Abstract
Background and Aim
Ezrin-Radixin-Moesin (ERM) proteins are cross-linkers between the plasma membrane and actin filaments. Radixin, the dominant ERM protein in hepatocytes, has been reported to selectively tether Mrp2 to the apical canalicular membrane. However it remains to be determined if this is its primary function.
Methods
An adenovirus-mediated siRNA was used to down-regulate radixin expression in collagen sandwich cultured rat hepatocytes and morphological and functional changes were characterized quantitatively.
Results
In control cultures, an extensive bile canalicular network developed with properly localized apical and basolateral transporters that provided for functional excretion of fluorescent cholephiles into the bile canalicular lumina. SiRNA induced suppression of radixin was associated with a marked reduction in the canalicular membrane structure as observed by differential interference contrast microscopy and F-actin staining, in contrast to control cells exposed to adenovirus encoding scrambled siRNA. Indirect immunofluorescence demonstrated that apical transporters (Mrp2, Bsep and Mdr1) dissociated from their normal location at the apical membrane and were found largely associated with Rab11-containing endosomes. Localization of the basolateral membrane transporter, Oatp2, was not affected. Consistent with this dislocation of apical transporters, the biliary excretion of GS-MF and CGamF was significantly decreased in the radixin-deficient cells but not in the control siRNA cells.
Conclusions
Radixin is essential for maintaining the polarized targeting and/or retaining of canalicular membrane transporters and is a critical determinant of the overall structure and function of the apical membrane of hepatocytes.
Keywords: ERM, siRNA, bile transporter, bile canaliculi, Rab11
Introduction
Hepatocytes are highly polarized epithelial cells whose apical canalicular domain is designed for the production of bile. This secretory process depends upon a group of membrane transporters at this apical pole that are members of the ABC superfamily of export pumps. These include the bile salt export pump (Bsep, Abcb11), the multidrug resistance protein (Mdr1, Abcb1), and the multidrug resistance associated protein 2 (Mrp2, Abcc2) among others. Under normal physiologic conditions, the transport of bile salts into bile generates bile salt dependent bile flow while bile salt independent flow is generated in large part by the excretion of glutathioine via Mrp2. Disorders that impair these transport proteins result in cholestatic liver injury1,2.
While the maintenance of secretory polarity of the hepatocyte is critical for its normal function, little is known about how these cells establish and maintain this functionally distinct apical domain3. The ERM (Ezrin, Radixin and Moesin) family of proteins plays an important role in regulating the structure and function of specific domains of the cell cortex by crosslinking membrane and actin cytoskeleton4. The dominant ERM protein in hepatocytes is radixin5, which is primarily localized at the canalicular membrane of hepatocytes5,6. At four weeks of age radixin-knock out mice demonstrate a selective loss of Mrp2 from the canalicular membrane and begin to develop conjugated hyperbilirubinemia, reminiscent of the Dubin-Johnson syndrome in man7. These findings suggest that radixin may be required for the tethering of Mrp2 to the apical canalicular domain. Radixin is also reduced and associated with redistribution of MRP2 within intracellular structures of hepatocytes in patients with Primary Biliary Cirrhosis (PBC)8. However, in contrast to radixin deficient mice, P-glycoproteins (MDR1, MDR3 and BSEP) are also redistributed to intracellular structures and colocalize with MRP2 in these patients with chronic cholestatic liver disease.
To clarify the role of radixin in the canalicular localization of bile transporters and the integrity of apical canalicular domain, we have used adenovirus-mediated siRNAs to suppress radixin expression in collagen sandwich cultured rat hepatocytes. This culture method has been described previously9,10 and sustains the expression of hepatocyte-specific proteins and the maintanace of bile canalicular structure and function. Our studies show that radixin deficiency results in a profound reduction in canalicular membrane structures and a dissociation of bile transporters from the apical canalicular membrane. This in turn leads to a functional impairment in the canalicular excretion of substrates for Mrp2 and Bsep. These results provide clear evidence that radixin is a critical requirement not just for the tethering of Mrp2 but for the normal maintenance of the canalicular membrane and the localization and function of its transport proteins.
Materials and Methods
Reagents
BD Adeno-X™ Expression Systems 2 was purchased from BD Biosciences (Bedford, MA). Alexa conjugated secondary antibodies, TO-PRO 3, CMFDA and Alexa 594 conjugated phalloidin were purchased from Molecular Probes (Eugene, OR). CGamF was a gift from Alan Hofmann, San Diego, CA. The following antibodies were used: mouse anti-Mrp2 (Alexis Biochemicals, San Diego, CA), rabbit anti-radixin (Cell Signaling Technology, Beverly, MA), goat anti-radixin (Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti-MDR (Signet Laboratories, Dedham, MA), rabbit anti-Bsep (Kamiya Biomedical Co, Seattle, WA), mouse anti-ZO-1 and rabbit anti-Rab11 (Zymed Laboratories, San Francisco, CA), and mouse anti-β-actin (Sigma, Saint Louis, MS). Rabbit anti-Oatp2 was kindly provided by Bruno Stieger, Zurich, Switzerland. Rabbit anti-Mrp3 was raised in our laboratory. Western blotting demonstrated its specificity for Mrp3 as opposed to Mrp2 by its presence in TR- rat liver and its up-regulation in cholestasis.
Construction of Recombinant siRNA-expressing Adenovirus
The siRNA sequences target rat radixin gene at positions 106–125 (siRDXA), 627–646 (siRDXB), 1011–1030 (siRDXC) and 1219–1238 (siRDXD), relative to the start codon. A scrambled sequence (5’-GAC TCC GAA CAT GTA ACG T-3’) was used for the control siRNA (siControl). The recombinant adenovirus encoding siRNA was constructed and amplified according to the instructions of BD Biosciences.
Cell Culture
Hepatocytes were isolated from rat liver by collagenase perfusion as described previously from this laboratory11. Cells were cultured on collagen-coated dishes or glass coverslips in Williams E medium with the addition of 5% fetal calf serum, 10mM Hepes buffer, 2mM L-glutamine, 1.8g/L glucose, 1μM dexamethasone, 4mg/L insulin, 100 U/ml penicillin and 100 μg/ml streptomycin and 10mg/L gentamicin. Cells were infected with adenovirus twice at 1h and 16h after seeding and were overlaid with gelled collagen 24 h after seeding.
