Abstract
The most salient feature of carbon monoxide (CO)-mediated cytoprotection is the suppression of inflammation and cell death. One of the important cellular targets of CO is the macrophage (mφ). Many studies have shown that exposure of mφ to CO results in the generation of an antiinflammatory phenotype; however, these reports have ignored the effect of CO alone on the cell before stimulation. Most investigations have focused on the actions of CO in modulating the response to noxious stimuli. We demonstrate here that exposure of mφ to CO results in a significant and transient burst of reactive oxygen species (ROS) arising from the mitochondria (mitochondria-deficient mφ do not respond to CO to produce ROS). The ROS promote rapid activation and stabilization of the transcription factor hypoxia-inducible factor 1α (HIF-1α), which regulates expression of genes involved in inflammation, metabolism, and cell survival. The increase in HIF-1α expression induced by CO results in regulated expression of TGF-β, a potent antiinflammatory cytokine. CO-induced HIF-1α and TGF-β expression are necessary to prevent anoxia/reoxygenation-induced apoptosis in mφ. Furthermore, blockade of HIF-1α using RNA interference and HIF-1α-cre-lox mφ resulted in a loss of TGF-β expression and CO-induced protection. A similar mechanism of CO-induced protection was operational in vivo to protect against lung ischemia-reperfusion injury. Taken together, we conclude that CO conditions the mφ via a HIF-1α and TGF-β-dependent mechanism and we elucidate the earliest events in mφ signaling that lead to and preserve cellular homeostasis at the site of injury.
Keywords: ischemia reperfusion, macrophage, heme oxygenase-1
There is increasing awareness of the salutary effects of CO at low concentrations (15–250 ppm) in preclinical animal models of disease, including shock (1), postoperative ileus (2), organ transplantation (3), airway hyperresponsiveness (4), necrotizing enterocolitis (5), and ischemia/reperfusion (I/R) injury (IRI) (6). Initially thought of as a highly toxic molecule, CO is presently considered a novel therapeutic. Elucidation of the molecular mechanism(s) that explain the actions of CO is still in its infancy. We and others have recently shown that CO increases the generation of reactive oxygen species (ROS), including superoxide and hydrogen peroxide, via its interaction with mitochondrial oxidases (7) that initiate immediate signal transduction and activates oxidant stress response factors (8, 9).
One of the key cellular targets of endogenous ROS is the stress response transcription factor, hypoxia-inducible factor 1α (HIF-1α), which regulates numerous genes involved in angiogenesis, metabolism, and cell survival (10, 11). HIF-1α is tightly regulated by two hemoproteins, prolyl 4-hydroxylase (PHD) (12, 13) and asparaginyl-hydroxylase factor inhibiting-HIF-1α, both of which function as cellular O2 sensors (14, 15). In the presence of hypoxia or elevated levels of ROS, PHD becomes hydroxylated, resulting in the activation and stabilization of HIF-1α (16, 17), which further regulates expression of stress response genes (18–20). This plasticity allows HIF-1α to behave as either protective or detrimental during inflammation, which continues to evoke discussion with strong evidence implicating HIF-1α as an important mediator in the immune response (19, 20). This concept is supported by the fact that macrophages (mφ) lacking HIF-1α show impaired bacterial killing and persistent inflammation (19). Additionally, expression of a constitutively active HIF-1α hybrid can protect cardiomyocytes against IRI (21).
One of the key genes regulated by ROS and HIF-1α (22, 23) is the pleiotropic cytokine, TGF-β. TGF-β belongs to a family of proteins that regulate cellular proliferation, differentiation, and matrix remodeling (24). Exogenous administration of TGF-β attenuates myocardial necrosis (25), enhances arterial oxygenation after lung transplantation (26), and promotes the restoration of epithelial monolayers in regenerating tubules in postischemic kidneys (27). Conversely, inhibition of TGF-β leads to growth deregulation in disease states such as cancer, cirrhosis, and fibrosis (28, 29). Some of the cytoprotective actions of TGF-β necessitate “deactivation” of mφ, i.e., suppressing excessive generation of mφ-derived ROS and subsequent cytokine release at the site of injury, so as to minimize further damage (31).
We hypothesized that CO exerts its effects in the cell rapidly, and therefore we explored events that occurred early into CO exposure that would explain subsequent protective effects. We demonstrate that CO rapidly stabilizes HIF-1α, leading to up-regulation of TGF-β, which are necessary events to rescue mφ from cell death and sustain tissue homeostasis in a model of IRI in mice.
Results
CO Induces HIF-1α Activation in mφ.
Exposure of mφ [RAW 264.7 and THP-1 (data not shown) to CO (250 ppm) and a CO-releasing molecule (CORM) (80 μM)] resulted in rapid up-regulation of HIF-1α, initially observed at 30 min with peak expression at 1–2 h by Western blot analysis (Fig. 1A). Mobility-shift assays showed DNA binding of HIF-1α after 15 min of exposure that was maintained for 30 min before returning to control levels (Fig. 1B). Mφ transiently transfected with pHIF-1α-luc and pTranslucent and exposed to CO gas or a CORM for 1 h induced HIF-1α promoter activity 3- to 4-fold compared with vector alone showing the functional consequences of CO (P < 0.01; Fig. 1C). Additionally the effects of CO were NO-independent, as blockade with N-nitro-l-arginine methyl ester had no effect. The relative role of ROS in the induction of HIF-1α are made clear by the ability of peroxide to induce HIF-1α and that the effects of CO on HIF-1α stabilization are abrogated in the presence of catalase and superoxide dismutase (Fig. 1C).
Fig. 1.
