platha WC, Clark DL, Colbourne F. 17 beta estradiol pretreatment reduces CA1 sector cell death and the spontaneous hyperthermia that follows forebrain ischemia in the gebril. Neuroscience. 2004:187–93. doi: 10.1016/j.neuroscience.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 28.Price JL, Ko A, Wade MJ, Tsou SK, McKeel D, Morris JC. Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer disease. Arch Neurol. 2001;58:1395–402. doi: 10.1001/archneur.58.9.1395. [DOI] [PubMed] [Google Scholar]
- 29.Pruessner JC, Collins DL, Pruessner M, Evans AC. Age and gender predict volume decline in the anterior and posterior hippocampus in early adulthood. J Neurosci. 2001;21:194–200. doi: 10.1523/JNEUROSCI.21-01-00194.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pruessner JC, Köhler S, Crane J, Pruessner M, Lord C, Byrne A, et al. Volumetry of temporopolar, perirhinal, entorhinal and parahippocampal cortex from high resolution MRI images considering the variability of the collateral sulcus. Cerebral Cortex. 2002;12:1342–53. doi: 10.1093/cercor/12.12.1342. [DOI] [PubMed] [Google Scholar]
- 31.Rasmussen T, Schliemann T, Sorensen JC, Zimmer J, West MJ. Memory impaired aged rats: no loss of principal hippocampal and subicular neurons. Neurobiol Aging. 1996;17:143–7. doi: 10.1016/0197-4580(95)02032-2. [DOI] [PubMed] [Google Scholar]
- 32.Rössler M, Zarski M, Bohl J, Ohm TG. Stage dependent and sector specific neuronal loss in hippocampus during Alzheimer’s disease. Acta Neuropathol. 2002;103:363–9. doi: 10.1007/s00401-001-0475-7. [DOI] [PubMed] [Google Scholar]
- 33.Sato Y, Yamanaka H, Toda T, Shinohara Y, Endo T. Comparison of hippocampal synaptosome proteins in young–adult and aged rats. Neurosci Lett. 2005;382:22–6. doi: 10.1016/j.neulet.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 34.Schuff N, Amend DL, Knowlton R, Norman D, Fein G, Weiner MW. Age-related metabolic changes and volume loss in the hippocampus by magnetic resonance spectroscopy and imaging. Neurobiol Aging. 1999;20:279–85. doi: 10.1016/s0197-4580(99)00022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shetty AK, Turner DA. Vulnerability of the dentate gyrus to aging and intracerebroventricular administration of kainic acid. Exp Neurol. 1999;158:491–503. doi: 10.1006/exnr.1999.7107. [DOI] [PubMed] [Google Scholar]
- 36.Shi L, Argenta AE, Winseck AK, Brunso-Bechtold JK. Stereological quantification of GAD 67 immunoreactive neurons and boutons in the hippocampus of middle-aged and old Fisher 344 × Brown Norway rats. J Comp Neurol. 2004;478:282–91. doi: 10.1002/cne.20303. [DOI] [PubMed] [Google Scholar]
- 37.Shi L, Linville MC, Tucker EW, Sonntag WE, Brunso-Bechtold JK. Differential effects of aging and insulin-like growth factor-1 on synapses in CA1 of rat hippocampus. Cerebral Cortex. 2005;15:571–7. doi: 10.1093/cercor/bhh158. [DOI] [PubMed] [Google Scholar]
- 38.Simic G, Kostovic I, Winblad B, Bogdanovic N. Volume and number of neurons of the human hippocampal formation in normal aging and Alzheimer’s disease. J Comp Neurol. 1997;379:482–94. doi: 10.1002/(sici)1096-9861(19970324)379:4<482::aid-cne2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 39.Small SA, Chawla MK, Buoncore M, Rapp PR, Barnes CA. Imaging correlates of brain function in monkeys and rats isolates a hippocampal subregion differentially vulnerable to aging. Proc Natl Acad Sci. 2004;101:7181–6. doi: 10.1073/pnas.0400285101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Age-related decline in MRI volumes of temporal lobe gray matter but not hippocampus. Neurobiol Aging. 1995;16:591–606. doi: 10.1016/0197-4580(95)00074-o. [DOI] [PubMed] [Google Scholar]
- 41.Sullivan EV, Marsh L, Pfefferbaum A. Preservation of hippocampal volume throughout adulthood in healthy men and women. Neurobiol Aging. 2005;26:1093–8. doi: 10.1016/j.neurobiolaging.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 42.Thibault O, Landfield PW. Increase in single L-type calcium channels in hippocampal neurons during aging. Science. 1996;272:1017–20. doi: 10.1126/science.272.5264.1017. [DOI] [PubMed] [Google Scholar]
- 43.Thomas DL, De Vita E, Roberts S, Turner R, Yousry TA, Ordidge RJ. High resolution fast spin echo imaging of the human brain at 4.7 T: implementation and sequence characteristics. Magn Reson Med. 2004;51:1254–64. doi: 10.1002/mrm.20106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Toghi H, Utsugisawa K, Yoshimura M, Yamagata M, Nagane Y. Heat shock cognate 70 messenger RNA expression in postmortem human hippocampus: regional differences and age-related changes. Neurosci Lett. 1995;196:89–92. doi: 10.1016/0304-3940(95)11854-p. [DOI] [PubMed] [Google Scholar]
- 45.Wang X, Pal R, Chen XW, Limpeanchob N, Kumar KN, Michaelis EK. High intrinsic oxidative stress may underlie selective vulnerability of the hippocampal CA1 region. Brain Res Mol Brain Res. 2005 doi: 10.1016/j.molbrainres.2005.07.018. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 46.Wegiel J, Kuchna I, Wisniewski T, de Leon MJ, Reisberg B, Pirttila T, et al. Vascular fibrosis and calcification in the hippocampus in aging, Alzheimer disease, and down syndrome. Acta Neuropathol. 2002;103:333–43. doi: 10.1007/s00401-001-0471-y. [DOI] [PubMed] [Google Scholar]
- 47.West MJ, Coleman PD, Flood DG, Troncoso JC. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer’s disease. Lancet. 1994;344:769–72. doi: 10.1016/s0140-6736(94)92338-8. [DOI] [PubMed] [Google Scholar]
- 48.West JM, Kawas CH, Stewart WF, Rodow GL, Troncoso JC. Hippocampal neurons in pre-clinical Alzheimer’s disease. Neurobiol Aging. 2004;25:1205–12. doi: 10.1016/j.neurobiolaging.2003.12.005. [DOI] [PubMed] [Google Scholar]