Abstract
Transforming growth factor-β (TGF-β) is a prototypical tumour-suppressor cytokine with cytostatic and pro-apoptotic effects on most target cells; however, mechanisms of its pro-survival/anti-apoptotic signalling in certain cell types and contexts remain unclear. In human lung fibroblasts, TGF-β1 is known to induce myofibroblast differentiation in association with the delayed activation of focal adhesion kinase (FAK) and protein kinase B (PKB/AKT). Here, we demonstrate that FAK and AKT are independently regulated by early activation of SMAD3 and p38 MAPK, respectively. Pharmacologic or genetic approaches that disrupt SMAD3 signalling block TGF-β1-induced activation of FAK, but not AKT; in contrast, disruption of early p38 MAPK signalling abrogates AKT activation, but does not alter FAK activation. TGF-β1 is able to activate AKT in cells expressing mutant FAK or in cells treated with an RGD-containing peptide that interferes with integrin signalling, inhibits FAK activation and induces anoikis (apoptosis induced by loss of adhesion signalling). TGF-β1 protects myofibroblasts from anoikis, in part, by activation of the PI3K-AKT pathway. Thus, TGF-β1 co-ordinately and independently activates the FAK and AKT protein kinase pathways to confer an anoikis-resistant phenotype to myofibroblasts. Activation of these pro-survival/anti-anoikis pathways in myofibroblasts likely contributes to essential roles of TGF-β1 in tissue fibrosis and tumour-promotion.
Keywords: Focal Adhesion Kinase, Protein kinase B, SMAD proteins, p38 MAPK, Transforming growth factor-beta, Apoptosis, Anoikis, Fibroblasts
1. Introduction
TGF-β1 is a 25 kDa dimeric polypeptide growth factor/ morphogen that functions in a cell-type and context-specific manner to regulate diverse cellular processes such as growth, differentiation, migration and apoptosis [1,2]. Importantly, TGF-β1 mediates divergent effects on epithelial and mesenchymal cells. In epithelial cells, TGF-β1 typically functions as a tumour-suppressor through growth-inhibition, cell-cycle arrest and induction of apoptosis [2]. In contrast, TGF-β1 promotes mesenchymal cell proliferation and survival [3–8]. These opposing actions on epithelial and mesenchymal cells favour net loss of epithelial cells and accumulation of myofibroblasts in injured tissues, a hallmark of human fibrotic diseases [9]. Additionally, myofibroblast activation in the tumour microenvironment is increasingly recognized as an important event in cancer progression and metastasis [10,11].
We have previously shown that myofibroblast differentiation by TGF-β1 is dependent on adhesion-mediated signalling through focal adhesion kinase (FAK), a nonreceptor protein tyrosine kinase that is upregulated by TGF-β1 [12]. FAK is also an important regulator of cell survival signalling [13]. Apoptosis induced by loss of adhesion or adhesion-mediated signalling (termed “anoikis”) is associated with deactivation of FAK [14,15]. A role of FAK-mediated signals in mesenchymal cell survival has been demonstrated in studies showing that fibroblasts in contractile gels are protected from apoptosis by activating antibodies to α5β1 integrins or through constitutive activation of FAK [16]. Other studies have shown, in a variety of cells, that activation of integrin-FAK signalling pathways can prevent apoptosis due to loss of cell adhesion [17].
Phosphatidylinositol 3′-OH kinase (PI3K) has also been shown to regulate a wide variety of cellular processes, including proliferation, metabolism, migration and survival; pro-survival signalling is primarily mediated through activation of the serine-threonine kinase, protein kinase B (PKB/AKT) [18]. Activation of the PKB/AKT pathway mediates growth factor-dependent survival of a wide variety of cell types through a number of downstream targets, including caspase-9, BAD, NF– B, fork-head family transcription factors and XIAP (X-linked inhibitor of apoptosis) [19,20]. In addition to receptor tyrosine kinase(s)-activating growth factor ligands, PI3K-AKT has also been shown to be activated by adhesion signalling via FAK [16,21,22] and integrin-linked kinase (ILK) [22].
We have previously shown that TGF-β1 protects myofibroblasts from serum deprivation-induced apoptosis through activation of the PI3K-AKT pathway via the p38 MAPK-dependent secretion of an autocrine growth factor [8]. This study was undertaken to determine the regulatory mechanisms of FAK and AKT by TGF-β1 via SMAD-dependent and -independent pathways, the potential interdependence of these pro-survival protein kinase pathways and their role(s) in the protection of myofibroblasts from anoikis.
2. Materials and methods
2.1. Cell culture
Normal primary human fetal lung fibroblasts (IMR-90; Institute for Medical Research, Camden, NJ, USA) were cultured as previously described [8] and studies performed at passage 5–9. Cells were plated on cell culture dishes or on 96-well cell culture plates and incubated with DMEM containing 10% fetal bovine serum (FBS) in 5% CO2–95% air. IMR-90 fibroblasts were growth-arrested for 24–48 h in DMEM containing 0.01% FBS prior to various treatments.
