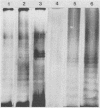
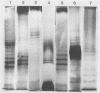
Abstract
Net acid-generating capacities of 39.74 kg of H2SO4 per ton (ca. 0.05 kg/kg) (pH 2.68) for the Lemoine copper mine tailings (closed ca. 8 years ago; located 40 km west of Chibougamau, Quebec, Canada) and 16.07 kg of H2SO4 per ton (ca. 0.02 kg/kg) (pH 3.01) for the Copper Rand tailings (in current use and 50 km distant [east] from those of Lemoine) demonstrate that these sulfide tailings can support populations of acidophilic thiobacilli. Oxidized regions in both tailings environments were readily visible, were extremely acidic (Lemoine, pH 2.36; Copper Rand, pH 3.07), and provided natural isolates for our study. A 10% (wt/vol) oxalic acid treatment, which solubilizes both ferric sulfate and ferric hydroxide precipitates (B. Ramsay, J. Ramsay, M. deTremblay, and C. Chavarie, Geomicrobiol. J. 6:171-177, 1988), enabled the recovery of intact bacterial cells from the tailings material and from liquid synthetic medium for lipopolysaccharide analysis. No viable cells could be cultured after this oxalic acid treatment. Sodium dodecyl sulfate-polyacrylamide gel electro-phoretic profiles of lipopolysaccharides extracted from the Lemoine tailings were complex, indicating a heterogeneous population of Thiobacillus ferrooxidans. Six T. ferrooxidans subspecies as identified by lipopolysaccharide analysis (i.e., lipopolysaccharide chemotypes) were eventually isolated from a total of 112 cultures from the Lemoine tailings. Using the same isolate and lipopolysaccharide typing techniques, we identified only a single lipopolysaccharide chemotype from 20 cultures of T. ferrooxidans isolated from the Copper Rand tailings. This homogeneity of lipopolysaccharide chemotype was much different from what was found for the older Lemoine tailings and may reflect a progressive lipopolysaccharide heterogeneity of Thiobacillus isolates as tailings leach and age.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldi F., Clark T., Pollack S. S., Olson G. J. Leaching of Pyrites of Various Reactivities by Thiobacillus ferrooxidans. Appl Environ Microbiol. 1992 Jun;58(6):1853–1856. doi: 10.1128/aem.58.6.1853-1856.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. C., Tributsch H. Bacterial leaching patterns on pyrite crystal surfaces. J Bacteriol. 1978 Apr;134(1):310–317. doi: 10.1128/jb.134.1.310-317.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester I. R., Meadow P. M. Heterogeneity of the lipopolysaccharide from Pseudomonas aeruginosa. Eur J Biochem. 1975 Oct 15;58(2):273–282. doi: 10.1111/j.1432-1033.1975.tb02373.x. [DOI] [PubMed] [Google Scholar]
- Harrison A. P., Jr, Jarvis B. W., Johnson J. L. Heterotrophic bacteria from cultures of autotrophic Thiobacillus ferrooxidans: relationships as studied by means of deoxyribonucleic acid homology. J Bacteriol. 1980 Jul;143(1):448–454. doi: 10.1128/jb.143.1.448-454.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison A. P., Jr The acidophilic thiobacilli and other acidophilic bacteria that share their habitat. Annu Rev Microbiol. 1984;38:265–292. doi: 10.1146/annurev.mi.38.100184.001405. [DOI] [PubMed] [Google Scholar]
- Hirt W. E., Vestal J. R. Physical and chemical studies of Thiobacillus ferroxidans lipopolysaccharides. J Bacteriol. 1975 Aug;123(2):642–650. doi: 10.1128/jb.123.2.642-650.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingledew W. J. Thiobacillus ferrooxidans. The bioenergetics of an acidophilic chemolithotroph. Biochim Biophys Acta. 1982 Nov 30;683(2):89–117. doi: 10.1016/0304-4173(82)90007-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane D. J., Stahl D. A., Olsen G. J., Heller D. J., Pace N. R. Phylogenetic analysis of the genera Thiobacillus and Thiomicrospira by 5S rRNA sequences. J Bacteriol. 1985 Jul;163(1):75–81. doi: 10.1128/jb.163.1.75-81.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- SILVERMAN M. P., LUNDGREN D. G. Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans. I. An improved medium and a harvesting procedure for securing high cell yields. J Bacteriol. 1959 May;77(5):642–647. doi: 10.1128/jb.77.5.642-647.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader J. A., Holmes D. S. Phenotypic switching of Thiobacillus ferrooxidans. J Bacteriol. 1988 Sep;170(9):3915–3923. doi: 10.1128/jb.170.9.3915-3923.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively J. M., Decker G. L., Greenawalt J. W. Comparative ultrastructure of the thiobacilli. J Bacteriol. 1970 Feb;101(2):618–627. doi: 10.1128/jb.101.2.618-627.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer P. C., Stumm W. Acidic mine drainage: the rate-determining step. Science. 1970 Feb 20;167(3921):1121–1123. doi: 10.1126/science.167.3921.1121. [DOI] [PubMed] [Google Scholar]
- Southam G., Beveridge T. J. Enumeration of Thiobacilli within pH-Neutral and Acidic Mine Tailings and Their Role in the Development of Secondary Mineral Soil. Appl Environ Microbiol. 1992 Jun;58(6):1904–1912. doi: 10.1128/aem.58.6.1904-1912.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki I., Takeuchi T. L., Yuthasastrakosol T. D., Oh J. K. Ferrous Iron and Sulfur Oxidation and Ferric Iron Reduction Activities of Thiobacillus ferrooxidans Are Affected by Growth on Ferrous Iron, Sulfur, or a Sulfide Ore. Appl Environ Microbiol. 1990 Jun;56(6):1620–1626. doi: 10.1128/aem.56.6.1620-1626.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestal J. R., Lundgren D. G., Milner K. C. Toxic and immunological differences among lipopolysaccharides from Thiobacillus ferrooxidans grown autotrophically and heterotrophically. Can J Microbiol. 1973 Nov;19(11):1335–1339. doi: 10.1139/m73-215. [DOI] [PubMed] [Google Scholar]
- Wang W. S., Korczynski M. S., Lundgren D. G. Cell envelope of an iron-oxidizing bacterium: studies of lipopolysaccharide and peptidoglycan. J Bacteriol. 1970 Oct;104(1):556–565. doi: 10.1128/jb.104.1.556-565.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]