Immunoblot
Cells were lysed in RIPA buffer (50mM Tris pH8.0, 150mM NaCl, 1% NP-40, 0.5% deoxycholate, 0.1%SDS, protease inhibitors) and centrifuged at 1,000g for 10min. The supernatants were fractionated on SDS-PAGE and analyzed by western blot and enhanced chemiluminescence method.
Light Microscopy
Differential interference contrast (DIC) microscopy was used to qunatitively assess the length of the bile canalicular channel using the software Openlab 3.1.5 (Improvision, Coventry, CV 4 7Hs, England). Images were obtained randomly from ten fields per coverslip (20x objective) and analyzed from 4 separate experiments.
Separate coverslips were fixed in 4% paraformaldehyde or in cold methanol for immunofluorescence. Primary antibodies were diluted in blocking buffer (1% BSA in PBS-0.05% Triton X100) and incubated on cell layers for 2 hrs at room temperature. Appropriate secondary antibodies were incubated for 1 hr and fluorescence labeling was observed with a Zeiss LSM 510 (Thornwood, NJ) using appropriate filters and the same gain levels for all conditions.
Electron Microscopy
After 5 days of culture, coverslips were coded (by WW) and hepatocyte monolayers were fixed with 2.0% Paraformaldehyde/2.5% Glutaraldehyde and postfixed with 1% osmium tetroxide. The cultures were blockstained with 2% uranyl acetate, dehydrated in acetone series and epon embedded. Thin sections were stained with lead citrate and uranyl acetate. Electron micrographs were acquired at 14,000 x primary magnification (by CR) on a Philips 410 electron microscope (FEI, Hillsboro, OR) and evaluated blindly by JLB and AM.
CMFDA and CGamF Excretion by Rat Hepatocytes
Hepatocytes were incubated with 5μM 5-chloromethylfluorescein diacetate (CMFDA) or 1μM cholylglycylamido-fluorescein (CGamF) diluted in HEPES buffer (0.35g/L KCl, 0.25g/L MgSO4, 0.18g/L CaCl2, 0.16g/L KH2PO4, 4.8g/L HEPES, 7.9g/L NaCl and 0.9g/L glucose, pH7.4) at 37°C for 15min. Cultures were immediately imaged (for CGamF) or were incubated for an additional 15min before being imaged (for CMFDA).
For quantitative assessment, hepatocytes were pre-treated with HEPES buffer or Ca2+-free HEPES buffer (0.35g/L KCl, 0.16g/L KH2PO4, 4.8g/L HEPES, 7.9g/L NaCl, 0.9g/L glucose and 10mM EGTA, pH7.4) at 37°C for 10min before incubation with 2μM CMFDA for 10min. Cells were then incubated in HEPES buffer at 37°C for 30min, washed and lysed in 1% Triton X-100 in PBS. The cell lysates were measured by Synergy HT microplate reader (Bio-Tek Instruments, VT) (λex =485nm, λem=530nm). Biliary Excretion Index (BEI) = [fluorescence Standard− fluorescence Ca2+− free]/fluorescence Standard × 100%, where the fluorescence represents the fluorescence units per mg of cell lysates12.
Data Analysis
Data represent mean ± S.D. Statistical significance was tested with paired t test assuming significance with p<0.05.
Results
Ad-siRNA effectively inhibits radixin expression in sandwich cultured rat hepatocytes
To address the requirement for radixin in normal hepatocyte function, we attempted to inhibit radixin expression in collagen sandwich cultured rat hepatocytes using adenovirus-mediated siRNA. We first tested the four siRNA target sequences in a normal rat liver cell line, Clone 9, in order to evaluate their ability to suppress radixin. Immunoblot analysis in Fig. 1A indicates that all four siRNAs individually and in combination reduced the level of radixin expression to 19–34% of Ad-siControl treated cells. The most potent siRNA, siRDXC, was selected for the subsequent studies in rat hepatocytes and is referred to as Ad-siRadixin. Fig. 1B illustrates that Ad-siRadixin progressively reduced radixin expression and generated significant knockdown 5 days after the initial infection. This down regulation of radixin expression was dose-dependent and MOI 50 resulted in reduction to 18% of Ad-siControl treated cells (Fig. 1C). No alteration was observed in the expression levels of β-actin (Fig. 1C), Mrp2, Bsep, Mdr1 and Mrp3 (a basolateral transporter) (Fig. 1D). Thus, Ad-siRadixin was able to potently and specifically suppress radixin expression in this sandwich cultured hepatocyte model.
Figure 1.
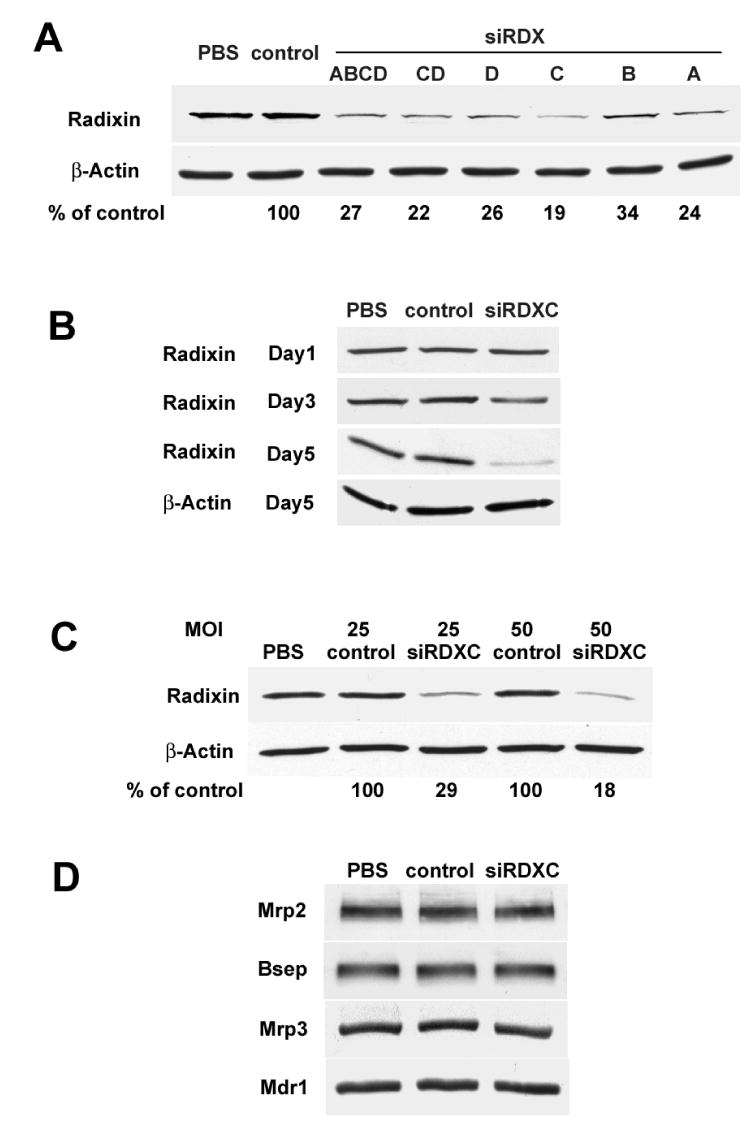
Ad-siRNA effectively inhibited radixin expression in collagen sandwich cultured rat hepatocytes. (A) Clone 9 cells were treated with PBS, Ad-siControl, or the four siRNAs individually or in various combinations at a virus dose of MOI 10. The cells were harvested 3 days after treatment and cell lysates were separated by PAGE, and blotted for radixin and normalized to β-actin. (B) Rat hepatocytes treated with PBS, Ad-siControl or Ad-siRDXC were harvested 1d, 3d and 5d after the treatment and cell lysates were blotted for radixin and β-actin. (C) Hepatocytes were treated with PBS, Ad-siControl or Ad-siRDXC with MOI 25 or 50, harvested on day 5, and cell lysates were blotted for radixin and β-actin. (D) The hepatocyte lysates were also blotted for Mrp2, Bsep, Mrp3 and Mdr1. A representative result of at least three experiments is shown.