Induction of HIF-1α expression and activity after administration of CO. (A) Kinetics of HIF-1α protein via Western blot analyses, in mφ exposed to CO or a CORM. α-Tubulin and β-actin were used as loading controls. (B) Detection of HIF-1α DNA binding by EMSA. (C) HIF-1α luciferase reporter activity in indicated groups. ∗, P < 0.01 vs. control; ∗#, P < 0.05 vs. CO. (D) Immunofluorescent detection of cytoplasmic and nuclear HIF-1α (green punctate dots, denoted by arrows) in situ in 3D-reconstructed isolated lung mφ (air control, Upper; panel-CO exposed, Lower). Radiographs and luciferase data are representative of three to five independent experiments. Images are representative of 10 fields of view from air- and CO-exposed animals (n = 3 each). (Scale bar: 1 μm.)
CO-induced HIF-1α expression also occurred in vivo. Wide-field 3D-reconstructed fluorescent images showed immuno-positive staining for HIF-1α in the nuclei (Fig. 1D) of isolated alveolar mφ (F4/80+; data not shown) of C57BL/6 mice exposed to CO or air for 2 h. A similar pattern was seen in vitro in RAW 264.7 mφ exposed to CO (Fig. 2C).
Fig. 2.
Role of mitochondria in CO-induced HIF-1α expression. (A) Mitochondria-deficient (ρ°) and WT mφs were characterized by their ability to generate ROS by 2′,7′-dichlorodihydrofluorescein diacetate (DCF) fluorescence. (Upper) WT. (Lower) ρ°. Cells were preloaded with DCF ± CO, and fluorescence was determined by FACS (filled, 0 min; unfilled dashed line, 5 min; unfilled bold line, 60 min). (B) Western blot showing kinetics of HIF-1α protein in ρ° cells exposed to CO; α-tubulin is shown as a loading control. (C) Immunofluorescent staining for HIF-1α (red, denoted by arrows) in cultured WT mφs (left-air and right-CO; Upper) and ρ° cells (left-air and right-CO; Lower). Images shown are representative of at least 10 fields of view. Results shown represent one of three independent experiments. (Scale bar: 1 μm.)
CO Induces ROS Generation in mφ.
CO efficiently increases oxidative stress (31–33). Mφ preloaded with DCF-DA and exposed to CO showed a transitory and significant burst of ROS after 5 min of CO that continued for 30–60 min (Fig. 2A Upper). The signal then rapidly declined, suggesting that the ROS generation by CO is tightly regulated. Mitochondria-deficient mφ (ρ°) exposed to CO produced insignificant levels of ROS (Fig. 2A Lower) compared with WT cells, implicating the mitochondria as the source of ROS. Residual or nonmitochondrial ROS production may likely be attributed to other oxidant sources such as NAD(P)H oxidase.
Mitochondria-Derived ROS Mediate CO Induced HIF-1α Activity.
PO2 and ROS control HIF-1α stability (34). We show that it is mitochondria-derived ROS that mediate CO-induced HIF-1α expression because CO was unable to induce HIF-1α expression at any time point in ρ° mφ (Western blot analysis (Fig. 2B) or nuclear translocation (Fig. 2C).
CO Induces the Expression of TGF-β.
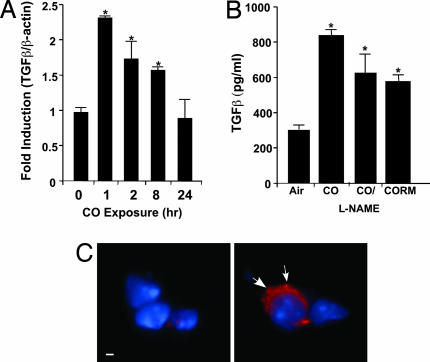
In addition to activating HIF-1α, CO modulated the expression of TGF-β, a mφ-derived cytokine regulated by HIF-1α that is essential for lung healing and remodeling after injury. Mφ exposed to CO showed increased expression of TGF-β with mRNA expression peaking after 1 h and returning to control levels over 24 h (Fig. 3A). Correlative protein expression in the media showed significant increases by 24 h in CO- or CORM-treated cells, which was NO-independent (Fig. 3B; P < 0.001). Immunofluorescent detection of TGF-β is seen in 3D reconstructed sections of freshly isolated CO-treated mouse lung mφ versus air-treated mφ (Fig. 3C). Similar effects were observed in vitro in RAW cells exposed to CO (data not shown).
Fig. 3.
Induction of TGF-β expression after exogenous administration of CO. (A) Kinetics of TGF-β mRNA expression in mxz by PCR analyses. Results were normalized to the β-actin (∗, P < 0.001). (B) Secretion of TGF-β in response to CO gas (± N-nitro-l-arginine methyl ester) or CORM in mφ (∗, P < 0.002). Shown is the mean ± SD of six wells from three independent experiments. (C) Immunofluorescent localization of TGF-β in mouse lung ± CO. Wide-field, 3D reconstruction of lung sections stained for TGF-β shows primarily localization in alveolar mφs. Images are representative of 10 sections from three or four mice per group. (Left) Air. (Right) CO. Blue are nuclei and red is intracellular TGF-β expression denoted by arrows. (Scale bar: 1 μm.)
Induction of TGF-β by CO Requires HIF-1α and Is Independent of IL-10.
There are HIF-1α consensus binding sites on the TGF-β promoter (35). To determine whether HIF-1α mediates CO-induced TGF-β expression, we generated a stable line of mφ expressing HIF-1α-miRNA (LMP-HIF-1α-miRNA). These cells when exposed to CO were unable to express HIF-1α protein and up-regulate TGF-β mRNA expression (Fig. 4) when compared with LMP vector-treated control cells. These data show that HIF-1α is critical for CO-induced TGF-β expression in mφ. The necessary role of HIF-1α and TGF-β in CO-induced protection was also observed in vivo (see Fig. 6). Exposure to CO alone does not induce IL-10 expression (data not shown) and thus TGF-β expression is most likely regulated by HIF-1α.
Fig. 4.
