Abstract
In recent years, much progress has been made with respect to the unravelling of the functions of peroxisomes in metabolism, and it is now well established that peroxisomes are indispensable organelles, especially in higher eukaryotes. Peroxisomes catalyse a number of essential metabolic functions including fatty acid β-oxidation, ether phospholipid biosynthesis, fatty acid α-oxidation and glyoxylate detoxification. The involvement of peroxisomes in these metabolic pathways necessitates the transport of metabolites in and out of peroxisomes. Recently, considerable progress has been made in the characterization of metabolite transport across the peroxisomal membrane. Peroxisomes posses several specialized transport systems to transport metabolites. This is exemplified by the identification of a specific transporter for adenine nucleotides and several half-ABC (ATP-binding cassette) transporters which may be present as hetero- and homo-dimers. The nature of the substrates handled by the different ABC transporters is less clear. In this review we will describe the current state of knowledge of the permeability properties of the peroxisomal membrane.
Keywords: fatty acid, genetic disease, metabolite, peroxisome, transport, Zellweger syndrome
Abbreviations: ABC, ATP-binding cassette; CPT, carnitine palmitoyltransferase; DHAS, dihydroxyacetone synthetase; DHCA, dihydroxycholestanoic acid; DNP, 2,4-dinitrophenol; G3PDH, glycerol-3-phosphate dehydrogenase; GOT, glutamate:aspartate aminotransferase; LACS, long-chain acyl-CoA synthetase; MCF, mitochondrial carrier family; MCFA, medium-chain fatty acid; MCT, monocarboxylate transporter; MDH, malate dehydrogenase; M-LP, Mpv17-like protein; PMP, peroxisomal membrane protein; ROS, reactive oxygen species; SCaMC, short calcium-binding mitochondrial carrier; THCA, trihydroxycholestanoic acid; XALD, X-linked adrenoleukodystrophy
INTRODUCTION
Much progress has been made in unravelling the role of peroxisomes in metabolism. In mammals, peroxisomes are now known to participate in fatty acid α- and β-oxidation, the biosynthesis of ether phospholipids and bile acids and in the degradation of purines, polyamines, L-pipecolic acid and D-amino acids [1]. Metabolic functions of peroxisomes in other organisms include methanol degradation (yeast), glyoxylate cycle (plants, yeasts), penicillin biosynthesis (fungi), hormone biosynthesis (plants) and karyogamy (fungi) [2–5]. Although it was previously thought that peroxisomes also play a role in cholesterol and dolichol synthesis, it has since been shown that this is not the case [6–11].
On the basis of our current knowledge of peroxisomal metabolism, predictions can be made with respect to the nature of the metabolites that need to pass across the peroxisomal membrane. In recent years, it has become clear that such metabolite transport is facilitated by at least several metabolite transporters. Indeed, a number of transporters have been identified in the peroxisomal membrane of various species. In the present paper, we review peroxisomal metabolite transport in relation to the metabolic pathways known to reside in this organelle, most notably fatty acid oxidation.
Fatty acid α- and β-oxidation are among the best studied metabolic functions of peroxisomes. Defects in either pathway are causative for a range of severe human disorders [12].
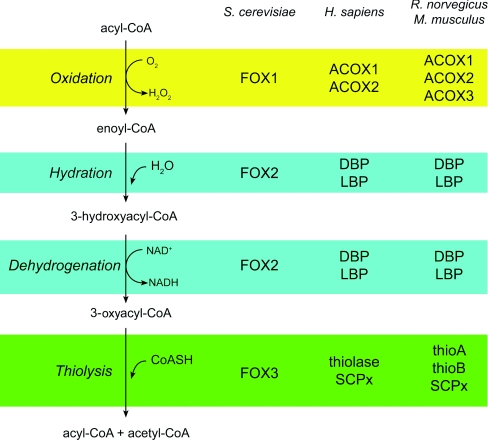
β-Oxidation is a cyclic process by which fatty acids are degraded from the C-terminal end (Figure 1). Each cycle of β-oxidation shortens the fatty acid carbon chain by two carbon atoms, releasing an acetyl-CoA unit (or a propionyl-CoA unit if a 2-methyl group is present). One cycle of β-oxidation in peroxisomes consists of four reactions. In the yeast Saccharomyces cerevisiae, peroxisomal β-oxidation is carried out by three proteins. In mammalian species, including plants, humans and rodents, each step of peroxisomal β-oxidation is usually carried out by multiple enzymes. However, the four reactions that constitute one cycle of β-oxidation are essentially identical regardless of the fatty acid substrate.
Figure 1. Peroxisomal β-oxidation.
H. sapiens, Homo sapiens; M. musculus, Mus musculus; R. norvegicus, Rattus norvegicus. ACOX, acyl-CoA oxidase; DBP, D-bifunctional enzyme; FOX1, acyl-CoA oxidase; FOX2, bifunctional enzyme; FOX3, 3-oxoacyl-CoA thiolase (3-ketoacyl-CoA thiolase); LBP, L-bifunctional enzyme; SCPx, sterol carrier protein X; thioA, thiolase A; thioB, thiolase B.
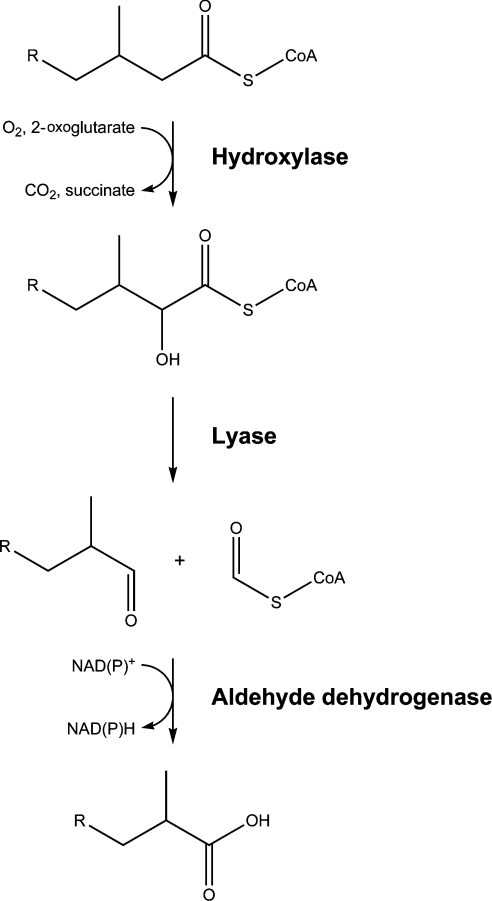
The presence of a 3-methyl group in certain fatty acids, such as phytanic acid (3,7,11,15-tetramethylhexadecanoic acid), interferes with β-oxidation. However, such fatty acids are a substrate for α-oxidation, which shortens the fatty acid by a single carbon atom (Figure 2). The resulting shortened fatty acid which now has the methyl group at the 2-position can enter β-oxidation and be degraded further. As shown, α-oxidation is carried out by three peroxisomal enzymes and yields a non-esterified (‘free’) fatty acid. This must then be reactivated to its CoA ester either within or outside of the peroxisome before entering β-oxidation (see [13,14] for reviews).
Figure 2. Peroxisomal α-oxidation.
Fatty acid import and the role of peroxisomal ABC (ATP-binding cassette) transporters
Available evidence holds that the uptake of fatty acids by peroxisomes does not take place in the form of a carnitine ester like in mitochondria. Indeed, C26:0-CoA and the CoA ester of the bile acid precursor trihydroxycholestanoic acid (which are exclusively β-oxidized in peroxisomes) are not converted into the respective carnitine esters by mitochondrial CPT1 (carnitine palmitoyltransferase I) [15], or by any other acyltransferase. In agreement with this, Jakobs and Wanders [16] reported that the addition of POCA {sodium 2-[5-(4-chlorphenyl)pentyl]-oxirane-2-carboxylate}, an inhibitor of CPT1, to human fibroblasts had virtually no effect on C26:0 β-oxidation, whereas it completely blocked C16:0 β-oxidation (which is performed exclusively by the mitochondrial β-oxidation system).
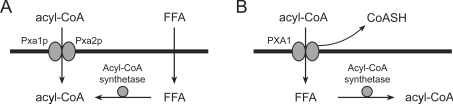
It is currently believed that fatty acids can enter the peroxisome either as non-esterified fatty acid or directly as a CoA ester. Most of our current understanding of the import of fatty acids destined for β-oxidation into the peroxisomal matrix comes from studies of the yeast S. cerevisiae. The peroxisomal membrane in S. cerevisiae contains two members of the superfamily of ABC transporters, Pxa1p and Pxa2p [17,18]. Hettema et al. [19] found that the β-oxidation of oleic acid (C18:1) is impaired in pxa1 and pxa2 gene-disruption mutants, but normal in cell lysates. This strongly suggested a function in a transport process required for peroxisomal β-oxidation. Moreover, since the β-oxidation of lauric acid (C12:0) was found to be completely normal in these knockouts, it was concluded that the defect occurs upstream of the β-oxidation process, presumably at the level of fatty acid import. These results suggest the existence of at least two distinct pathways for the import of fatty acids: (i) a Pxa1p/Pxa2p-independent pathway, which is utilized by lauric acid, and (ii) a Pxa1p/Pxa2p-dependent pathway, by which oleic acid is imported. It was also reported that the β-oxidation of lauric acid is impaired in a gene-disruption mutant which lacks Faa2p, a peroxisomal acyl-CoA synthetase, whereas oleic acid is normally β-oxidized by these cells [19,20]. This led to a model which proposes that lauric acid is imported as a non-esterified fatty acid that requires activation to its CoA ester by Faa2p, whereas oleic acid is activated in the extraperoxisomal space and imported as a CoA ester directly (Figure 3). This model was supported further by the observation that redirection of Faa2p to the cytosol [by disruption of its PTS1 (peroxisomal targeting signal type 1)] causes lauric acid β-oxidation to become fully Pxa1p/Pxa2p-dependent [19].
Figure 3. Models for the import of fatty acids into the peroxisome.
(A) Two routes for fatty acid import into peroxisomes of S. cerevisiae. (B) Model as proposed by Fulda et al. [21] for the import of fatty acids into peroxisomes of A. thaliana. FFA, non-esterified (‘free’) fatty acid.
Most fatty acids appear to be imported through both pathways to various extents. Two factors are probably decisive for which pathway is utilized by a specific fatty acid. First, the physiochemical properties of a fatty acid, in particular its solubility in a non-polar solvent, play an important role. It is well known that the permeability coefficient of small molecules is strongly correlated with their solubility in non-polar solvents relative to their solubility in water. Secondly, the substrate specificity and subcellular localization of the acyl-CoA synthetases present in a cell is a determining factor. As mentioned, expression of a cytosolic variant of the acyl-CoA synthetase Faa2p in S. cerevisiae shifts peroxisomal C12:0 import almost entirely to the Pxa1p/Pxa2p-dependent pathway.