Cell viability of 5 day cultures was examined using the lactate dehydrogenase (LDH) assay (Thermo Electron, Louisville, CO). LDH activity in the media of 5 day cultures of PBS, Ad-siControl, and Ad-siRadixin treated cells was not significantly different (12.20 ± 4.09, 13.48 ± 2.89 and 16.40 ± 2.30 U/L, respectively) (mean ± S.D. of five experiments).
Ad-siRadixin reduced the canalicular structures of collagen sandwich cultured hepatocytes
To assess the role of radixin in the structure of the apical canalicular domain, the formation of the bile canalicular network was examined by DIC optics over a 5 day period following administration of the Ad-siRadixin. As shown in Fig. 2A, one day after seeding, hepatocytes formed uniform monolayers and there was little or no sign of apical domain formation. By day 3, a continuous, anastomotic network was established in all the cultures along cell borders. By day 5, the canalicular lumina assumed a more refined and homogeneous appearance in PBS and Ad-siControl treated cells. Quantitative assessment of the lengths of these canalicular channels was 439 ± 26 and 457 ± 25μm/field, respectively (mean ± S.D. of four experiments). However, in striking contrast, the canalicular structures in the Ad-siRadixin treated cells degenerated by 56% to 195 ± 25 μm/field (mean±S.D. of four experiments, p<0.0001). In some regions, only a few canalicular structures could be observed. Notably, this degeneration of canalicular structures occurred in parallel over the same time course as the down-regulation of radixin as showed by immunoblot (Fig. 1B). Immunofluorescence demonstrated that radixin was concentrated at the apical domains of PBS or Ad-siControl treated cells, whereas the signal was diminished in Ad-siRadixin treated cells (Fig. 2B). Actin localization (Fig. 2C) in PBS and Ad-siControl treated cells displayed a normal ‘railroad track’ alignment of actin filaments bordering the canalicular membrane. Often the lumen appeared to be more highly dilated than the Ad-siControl treated cells. In radixin-deficient cells, actin staining appeared as single lines with little or no separation (Fig. 2C). In some areas the pattern was punctuate and disrupted. Localization of the tight junction proteins, ZO-1 and claudin 3, demonstrated a similar pattern of labeling (data not shown).
Figure 2.
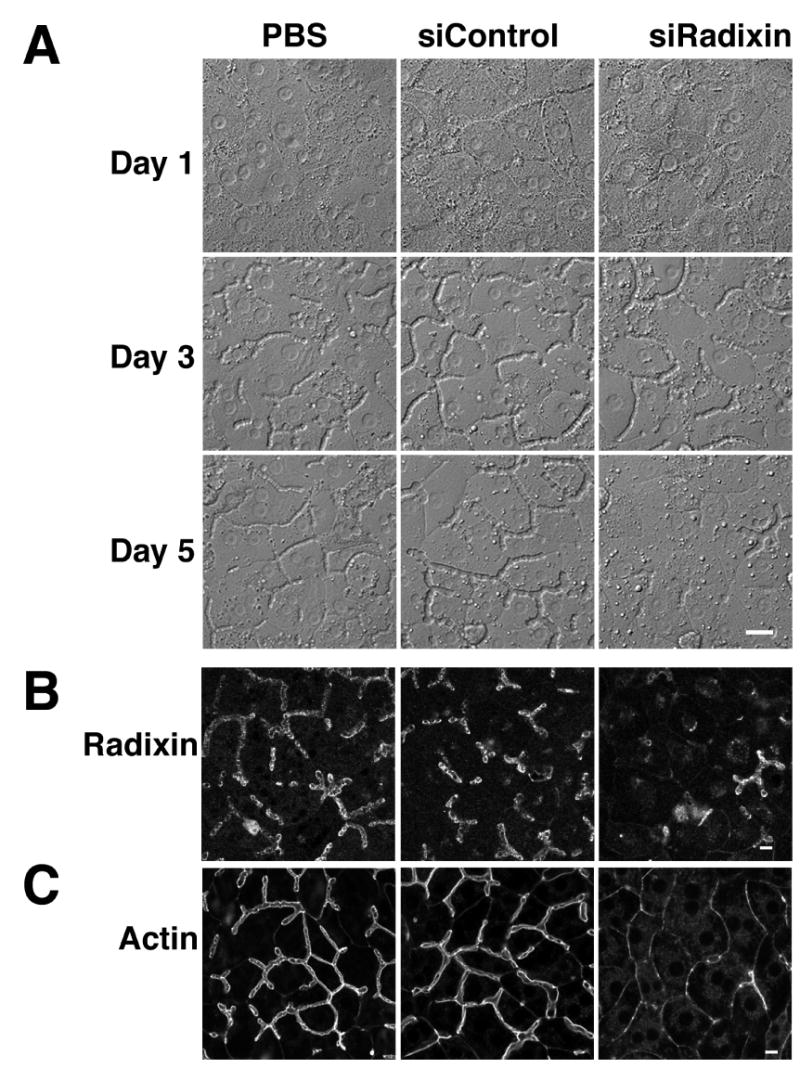
Ad-siRadixin reduced canalicular structures in sandwich cultured hepatocytes. DIC images of hepatocytes that were treated with PBS, Ad-siControl or Ad-siRadixin were acquired on day 1, day 3 and day 5 (A). Five day cultures of cells treated with PBS, Ad-siControl or Ad-siRadixin were labeled with anti-radixin antibody (B) or Alexa 594 phalloidin for actin filaments (C). Both demonstrate loss of apical canalicular structures after Ad-siRadixin treatment. Bar = 10 μm
Ultrastructural analysis of PBS and Ad-siControl treated hepatocytes revealed the presence of dilated bile canaliculi as previously described in collagen sandwich cultured hepatocytes10,26 (Fig. 3A,B). The significant dilation of the sealed “vacuoles” resulted in loss of microvilli, as commonly seen in cholestasis. After Ad-siRadixin treatment the lumina of many bile canaliculi were largely collapsed (Fig. 3C). Tight junctions appeared normal and the presence of microvilli suggests that the collapse of the lumen may be due to deficient excretion of osmotically active bile components into these sealed spaces. Blind evaluation (JLB and AM) consistently identified the Ad-siRadixin micrographs.