Induction of TGF-β expression by CO is HIF-1α-dependent. A stable HIF-1α-miRNA mφ cell line was generated to study the relationship between HIF-1α activation and TGF-β expression. (A) CO is unable to induce HIF-1α in HIF-1α-miRNA-infected mφ (open bars) compared with LMP vector control (filled bars) as shown by Western blot (Inset) (2 h) and PCR (0–4 h) analyses. (B) The ability of a HIF-1α-miRNA mφ cell line (filled bars) to express TGF-β in response to CO was compared with control and LMP vector control cells (empty bars) by Western blot. β-Actin was used as a loading control. Results shown mean ± SD from three independent experiments of n = 3 per experiment (∗, P < 0.001 vs. LMP-miRNA control).
Fig. 6.
CO-dependent HIF-1α and TGF-β expression are necessary to prevent lung IRI. (A) C57BL/6 mice were exposed to CO alone (250 ppm) for 2 and 24 h (n = 4 per group). Lungs were harvested and stained for HIF-1α (2-h exposure) or TGF-β (24-h exposure). Images are representative of six to eight sections per lung. IgG isotype control is shown. (B–G) Silencing of HIF-1α abrogates CO-induced protection against lung IRI. HIF-1α-siRNA or saline were administered to mice intratracheally as described in Methods. CO was administered 1 h before IRI. Lung injury was assessed 48 h after reperfusion by TUNEL staining. TUNEL-positive cells can be seen in C–F. (B) Air/HIF-1α-siRNA. (C) Air/I/R. (D) CO/I/R. (E) Air/I/R/HIF-1α-siRNA. (F) CO/I/R/HIF-1α-siRNA. Note that CO-treated animals (D) show a marked reduction in TUNEL-positive cells compared with those in C, E, and F. Images shown are representative of 8–10 sections from n = 3–5 animals per group. (G) Histogram representation of B–F. Results are mean ± SD of four to six mice per group (∗, P < 0.01 vs. CO). (H) TGF-β expression from IRI-injured lungs. Air+I/R-injured mice (Upper Left), CO+I/R-injured mice (Lower Left), air+HIF-1α-siRNA+I/R-injured mice (Upper Right) and CO+HIF-1α-siRNA+I/R-injured mice (Lower Right). Note that the majority of positive staining is localized to mφ (arrows) and a greater number of TGF-β-positive mφ is present in lungs exposed to CO+I/R (Lower Left) when compared with that of CO+HIF-1α-siRNA+I/R-injured mice (Lower Right). Images are representative of 8–10 sections from three to five animals per group. (I) TGF-β expression by CO is required for protection. CO was unable to protect against IRI in mice treated with TGF-β-siRNA as described in H. Results are mean ± SD of four to six mice per group (∗, P < 0.002 vs. CO). (Scale bar: 40 μm.)
CO Increases HIF-1α Activity and Promotes Survival of mφ Exposed to Anoxia-Reoxygenation (A/R).
HIF-1α activation markedly enhances cell survival in many models of hypoxia and cancer (19, 36–38). Mφs exposed to A/R (8 h of anoxia followed by 16 h of reoxygenation) demonstrated a 5-fold increase in propidium iodide staining compared with untreated (Fig. 5A; P < 0.01) In contrast, mφs exposed to the same A/R regimen treated with CO only during reoxygenation were protected from cell death (Fig. 5A; P < 0.01). Similar effects were observed when measuring caspase-3 activity as a marker of apoptosis. A/R induced a 6-fold increase in activity [5.8 ± 0.3% in control to 33.6 ± 10% (P < 0.009)], which was abrogated in CO-treated cells to 4.8 ± 0.3% (P < 0.01). CO-induced TGF-β is at least in part responsible for the cytoprotection. Conditioned media from RAW cells exposed to CO for 24 h and placed onto cells exposed to A/R were completely protected from apoptosis (Fig. 5A), similar to the effects observed with CO treatment during reoxygenation. CO induces secretion of TGF-β, which then mediates protection against A/R; neutralization of TGF-β with an antibody partially but significantly abrogated the effects observed with CO (Fig. 5A). Finally, addition of recombinant TGF-β to RAW cells in the absence of CO prevented AR-induced apoptosis, mimicking the CO effects (Fig. 5A). These results show that TGF-β is a critical mediator in the protective effects of CO in preventing A/R-induced cell death.
Fig. 5.
Role of HIF-1α in CO-induced inhibition of apoptosis. (A) Mφs were exposed to a regimen of A/R to induce cell death (as described in Methods) in the presence of CO, conditioned media from cells exposed to CO for 24 h (CM), recombinant TGF-β, and a combination of CO plus neutralizing TGF-β (λTGF-β). Apoptosis was assessed by FACS analyses of propidium iodide. Results represent mean ± SD of four independent experiments. (B) A/R-induced apoptosis in mφs deficient in HIF-1α, Ad-CVL (BMDM from HIF-1α-Loxp mice + Ad-Cre), and vector control Ad-Y5 ± CO. Results represent mean ± SD from four independent experiments. (C) CO augments A/R-induced TGF-β expression, which is independent of IL-10. RAW 264.7 cells were pretreated with a neutralizing antibody to IL-10 (λIL-10) and then exposed to A/R ± CO as described above. Results are mean ± SD of four to six wells from three independent experiments ∗, P < 0.02 vs. Untx; ∗#, P < 0.001 vs. Air+A/R.