The model described appears to explain the translocation of most fatty acids successfully. However, a few results have been reported that do not appear to be consistent with this model, suggesting that it may not be entirely correct.
Fulda et al. [21] reported the involvement of the peroxisomal full ABC transporter PXA1 and two peroxisomal long-chain acyl-CoA synthetases (LACS6 and LACS7) in the import of fatty acids into peroxisomes of Arabidopsis thaliana. Surprisingly, it was found that a β-oxidation defect occurs in plants lacking PXA1, but also in double-knockout mutants lacking both LACS6 and LACS7. An acyl-CoA synthetase single-knockout mutant (in which either LACS6 or LACS7 was disrupted) did not exhibit an observable phenotype. This suggested to the authors that the import of fatty acids for β-oxidation in this plant does not occur via two independent pathways, but requires the concerted action of both PXA1 and peroxisomal acyl-CoA synthetases. They propose a modified model where PXA1 transports and hydrolyses acyl-CoA esters, releasing a non-esterified fatty acid into the peroxisomal matrix which then needs to be re-activated by peroxisomal LACS (Figure 3B).
In this context, it is of interest to note that A. thaliana PXA1 is somewhat unusual when compared with its yeast and mammalian orthologues. While the orthologous peroxisomal ABC transporters in other organisms are half-ABC transporters (which are thought to dimerize to produce a functional transporter) the A. thaliana PXA1 is a full ABC transporter (which is believed to operate as a monomer). The significance of this marked difference is unknown, but clearly the situation in A. thaliana is not necessarily representative for other organisms.
Alternatively, the results of Fulda et al. [21] may also be explained if A. thaliana PXA1 would be involved in the transport of a cofactor required to sustain the activity of peroxisomal LACS (such as CoA, adenine nucleotides or phosphate). More insight into the actual substrate of A. thaliana PXA1 is clearly required to comprehend the significance of the observed results.
In mammalian species, four members of the ABC transporter superfamily have been identified in the peroxisomal membrane [ALDP/ABCD1, ALDR/ABCD2, PMP70 (70 kDa peroxisomal membrane protein)/ABCD3 and PMP69/ABCD4]. Of these, ABCD1 has been studied most intensively. Mutations in the gene ABCD1 lead to the severe human disorder XALD (X-linked adrenoleukodystrophy). It is thought that ABCD1 is involved in the import of CoA esters of very-long-chain fatty acids into the peroxisome, in a similar fashion as performed by Pxa1p/Pxa2p in S. cerevisiae. All of the four human peroxisomal ABC transporters are half-ABC transporters and are thought to dimerize to form functional transporters. Whether ABCD1 functions as a homo- or hetero-dimer remains in debate. Although it has been shown that various combinations of heterodimers may form in vitro [22,23], others have reported that ABCD1, ABCD2 and ABCD3 are predominantly homodimeric in vivo [24].
The function of the other three mammalian peroxisomal ABC transporters has remained largely unresolved. Various lines of evidence suggest that the function of ABCD2 overlaps to a significant extent with that of ABCD1, which is mainly based on two observations: (i) overexpression or induction of the transcription of ABCD2 partially relieves the biochemical phenotype observed in ABCD1-knockout cell lines, and (ii) the biochemical abnormalities observed in Abcd2-knockout mice resemble those observed in Abcd1-knockout mice, although they are less pronounced [25]. Furthermore, the biochemical abnormalities found in Abcd1-knockout mice can be restored by overexpression of Abcd2 [26].
Abcd3-knockout mice were found to accumulate the bile acid precursors THCA (trihydroxycholestanoic acid) and DHCA (dihydroxycholestanoic acid) (P. Vreken, G. H. K. Jimenez-Sanchez, D. Valle, S. Ferdinandusse and R. J. A. Wanders, unpublished work), strongly suggesting that Abcd3 is involved in the import of these compounds into the peroxisomes, presumably in the form of the corresponding CoA esters. Furthermore, the accumulation of pristanic acid in these mice suggests that Abcd3 may specifically be involved in the import of branched-chain substrates into the peroxisome. The function of ABCD4 remains unknown at this time.
Activation of imported non-esterified fatty acids: ANT1/PMP34
In 1990, McCammon et al. [27] identified a gene in the methylotrophic yeast Candida boidinii that codes for a peroxisomal membrane protein of 47 kDa (PMP47). Although its function was unclear at the time, it was soon recognized on the basis of primary sequence homology to be a member of the MCF (mitochondrial carrier family) (SLC25) [28]. Since then, orthologues have been identified in A. thaliana (PMP38) [29], humans (PMP34) [30] and S. cerevisiae (YPR128C/ANT1) [31].
The first indication of its function came from a study by Nakagawa et al. [32], who showed that the C. boidinii protein is required for the β-oxidation of lauric acid (C12:0), but not for the β-oxidation of oleic acid (C18:1). MCFAs (medium-chain fatty acids), such as lauric acid, are activated in the peroxisomal matrix by Faa2p, whereas oleic acid is most likely to be imported into the peroxisome as a CoA ester. Their results suggested that the protein is involved in the transport of a factor that is required for the intraperoxisomal activation of fatty acids. Subsequently, using the peroxisomal protein luciferase to detect intraperoxisomal ATP in vivo, van Roermund et al. [31] showed that the S. cerevisiae protein is probably involved in the transport of ATP. Ultimately, direct evidence that the human and S. cerevisiae proteins are indeed involved in the transport of adenine nucleotides was gained by reconstitution of the purified protein in liposomes, and following the uptake of radiolabelled substrates by these proteoliposomes [33,34].
As discussed, the model of Fulda et al. [21] for fatty acid import into the peroxisome via the A. thaliana peroxisomal ABC transporter PXA1 relies on the intraperoxisomal activation of fatty acids. However, in S. cerevisiae, the β-oxidation of fatty acids that utilize the Pxa1p/Pxa2p acyl-CoA import pathway (such as oleic acid, C18:1) is unaffected in ant1Δ cells, suggesting that an imported fatty acid is released into the lumen of the peroxisome as a CoA ester in this organism, and not as a non-esterified fatty acid as suggested by Fulda et al. [21].
Recently, more detailed studies of the properties of the S. cerevisiae protein suggest that it also participates in generating a pH gradient across the peroxisomal membrane [35,36].
Lastly, the proper localization of DHAS (dihydroxyacetone synthetase) was found to be disturbed in methanol-grown C. boidinii cells in which PMP47 was disrupted [37]. The localization of other peroxisomal proteins appeared to proceed normally. The role of PMP47 in the peroxisomal localization of DHAS has never been resolved.
Reducing equivalents: NAD+/NADH
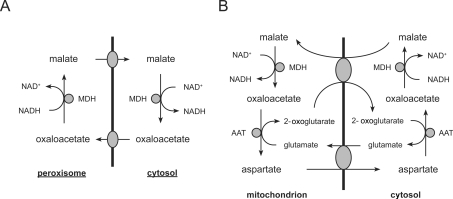
NAD+ is consumed during peroxisomal β-oxidation, yielding NADH. Persuasive evidence that the peroxisomal membrane is impermeant to NAD+ and NADH in vivo has come from certain S. cerevisiae gene disruption mutants. Van Roermund et al. [38] observed that disruption of the gene encoding peroxisomal MDH (malate dehydrogenase) (MDH3) blocks β-oxidation, whereas β-oxidation was found to proceed at a normal rate in cell lysates prepared from mdh3Δ cells. Moreover, they found an accumulation of the 3-hydroxyacyl-CoA β-oxidation intermediate, which indicates that the defect lies at the level of (NAD+-dependent) 3-hydroxyacyl-CoA dehydrogenase. Taken together, these results strongly suggest that NAD+ is not able to permeate the peroxisomal membrane, and that NAD+ is regenerated by malate dehydrogenase to meet the demand of β-oxidation.
Presumably, the substrates and products of Mdh3p, i.e. malate and oxaloacetate, are shuttled across the peroxisomal membrane (Figure 4A). A similar shuttle operates in mitochondria (Figure 4B). However, in cases where the mitochondrial membrane is impermeant to oxaloacetate, such as in rat heart [39], transport appears to occur instead in the form of aspartate which is generated by a mitochondrial GOT (glutamate:aspartate aminotransferase). After translocation, oxaloacetate is regenerated by cytosolic GOT. Interestingly, it has been demonstrated that peroxisomes of S. cerevisiae and various plant species [40–42] also contain aspartate aminotransferase, suggesting that a similar malate/aspartate shuttle may operate in peroxisomes. However, S. cerevisiae gene-disruption mutants lacking the peroxisomal aspartate aminotransferase (Aat2p) do not exhibit a β-oxidation defect [42]. In addition, expression of AAT2 is not enhanced by growth on oleate-containing medium [42], in contrast with Mdh3p and most other enzymes that perform a metabolic function required for fatty acid β-oxidation in S. cerevisiae. Therefore Aat2p does not appear to play an essential role in the malate/oxaloacetate redox shuttle.
Figure 4. Schematic representation of the regeneration of NAD+ in peroxisomes (A) and in mitochondria (B).
Abbreviation: AAT, glutamate:aspartate aminotransferase.
Mammalian peroxisomes apparently do not contain MDH. However, several alternative pathways can be envisioned that would be able to provide a supply of NAD+. The presence of NAD+-linked G3PDH (glycerol-3-phosphate dehydrogenase) activity has been demonstrated in peroxisomes of mammalian cells [43] and in the glycosomes of the trypanosome Trypanosoma brucei [44]. Therefore a shuttle based on G3PDH is also conceivable (Figure 5A). No experimental evidence is available to indicate whether this shuttle is responsible for the transfer of reducing equivalents in vivo.
Figure 5. Alternative shuttles for the regeneration of NAD+ in mammalian peroxisomes.
LDH, lactate dehydrogenase.