Figure 3.
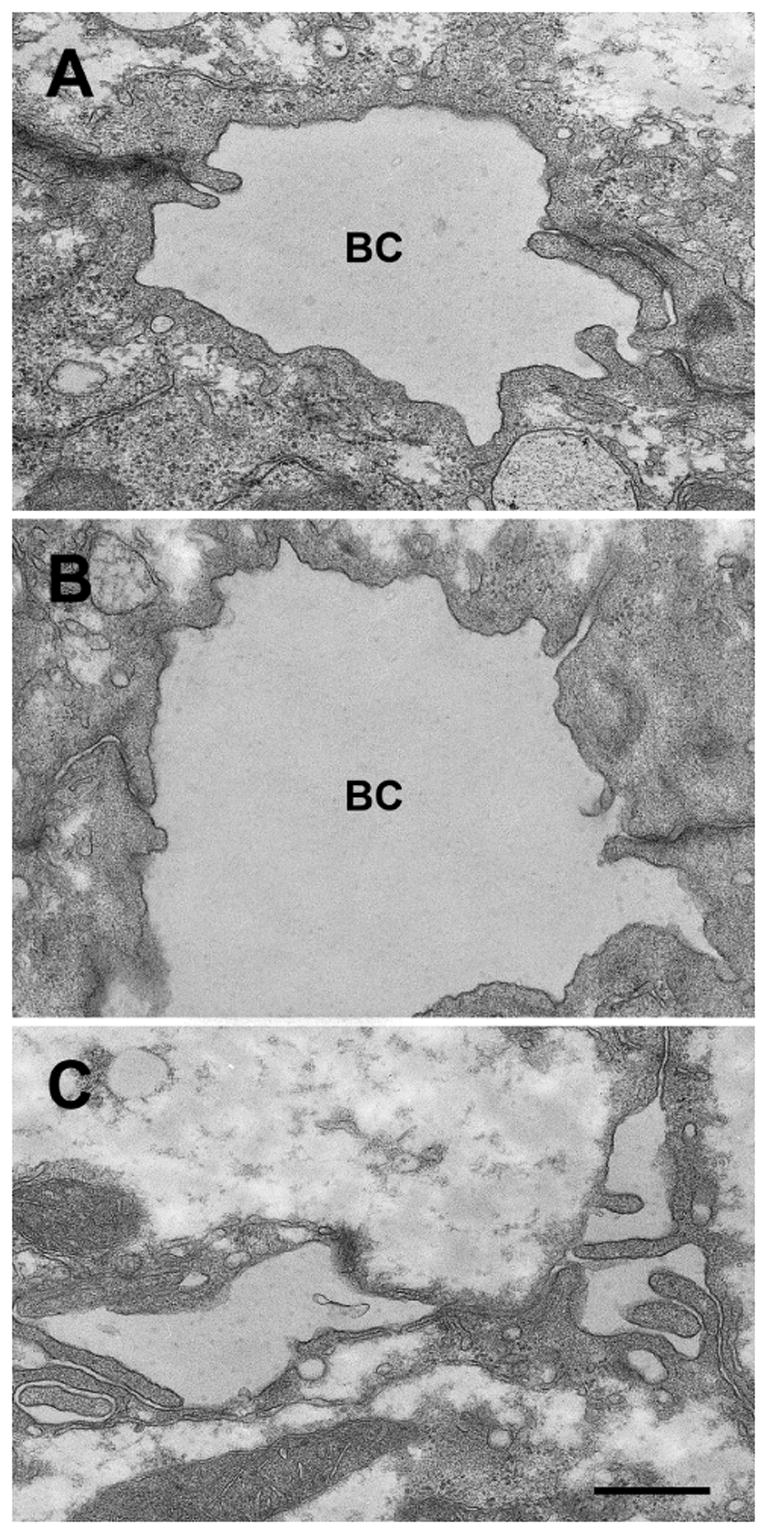
Electron micrographs of sandwich cultured hepatocytes. Hepatocytes were treated with PBS, Ad-siControl or Ad-siRadixin and cultured for 5 days. Hepatocytes treated with PBS (A) or Ad-siControl (B) developed dilated bile canaliculi. Tight junctions are intact and few microvilli are observed. The bile canalicular lumina of Ad-siRadixin treated cells were largely collapsed (C). The tight junctions remain intact and the loss of severe dilation results in the appearance of microvilli. BC, bile canaliculi. Bar = 2μm
Ad-siRadixin alters the distribution of apical membrane transporters in hepatocytes
Localization of membrane transporters was evaluated in five day cultures of hepatocytes by indirect immunofluorescence. As shown in Fig. 4, Mrp2, Bsep and Mdr1 were primarily localized on apical membranes in PBS and Ad-siControl treated cells. Remarkably in Ad-siRadixin treated cells, strong intracellular accumulation of the apical transporters was observed primarily in a perinuclear distribution and only in areas that lacked intact bile canaliculi. Residual stubby canaliculi were also labeled. All apical membrane proteins tested (Mrp2, Bsep, Mdr1, DPPIV), but not actin or ZO1, were found in this intracellular compartment. In contrast, staining for a basolateral transporter, Oatp2, was unchanged compared to control cells (Fig. 4). These findings indicate that radixin is selectively required for apical, but not basolateral, targeting/retaining of transport proteins in hepatocytes and that radixin deficiency results in a generalized impairment of the localization of apical membrane proteins.