To further test the role of HIF-1α in the protection of CO against A/R, we performed identical experiments as above using bone marrow-derived mφ (BMDM) obtained from WT and HIF-1α-loxp mice. After purification and differentiation, BMDM were infected with an Adeno-Cre recombinase (CVL) virus to knock down HIF-1α. Efficiency of infection was >85%. Controls included WT cells infected with Y5. All cells were then exposed to A/R ± CO. Unlike animals with WT-infected cells, CO was unable to protect animals with HIF-1α-deficient mφ against A/R-induced cell death (Fig. 5B). Alveolar mφs isolated from HIF-1α-loxp mice and treated with the same regimen as described above yielded similar results (data not shown). These data further support a role for HIF-1α in mediating CO-induced cytoprotection against A/R-induced cell death. Based on reports of CO modulating IL-10, and IL-10 regulating TGF-β expression, we evaluated a potential role for IL-10 in the A/R model. We observed an increase in TGF-β expression in response to A/R, which was augmented in the presence of CO. These effects were independent of IL-10, as addition of an IL-10 neutralizing antibody had no effect on CO-induced TGF-β expression (Fig. 5C).
CO Inhibits IRI in the Lung: The Role of HIF-1α and TGF-β.
HIF-1α and TGF-β play vital roles in attenuating IRI (39–41). Based on these reports, we tested whether the molecules identified in our in vitro findings in mφ were involved in vivo in a model of lung IRI where CO has been shown to be an effective treatment (42, 43). Unlike the previous reports that did not evaluate the effects of CO during the pretreatment period (42, 43), we show that CO exposure to noninjured mice resulted in increased expression of both HIF-1α and TGF-β, primarily seen in alveolar mφ. We exposed mice to CO alone before IRI to ascertain whether HIF-1α and TGF-β were involved in preconditioning the lung to induce resistance to subsequent injury. CO exposure to uninjured mice resulted in increased expression of both HIF-1α and TGF-β, which was primarily localized to alveolar mφ (Fig. 6A). We demonstrate that CO attenuated I/R-induced cell death as measured by TUNEL staining (>50% over untreated animals) compared with I/R alone (Fig. 6 B–G). Additionally, pretreatment with HIF-1α-siRNA intratracheally negated CO-induced protection, implicating CO-induced HIF-1α in mediating lung cytoprotection. There were also significantly more TGF-β-positive mφs [colocalization of mφ marker (F4/80+; data not shown) and TGF-β] in lungs exposed to I/R in the presence of CO (Fig. 6H) compared with I/R alone, supporting a protective role of TGF-β similar to the in vitro studies. Fewer TGF-β-positive mφs were observed in CO-treated, IR-injured animals where HIF-1α was silenced with siRNA (Fig. 6H), confirming the in vivo role of HIF-1α in regulating TGF-β. Silencing of TGF-β in the lung with specific siRNA (TGF-β null mice are embryonic lethals) resulted in a similar abrogation of CO-induced protection (Fig. 6I), validating in vivo the requirement for TGF-β induction by CO.
Discussion
Protection by exogenous CO in lung IRI (43, 44) involves modulation of p38 and ERK1/2 MAPK as well as downstream activation of EGR-1, caspases, and PAI-1 (34, 43, 44). In addition, exogenous CO (15 ppm) prevented endothelial cell apoptosis via p38/STAT3-mediated inhibition of Fas and caspase-3 (42). CO (1,000 ppm) preconditioning for 16–24 h before lung transplantation suppressed ERK1/2-mediated proinflammatory and prothrombotic factor secretion (43). In all of these cases, the effects of CO were in the presence of a stressor. In this study, we elucidate the sequence of events that occur earlier during preconditioning with CO and define the specific downstream signaling and gene regulation that result in the observed inhibition of subsequent IRI.
We document one of the earliest events observed in cells and tissues exposed to a low concentration of CO, i.e., transitory production of ROS within 5–10 min of exposure. More importantly, that the CO-derived ROS that have also been shown to increase peroxisome proliferator-activated receptor γ (PPARγ) (31), rapidly stabilize HIF-1α within minutes, leading to regulated TGF-β expression that subsequently protects mφ and lung tissue against A/R injury and IRI. Mφs are a major target of CO in this model and show the highest expression of HIF-1α and TGF-β. CO rapidly conditions the mφ through HIF-1α and secretion of TGF-β into a “prosurvival” mode, preventing tissue damage ordinarily elicited by IRI. PPARγ is increased after 2–3 h of CO, in stark contrast to CO-induced HIF-1α activation, which is observed as early as 15–30 min postexposure. Ongoing studies are evaluating potential PPARγ regulation by HIF-1α. If no link is found, these two molecules may function in different signaling cascades, each mediating its own effects.
We and others (1, 2, 5, 31, 42, 43) have shown that administering low concentrations of CO (15–500 ppm) has profound cytoprotective effects in vivo in rodents and swine and in vitro, modulating vasodilatation, inflammation, apoptosis, and immune tolerance. These concentrations are well tolerated with no adverse effects on the animals noted. These effects are mediated by activation of MAPKs (42, 43), peroxisome proliferator-activated receptor γ (31), and STAT (41), as well as the heme-containing molecules soluble guanylate cyclase (44) and the mitochondrial oxidases (7, 31, 45). The latter conclusion is most strongly supported by our demonstration that CO is ineffective when using a mitochondria-deficient mφ line (ρ°). Recent reports suggest that increases in ROS arising from mitochondria (9, 16) and/or from NAD(P)H oxidase (45) also stabilize HIF-1α (46) to regulate downstream gene expression. Generation of endogenous ROS, especially hydrogen peroxide, has been postulated as a means of “cellular communication” (47), which is supported in Fig. 1. Activation of HIF-1α by ROS appears to act upstream of prolyl hydroxylases (48) in the mitochondria at the Rieske Fe-S clusters on complex III (49). Interfering with these clusters may be one possible target for CO because of the affinity of CO for iron. Another possibility to explain the effects of CO on HIF-1α is that low concentrations of NO promote HIF-1α stability (50), perhaps by binding to the prolyl hydroxylases and interfering with O2 sensing. However, NO in this model, does not play a key role in mediating CO-induced HIF-1α activation.