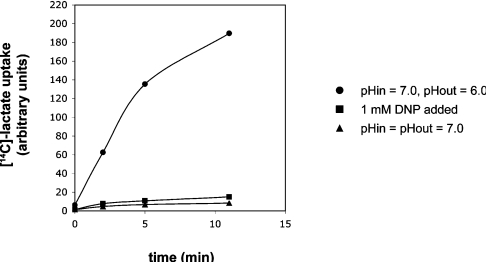
Alternatively, a shuttle can be postulated based upon peroxisomal lactate dehydrogenase (Figure 5B), which has been shown to be present in the peroxisomal matrix despite the fact that it does not possess a known peroxisomal targeting signal [45]. A partial peroxisomal localization of the MCTs (monocarboxylate transporters) Mct1 and Mct2 was found in rat liver [46,47]. It was also reported that the β-oxidation of a purified peroxisomal fraction in vitro is stimulated when pyruvate is supplied, and that this stimulation is partially inhibited by the addition of α-cyano-4-hydroxycinnamate, which is a known inhibitor of MCT proteins [46,47]. Osmundsen [48] also found a slight stimulation of β-oxidation upon the addition of pyruvate to isolated rat liver peroxisomes in vitro. The MCT family of transporters is thought to mediate the symport of protons and monocarboxylates across a membrane. However, some of our own findings indicate that lactate is able to diffuse across a lipid bilayer at a significant rate even without the aid of a transporter in its protonated, neutral form (Figure 6). Furthermore, this diffusion of lactate becomes directional when a ΔpH is applied, since a higher concentration of protonated lactate then exists at the acidic side of the membrane. This was readily demonstrated by monitoring the uptake of radiolabelled lactate into liposomes when a pH gradient was established across the membrane. The lactate uptake could be inhibited strongly by the addition of DNP (2,4-dinitrophenol), which dissipates the pH gradient. We found that pyruvate behaves similarly. These observations indicate that a transporter protein might not necessarily be required to mediate the transfer of lactate and pyruvate. Whether the rate of diffusion is sufficient to adequately supply NAD+ for β-oxidation under in vivo conditions remains to be established. Based on these findings, we conclude that caution should be taken in the interpretation of the results of in vitro experiments with isolated peroxisomes, such as those reported by Osmundsen [48], Brooks [46] and McClelland et al. [47].
Figure 6. Proton-coupled uptake of [14C]lactate by liposomes in the presence of an inwardly directed proton gradient (pHout=6.0, pHin=7.0), in the absence of a proton gradient (pHin=pHout=7.0), or after dissipation of the proton gradient by the addition of the protonophorous uncoupler DNP.
The liposomes were prepared from egg-yolk phosphatidylcholine, and the uptake of lactate was determined as described in [120]. The amount of radiolabelled lactate taken up by the proteoliposomes was measured at the indicated time points. An inwardly directed pH gradient was established across the liposomal membranes by the addition of HCl as required to lower the pH of the reaction medium to 6.0 at the start of the assay.
Reducing equivalents: NADP+/NADPH
Peroxisomal β-oxidation of unsaturated fatty acids with a double bond at an even position requires the activity of NADPH-dependent 2,4-dienoyl-CoA reductase [49]. Studies of S. cerevisiae gene-disruption mutants have yielded compelling evidence that the NADPH required for this reaction is supplied by peroxisomal isocitrate dehydrogenase (Idp3p).
Van Roermund et al. [50] and Henke et al. [51] have demonstrated that S. cerevisiae idp3Δ cells are deficient in the β-oxidation of fatty acids with double bonds at an even position, such as petroselenic acid (C18:1n−6), linoleic acid (C18:2n−6) and arachidonic acid (C20:4n−6). Oleic acid β-oxidation proceeds normally in these knockouts, indicating that the defect is specific for fatty acids with a double bond at an even position. Furthermore, van Roermund et al. [50] found that idp3Δ cells accumulate the 2,4-dienoyl-CoA precursor of the NADPH-dependent reaction.
These findings strongly suggested the existence of an isocitrate/2-oxoglutarate (2-ketoglutarate) shuttle in peroxisomes that is essential for the regeneration of NADPH. Very recently, the existence of a isocitrate/2-oxoglutarate antiporter has been demonstrated in mammalian peroxisomes [52]. Since mammalian peroxisomes also contain isocitrate dehydrogenase [53], it is very likely that a similar shuttle is responsible for the generation of NADPH in mammalian peroxisomes.
Export of β-oxidation products
Depending on the substrate that enters β-oxidation, several products may emerge. Degradation of a carboxyl chain yields acetyl-CoA and, if a 2-methyl-side chain is present, propionyl-CoA. Van Roermund et al. [38] used the model organism S. cerevisiae to study the transfer of acetyl and propionyl units from peroxisomes to mitochondria. In this organism, three pathways can be envisioned to provide an export route for acetyl units from the peroxisome: (i) entry into the glyoxylate cycle via peroxisomal citrate synthetase (Cit2p), (ii) conversion into a carnitine ester by peroxisomal carnitine acetyltransferase (Cat2p), and (iii) hydrolysis of acetyl-CoA to acetate and CoASH, followed by export of the acetate. It was observed that a double-knockout mutant lacking both the CIT2 and CAT2 genes exhibits a β-oxidation defect. In contrast, a normal β-oxidation activity was found in cell lysates of these cells, which suggested that the defect might lie at the level of membrane translocation. The single-gene-disruption mutants cit2Δ and cat2Δ each display only a slight decrease in the rate of β-oxidation, indicating that both proposed routes can contribute significantly to the export of acetyl-CoA. These results strongly suggest that acetyl- and propionyl-CoA are unable to traverse the peroxisomal membrane, but are converted into carnitine esters before transport out of the peroxisome. It should be noted that acetyl units may also leave the peroxisome through the glyoxylate cycle. A third route for the export of acyl units from the peroxisome involves the hydrolytic cleavage of acyl-CoA to free CoASH and a fatty acid by peroxisomal thioesterases. Mammalian peroxisomes contain several acyl-CoA thioesterases, which are capable of catalysing the hydrolysis of a wide range of substrates, including the CoA esters of long-, medium- and short-chain fatty acids, bile acids, branched-chain fatty acids and succinate [54,55]. Leighton et al. [56] reported that, in human hepatocytes, peroxisomal β-oxidation yields considerable amounts of free acetate via the action of an intraperoxisomal acetyl-CoA hydrolase activity, suggesting that, in these cells, acetyl units are predominantly exported from the peroxisome in the form of free acetate.
To obtain more insight in the transfer of propionyl units from peroxisomes to mitochondria in man, Jakobs and Wanders [57] supplied [1-14C]pristanic acid to fibroblasts of patients with a defect in the gene encoding mitochondrial carnitine/acylcarnitine translocase (CACT/SLC25A20) or CPT2 (CPT2). They observed that 14CO2 formation was completely deficient in the CACT-deficient cells, but not in the CPT2-deficient cells. This suggested that CACT participates in the transfer of propionyl units into the mitochondrion where they can be oxidized to yield CO2. It is therefore likely that acetyl- and propionyl-CoA units are exported from the peroxisome as carnitine esters in both yeast and humans. The transporter involved in the export of carnitine esters from the peroxisome remains to be identified, although it has been suggested that the human peroxisomal membrane contains an orthologue of murine OCTN3 [58], which has been reported to transport carnitine esters [59,60].
The distribution of glyoxylate cycle enzymes between the cytosol and peroxisome suggests the translocation of glyoxylate cycle intermediates across the peroxisomal membrane. Acetyl units produced by β-oxidation can enter the glyoxylate cycle on the peroxisomal side via Cit2p, which results in the release of a succinate moiety on the cytosolic side by cytosolic isocitrate lyase. Succinate can subsequently enter the mitochondrion via the mitochondrial fumarate/succinate transporter Acr1p for further oxidation, ultimately yielding CO2 and water [61].
In higher eukaryotes, including humans, there are only two mechanisms by which fatty acids can exit the peroxisome: (i) via a carnitine-dependent route, or (ii) via a thioesterase-dependent route. Which of these two routes is actually taken by a particular fatty acid depends first on whether or not the corresponding acyl-CoA ester can be converted into a carnitine ester, and, secondly, on the relative activity of the carnitine acyltransferase on one hand and that of the acyl-CoA thioesterase on the other hand. In human fibroblasts, for instance, most of the acetyl-CoA produced in peroxisomes follows the carnitine-dependent route, as may be concluded from the work of Jakobs and Wanders [57]. In contrast, in hepatocytes, most of the acetyl-CoA produced in peroxisomes follows the second route, as concluded from the findings of Leighton et al. [56]. The acetate formed in hepatic peroxisomes can be released and taken up and oxidized by other cells in the same way as ketone bodies produced in hepatocytes from fatty acids are exported to extrahepatic tissues for oxidation.
Acyl-CoA esters that cannot be converted into their respective carnitine esters, such as succinyl-CoA [54], require the thioesterase-dependent route to exit peroxisomes.
The biosynthesis of the acid intermediates choloyl-CoA and chenodeoxycholoyl-CoA proceeds via peroxisomal β-oxidation, and export of the cholate and chenodeoxycholate groups occurs after conjugation to glycine or taurine, followed by secretion into bile.
Import of other β-oxidation substrates
Jedlitschky et al. [62] observed that isolated peroxisomes are able to degrade the leukotriene ω-hydroxy-N-acetyl-leukotriene E4 (in the presence of appropriate cofactors) only when a microsomal fraction was added to provide an acyl-CoA synthetase activity. Schepers et al. [63] made a similar observation using prostaglandin E2. This may imply that these compounds, and perhaps related compounds, are activated in microsomes and subsequently imported into the peroxisome. How the transport of the CoA esters across the peroxisomal membrane is accomplished remains unclear. Ferdinandusse et al. [64] reported that the levels of leukotrienes B4 and E4 and their oxidation products are normal in the urine of XALD patients. Similarly, Mayatepek et al. [65] observed a normal leukotriene profile in the bile of XALD patients, suggesting that the ABC transporter ABCD1 is not required for the import of these compounds into the peroxisome.
The activation of the bile acid precursors to their respective CoA esters is required before β-oxidation. Mihalik et al. [66] reported that peroxisomal very-long-chain acyl-CoA synthetase is at least capable of this reaction. In contrast, both Prydz et al. [67] and Koibuchi et al. [68] found that THCA-CoA synthetase activity is localized exclusively to microsomes. Hence, whether bile acid precursors are imported into peroxisomes as CoA esters remains to be established. A strong indication, however, that the peroxisomal half-ABC transporter ABCD3 is involved in the import of the bile acid precursors DHCA and THCA into peroxisomes is provided by the observation that Abcd3-knockout mice accumulate β-methyl-substituted fatty acids such as pristanic acid and di- and tri-hydroxycholestanoic acid [68a].
pH of the peroxisomal lumen in vivo
Several groups have reported the existence of a pH gradient across the peroxisomal membrane in vivo, suggesting a restricted permeability of the peroxisomal membrane. However, the observed intraperoxisomal pH varies considerably. Early work by Nicolay et al. [69] using 31P-NMR suggested an acidic pH for the peroxisomal compartment of methanol-grown cells of the yeast Hansenula polymorpha. Their results were supported by Waterham et al. [70] using DAMP {N-(3-[(2,4-dinitrophenyl)-amino]propyl)-N-(3-aminopropyl)methylamine}-labelling of spheroplasts. More recently, Lasorsa et al. [35] also reported observing a low pH in the peroxisomes of S. cerevisiae cells grown on oleic acid, using a pH-sensitive fluorescent probe that is targeted to the peroxisome.