Figure 4.
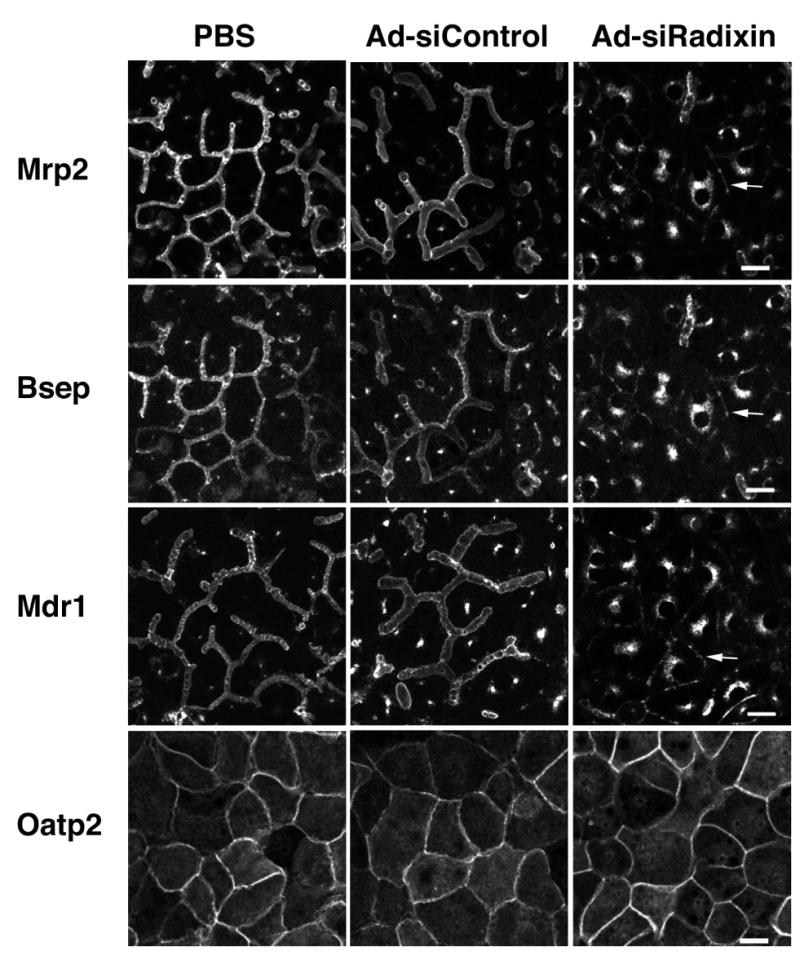
Ad-siRadixin altered the localization of apical bile transporters in hepatocytes. Hepatocytes were treated with PBS, Ad-siControl or Ad-siRadixin and cultured for 5 days. Cells were double labeled with anti-Mrp2 and anti-Bsep antibodies (top 2 rows), with anti-Mdr1 (third row), or anti-Oatp2 antibodies (bottom row). Note that all three apical membrane transporters, but not the basolateral membrane protein, showed significant intracellular localization in knock down areas after Ad-siRadixin treatment. Arrow points to collapsed canaliculi. Bar =10 μm
The intracellular fraction of Mrp2 colocalizes with Rab11
Because the intracellular localization of apical transporters in radixin knock down cells was reminiscent of Rab11-containing endosomes described for Bsep in WIF-B9 cells13 , we performed double labeling immunofluorescence for Mrp2 and Rab11 in the collagen sandwiched hepatocytes. In control cells, Rab 11 was localized to a subapical compartment and a perinuclear compartment that were both distinct from the Mrp2-positive canaliculi (Fig 5). However, in Ad-siRadixin treated cells Mrp2 largely co-localized with Rab11 in an expanded perinuclear compartment in the knock down areas (as defined by the lack of dilated canaliculi) (Fig 5). This perinuclear accumulation of apical membrane transporters did not colocalize with mannosidase II, a Golgi marker (data not shown). Thus, radixin suppression appears to increase the accumulation of Mrp2 in recycling endosomes coincident with its apical reduction.
Figure 5.
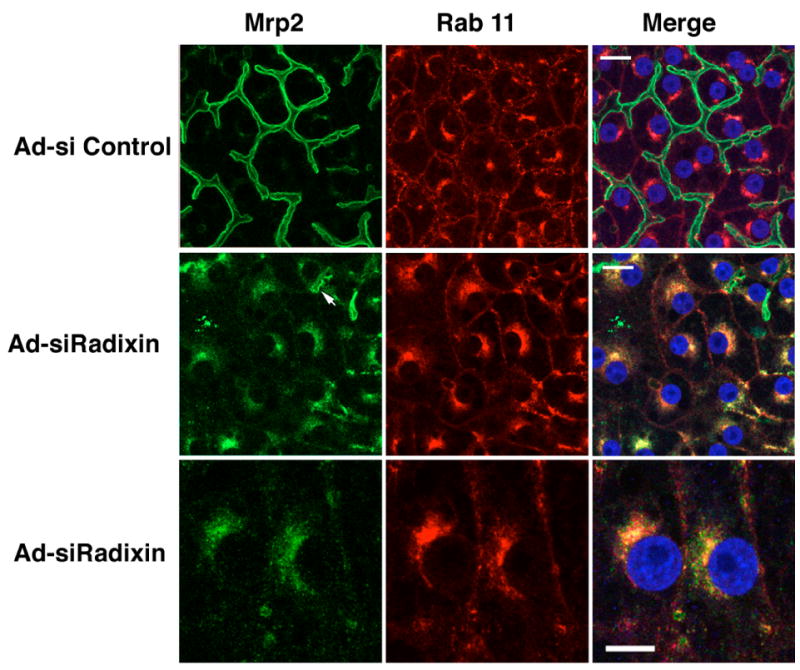
The intracellular fraction of Mrp2 was colocalized with Rab11. In Ad-siControl cells Mrp2 (green) was localized to the dilated bile canaliculi. Occasional, weak staining was seen intracelluarly. Rab11 (red) was found in a subapical and perinuclear compartment in these cells. The merged image demonstrates the separation of these compartments. In contrast, in Ad-siRadixin treated cells, knock down areas (as defined by the lack of intact, dilated bile canaliculi) revealed extensive perinuclear co-localization of both Mrp2 and Rab11. Occasional, stubby canaliculi were seen labeled for Mrp2 (arrow). Bar = 10 μm. High magnification of the knock down area demonstrated that Mrp2 was largely co-localized with Rab11 in the intracellular compartment. Bar = 5 μM
Ad-siRadixin decreases the rate of biliary excretion of GS-MF and CGamF
The excretory function of Mrp2 and Bsep in radixin-deficient hepatocytes was assessed using CMFDA and CGamF, respectively. In PBS or Ad-siControl treated cells, GS-MF (Mrp2 substrate derived from CMFDA) and CGamF (Bsep substrate) were efficiently transported and accumulated in canalicular lumina with little retention in cytosol (Fig. 6A). In contrast, both fluorescent substrates accumulated intracellularly in Ad-siRadixin treated cells.