The potential relevance of the effects of CO as studied here to that generated endogenously by heme oxygenase (HO)-1 was recently supported by D'Amico et al. (45) where comparisons were made between exogenous CO at concentrations similar to ones used here with those generated endogenously by HO. They found remarkable similarities in the effects on cellular respiration. Similar to our in vitro findings, exogenous CO disrupted mitochondrial respiration that led to increased ROS, an effect that was magnified with decreases in cellular PO2. In our study CO was administered under normoxic conditions, which may then account for the transitory nature of the ROS burst. The transient effects on ROS generation might also be explained by an increase in the activity of antioxidant enzymes such as MnSOD and glutathione, which are activated in response to CO (unpublished observations).
IRI is associated with cell death from oxygen deprivation and uncontrolled release of ROS and inflammatory cytokines during reperfusion. Elevated HIF-1α and TGF-β expression imparts anti apo pto tic, ant-fibrotic, and antiinflammatory properties in heart and kidney models of IRI (51–56). We and others have shown that TGF-β can function to promote activation of prosurvival genes such as HO-1, acting to block inflammation (29, 51, 55). The ability to up-regulate HIF-1α and TGF-β before the onset of IRI would ameliorate the undesirable consequences of IRI. In this study, we have elucidated a sequence of events that occurs in response to CO resulting in a rapid phenotypic switch toward a protective phenotype that prevents lung IRI. These studies support the concept that HIF-1α and TGF-β function as protective molecules in preparing the cells for imminent injury.
Elucidation of the signaling pathways involved in the protective effects of CO is important for both our basic understanding of the mechanism of action of CO and the creation of new therapeutic approaches to IRI. Activation of HIF-1α and TGF-β to impart therapeutic effects in preventing injury sustained during IRI would be important clinically during surgical procedures, organ transplantation, balloon angioplasty, or hemorrhagic shock. The fact that within 15 min of exposure CO markedly induces HIF-1α activation, preceding all reported signaling events to date, positions HIF-1α as a central regulator in conditioning of the mφ. We observed that CO did not reduce the influx of mφ to the site of injury, but rather reprogrammed their state of activation toward one of protection versus aggression. Harnessing the immune system is in part how CO and HO-1 act to maintain homeostasis. Taken together, these data provide critical information related to the biology of CO and the mechanisms of its action. Clinical testing has begun evaluating the efficacy of CO in organ transplantation to prevent the sequelae of IRI that lead to chronic rejection.
Materials and Methods
Cell Culture.
Murine RAW 264.7 mφ, human THP-1 mφ, and HEK293 cells from ATCC (Rockville, MD) were maintained in DMEM supplemented with 10% FBS and penicillin/streptomycin (Invitrogen, Carlsbad, CA). Primary bone marrow-derived mφ were harvested as described (31) from myelogenic specific HIF-1α Double Floxed (loxP) B.129/C57BL/6 mice (generous gift from Randall Johnson, University of California at San Diego, La Jolla, CA). RAW and THP-1 mitochondria-deficient mφ (ρ° cells) were generated and maintained as described (17). Confirmation of mitochondria deficiency was assessed by the absence of cytochrome oxidase-2 transcript by PCR (R & D Systems, Minneapolis, MN).
Adenoviral Infections of BMDM.
Peripheral blood mononuclear cells were differentiated into mφ and infected with recombinant adenovirus for Cre recombinase or control virus (Y5) at a concentration of 50 multiplicity of infection per cell. Infections were performed as described (31). Experiments were performed on day 5. Cre activity in knocking down HIF-1α loxP BMDM was assessed by PCR (Qiagen, Valencia, CA).
Reagents.
Antibodies for HIF-1α, TGF-β, and β-actin were purchased from Novus Biochemicals (Littleton, CO), R&D Systems, and Cell Signaling Technology (Beverly, MA), respectively, and visualized with HRP-conjugated anti-mouse antibodies (Cell Signaling Technology). DAPI was from Molecular Probes, Eugene, OR. CORM was purchased from Sigma (St. Louis, MO). Recombinant human and neutralizing TGF-β antibodies were from R & D Systems. HIF-1α-siRNA and TGF-β-siRNA were purchased from Dharmacon (Lafayette, CO). RT-PCR and RNAeasy kits were from Ambion (Austin, TX) and Qiagen, respectively. HIF-1α oligonucleotides with consensus sequence, 5′-TCT GTA CGT GAC CAC ACT CAC CTC–3′, was from Santa Cruz Biotechnology (Santa Cruz, CA).
Transfections and Transient Reporter Assays.
THP-1 mφs were transfected with 1 μg/ml pHIF-1α-luc and pTranslucent responsive plasmids (Panomics, Freemont, CA) using FuGene 6 (Roche Diagnostics, Alameda, CA) according to the manufacturer's direction. After 24–36 h, cells were washed with serum-free media and exposed to CO or CORM for 0–2 h, and luciferase activity (Promega, Madison, WI) was assessed as described (31). Luminescence was normalized to total protein levels and presented as fold induction against control pTranslucent reporter activity.
HIF-1α and TGF-β Silencing.
THP-1 mφs were transfected with HIF-1α or TGF-β siRNA-SMARTpool reagent (Dharmacon) with FuGene (Roche Diagnostics). Four siRNA sequences were tested, and one was chosen for further experimentation based on its ability to block CO and hypoxia-induced HIF-1α and or TGF-β expression by Western blot.
Stable Transfection with mir-HIF-1α-shRNA.
shRNAmir (microRNA-adapted shRNA) against mouse HIF-1α was generated in pSM2 vector (Open Biosystems, Huntsville, AL). shRNAmirHIF-1α was subcloned into MSCV-LTRmiR30-PIG (LMP) vector (Open Biosystems) with XhoI and EcoRI (Invitrogen) restriction enzymes. Confirmation was verified by restriction site analysis and sequencing. For production of retrovirus, HEK293 cells were transfected with shRNAmirHIF-1αLMP, VSVG, and Gag-Pol plasmids at ratio 2:1:1 using Lipofectamine 2000 (Invitrogen). After 12 h, medium containing the virus was centrifuged at 2,000 × g for 10 min at 4°C, and the supernatant was filtered by using 0.45-μm filters (Millipore, Phoenix, AZ). After 14-h incubation with viruses, RAW cells were selected with 5 μg/ml of puromycin (Sigma) for 2 weeks and the expression of GFP and knockdown was tested by Western blot.