In contrast, Dansen et al. [71] also used peroxisomally targeted fluorescent probes to measure the pH of human fibroblasts, which they reported to be approx. 8.2. Using a similar technique, van der Lende et al. [72] found that peroxisomes in the filamentous fungus Penicillium chrysogenum are slightly alkaline.
Similarly, van Roermund et al. [36] observed an alkaline pH in S. cerevisiae cells grown on oleic acid/glycerol medium. Furthermore, the same authors have provided evidence to suggest that the observed pH gradient may assist in the import of fatty acids via the non-esterified fatty acid route.
PEX11
A number of proteins have been identified that appear to play a role in the biogenesis of peroxisomes, which have been designated ‘peroxins’ or PEX proteins. The role of the individual PEX proteins in peroxisomal biogenesis is largely unknown. The categorization ‘PEX’ gene has commonly been given to genes when aberrant peroxisome morphology was observed in a gene-disruption mutant. The possibility remains that some of the peroxins primarily serve a role in metabolism. In support of this possibility, abnormal morphology is also observed in oleate-grown S. cerevisiae gene-disruption mutants lacking for instance acyl-CoA oxidase (fox1Δ), 3-oxoacyl-CoA thiolase (fox3Δ) or the adenine nucleotide carrier (ant1Δ).
The role of Pex11p is under debate. All reports seem to agree that disruption of the PEX11 gene leads to a ‘giant peroxisome’ phenotype in which a reduced number of enlarged peroxisomes are formed. Conversely, overexpression results in an increased number of peroxisomes of reduced size [73–75].
Van Roermund et al. [76] suggested that Pex11p primarily performs a metabolic function in the degradation of MCFAs in S. cerevisiae, and that the observed morphology is a consequence of the metabolic defect. This finding suggests a role for PEX11 upstream of β-oxidation, most likely at the level of fatty acid activation to a CoA ester or transport of the fatty acid into the peroxisome.
In contrast, Li et al. [77,78] reported that the defect lies at the level of peroxisomal proliferation, at least in mice. However, the situation is complicated by the existence of three PEX11 isoforms in humans and mice: PEX11α, PEX11β and PEX11γ. Recently, two additional proteins with extensive homology with Pex11p have been identified in S. cerevisiae as well, named Pex25p and Pex27p [79]. Rottensteiner et al. [79] have proposed a role in biogenesis and proliferation for these proteins, based mainly on the finding that peroxisomal morphology and protein import is disturbed in gene-disruption mutants.
Evidence for a peroxisomal channel
Early investigations into the permeability of the peroxisomal membrane indicated that the membrane of isolated peroxisomes is highly permeant to low-molecular-mass compounds, including many substrates and products of peroxisomal metabolism [80,81]. To account for these observations, the existence of a peroxisomal channel-forming protein, or ‘porin’ was suggested [80,82].
Evidence in support of the existence of a peroxisomal channel was gained mainly by patch–clamp and black lipid bilayer experiments. The presence of channel-forming activity in peroxisomes obtained from rat and mouse liver [83,84], peroxisomes from the yeast H. polymorpha, and various plant species [85–88] has been described, although the properties of the reported channel activities has been found to vary considerably between species and methods.
A particularly interesting observation was made by Reumann et al. [85], who reported that the chloride-conductance of a channel activity found in spinach leaf peroxisomes is strongly inhibited by the addition of small amounts of specific anions. A particularly high affinity for certain dicarboxylic anions was observed, including 2-oxoglutarate, oxaloacetate, malate and succinate. This led them to suggest that the detected channel exhibits notable substrate specificity, which agrees strikingly well with our understanding of the malate/oxaloacetate and isocitrate/α-oxoglutarate shuttles (see above). The estimated channel diameter of approx. 0.5 nm is too small to allow the passage of large cofactors, such as NADH, CoASH or ATP. They also reported the presence of a similar channel in castor bean glyoxysomes [88].
Recently, Antonenkov et al. [89] reported detecting two distinct channels in purified mouse liver peroxisomes using planar lipid bilayer techniques with conductances of 1.3 and 2.5 nS. They hypothesize that these channel proteins facilitate the passage of low-molecular-mass compounds, whereas the transport of larger metabolites is performed by specific transporters.
Although numerous attempts have been made to purify and identify the putative peroxisomal channel, only partially purified fractions have been described [80,82].
Recently, however, a peroxisomal membrane protein with significant homology with channel-forming proteins was cloned from smooth brome grass (Bromus inermis Leyss) [90], which may account for some of the electrophysiological observations, particularly those made by Reumann et al. [85–88] as mentioned above. This protein is discussed in more detail below. However, although orthologues can be identified in certain plant species, it does not appear to have any orthologues in other species, and it is therefore unlikely to represent the putative peroxisomal channel in mammalian species. The expression pattern of this protein suggests it may serve a function in the transport of intermediates of the glyoxylate cycle, which is absent in mammals.
PMP22-related proteins
Early investigations into the constituents of the peroxisomal membrane identified a 22 kDa protein as an abundant component [91–93]. Combined, PMP70 and PMP22 constitute roughly half of the protein content of the membrane of rat liver peroxisomes [94]. Several related 22 kDa proteins have been identified in various species: rat Pmp22 [95], mouse Mpv17 [96], mouse Pmp22/Pxmp2 [97,98], mouse M-LP (Mpv17-like protein) [99], A. thaliana PMP22 [100], human PMP22 [101], human ML-P [102], human MPV17 [103] and S. cerevisiae YLR251W/Sym1p [104]. A splicing variant of mouse ML-P has also been reported [105].
All PMP22-related proteins are localized to peroxisomes, with the exception of the yeast protein Sym1p (W. F. Visser, unpublished work, and [104]), and human MPV17 [103], which are localized to the mitochondrial inner membrane, and the alternative splice variant of mouse ML-P which is localized to the cytosol [105]. Very recently, defects in human MPV17 have been identified as a cause of MDDS (mitochondrial DNA depletion syndrome) [102,106].
Van Veldhoven et al. [80] speculated that a peroxisomal 22 kDa protein might posses pore-forming activity, based on the observation that a protein fraction containing a 22 kDa PMP and other PMPs was shown to confer sucrose permeability when reconstituted in liposome membranes. However, the 22 kDa protein was not identified further, and their preparation contains multiple proteins.
Later studies have been performed to gain more insight into the function of the PMP22-related proteins. Zwacka et al. [96] described that an Mpv17-knockout mouse developed glomerulosclerosis, nephrotic syndrome [96], hypertension [107] and sensineural deafness [108]. Peroxisomal biogenesis appears to be normal in these mice, but a reduced level of ROS (reactive oxygen species) was observed. Overexpression of murine Mpv17 in transfected cells resulted in increased intracellular ROS. Consequently, a role in ROS regulation was proposed. In line with this hypothesis, Wagner et al. [109] found altered levels of antioxidant enzymes in these mice. Furthermore, transfection of COS-7 cells with another PMP22-related protein, murine M-LP, was reported to elevate the expression of SOD2 (manganese superoxide dismutase) [110]. Reuter et al. [111] found that the expression of MMP-2 (matrix metalloproteinase 2) is increased in the Mpv17-knockout mouse strain.
The mitochondrial yeast orthologue (Sym1p) has also been functionally characterized to some extent. sym1Δ cells were found to exhibit a growth defect on ethanol at elevated temperature [104]. Dysregulation of several ethanol-repressed genes was observed when the cells were cultured at 37 °C, compared with wild-type [104]. Expression of the murine Mpv17 in sym1Δ cells was found to complement the phenotype. Unfortunately, the subcellular localization of Mpv17 in these cells was not determined because attempts to produce an epitope-tagged protein were found to inactivate it, as was evident from the inability of the tagged protein to rescue the phenotype mentioned.
The putative S. cerevisiae ORF (open reading frame) YOR292C also bears significant sequence homology with other PMP22-related genes. The gene product from YOR292C was localized to the vacuole in a large-scale localization study [112]. However, in the light of the sensitivity of Mpv17 to the presence of an epitope tag, the influence of the rather large GFP (green fluorescent protein) fused to YOR292C in this study could have influenced the result. Furthermore, a putative ORE (oleate-responsive element) box can be identified in its promoter region (approx. 350 bp upstream of the start codon), suggesting a role for this protein in fatty acid metabolism.
Secretory pathway Ca2+/Mn2+-ATPase
Very recently, Southall et al. [113] reported that a YFP (yellow fluorescent protein)-tagged member of the secretory pathway family of Ca2+/Mn2+-ATPase proteins localizes to peroxisomes in Drosophila melanogaster. They found that overexpression of this protein alters calcium levels and excretion rates of cells, confirming that the protein is involved in calcium transport. However, the role of this protein in the peroxisomal membrane is unclear. Southall et al. [113] speculate that it may be required to provide Ca2+ and/or Mn2+ for matrix enzymes that depend on these ions, such as superoxide dismutase.
A peroxisomal channel in plants
Wu et al. [90] found a protein that localizes to peroxisomes in smooth brome grass that bears significant identity with various channel-forming proteins from other plant species. The subcellular localization of the native protein was determined using immunogold electron microscopy. The precise function of the protein was not established, but its expression were found to vary in response to cold and drought stress, the plant hormone ABA (abscisic acid), and during embryogesis. These observations lead Wu et al. [90] to propose that the channel protein could be required to allow gluconeogenesis via the products of peroxisomal β-oxidation and the glyoxylate cycle, possibly by transporting fatty acids or succinate. The channel appears not to have any orthologues in species other than plants, suggesting that it serves a function specific to these organisms.
PMP24
Reguenga et al. [114] isolated and identified a peroxisomal membrane protein of 24 kDa from rat liver. They subsequently identified a human orthologue in a cDNA library. The function of the protein is unknown. However, the protein does posses significant identity with certain bacterial transporters (e.g. 29% identity with a permease from Bacteroides thetaiotaomicron; GenBank® accession number AAO79343). Recently, Wu et al. [115] identified the gene in a screen for CpG sites that are differentially methylated and transcriptionally silenced in a comparison of two prostate cancer cell lines.