2.2. Reagents
TGF-β1 derived from porcine platelets was obtained from R and D Systems, Minneapolis, MN. SB203580 was from Calbiochem, La Jolla, CA, USA. LY294002 was obtained from Cell Signalling Technology, Beverly MA. SB431542 was from Tocris Bioscience, Ellisville, MO, USA. Rabbit polyclonal antibodies to phospho-Ser473AKT, phospho-Thr308AKT, total AKT, and total FAK were from Cell Signalling Technology. Mouse monoclonal antibody to phosphatidylserine (clone 1H6) was from Upstate Biotechnology, Lake Placid, NY, USA. Mouse monoclonal antibody to β actin and the soluble RGD-containing fibronectin peptide (GRGDS) were from Sigma, St. Louis, MO, USA. The non-RGD-containing peptide (GRADSP) was from Calbiochem. Mouse monoclonal antibody to single-stranded DNA (ssDNA) was from Chemicon International, Temecula, CA, USA. Rabbit polyclonal antibody to phospho-Y397 FAK was from BioSource International Inc, Camarillo, CA, USA. Antibody to phospho-SMAD3(Ser433/435) was from Cell Signalling Technology. Rabbit polyclonal antibody to total SMAD3 was from Zymed Laboratories, San Francisco, CA, USA. Antibody to GAPDH was from Abcam, Cambridge, MA, USA. Secondary HRP-conjugated anti-mouse and anti-rabbit antibodies were obtained from Pierce, Rockford, IL, USA.
2.3. Generation of stable cell lines expressing Y397-mutant FAK
Plasmid transfections of IMR-90 cells with a FAK mutant construct in which the tyrosine-397 phosphorylation site was substituted with phenylalanine (Y397F-FAK) were performed using the cationic lipid reagent LipofectAMINE, Invitrogen, Carlsbad, CA, USA, according the manufacturer's instructions. The optimal ratio of DNA (μg) to LipofectAMINE (μl) was determined to be 1:5 for IMR-90 cells. Cells were incubated with DNA-lipid complexes in serum-free Opti-MEM I medium (Invitrogen) for 4–5 h prior to introducing 10% FBS for 16–20 h. The next day, transfection medium was replaced by DMEM supplemented with 10% FBS and geneticin (Invitrogen). Geneticin concentrations were 400 μg/ml for selection transfected cells and 200 μg/ml for maintenance of stable transfectants prior to treatments.
2.4. siRNA and transfections
RNA interference was accomplished by transfecting cells with siGENOME SMARTPOOL siRNA (Dharmacon Inc., Lafayette, CO, USA; Catalogue # 020067). Transfections were carried out using TransIT-siQUEST lipid reagent according to the manufacturer's instructions (Mirus, Madison WI, USA). Briefly, for each transfection in 35 mm cell culture dishes, 5 μl of lipid reagent was mixed with 250 μl of serum free media for 20 min, followed by the addition pooled siRNA for another 20 min. This lipid–reagent/siRNA mixture was then slowly added to 750 μl of DMEM supplemented with 5% FBS. IMR-90 cells were then incubated with this mixture for 48–72 h and media changed to serum-free DMEM for another 24 h prior to treatment. For negative controls, cells were cultured, transfected, and treated in a similar manner using the non-targeting pooled siRNA (siControl) from Dharmacon Inc.
2.5. Western immunoblotting
Cell lysates were prepared in RIPA buffer and subjected to SDS-PAGE and Western blot analyses performed as described previously [8].
2.6. Assays for apoptosis/anoikis
2.6.1. ELISA for ssDNA
This assay is based on the susceptibility of double-stranded DNA in apoptotic cells to form single strands when exposed to formamide [23] and was performed as previously described [8].
2.6.2. Anti-phosphatidylserine immunofluorescence
Immunofluorescent staining procedures were as previously described with minor modifications [8]. IMR-90 cells plated on 35 mm tissue culture dishes were grown to 50% confluence and growth-arrested in serum-free media for 48 h prior to treatment. Cells were then fixed in 5% formaldehyde and washed three times with cold phosphate-buffered saline (PBS), but were not permeabilized. Nonspecific binding sites were blocked with 1% bovine serum albumin (BSA) for 15 min prior to the addition of antibody to phosphatidylserine (1:25 dilution) for 1 h followed by fluorescein isothiocyanate (FITC)-conjugated secondary antibody (1:40 dilution) for 1 h. Nuclear counterstaining was with DAPI and cells were visualized/photographed using a Zeiss fluorescence microscope.
2.6.3. Caspase-3 activation
Caspase-3 activation was assessed using the Caspase-3 Fluorometric Assay Kit (Assay Designs, Inc) according to manufacturer's protocol. 50 μl of whole cell lysate for each experimental condition was placed in a 96-well plate with 150 μl of fluorometric caspase substrate in reaction buffer and incubated for 3 h. Fluorescence intensity was read at an excitation wavelength of 360 nm and emission wavelength of 440 nm.