Figure 6.
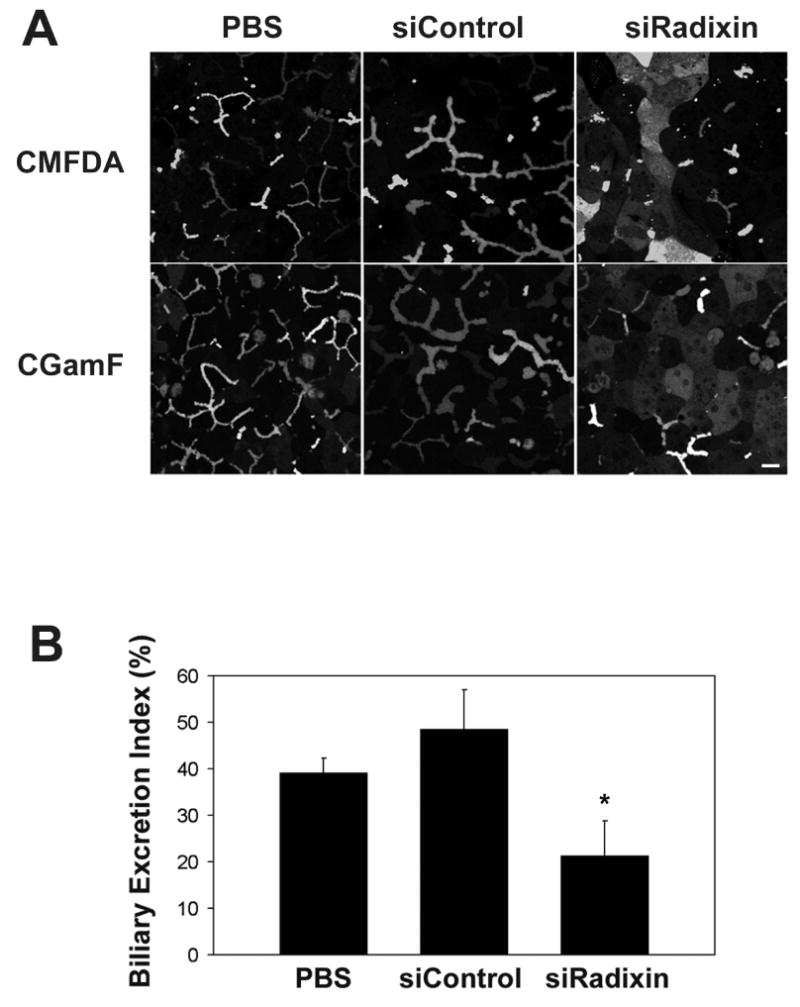
Ad-siRadixin decreased the rate of biliary excretion of GS-MF and CGamF. Hepatocytes were treated with PBS, Ad-siControl or Ad-siRadixin and cultured for 5 days. (A) Cells were incubated with CMFDA or CGamF and visualized by confocal microscopy. Bar = 10 μm (B) Hepatocytes were treated with standard or Ca2+-free HEPES buffer before the bile excretion assay. The Biliary Excretion Index (BEI) was measured as the percentage of GS-MF fluorescence released into canalicular lumina. Data represent the means ± S.D. of five experiments. p>0.05, PBS compared to Ad-siControl treated cells. * p<0.05, Ad-siRadixin compared to PBS or Ad-siControl treated cells.
To quantitate these differences in fluorescent dye excretion, hepatocytes were treated with standard or Ca2+-free HEPES buffer before the bile excretion assay. Ca2+-free treatment disturbed the integrity of the tight junction barrier surrounding bile canaliculi and thus permitted the release of bile components as described12. The Biliary Excretion Index (BEI) was measured as the percentage of GS-MF fluorescence released into canalicular lumina. BEI of PBS and Ad-siControl treated cells was 39.2% and 48.6% (p>0.05), respectively, whereas the value was reduced by nearly 50% to 21.3% in Ad-siRadixin treated cells (p<0.05) (Fig. 6B). These results indicate that radixin is required to maintain normal bile excretory function in hepatocytes.
Discussion
The principal finding in this study is that radixin is required for the maintenance of the structure of the apical bile canalicular membrane as well as the localization and function of apical canalicular membrane transport proteins. When this ERM protein is suppressed in cell culture, the once-established bile canalicular domain is markedly disrupted. This impairment in the normal configuration of the apical canalicular membrane was confirmed by the altered distribution of actin filaments. Actin filaments have long been known to be required for the maintenance of the canalicular domain. Microfilament-perturbing agents result in loss of the normal microvilli in the canalicular membrane14. Radixin, a cross-linker of actin filaments and plasma membrane, is a known component of the hepatocellular microvilli6. Loss of microvilli in radixin-deficient mice7 is consistent with our study and indicates that radixin is essential for maintaining normal hepatic canalicular membrane morphology. The specificity of our findings for radixin is emphasized by the lack of significant disturbance of the bile canalicular structure and secretory function in the PBS and control siRNA treated cells and by the absence of significant differences in LDH released into the media in these cell culture systems.