Short calcium-binding MCF member in rabbit (‘Efinal’)
Weber et al. [116] identified a putative calcium-binding member of the MCF (SLC25) of solute carriers from a rabbit small intestinal cDNA library, which they named ‘Efinal’. Using immunoelectron microscopy with an antibody generated against a fragment of the native protein they found a bimodal peroxisomal/mitochondrial localization. A function of the protein was not established. However, the calcium-binding properties inferred from the presence of four EF-hand motifs in the N-terminus were confirmed. Later studies by others identified similar proteins in other organisms. All consist of a N-terminal domain containing four EF-hand motifs and a C-terminal domain possessing all the characteristics of MCF proteins. The name short calcium-binding mitochondrial carrier (SCaMC) has been suggested for members of this subfamily [117]. So far, other SCaMC members identified were reported to localize exclusively to mitochondria [117,118]. Fiermonte et al. [119] recently characterized three closely related human SCaMC proteins in vitro and found that they catalyse a calcium-stimulated Mg-ATP/Pi antiport reaction.
Concluding remarks
We now know that a number of specific transporters facilitate metabolite transport into and out of the peroxisome. In particular, considerable progress has been made in recent years towards identifying the various peroxisomal transporters that are required to sustain peroxisomal fatty acid degradation via the α- and β-oxidation pathways, including ABCD1 and Ant1p/PMP34. The existence of model organisms (S. cerevisiae mutants, knockout mice) and in vitro transport studies have contributed greatly to elucidating the role of the transporters involved. Much less is known about the transport of metabolites required to support other metabolic pathways residing in mammalian peroxisomes, including ether phospholipid and bile acid biosynthesis, the degradation of D-amino acids, purines and polyamines. Predictions can be made regarding the nature of the transporters that may exist in the peroxisomal membrane based upon the subcellular distribution of enzymes involved in these pathways. It then becomes obvious that our understanding of peroxisomal metabolite transport is still limited. It is very likely that future research will reveal additional transporters, beyond the few that have been identified to date. In addition, some of the transporters known to reside in the peroxisomal membrane remain poorly characterized. Clearly, resolving the function of these transporters will be important to establish the metabolic interactions between the peroxisome and other intracellular compartments.
Acknowledgments
This work was supported by the FP6 (6th Framework Programme) European Union Project ‘Peroxisome’ (grant no. LSHG-CT-2004-512018).
References
- 1.Wanders R. J., Waterham H. R. Biochemistry of mammalian peroxisomes revisited. Annu. Rev. Biochem. 2006;75:295–332. doi: 10.1146/annurev.biochem.74.082803.133329. [DOI] [PubMed] [Google Scholar]
- 2.Veenhuis M. Peroxisome biogenesis and function in Hansenula polymorpha. Cell Biochem. Funct. 1992;10:175–184. doi: 10.1002/cbf.290100307. [DOI] [PubMed] [Google Scholar]
- 3.Müller W. H., Van der Krift T. P., Krouwer A. J. J., Wösten H. A. B., Van der Voort L. H. M., Smaal E. B., Verkleij A. J. Localization of the pathway of the penicillin biosynthesis in Penicillium chrysogenum. EMBO J. 1991;10:489–495. doi: 10.1002/j.1460-2075.1991.tb07971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berteaux-Lecellier V., Picard M., Thompson-Coffe C., Zickler D., Panvier-Adoutte A., Simonet J. M. A nonmammalian homolog of the PAF1 gene (Zellweger syndrome) discovered as a gene involved in caryogamy in the fungus Podospora anserina. Cell. 1995;81:1043–1051. doi: 10.1016/s0092-8674(05)80009-1. [DOI] [PubMed] [Google Scholar]
- 5.Theodoulou F. L., Holdsworth M., Baker A. Peroxisomal ABC transporters. FEBS Lett. 2006;580:1139–1155. doi: 10.1016/j.febslet.2005.12.095. [DOI] [PubMed] [Google Scholar]
- 6.Hogenboom S., Tuyp J. J., Espeel M., Koster J., Wanders R. J., Waterham H. R. Phosphomevalonate kinase is a cytosolic protein in humans. J. Lipid Res. 2004;45:697–705. doi: 10.1194/jlr.M300373-JLR200. [DOI] [PubMed] [Google Scholar]
- 7.Hogenboom S., Tuyp J. J., Espeel M., Koster J., Wanders R. J., Waterham H. R. Mevalonate kinase is a cytosolic enzyme in humans. J. Cell Sci. 2004;117:631–639. doi: 10.1242/jcs.00910. [DOI] [PubMed] [Google Scholar]
- 8.Hogenboom S., Tuyp J. J., Espeel M., Koster J., Wanders R. J., Waterham H. R. Human mevalonate pyrophosphate decarboxylase is localized in the cytosol. Mol. Genet. Metab. 2004;81:216–224. doi: 10.1016/j.ymgme.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Hogenboom S., Romeijn G. J., Houten S. M., Baes M., Wanders R. J., Waterham H. R. Peroxisome deficiency does not result in deficiency of enzymes involved in cholesterol biosynthesis. Adv. Exp. Med. Biol. 2003;544:329–330. doi: 10.1007/978-1-4419-9072-3_42. [DOI] [PubMed] [Google Scholar]
- 10.Hogenboom S., Romeijn G. J., Houten S. M., Baes M., Wanders R. J., Waterham H. R. Absence of functional peroxisomes does not lead to deficiency of enzymes involved in cholesterol biosynthesis. J. Lipid Res. 2002;43:90–98. [PubMed] [Google Scholar]
- 11.Hogenboom S., Wanders R. J., Waterham H. R. Cholesterol biosynthesis is not defective in peroxisome biogenesis defective fibroblasts. Mol. Genet. Metab. 2003;80:290–295. doi: 10.1016/S1096-7192(03)00143-4. [DOI] [PubMed] [Google Scholar]
- 12.Wanders R. J., van Roermund C. W., Visser W. F., Ferdinandusse S., Jansen G. A., van den Brink D. M., Gloerich J., Waterham H. R. Peroxisomal fatty acid α- and β-oxidation in health and disease: new insights. Adv. Exp. Med. Biol. 2003;544:293–302. doi: 10.1007/978-1-4419-9072-3_37. [DOI] [PubMed] [Google Scholar]
- 13.van den Brink D. M., Wanders R. J. Phytanic acid: production from phytol, its breakdown and role in human disease. Cell. Mol. Life Sci. 2006;63:1752–1765. doi: 10.1007/s00018-005-5463-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casteels M., Foulon V., Mannaerts G. P., Van Veldhoven P. P. α-Oxidation of 3-methyl-substituted fatty acids and its thiamine dependence. Eur. J. Biochem. 2003;270:1619–1627. doi: 10.1046/j.1432-1033.2003.03534.x. [DOI] [PubMed] [Google Scholar]
- 15.Wanders R. J., Vreken P., Ferdinandusse S., Jansen G. A., Waterham H. R., van Roermund C. W., Van Grunsven E. G. Peroxisomal fatty acid α- and β-oxidation in humans: enzymology, peroxisomal metabolite transporters and peroxisomal diseases. Biochem. Soc. Trans. 2001;29:250–267. doi: 10.1042/0300-5127:0290250. [DOI] [PubMed] [Google Scholar]
- 16.Jakobs B. S., Wanders R. J. Conclusive evidence that very-long-chain fatty acids are oxidized exclusively in peroxisomes in human skin fibroblasts. Biochem. Biophys. Res. Commun. 1991;178:842–847. doi: 10.1016/0006-291x(91)90967-c. [DOI] [PubMed] [Google Scholar]
- 17.Shani N., Watkins P. A., Valle D. PXA1, a possible Saccharomyces cerevisiae ortholog of the human adrenoleukodystrophy gene. Proc. Natl. Acad. Sci. U.S.A. 1995;92:6012–6016. doi: 10.1073/pnas.92.13.6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shani N., Valle D. A Saccharomyces cerevisiae homolog of the human adrenoleukodystrophy transporter is a heterodimer of two half ATP-binding cassette transporters. Proc. Natl. Acad. Sci. U.S.A. 1996;93:11901–11906. doi: 10.1073/pnas.93.21.11901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hettema E. H., van Roermund C. W., Distel B., van den Berg M., Vilela C., Rodrigues-Pousada C., Wanders R. J., Tabak H. F. The ABC transporter proteins Pat1 and Pat2 are required for import of long-chain fatty acids into peroxisomes of Saccharomyces cerevisiae. EMBO J. 1996;15:3813–3822. [PMC free article] [PubMed] [Google Scholar]
- 20.Verleur N., Hettema E. H., van Roermund C. W., Tabak H. F., Wanders R. J. Transport of activated fatty acids by the peroxisomal ATP-binding-cassette transporter Pxa2 in a semi-intact yeast cell system. Eur. J. Biochem. 1997;249:657–661. doi: 10.1111/j.1432-1033.1997.00657.x. [DOI] [PubMed] [Google Scholar]
- 21.Fulda M., Schnurr J., Abbadi A., Heinz E., Browse J. Peroxisomal Acyl-CoA synthetase activity is essential for seedling development in Arabidopsis thaliana. Plant Cell. 2004;16:394–405. doi: 10.1105/tpc.019646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu L. X., Janvier K., Berteaux-Lecellier V., Cartier N., Benarous R., Aubourg P. Homo- and heterodimerization of peroxisomal ATP-binding cassette half-transporters. J. Biol. Chem. 1999;274:32738–32743. doi: 10.1074/jbc.274.46.32738. [DOI] [PubMed] [Google Scholar]
- 23.Shani N., Sapag A., Watkins P. A., Valle D. An S. cerevisiae peroxisomal transporter, orthologous to the human adrenoleukodystrophy protein, appears to be a heterodimer of two half ABC transporters: Pxa1p and Pxa2p. Ann. N.Y. Acad. Sci. 1996;804:770–772. doi: 10.1111/j.1749-6632.1996.tb18697.x. [DOI] [PubMed] [Google Scholar]