CO Exposure.
Cells were exposed to CO as described (30).
FACS Analyses of 2′,7′-Dichlorodihydrofluorescein Diacetate and Propidium Iodide Staining as Markers of ROS Production and Cell Death.
RAW 264.7 and THP-1 mφs were incubated with 0.05 mM DCFH-DA (Invitrogen) for 30 min at 37°C before the harvest time point. The cells were then washed, exposed to air or CO for 5–60 min, and resuspended in FACS buffer (PBS + 1% FBS), and the intensity of fluorescence was measured as described (33). Cell cycle and DNA content as markers of cell death were detected with propidium iodide and the data were analyzed with CellQuest software (BD, San Diego, CA).
Western Blot Analysis.
Cellular protein extracts, concentrations, and the methodology used for Western analyses were obtained as described (31). The membranes were incubated with HIF-1α and TGF-β antibodies followed by incubation with HRP-conjugated anti-mouse and anti-rabbit secondary antibodies, respectively. Signal development was carried out with an enhanced chemiluminescence detection kit (Pierce, Rockford, IL).
Immunofluorescence Staining.
Cells were grown on Met-Tek slides (Met-Tek, Ashland, MA), exposed as described, and fixed in ice-cold methanol/acetic acid (75%: 25%). Cells were then permeabilized with 0.1% Nonidet P-40 (Sigma) and incubated with nonimmune IgG. Expression of HIF-1α, and TGF-β were detected with antibodies as described and visualized via conjugation to Alexa-488 and Alexa-592 antibodies (Molecular Probes). Nuclei were stained and visualized with DAPI. Micrographs were obtained with an Axiovert 200M Apotome wide-field microscope (Zeiss, Thornwood, NY) and Axiovision software. Lungs from mice treated as described were immersed in either zinc or mercury fixative (BD Pharmingen), sectioned in 5-μm slices, and stained for HIF-1α and TGF-β. Primary antibodies were visualized by using HRP-conjugated anti-mouse antibody, and images were captured by using the Zeiss light microscope.
EMSA.
Cultured mφs were exposed to CO for 0–2 h after which the cells were lysed, nuclear protein was extracted, and electrophoresis was performed as described (31).
SemiQuantitative RT-PCR.
Primers for HIF-1α and TGF-β were purchased from Santa Cruz Biotechnology and R & D Systems, respectively. Total RNA was extracted and reverse-transcribed per the manufacturer's instructions (Ambion) on an iCycler (Bio-Rad, Carlsbad, CA) with the following conditions: start at 94°C for 4 min followed by 30–35 cycles of amplification (94°C for 45 s, 55°C for 45 s, and 72°C for 45 s), with a final extension of 72°C for 10 min. Amplified PCR products were fractionated with a 1% agarose gel.
Real-Time PCR.
Total RNA (1 μg) was reverse-transcribed into cDNA by Moloney murine leukemia virus enzyme (Promega, Mannheim, Germany) with random hexamers (1 μg/μg total RNA. All PCRs were performed with the SYBR Green kit (BioRad, Hercules, CA). Primers were purchased from Biosource International, Camarillo, CA. A Mx3000P QPCR System (Stratagene, La Jolla, CA) was used with the following cycling conditions: initial denaturation at 95°C for 10 min, followed by 40 cycles at 94°C for 30 s, 58°C for 15 s, and 72°C for 30 s and a 10-min terminal incubation at 72°C. Expression of target genes was normalized to β-actin or tubulin expression levels.
In Vivo siRNA Administration and Murine Lung IRI.
Normal C57BL/6 male mice at 6–8 weeks weighing 20–25 g (Jackson Laboratory, Bar Harbor, ME) were anesthetized with methoxyfluorane (Sigma). Animal protocols were approved by the Beth Israel Deaconess Medical Center and Yale University institutional animal care and use committees. After anesthetization 3 nmol/20 g in a volume of 50 μl of HIF-1α or TGF-β siRNA (Dharmacon) was administered intranasally. This protocol was repeated twice at 48 and 24 h before I/R. Lung IRI was induced as described (42). Lungs were extracted for TUNEL analyses 24–48 h post-IRI. Quantitation of TUNEL-positive cells was performed by counting the number of positive cells in 8–10 random fields of view per section.
Acknowledgments
We thank Martin Bilban for assisting with the silencing technology and the Julie Henry Fund at the Transplant Center of the Beth Israel Deaconess Medical Center for their support. This work was supported by National Institutes of Health Grants HL-071797 and HL-076167 (to L.E.O.).
Abbreviations
- mφ
macrophage
- BMDM
bone marrow-derived mφ
- HO
heme oxygenase
- HIF-1α
hypoxia-inducible factor 1α
- ROS
reactive oxygen species
- I/R
ischemia/reperfusion
- IRI
I/R injury
- CORM
CO-releasing molecule
- A/R
anoxia/reoxygenation.
Footnotes
Conflict of interest statement: L.E.O. is a paid consultant of Linde Healthcare.
This article is a PNAS direct submission.