The requirement of ERM proteins for normal apical organization appears to be a common property in epithelial cells. The single ERM protein in Dropsophila, Dmoesin, is enriched in the rhabdomere base of the photoreceptor. Down-regulation of Dmoesin profoundly disrupts the cortical cytoskeleton and apical membrane organization15. Radixin-deficient mice also suffer from deafness due to the loss of stereocilia, the specially developed giant microvilli in cochlear sensory hair cells16. Ezrin is critical for the assembly of the apical terminal web region of developing mouse intestine, which is particularly important for the propagation of polarity during the formation and expansion of secondary lumina17. Ezrin is also abundantly expressed at the actin cortical layers of parietal cells in mouse stomach18. Ezrin knockdown mice suffer from severe achlorhydria. The loss of gastric acid secretion is caused by defects in the formation/expansion of gastric canalicular apical membranes.
A second important finding in the present study is that radixin is required not only for the localization of Mrp2 to the apical membrane, but also for other canalicular membrane proteins including Bsep and Mdr1. When Mrp2 is retrieved from the plasma membrane after radixin suppression, Bsep and Mdr1 also relocate to the intracellular pool. Consistent with this morphologic finding, the excretion of both Mrp2 and Bsep substrates into the bile canalicular lumen is reduced by the deficiency of radixin. Phalloidin induced changes in actin filament organization also alter the localization of canalicular export pumps19, supporting the importance of normal canalicular organization for the polarized distribution of apical transporters. Our findings also help explain the observation that MDR1 colocalizes with abnormally redistributed MRP2 in regions where radixin is largely reduced in livers of PBC patients8. In cholestasis it is yet to be determined whether a loss of radixin precedes the loss of microvilli, canalicular lumen dilation, and dissociation of apical transporters, or whether these all occur simultaneously. However, it is of interest to note that in radixin knock-out mice, these cholestatic changes also occurred in older animals7. Perhaps other compensatory factors are present in younger mice, preventing the demonstration of cholestasis. Nevertheless, the current study emphasizes the critical importance of radixin in the localization and function of canalicular transporters and strongly suggests that deficient radixin function must be part of the pathophysiology of cholestasis.
Our finding that the increased intracellular fraction of Mrp2 colocalizes with Rab11 GTPase, a recycling endosome marker, suggests that Mrp2 undergoes endocytosis upon the disruption of canaliculi induced by radixin suppression. As the redistributed apical transporters do not colocalize with Golgi markers, they must not be produced solely by de novo protein synthesis. Instead, it is most likely that they are retrieved from the canalicular membrane. Apical membrane recycling involving Rab11 endosomal compartments has been described with Bsep in WIF-B9 cells13. Mrp2, as well as Bsep and Mdr1, have all been identified in intracellular vesicular structures in addition to the canalicular membrane in rat liver20,21. Furthermore, Mrp2 and Bsep are regulated rapidly by retrieval from and/or insertion into the canalicular membrane via intracellular vesicles21,22,23,24,25. In support of our finding, a recent study in WIF-B9 cells colocalized apical ABC transporters and Rab11 in polarized cells and restricted these transporters to Rab11-endosomes in non-polarized cells where no canalicular pole was formed27. It remains to be determined if the Rab11 endosome mediates an Mrp2 trafficking pathway in normal hepatocytes in vivo.
In summary, the present study indicates that down-regulation of radixin in sandwich-cultured hepatocytes by adenovirus-mediated siRNA disorganizes the structure of the bile canaliculus, which leads to impairment in bile excretory function due to both a reduction in the total amount of apical membrane as well as retrieval of bile acid and organic solute transporters from the canalicular membrane to intracellular compartments. These observations indicate that radixin is a critical determinant of the structure and function of the apical membrane of hepatocytes and is not just a tethering protein for Mrp2. Rather, radixin is also essential for maintaining polarized targeting and/or retaining of other critical bile export pumps on the canalicular membrane. These findings prompt revision of current concepts regarding the role of radixin in the hepatocyte and provide new insights into the molecular events in cholestasis. Further studies on the alteration of radixin’s function in cholestasis should broaden our understanding of the pathogenesis of cholestatic syndromes.
Abbreviations
- siRDX
siRadixin
- Mrp2
multidrug resistance associated protein 2
- Mrp3
multidrug resistance associated protein 3
- Bsep
bile salt export pump
- Mdr1
multidrug resistance protein 1
- Oatp2
organic anion transporting polypeptide 2
- PBC
Primary Biliary Cirrhosis
- MOI
multiplicity of infection
- DIC
differential interference contrast
- CMFDA
5-chloromethylfluorescein diacetate
- GS-MF
glutathione-methylfluorescein
- CGamF
cholylglycylamido-fluorescein
- LDH
lactate dehydrogenase
Footnotes
Supported by National Institutes of Health grant DK 25636 and P30 -34989 (to J.L.B.).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Trauner M, Boyer JL. Cholestatic syndromes. Curr Opin Gastroenterol. 2004;20:220–230. doi: 10.1097/00001574-200405000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Pauli-Magnus C, Meier PJ. Hepatocellular transporters and cholestasis. J Clin Gastroenterol. 2005;39:S103–110. doi: 10.1097/01.mcg.0000155550.29643.7b. [DOI] [PubMed] [Google Scholar]
- 3.Wang L, Boyer JL. The maintenance and generation of membrane polarity in hepatocytes. Hepatology. 2004;39:892–899. doi: 10.1002/hep.20039. [DOI] [PubMed] [Google Scholar]
- 4.Bretscher A, Edwards K, Fehon R. ERM proteins and merlin: integrators at the cell cortex. Nature Reviews. 2002;3:586–599. doi: 10.1038/nrm882. [DOI] [PubMed] [Google Scholar]