- 24.Guimaraes C. P., Domingues P., Aubourg P., Fouquet F., Pujol A., Jimenez-Sanchez G., Sa-Miranda C., Azevedo J. E. Mouse liver PMP70 and ALDP homomeric interactions prevail in vivo. Biochim. Biophys. Acta. 2004;1689:235–243. doi: 10.1016/j.bbadis.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Ferrer I., Kapfhammer J. P., Hindelang C., Kemp S., Troffer-Charlier N., Broccoli V., Callyzot N., Mooyer P., Selhorst J., Vreken P., et al. Inactivation of the peroxisomal ABCD2 transporter in the mouse leads to late-onset ataxia involving mitochondria, Golgi and endoplasmic reticulum damage. Hum. Mol. Genet. 2005;14:3565–3577. doi: 10.1093/hmg/ddi384. [DOI] [PubMed] [Google Scholar]
- 26.Pujol A., Ferrer I., Camps C., Metzger E., Hindelang C., Callizot N., Ruiz M., Pampols T., Giros M., Mandel J. L. Functional overlap between ABCD1 (ALD) and ABCD2 (ALDR) transporters: a therapeutic target for X-adrenoleukodystrophy. Hum. Mol. Genet. 2004;13:2997–3006. doi: 10.1093/hmg/ddh323. [DOI] [PubMed] [Google Scholar]
- 27.McCammon M. T., Dowds C. A., Orth K., Moomaw C. R., Slaughter C. A., Goodman J. M. Sorting of peroxisomal membrane protein PMP47 from Candida boidinii into peroxisomal membranes of Saccharomyces cerevisiae. J. Biol. Chem. 1990;265:20098–20105. [PubMed] [Google Scholar]
- 28.Jank B., Habermann B., Schweyen R. J., Link T. A. PMP47, a peroxisomal homologue of mitochondrial solute carrier proteins. Trends Biochem. Sci. 1993;18:427–428. [PubMed] [Google Scholar]
- 29.Fukao Y., Hayashi Y., Mano S., Hayashi M., Nishimura M. Developmental analysis of a putative ATP/ADP carrier protein localized on glyoxysomal membranes during the peroxisome transition in pumpkin cotyledons. Plant Cell Physiol. 2001;42:835–841. doi: 10.1093/pcp/pce108. [DOI] [PubMed] [Google Scholar]
- 30.Wylin T., Baes M., Brees C., Mannaerts G. P., Fransen M., Van Veldhoven P. P. Identification and characterization of human PMP34, a protein closely related to the peroxisomal integral membrane protein PMP47 of Candida boidinii. Eur. J. Biochem. 1998;258:332–338. doi: 10.1046/j.1432-1327.1998.2580332.x. [DOI] [PubMed] [Google Scholar]
- 31.van Roermund C. W., Drissen R., van Den Berg M., Ijlst L., Hettema E. H., Tabak H. F., Waterham H. R., Wanders R. J. Identification of a peroxisomal ATP carrier required for medium-chain fatty acid β-oxidation and normal peroxisome proliferation in Saccharomyces cerevisiae. Mol. Cell. Biol. 2001;21:4321–4329. doi: 10.1128/MCB.21.13.4321-4329.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakagawa T., Imanaka T., Morita M., Ishiguro K., Yurimoto H., Yamashita A., Kato N., Sakai Y. Peroxisomal membrane protein Pmp47 is essential in the metabolism of middle-chain fatty acid in yeast peroxisomes and is associated with peroxisome proliferation. J. Biol. Chem. 2000;275:3455–3461. doi: 10.1074/jbc.275.5.3455. [DOI] [PubMed] [Google Scholar]
- 33.Visser W. F., van Roermund C. W., Waterham H. R., Wanders R. J. Identification of human PMP34 as a peroxisomal ATP transporter. Biochem. Biophys. Res. Commun. 2002;299:494–497. doi: 10.1016/s0006-291x(02)02663-3. [DOI] [PubMed] [Google Scholar]
- 34.Palmieri L., Rottensteiner H., Girzalsky W., Scarcia P., Palmieri F., Erdmann R. Identification and functional reconstitution of the yeast peroxisomal adenine nucleotide transporter. EMBO J. 2001;20:5049–5059. doi: 10.1093/emboj/20.18.5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lasorsa F. M., Scarcia P., Erdmann R., Palmieri F., Rottensteiner H., Palmieri L. The yeast peroxisomal adenine nucleotide transporter: characterization of two transport modes and involvement in ΔpH formation across peroxisomal membranes. Biochem. J. 2004;381:581–585. doi: 10.1042/BJ20040856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Roermund C. W., de Jong M., Ijlst L., van Marle J., Dansen T. B., Wanders R. J., Waterham H. R. The peroxisomal lumen in Saccharomyces cerevisiae is alkaline. J. Cell Sci. 2004;117:4231–4237. doi: 10.1242/jcs.01305. [DOI] [PubMed] [Google Scholar]
- 37.Sakai Y., Saiganji A., Yurimoto H., Takabe K., Saiki H., Kato N. The absence of Pmp47, a putative yeast peroxisomal transporter, causes a defect in transport and folding of a specific matrix enzyme. J. Cell Biol. 1996;134:37–51. doi: 10.1083/jcb.134.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Roermund C. W., Elgersma Y., Singh N., Wanders R. J., Tabak H. F. The membrane of peroxisomes in Saccharomyces cerevisiae is impermeable to NAD(H) and acetyl-CoA under in vivo conditions. EMBO J. 1995;14:3480–3486. doi: 10.1002/j.1460-2075.1995.tb07354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gimpel J. A., de Haan E. J., Tager J. M. Permeability of isolated mitochondria to oxaloacetate. Biochim. Biophys. Acta. 1973;292:582–591. doi: 10.1016/0005-2728(73)90006-6. [DOI] [PubMed] [Google Scholar]
- 40.Gebhardt J. S., Wadsworth G. J., Matthews B. F. Characterization of a single soybean cDNA encoding cytosolic and glyoxysomal isozymes of aspartate aminotransferase. Plant Mol. Biol. 1998;37:99–108. doi: 10.1023/a:1005973019045. [DOI] [PubMed] [Google Scholar]
- 41.Schultz C. J., Hsu M., Miesak B., Coruzzi G. M. Arabidopsis mutants define an in vivo role for isoenzymes of aspartate aminotransferase in plant nitrogen assimilation. Genetics. 1998;149:491–499. doi: 10.1093/genetics/149.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verleur N., Elgersma Y., Van Roermund C. W., Tabak H. F., Wanders R. J. Cytosolic aspartate aminotransferase encoded by the AAT2 gene is targeted to the peroxisomes in oleate-grown Saccharomyces cerevisiae. Eur. J. Biochem. 1997;247:972–980. doi: 10.1111/j.1432-1033.1997.00972.x. [DOI] [PubMed] [Google Scholar]
- 43.McGroarty E., Tolbert N. E. Enzymes in peroxisomes. J. Histochem. Cytochem. 1973;21:949–954. doi: 10.1177/21.11.949. [DOI] [PubMed] [Google Scholar]
- 44.Kohl L., Drmota T., Thi C. D., Callens M., Van Beeumen J., Opperdoes F. R., Michels P. A. Cloning and characterization of the NAD-linked glycerol-3-phosphate dehydrogenases of Trypanosoma brucei brucei and Leishmania mexicana mexicana and expression of the trypanosome enzyme in Escherichia coli. Mol. Biochem. Parasitol. 1996;76:159–173. doi: 10.1016/0166-6851(95)02556-1. [DOI] [PubMed] [Google Scholar]
- 45.Baumgart E., Fahimi H. D., Stich A., Volkl A. L-Lactate dehydrogenase A4- and A3B isoforms are bona fide peroxisomal enzymes in rat liver: evidence for involvement in intraperoxisomal NADH reoxidation. J. Biol. Chem. 1996;271:3846–3855. doi: 10.1074/jbc.271.7.3846. [DOI] [PubMed] [Google Scholar]
- 46.Brooks G. A. Lactate shuttles in nature. Biochem. Soc. Trans. 2002;30:258–264. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 47.McClelland G. B., Khanna S., Gonzalez G. F., Butz C. E., Brooks G. A. Peroxisomal membrane monocarboxylate transporters: evidence for a redox shuttle system? Biochem. Biophys. Res. Commun. 2003;304:130–135. doi: 10.1016/s0006-291x(03)00550-3. [DOI] [PubMed] [Google Scholar]
- 48.Osmundsen H. Factors which can influence β-oxidation by peroxisomes isolated from livers of clofibrate treated rats: some properties of peroxisomal fractions isolated in a self-generated Percoll gradient by vertical rotor centrifugation. Int. J. Biochem. 1982;14:905–914. doi: 10.1016/0020-711x(82)90074-x. [DOI] [PubMed] [Google Scholar]
- 49.Hiltunen J. K., Filppula S. A., Koivuranta K. T., Siivari K., Qin Y. M., Hayrinen H. M. Peroxisomal β-oxidation and polyunsaturated fatty acids. Ann. N.Y. Acad. Sci. 1996;804:116–128. doi: 10.1111/j.1749-6632.1996.tb18612.x. [DOI] [PubMed] [Google Scholar]
- 50.van Roermund C. W., Hettema E. H., Kal A. J., van den Berg M., Tabak H. F., Wanders R. J. Peroxisomal β-oxidation of polyunsaturated fatty acids in Saccharomyces cerevisiae: isocitrate dehydrogenase provides NADPH for reduction of double bonds at even positions. EMBO J. 1998;17:677–687. doi: 10.1093/emboj/17.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Henke B., Girzalsky W., Berteaux-Lecellier V., Erdmann R. IDP3 encodes a peroxisomal NADP-dependent isocitrate dehydrogenase required for the β-oxidation of unsaturated fatty acids. J. Biol. Chem. 1998;273:3702–3711. doi: 10.1074/jbc.273.6.3702. [DOI] [PubMed] [Google Scholar]
- 52.Visser W. F., van Roermund C. W., Ijlst L., Hellingwerf K. J., Waterham H. R., Wanders R. J. First identification of a 2-ketoglutarate/isocitrate transport system in mammalian peroxisomes and its characterization. Biochem. Biophys. Res. Commun. 2006;348:1224–1231. doi: 10.1016/j.bbrc.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 53.Geisbrecht B. V., Gould S. J. The human PICD gene encodes a cytoplasmic and peroxisomal NADP(+)-dependent isocitrate dehydrogenase. J. Biol. Chem. 1999;274:30527–30533. doi: 10.1074/jbc.274.43.30527. [DOI] [PubMed] [Google Scholar]
- 54.Westin M. A., Hunt M. C., Alexson S. E. The identification of a succinyl-CoA thioesterase suggests a novel pathway for succinate production in peroxisomes. J. Biol. Chem. 2005;280:38125–38132. doi: 10.1074/jbc.M508479200. [DOI] [PubMed] [Google Scholar]
- 55.Hunt M. C., Alexson S. E. The role Acyl-CoA thioesterases play in mediating intracellular lipid metabolism. Prog. Lipid Res. 2002;41:99–130. doi: 10.1016/s0163-7827(01)00017-0. [DOI] [PubMed] [Google Scholar]