References
- 1.Mazzola S, Forni M, Albertini M, Bacci ML, Zannoni A, Gentilini F, Lavitrano M, Bach FH, Otterbein LE, Clement MG. FASEB J. 2005;14:2045–2047. doi: 10.1096/fj.05-3782fje. [DOI] [PubMed] [Google Scholar]
- 2.Moore BA, Overhaus M, Whitcomb J, Ifedigbo E, Choi AM, Otterbein LE, Bauer AJ. Crit Care Med. 2005;6:1317–1326. doi: 10.1097/01.ccm.0000166349.76514.40. [DOI] [PubMed] [Google Scholar]
- 3.Neto JS, Nakao A, Kimizuka K, Romanosky AJ, Stolz DB, Uchiyama T, Nalesnik MA, Otterbein LE, Murase N. Am J Physiol. 2004;287:F979–F989. doi: 10.1152/ajprenal.00158.2004. [DOI] [PubMed] [Google Scholar]
- 4.Ameredes BT, Otterbein LE, Kohut LK, Gligonic AL, Calhoun WJ, Choi AM. Am J Physiol. 2003;285:L1270–L1276. doi: 10.1152/ajplung.00145.2003. [DOI] [PubMed] [Google Scholar]
- 5.Zuckerbraun BS, Otterbein LE, Boyle P, Jaffe R, Upperman J, Zamora R, Ford HR. Am J Physiol. 2005;289:G607–G613. doi: 10.1152/ajpgi.00055.2005. [DOI] [PubMed] [Google Scholar]
- 6.Nakao A, Neto JS, Kanno S, Stolz DB, Kimizuka K, Liu F, Bach FH, Billiar TR, Choi AM, Otterbein LE, Murase N. Am J Transplant. 2005;5:282–291. doi: 10.1111/j.1600-6143.2004.00695.x. [DOI] [PubMed] [Google Scholar]
- 7.Taille C., El-Benna J, S, Boczkowski MJ, Motterlini R. J Biol Chem. 2005;280:25350–25360. doi: 10.1074/jbc.M503512200. [DOI] [PubMed] [Google Scholar]
- 8.Emerling BM, Platanias LC, Black E, Nebreda AR, Davis RJ, Chandel NS. Mol Cell Biol. 2005;25:4853–4862. doi: 10.1128/MCB.25.12.4853-4862.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Proc Natl Acad Sci USA. 1998;95:11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bacon AL, Harris AL. Ann Med. 2004;36:530–539. doi: 10.1080/07853890410018231. [DOI] [PubMed] [Google Scholar]
- 11.Stoeltzing O, McCarty MF, Wey JS, Fan F, Liu W, Belcheva A, Bucana CD, Semenza GL, Ellis LM. J Natl Cancer Inst. 2004;96:946–956. doi: 10.1093/jnci/djh168. [DOI] [PubMed] [Google Scholar]
- 12.Bruick RK, McKnight SL. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- 13.Yu F, White SB, Zhao Q, Lee FS. Proc Natl Acad Sci USA. 2001;98:9630–9635. doi: 10.1073/pnas.181341498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hewitson KS, McNeill LA, Riordan MV, Tian YM, Bullock AN, Welford RWD, Elkins JM, Oldham NJ, Battacharya S, Gleadle J. J Biol Chem. 2002;277:26351–26355. doi: 10.1074/jbc.C200273200. [DOI] [PubMed] [Google Scholar]
- 15.Lando D, Peet DJ, Gorman JJ, Whelan DA, Whitelaw ML, Bruick RK. Genes Dev. 2002;16:1466–1471. doi: 10.1101/gad.991402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schroedl C, McClintock DS, Budinger GR, Chandel NS. Am J Physiol. 2002;283:L922–L931. doi: 10.1152/ajplung.00014.2002. [DOI] [PubMed] [Google Scholar]
- 17.Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Proc Natl Acad Sci USA. 1998;95:11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Albina JE, Mastrofrancesco B, Vessella JA, Louis CA, Henry WL, Jr, Reichner JS. Am J Physiol. 2001;281:C1971–C1977. doi: 10.1152/ajpcell.2001.281.6.C1971. [DOI] [PubMed] [Google Scholar]
- 19.Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, et al. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blouin CC, Page EL, Soucy GM, Richard DE. Blood. 2004;103:1124–1130. doi: 10.1182/blood-2003-07-2427. [DOI] [PubMed] [Google Scholar]
- 21.Date T, Mochizuki S, Belanger AJ, Yamakawa M, Luo Z, Vincent KA, Cheng SH, Gregory RJ, Jiang C. Am J Physiol. 2005;288:C314–C320. doi: 10.1152/ajpcell.00374.2004. [DOI] [PubMed] [Google Scholar]
- 22.Zarember KA, Malech HL. J Clin Invest. 2005;115:1702–1704. doi: 10.1172/JCI25740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hong YH, Peng HB, La Fata V, Liao JK. J Immunol. 1997;159:2418–2423. [PubMed] [Google Scholar]
- 24.Massague J, Blain SW, Lo RS. Cell. 2000;103:295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- 25.Chen H, Li D, Saldeen T, Mehta JL. Am J Physiol. 2003;284:H1612–H1617. doi: 10.1152/ajpheart.00992.2002. [DOI] [PubMed] [Google Scholar]
- 26.Daddi N, Kanaan SA, Suda T, Tagawa T, D'Ovidio F, Grapperhaus K, Kozower BD, Ritter JH, Mohanakumar T, Patterson GA. J Heart Lung Transplant. 2003;22:1323–1334. doi: 10.1016/j.healun.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 27.Docherty NG, Perez-Barriocanal F, Balboa NE, Lopez-Novoa JM. Ren Fail. 2002;24:391–406. doi: 10.1081/jdi-120006767. [DOI] [PubMed] [Google Scholar]
- 28.Fogar P, Pasquali C, Basso D, Floreani A, Piva MG, De Paoli M, Melis A, Sperti C, Pedrazzoli S, Plebani M. J Med. 1998;29:277–287. [PubMed] [Google Scholar]