- 5.Fouassier L, Duan CY, Feranchak AP, Yun C, Sutherland E, Simon F, Fitz JG, Doctor B. Ezrin-radixin-moesin-binding phosphoprotein 50 is expressed at the apical membrane of rat liver epithelia. Hepatology. 2001;33:166–176. doi: 10.1053/jhep.2001.21143. [DOI] [PubMed] [Google Scholar]
- 6.Amieva MR, Wilgenbus KK, Furthmayr H. Radixin is a component of hepatocyte microvilli in situ. Exp Cell Res. 1994;210:140–144. doi: 10.1006/excr.1994.1021. [DOI] [PubMed] [Google Scholar]
- 7.Kikuchi S, Hata M, Fukumoto K, Yamane Y, Matsui T, Tamura A, Yonemura S, Yamagishi H, Keppler D, Tsukita S, Tsukita S. Radixin deficiency causes conjugated hyperbilirubinemia with loss of Mrp2 from bile canalicular membranes. Nature Genetics. 2002;31:320–325. doi: 10.1038/ng905. [DOI] [PubMed] [Google Scholar]
- 8.Kojima H, Nies AT, König J, Hagmann W, Spring H, Uemura M, Fukui H, Keppler D. Changes in the expression and localization of hepatocellular transporters and radixin in primary biliary cirrhosis. J Hepatology. 2003;39:693–702. doi: 10.1016/s0168-8278(03)00410-0. [DOI] [PubMed] [Google Scholar]
- 9.Chandra P, Lecluyse EL, Brouwer KL. Optimization of culture conditions for determining hepatobiliary disposition of taurocholate in sandwich-cultured rat hepatocytes. In Vitro Cell Dev Biol Anim. 2001;37:380–385. doi: 10.1007/BF02577575. [DOI] [PubMed] [Google Scholar]
- 10.Talamini MA, Kappus B, Hubbard A. Repolarization of hepatocytes in culture. Hepatology. 1997;25:167–172. doi: 10.1002/hep.510250131. [DOI] [PubMed] [Google Scholar]
- 11.Boyer JL, Phillips JM, Graf J. Preparation and specific applications of isolated hepatocyte couplets. Methods Enzymol. 1990;192:501–516. doi: 10.1016/0076-6879(90)92090-z. [DOI] [PubMed] [Google Scholar]
- 12.Liu X, Chism JP, LeCluyse EL, Brouwer KR, Brouwer KLR. Correlation of biliary excretion in sandwich-cultured rat hepatocytes and in vivo in rats. Drug Metab Dispos. 1999;27:637–44. [PubMed] [Google Scholar]
- 13.Wakabayashi Y, Lippincott-Schwartz J, Arias IM. Intracellular trafficking of bile salt export pump (ABCB11) in polarized hepatic cells: constitutive cycling between the canalicular membrane and rab11-positive endosomes. Mol Biol Cell. 2004;15:3485–3496. doi: 10.1091/mbc.E03-10-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elias EZ, Hruban JB, Boyer JL. Phalloidin-induced cholestasis: a microfilament-mediated change in junctional complex permeability. Proc Natl Aca Sci USA. 1980;77:2229–2233. doi: 10.1073/pnas.77.4.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karagiosis SA, Ready DF. Moesin contributes an essential structural role in Drosophila photoreceptor morphogenesis. Development. 2004;131:725–732. doi: 10.1242/dev.00976. [DOI] [PubMed] [Google Scholar]
- 16.Kitajiri S, Fukumoto K, Hata M, Sasaki H, Katsuno T, Nakagawa T, Ito J, Tsukita S, Tsukita S. Radixin deficiency causes deafness associated with progressive degeneration of cochlear stereocilia. J Cell Biol. 2004;166:559–570. doi: 10.1083/jcb.200402007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saotome I, Curto M, McClatchey AI. Ezrin is essential for epithelial organization and villus morphogenesis in the developing intestine. Develop Cell. 2004;6:855–864. doi: 10.1016/j.devcel.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Tamura A, Kikuchi S, Hata M, Katsuno T, Matsui T, Hayashi H, Suzuki Y, Noda T, Tsukita S, Tsukita S. Achlorhydria by ezrin knockdown : defects in the formation/expansion of apical canaliculi in gastric parietal cells. J Cell Biol. 2005;169:21–28. doi: 10.1083/jcb.200410083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rost D, Kartenbeck J, Keppler D. Changes in the localization of the rat canalicular conjugate export pump mrp2 in phalloidin-induced cholestasis. Hepatology. 1999;29:814–821. doi: 10.1002/hep.510290319. [DOI] [PubMed] [Google Scholar]
- 20.Soroka CJ, Pate MK, Boyer JL. Canalicular export pumps traffic with polymeric immunoglobulin A receptor on the same microtubule-associated vesicle in rat liver. J Biol Chem. 1999;274:26416–26424. doi: 10.1074/jbc.274.37.26416. [DOI] [PubMed] [Google Scholar]
- 21.Kipp H, Pichetshote N, Arias IM. Transporters on Demand. Intrahepatic pools of canalicular ATP binding cassette transporters in rat liver. J Biol Chem. 2001;276:7218–7224. doi: 10.1074/jbc.M007794200. [DOI] [PubMed] [Google Scholar]
- 22.Schmitt M, Kubitz R, Lizun S, Wettstein M, Häussinger D. Regulation of the dynamic localization of the rat Bsep gene-encoded bile salt export pump by anisoosmolarity. Hepatology. 2001;33:509–518. doi: 10.1053/jhep.2001.22648. [DOI] [PubMed] [Google Scholar]
- 23.Beuers U, Bilzer M, Chittattu A, Kullak-Ublick G, Keppler D, Paumgartner G, Dombrowski F. Tauroursodeoxycholic acid inserts the apical conjugate export pump, Mrp2, into canalicular membranes and stimulates organic anion secretion by protein kinase C-dependent mechanisms in cholestatic rat liver. Hepatology. 2001;33:1206–1216. doi: 10.1053/jhep.2001.24034. [DOI] [PubMed] [Google Scholar]
- 24.Misra S, Varticovski L, Arias IM. Mechanisms by which cAMP increases bile acid secretion in rat liver and canalicular membrane vesicles. Am J Physiol Gastrointest Liver Physiol. 2003;285:G316–G324. doi: 10.1152/ajpgi.00048.2003. [DOI] [PubMed] [Google Scholar]
- 25.Mottino AD, Crocenzi FA, Pozzi EJS, Veggi LM, Roma MG, Vore M. Role of microtubules in estradiol-17β-D-glucuronide-induced alteration of canalicular Mrp2 localization and activity. Am J Physiol Gastrointest Liver Physiol. 2005;288:G327–36. doi: 10.1152/ajpgi.00227.2004. [DOI] [PubMed] [Google Scholar]
- 26.Lecluyse EL, Fix JA, Audus KL, Hochman JH. Regeneration and maintenance of bile canalicular networks in collagen-sandwiched hepatocytes. Toxicol In Vitro. 2000;14:117–32. doi: 10.1016/s0887-2333(99)00096-x. [DOI] [PubMed] [Google Scholar]
- 27.Wakabayashi Y, Dutt P, Lippincott-Schwartz J, Arias IM. Rab11a and myosin Vb are required for bile canalicular formation in WIF-B9 cells. Proc Natl Acad Sci USA. 2005;102:15087–15092. doi: 10.1073/pnas.0503702102. [DOI] [PMC free article] [PubMed] [Google Scholar]