- 56.Leighton F., Bergseth S., Rortveit T., Christiansen E. N., Bremer J. Free acetate production by rat hepatocytes during peroxisomal fatty acid and dicarboxylic acid oxidation. J. Biol. Chem. 1989;264:10347–10350. [PubMed] [Google Scholar]
- 57.Jakobs B. S., Wanders R. J. Fatty acid β-oxidation in peroxisomes and mitochondria: the first, unequivocal evidence for the involvement of carnitine in shuttling propionyl-CoA from peroxisomes to mitochondria. Biochem. Biophys. Res. Commun. 1995;213:1035–1041. doi: 10.1006/bbrc.1995.2232. [DOI] [PubMed] [Google Scholar]
- 58.Lamhonwah A. M., Ackerley C. A., Tilups A., Edwards V. D., Wanders R. J., Tein I. OCTN3 is a mammalian peroxisomal membrane carnitine transporter. Biochem. Biophys. Res. Commun. 2005;338:1966–1972. doi: 10.1016/j.bbrc.2005.10.170. [DOI] [PubMed] [Google Scholar]
- 59.Tamai I., Ohashi R., Nezu J. I., Sai Y., Kobayashi D., Oku A., Shimane M., Tsuji A. Molecular and functional characterization of organic cation/carnitine transporter family in mice. J. Biol. Chem. 2000;275:40064–40072. doi: 10.1074/jbc.M005340200. [DOI] [PubMed] [Google Scholar]
- 60.Duran J. M., Peral M. J., Calonge M. L., Ilundain A. A. OCTN3: a Na+-independent L-carnitine transporter in enterocytes basolateral membrane. J. Cell. Physiol. 2005;202:929–935. doi: 10.1002/jcp.20193. [DOI] [PubMed] [Google Scholar]
- 61.Palmieri L., Lasorsa F. M., De Palma A., Palmieri F., Runswick M. J., Walker J. E. Identification of the yeast ACR1 gene product as a succinate-fumarate transporter essential for growth on ethanol or acetate. FEBS Lett. 1997;417:114–118. doi: 10.1016/s0014-5793(97)01269-6. [DOI] [PubMed] [Google Scholar]
- 62.Jedlitschky G., Huber M., Volkl A., Muller M., Leier I., Muller J., Lehmann W. D., Fahimi H. D., Keppler D. Peroxisomal degradation of leukotrienes by β-oxidation from the ω-end. J. Biol. Chem. 1991;266:24763–24772. [PubMed] [Google Scholar]
- 63.Schepers L., Casteels M., Vamecq J., Parmentier G., Van Veldhoven P. P., Mannaerts G. P. β-Oxidation of the carboxyl side chain of prostaglandin E2 in rat liver peroxisomes and mitochondria. J. Biol. Chem. 1988;263:2724–2731. [PubMed] [Google Scholar]
- 64.Ferdinandusse S., Meissner T., Wanders R. J., Mayatepek E. Identification of the peroxisomal β-oxidation enzymes involved in the degradation of leukotrienes. Biochem. Biophys. Res. Commun. 2002;293:269–273. doi: 10.1016/S0006-291X(02)00214-0. [DOI] [PubMed] [Google Scholar]
- 65.Mayatepek E., Ferdinandusse S., Meissner T., Wanders R. J. Analysis of cysteinyl leukotrienes and their metabolites in bile of patients with peroxisomal or mitochondrial β-oxidation defects. Clin. Chim. Acta. 2004;345:89–92. doi: 10.1016/j.cccn.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 66.Mihalik S. J., Steinberg S. J., Pei Z., Park J., Kim D. G., Heinzer A. K., Dacremont G., Wanders R. J., Cuebas D. A., Smith K. D., et al. Participation of two members of the very long-chain acyl-CoA synthetase family in bile acid synthesis and recycling. J. Biol. Chem. 2002;277:24771–24779. doi: 10.1074/jbc.M203295200. [DOI] [PubMed] [Google Scholar]
- 67.Prydz K., Kase B. F., Bjorkhem I., Pedersen J. I. Subcellular localization of 3α,7α-dihydroxy- and 3α,7α,12α-trihydroxy-5β-cholestanoyl-coenzyme A ligase(s) in rat liver. J. Lipid Res. 1988;29:997–1004. [PubMed] [Google Scholar]
- 68.Koibuchi Y., Yamada J., Watanabe T., Kurosawa T., Tohma M., Suga T., Tohma S. Study on stereospecificity of enzyme reaction related to peroxisomal bile acid synthesis in rat liver. Chem. Pharm. Bull. 1992;40:446–448. doi: 10.1248/cpb.40.446. [DOI] [PubMed] [Google Scholar]
- 68a.Jimenez-Sanchez G. H. K., Hebron K. J., Mihalik S., Watkins P., Espeel M., Moser A., Thomas G., Roels F., Vallee D. Defective phytanic and pristanic acid metabolism in 70 kDa peroxisomal membrane protein (PMP70) deficient mice results in defective nonshivering thermogenesis and dicarboxylic aciduria. Am. J. Hum. Genet. 2000;67:65. [Google Scholar]
- 69.Nicolay K., Veenhuis M., Douma A. C., Harder W. A 31P NMR study of the internal pH of yeast peroxisomes. Arch. Microbiol. 1987;147:37–41. doi: 10.1007/BF00492902. [DOI] [PubMed] [Google Scholar]
- 70.Waterham H. R., Keizer-Gunnink I., Goodman J. M., Harder W., Veenhuis M. Immunocytochemical evidence for the acidic nature of peroxisomes in methylotrophic yeasts. FEBS Lett. 1990;262:17–19. doi: 10.1016/0014-5793(90)80142-6. [DOI] [PubMed] [Google Scholar]
- 71.Dansen T. B., Wirtz K. W., Wanders R. J., Pap E. H. Peroxisomes in human fibroblasts have a basic pH. Nat. Cell Biol. 2000;2:51–53. doi: 10.1038/71375. [DOI] [PubMed] [Google Scholar]
- 72.van der Lende T. R., Breeuwer P., Abee T., Konings W. N., Driessen A. J. Assessment of the microbody luminal pH in the filamentous fungus Penicillium chrysogenum. Biochim. Biophys. Acta. 2002;1589:104–111. doi: 10.1016/s0167-4889(02)00162-3. [DOI] [PubMed] [Google Scholar]
- 73.Marshall P. A., Krimkevich Y. I., Lark R. H., Dyer J. M., Veenhuis M., Goodman J. M. Pmp27 promotes peroxisomal proliferation. J. Cell Biol. 1995;129:345–355. doi: 10.1083/jcb.129.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marshall P. A., Dyer J. M., Quick M. E., Goodman J. M. Redox-sensitive homodimerization of Pex11p: a proposed mechanism to regulate peroxisomal division. J. Cell Biol. 1996;135:123–137. doi: 10.1083/jcb.135.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Erdmann R., Blobel G. Giant peroxisomes in oleic acid-induced Saccharomyces cerevisiae lacking the peroxisomal membrane protein Pmp27p. J. Cell Biol. 1995;128:509–523. doi: 10.1083/jcb.128.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van Roermund C. W., Tabak H. F., van Den Berg M., Wanders R. J., Hettema E. H. Pex11p plays a primary role in medium-chain fatty acid oxidation, a process that affects peroxisome number and size in Saccharomyces cerevisiae. J. Cell Biol. 2000;150:489–498. doi: 10.1083/jcb.150.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li X., Gould S. J. PEX11 promotes peroxisome division independently of peroxisome metabolism. J. Cell Biol. 2002;156:643–651. doi: 10.1083/jcb.200112028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li X., Baumgart E., Dong G. X. PEX11α is required for peroxisome proliferation in response to 4-phenylbutyrate, but is dispensable for peroxisome proliferator-activated receptor α-mediated peroxisome proliferation. Mol. Cell. Biol. 2002;22:8226–8240. doi: 10.1128/MCB.22.23.8226-8240.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rottensteiner H., Stein K., Sonnenhol E., Erdmann R. Conserved function of pex11p and the novel pex25p and pex27p in peroxisome biogenesis. Mol. Biol. Cell. 2003;14:4316–4328. doi: 10.1091/mbc.E03-03-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Van Veldhoven P. P., Just W. W., Mannaerts G. P. Permeability of the peroxisomal membrane to cofactors of β-oxidation: evidence for the presence of a pore-forming protein. J. Biol. Chem. 1987;262:4310–4318. [PubMed] [Google Scholar]
- 81.De Duve C., Baudhuin P. Peroxisomes (microbodies and related particles) Physiol. Rev. 1966;46:323–357. doi: 10.1152/physrev.1966.46.2.323. [DOI] [PubMed] [Google Scholar]
- 82.Sulter G. J., Verheyden K., Mannaerts G., Harder W., Veenhuis M. The in vitro permeability of yeast peroxisomal membranes is caused by a 31 kDa integral membrane protein. Yeast. 1993;9:733–742. doi: 10.1002/yea.320090707. [DOI] [PubMed] [Google Scholar]
- 83.Labarca P., Wolff D., Soto U., Necochea C., Leighton F. Large cation-selective pores from rat liver peroxisomal membranes incorporated to planar lipid bilayers. J. Membr. Biol. 1986;94:285–291. doi: 10.1007/BF01869724. [DOI] [PubMed] [Google Scholar]
- 84.Lemmens M., Verheyden K., Van Veldhoven P. P., Vereecke J., Mannaerts G. P., Carmeliet E. Single-channel analysis of a large conductance channel in peroxisomes from rat liver. Biochim. Biophys. Acta. 1989;984:351–359. doi: 10.1016/0005-2736(89)90302-7. [DOI] [PubMed] [Google Scholar]
- 85.Reumann S., Maier E., Heldt H. W., Benz R. Permeability properties of the porin of spinach leaf peroxisomes. Eur. J. Biochem. 1998;251:359–366. doi: 10.1046/j.1432-1327.1998.2510359.x. [DOI] [PubMed] [Google Scholar]
- 86.Reumann S., Maier E., Benz R., Heldt H. W. A specific porin is involved in the malate shuttle of leaf peroxisomes. Biochem. Soc. Trans. 1996;24:754–757. doi: 10.1042/bst0240754. [DOI] [PubMed] [Google Scholar]
- 87.Reumann S., Maier E., Benz R., Heldt H. W. The membrane of leaf peroxisomes contains a porin-like channel. J. Biol. Chem. 1995;270:17559–17565. doi: 10.1074/jbc.270.29.17559. [DOI] [PubMed] [Google Scholar]