- 29.Biswas S, Criswell TL, Wang SE, Arteaga CL. Clin Cancer Res. 2006;12:4142–4146. doi: 10.1158/1078-0432.CCR-06-0952. [DOI] [PubMed] [Google Scholar]
- 30.Tsunawaki S, Sporn M, Ding A, Nathan C. Nature. 1988;334:260–262. doi: 10.1038/334260a0. [DOI] [PubMed] [Google Scholar]
- 31.Bilban M, Bach FH, Otterbein SL, Ifedigbo E, de Costa d'Avila J, Esterbauer H, Chin BY, Usheva A, Robson SC, Wagner O, Otterbein LE. Immunity. 2006;24:601–610. doi: 10.1016/j.immuni.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 32.Chin BY, Trush MA, Choi AM, Risby TH. Am J Physiol. 2003;284:L473–L480. doi: 10.1152/ajplung.00297.2002. [DOI] [PubMed] [Google Scholar]
- 33.Piantadosi CA, Carraway MS, Suliman HB. Free Radical Biol Med. 2006;40:1332–1339. doi: 10.1016/j.freeradbiomed.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 34.Jiang BH, Zheng JZ, Leung SW, Roe R, Semenza GL. J Biol Chem. 1997;272:19253–19260. doi: 10.1074/jbc.272.31.19253. [DOI] [PubMed] [Google Scholar]
- 35.Sanchez-Elsner T, Ramirez JR, Sanz-Rodriguez F, Varela E, Bernabeu C, Botella LM. J Mol Biol. 2004;336:9–24. doi: 10.1016/j.jmb.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 36.Li J, Shi M, Cao Y, Yuan W, Pang T, Li B, Sun Z, Chen L, Zhao RC. Biochem Biophys Res Commun. 2006;342:1341–1351. doi: 10.1016/j.bbrc.2006.02.094. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki A, Kusakai G, Shimojo Y, Ogura T, Kobayashi M, Esumi H. J Biol Chem. 2005;280:31557–31563. doi: 10.1074/jbc.M503714200. [DOI] [PubMed] [Google Scholar]
- 38.Cai Z, Manalo DJ, Wei G, Rodriguez ER, Fox-Talbot K, Lu H, Zweier JL, Semenza GL. Circulation. 2003;108:79–85. doi: 10.1161/01.CIR.0000078635.89229.8A. [DOI] [PubMed] [Google Scholar]
- 39.Natarajan R, Salloum FN, Fisher BJ, Kukreja RC, Fowler AA. Circ Res. 2006;98:133–140. doi: 10.1161/01.RES.0000197816.63513.27. [DOI] [PubMed] [Google Scholar]
- 40.Gross CE, Bednar MM, Howard DB, Sporn MB. Stroke. 1993;24:558–562. doi: 10.1161/01.str.24.4.558. [DOI] [PubMed] [Google Scholar]
- 41.Lefer AM, Ma XL, Weyrich AS, Scalia R. Proc Natl Acad Sci USA. 1993;90:1018–10122. doi: 10.1073/pnas.90.3.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang X, Shan P, Alam J, Fu XY, Lee PJ. J Biol Chem. 2005;280:8714–8721. doi: 10.1074/jbc.M408092200. [DOI] [PubMed] [Google Scholar]
- 43.Mishra S, Fujita T, Lama VN, Nam D, Liao H, Okada M, Minamoto K, Yoshikawa Y, Harada H, Pinsky DJ. Proc Natl Acad Sci USA. 2006;103:5191–5196. doi: 10.1073/pnas.0600241103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verma A, Hirsch DJ, Glatt CE, Ronnett GV, Snyder SH. Science. 1993;259:381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- 45.D'Amico G, Lam F, Hagen T, Moncada S. J Cell Sci. 2006;119:2291–2298. doi: 10.1242/jcs.02914. [DOI] [PubMed] [Google Scholar]
- 46.Ateghang B, Wartenberg M, Gassman M, Sauer H. J Cell Sci. 2006;119:1043–1052. doi: 10.1242/jcs.02798. [DOI] [PubMed] [Google Scholar]
- 47.Droge W. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 48.Brunelle JK, Bell EL, Quesada NM, Vercauteren K, Tiranti V, Zeviani M, Scarpulla RC, Chandel NS. Cell Metab. 2005;1:409–414. doi: 10.1016/j.cmet.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 49.Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT. Cell Metab. 2005;1:401–408. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 50.Hagen T, Taylor CT, Lam F, Moncada S. Science. 2003;302:1975–1978. doi: 10.1126/science.1088805. [DOI] [PubMed] [Google Scholar]
- 51.Chin BY, Petrache I, Choi AM, Choi ME. J Biol Chem. 1999;274:11362–11368. doi: 10.1074/jbc.274.16.11362. [DOI] [PubMed] [Google Scholar]
- 52.Bergeron M, Gidday JM, Yu AY, Smenza GL, Ferriero DM, Sharp FR. Ann Neurol. 2000;48:285–296. [PubMed] [Google Scholar]
- 53.Zhang B, Tanaka J, Yang L, Sakanaka M, Hata R, Maeda N, Mitsuda N. Neuroscience. 2004;126:433–440. doi: 10.1016/j.neuroscience.2004.03.057. [DOI] [PubMed] [Google Scholar]
- 54.Yang BC, Zander DS, Mehta JL. J Pharmacol Exp Ther. 1999;291:733–738. [PubMed] [Google Scholar]
- 55.Kuruvilla AP, Shah R, Hochwald GM, Liggit HD, Palladino MA, Thorbecke GJ. Proc Natl Acad Sci USA. 1991;88:2918–2921. doi: 10.1073/pnas.88.7.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Horowitz JC, Lee DY, Waghray M, Keshamouni VG, Thomas PE, Zhang H, Cui Z, Thannickal VJ. J Biol Chem. 2004;279:1359–1367. doi: 10.1074/jbc.M306248200. [DOI] [PMC free article] [PubMed] [Google Scholar]