- 88.Reumann S., Bettermann M., Benz R., Heldt H. W. Evidence for the presence of a porin in the membrane of glyoxysomes of castor bean. Plant Physiol. 1997;115:891–899. doi: 10.1104/pp.115.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Antonenkov V. D., Rokka A., Sormunen R. T., Benz R., Hiltunen J. K. Solute traffic across mammalian peroxisomal membrane–single channel conductance monitoring reveals pore-forming activities in peroxisomes. Cell. Mol. Life Sci. 2005;62:2886–2895. doi: 10.1007/s00018-005-5233-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu G., Robertson A. J., Zheng P., Liu X., Gusta L. V. Identification and immunogold localization of a novel bromegrass (Bromus inermis Leyss) peroxisome channel protein induced by ABA, cold and drought stresses, and late embryogenesis. Gene. 2005;363:77–84. doi: 10.1016/j.gene.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 91.Koster A., Heisig M., Heinrich P. C., Just W. W. In vitro synthesis of peroxisomal membrane polypeptides. Biochem. Biophys. Res. Commun. 1986;137:626–632. doi: 10.1016/0006-291x(86)91124-1. [DOI] [PubMed] [Google Scholar]
- 92.Hashimoto T., Kuwabara T., Usuda N., Nagata T. Purification of membrane polypeptides of rat liver peroxisomes. J. Biochem. (Tokyo) 1986;100:301–310. doi: 10.1093/oxfordjournals.jbchem.a121716. [DOI] [PubMed] [Google Scholar]
- 93.Suzuki Y., Orii T., Takiguchi M., Mori M., Hijikata M., Hashimoto T. Biosynthesis of membrane polypeptides of rat liver peroxisomes. J. Biochem. (Tokyo) 1987;101:491–496. doi: 10.1093/oxfordjournals.jbchem.a121935. [DOI] [PubMed] [Google Scholar]
- 94.Hartl F. U., Just W. W. Integral membrane polypeptides of rat liver peroxisomes: topology and response to different metabolic states. Arch. Biochem. Biophys. 1987;255:109–119. doi: 10.1016/0003-9861(87)90300-6. [DOI] [PubMed] [Google Scholar]
- 95.Kaldi K., Diestelkotter P., Stenbeck G., Auerbach S., Jakle U., Magert H. J., Wieland F. T., Just W. W. Membrane topology of the 22 kDa integral peroxisomal membrane protein. FEBS Lett. 1993;315:217–222. doi: 10.1016/0014-5793(93)81167-x. [DOI] [PubMed] [Google Scholar]
- 96.Zwacka R. M., Reuter A., Pfaff E., Moll J., Gorgas K., Karasawa M., Weiher H. The glomerulosclerosis gene Mpv17 encodes a peroxisomal protein producing reactive oxygen species. EMBO J. 1994;13:5129–5134. doi: 10.1002/j.1460-2075.1994.tb06842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bryant D. D., Wilson G. N. Differential evolution and expression of murine peroxisomal membrane protein genes. Biochem. Mol. Med. 1995;55:22–30. doi: 10.1006/bmme.1995.1027. [DOI] [PubMed] [Google Scholar]
- 98.Luers G. H., Otte D. M., Subramani S., Franz T. Genomic organization, chromosomal localization and tissue specific expression of the murine Pxmp2 gene encoding the 22 kDa peroxisomal membrane protein (Pmp22) Gene. 2001;272:45–50. doi: 10.1016/s0378-1119(01)00531-5. [DOI] [PubMed] [Google Scholar]
- 99.Iida R., Yasuda T., Tsubota E., Matsuki T., Kishi K. Cloning, mapping, genomic organization, and expression of mouse M-LP, a new member of the peroxisomal membrane protein Mpv17 domain family. Biochem. Biophys. Res. Commun. 2001;283:292–296. doi: 10.1006/bbrc.2001.4769. [DOI] [PubMed] [Google Scholar]
- 100.Tugal H. B., Pool M., Baker A. Arabidopsis 22-kilodalton peroxisomal membrane protein: nucleotide sequence analysis and biochemical characterization. Plant Physiol. 1999;120:309–320. doi: 10.1104/pp.120.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Brosius U., Dehmel T., Gartner J. Two different targeting signals direct human peroxisomal membrane protein 22 to peroxisomes. J. Biol. Chem. 2002;277:774–784. doi: 10.1074/jbc.M108155200. [DOI] [PubMed] [Google Scholar]
- 102.Iida R., Yasuda T., Tsubota E., Takatsuka H., Matsuki T., Kishi K. Human Mpv17-like protein is localized in peroxisomes and regulates expression of antioxidant enzymes. Biochem. Biophys. Res. Commun. 2006;344:948–954. doi: 10.1016/j.bbrc.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 103.Spinazzola A., Viscomi C., Fernandez-Vizarra E., Carrara F., D'Adamo P., Calvo S., Marsano R. M., Donnini C., Weiher H., Strisciuglio P., et al. MPV17 encodes an inner mitochondrial membrane protein and is mutated in infantile hepatic mitochondrial DNA depletion. Nat. Genet. 2006;38:570–575. doi: 10.1038/ng1765. [DOI] [PubMed] [Google Scholar]
- 104.Trott A., Morano K. A. SYM1 is the stress-induced Saccharomyces cerevisiae ortholog of the mammalian kidney disease gene Mpv17 and is required for ethanol metabolism and tolerance during heat shock. Eukaryotic Cell. 2004;3:620–631. doi: 10.1128/EC.3.3.620-631.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Iida R., Yasuda T., Tsubota E., Takatsuka H., Masuyama M., Matsuki T., Kishi K. A novel alternative spliced Mpv17-like protein isoform localizes in cytosol and is expressed in a kidney- and adult-specific manner. Exp. Cell Res. 2005;302:22–30. doi: 10.1016/j.yexcr.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 106.Karadimas C. L., Vu T. H., Holve S. A., Chronopoulou P., Quinzii C., Johnsen S. D., Kurth J., Eggers E., Palenzuela L., Tanji K., et al. Navajo neurohepatopathy is caused by a mutation in the MPV17 gene. Am J. Hum. Genet. 2006;79:544–548. doi: 10.1086/506913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Clozel M., Hess P., Fischli W., Loffler B. M., Zwacka R. M., Reuter A., Weiher H. Age-dependent hypertension in Mpv17-deficient mice, a transgenic model of glomerulosclerosis and inner ear disease. Exp. Gerontol. 1999;34:1007–1015. doi: 10.1016/s0531-5565(99)00074-1. [DOI] [PubMed] [Google Scholar]
- 108.Meyer zum Gottesberge A. M., Reuter A., Weiher H. Inner ear defect similar to Alport's syndrome in the glomerulosclerosis mouse model Mpv17. Eur. Arch. Otorhinolaryngol. 1996;253:470–474. doi: 10.1007/BF00179952. [DOI] [PubMed] [Google Scholar]
- 109.Wagner G., Stettmaier K., Bors W., Sies H., Wagner E. M., Reuter A., Weiher H. Enhanced γ-glutamyl transpeptidase expression and superoxide production in Mpv17−/− glomerulosclerosis mice. Biol. Chem. 2001;382:1019–1025. doi: 10.1515/BC.2001.128. [DOI] [PubMed] [Google Scholar]
- 110.Iida R., Yasuda T., Tsubota E., Takatsuka H., Masuyama M., Matsuki T., Kishi K. M-LP, Mpv17-like protein, has a peroxisomal membrane targeting signal comprising a transmembrane domain and a positively charged loop and up-regulates expression of the manganese superoxide dismutase gene. J. Biol. Chem. 2003;278:6301–6306. doi: 10.1074/jbc.M210886200. [DOI] [PubMed] [Google Scholar]
- 111.Reuter A., Nestl A., Zwacka R. M., Tuckermann J., Waldherr R., Wagner E. M., Hoyhtya M., Meyer zum Gottesberge A. M., Angel P., Weiher H. Expression of the recessive glomerulosclerosis gene Mpv17 regulates MMP-2 expression in fibroblasts, the kidney, and the inner ear of mice. Mol. Biol. Cell. 1998;9:1675–1682. doi: 10.1091/mbc.9.7.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Huh W. K., Falvo J. V., Gerke L. C., Carroll A. S., Howson R. W., Weissman J. S., O'Shea E. K. Global analysis of protein localization in budding yeast. Nature. 2003;425:686–691. doi: 10.1038/nature02026. [DOI] [PubMed] [Google Scholar]
- 113.Southall T. D., Terhzaz S., Cabrero P., Chintapalli V. R., Evans J. M., Dow J. A., Davies S.-A. Novel sub-cellular locations and functions for secretory pathway Ca2+/Mn2+ ATPases. Physiol. Genomics. 2006;26:35–45. doi: 10.1152/physiolgenomics.00038.2006. [DOI] [PubMed] [Google Scholar]
- 114.Reguenga C., Oliveira M. E., Gouveia A. M., Eckerskorn C., Sa-Miranda C., Azevedo J. E. Identification of a 24 kDa intrinsic membrane protein from mammalian peroxisomes. Biochim. Biophys. Acta. 1999;1445:337–341. doi: 10.1016/s0167-4781(99)00061-5. [DOI] [PubMed] [Google Scholar]
- 115.Wu M., Ho S. M. PMP24, a gene identified by MSRF, undergoes DNA hypermethylation-associated gene silencing during cancer progression in an LNCaP model. Oncogene. 2004;23:250–259. doi: 10.1038/sj.onc.1207076. [DOI] [PubMed] [Google Scholar]
- 116.Weber F. E., Minestrini G., Dyer J. H., Werder M., Boffelli D., Compassi S., Wehrli E., Thomas R. M., Schulthess G., Hauser H. Molecular cloning of a peroxisomal Ca2+-dependent member of the mitochondrial carrier superfamily. Proc. Natl. Acad. Sci. U.S.A. 1997;94:8509–8514. doi: 10.1073/pnas.94.16.8509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.del Arco A., Satrustegui J. Identification of a novel human subfamily of mitochondrial carriers with calcium-binding domains. J. Biol. Chem. 2004;279:24701–24713. doi: 10.1074/jbc.M401417200. [DOI] [PubMed] [Google Scholar]
- 118.Mashima H., Ueda N., Ohno H., Suzuki J., Ohnishi H., Yasuda H., Tsuchida T., Kanamaru C., Makita N., Iiri T., et al. A novel mitochondrial Ca2+-dependent solute carrier in the liver identified by mRNA differential display. J. Biol. Chem. 2003;278:9520–9527. doi: 10.1074/jbc.m208398200. [DOI] [PubMed] [Google Scholar]
- 119.Fiermonte G., De Leonardis F., Todisco S., Palmieri L., Lasorsa F. M., Palmieri F. Identification of the mitochondrial ATP-Mg/Pi transporter. J. Biol. Chem. 2004;279:30722–30730. doi: 10.1074/jbc.M400445200. [DOI] [PubMed] [Google Scholar]
- 120.Visser W. F., van Roermund C. W., Ijlst L., Hellingwerf K. J., Waterham H. R., Wanders R. J. First identification of a 2-ketoglutarate/isocitrate transport system in mammalian peroxisomes and its characterization. Biochem. Biophys. Res. Commun. 2006;348:1224–1231. doi: 10.1016/j.bbrc.2006.07.049. [DOI] [PubMed] [Google Scholar]