2.7. Induction of cellular anoikis
IMR-90 cells were grown to 80–90% confluence in 100 mm tissue culture plastic dishes, growth-arrested for 24–48 h in serum-free media and treated with/without TGF-β1 for 24 h. Cell anoikis was induced by the exogenous addition of RGD-containing peptides, which interfere with adhesion signalling, or by placing cells in suspension for defined time-intervals.
2.7.1. RGD-containing peptides
Peptides containing the RGD motif (GRGDS) and control peptide (GRADSP) were reconstituted in DMEM to the final desired concentration. IMR-90 cells were treated with/without TGF-β1 in the presence/absence of synthetic peptides for 24 h. Cells were then analyzed by different apoptosis assays as described above.
2.7.2. Cell suspension
IMR-90 cells were trypsinized, pelleted by centrifugation and re-suspended in 10 ml of serum-free DMEM media. Suspended cells were then placed on a rocker in a 37 °C incubator at 5% CO2–95% air for defined time-periods (0–6 h). At the end of the incubation period, cells were again pelleted by centrifugation and seeded onto 96-well plates for analyses of apoptosis (as described above) or plated onto 35-mm tissue culture dishes for assessment of the ability of cell to re-adhere on tissue culture plastic (described under “cell survival studies” below).
2.8. Cell survival (anoikis-resistance) studies
IMR-90 fibroblasts induced to undergo anoikis by either soluble RGD-peptides or by cell suspension were assessed for their ability to re-adhere to tissue culture plastic. This assay is based on the principle that non-viable/ apoptotic cells fail to attach to tissue culture plastic. Cells were allowed to re-adhere for 3 h prior to trypsinization of attached cells and cell numbers were measured by Coulter counting (Coulter-Z series, Hialeah, FL, USA).
2.9. Statistical and densitometric analyses
Statistical analysis was performed using Student's t test when comparing two groups and one-way analysis of variance (ANOVA) with Bonferroni post-test when comparing three or more experimental conditions. This analysis was done using GraphPad Prism version 3.0 for Windows, GraphPad Software, San Diego (www.graphpad.com). Densitometric analyses of Western blots were performed using the public domain NIH Image program available on the Internet at rsb.info.nih.gov/nih-image.
3. Results
3.1. TGF-β1-induced FAK, but not AKT, activation is dependent on SMAD3
TGF-β1 signalling in fibroblasts involves early receptor(s)-mediated SMAD-dependent and -independent pathways with more delayed activation of the nonreceptor protein kinases, FAK and AKT [8,12]. Our previous studies showed that AKT activation by TGF-β1 in human lung fibroblasts is dependent on early p38-MAPK [8]. Additionally, FAK activation by TGF-β1 is mediated via a transcription-dependent process that is associated with upregulation of fibronectin and its integrin receptor subunits [12]. Crosstalk between these two pro-survival pathways and the specific role(s) of SMAD3, a key regulator of TGF-β1 pro-fibrotic signalling [24], in the delayed activation of FAK and AKT have not been defined.
To investigate the role of SMAD3 on the activation of these protein kinases, we used a combination of pharmacologic and genetic approaches to inhibit SMAD3 activity. First, we studied the effects of the type 1 TGF-β receptor (ALK5) inhibitor, SB431542, on the early activation of the SMAD3 and p38 MAPK by TGF-β1 in human lung fibroblasts (IMR-90). IMR-90 fibroblasts were stimulated with TGF-β1 for 45 min in the presence/absence of increasing concentrations of SB431542 (0–10 μM). We found that SB431542, at a concentration of 1 μM, induced a >90% inhibition of the TGF-β1-stimulated phosphorylation of SMAD3 with an IC50 between 0.1 and 0.5 μM (Fig. 1A and B). However, SB431542, at the same dose (1 μM), reduced TGF-β1-stimulated activation of p38 MAPK by only 40%; the IC50 for p38 MAPK was approximately 5-fold higher than that for the inhibition of SMAD3 activation (Fig. 1A and B).
Fig. 1.

Pharmacologic inhibition of SMAD3 phosphorylation blocks activation of FAK, but not AKT, induced by TGF-β1. (A) Quiescent IMR-90 cells were treated with/without TGF-β1 (2 ng/ml) in the presence or absence of increasing concentrations of the TGF-βR1(ALK5) inhibitor, SB431542. Cell lysates were collected after 45 min and assessed by SDS-PAGE followed by western immunoblotting for phospho-SMAD3 and phospho-p38 MAPK. The blots were then striped and probed for total SMAD3 and p38 MAPK, respectively. (B) Band densities were determined for TGF-β1-stimulated phospho-SMAD3 and phospho-p38 MAPK in the absence and presence of the indicated concentration of SB431542. The band densities for TGF-β1 stimulated phospho-SMAD3 and phospho-p38 MAPK in the absence of inhibitor were normalized to 100%, and the band densities of the respective proteins in the presence of the indicated concentrations of SB431542 were calculated as “% of TGF-β1 stimulated” and plotted on a non-linear scale against the concentration of SB431542. Values are mean±SEM; n=3 independent experiments. (C) Quiescent IMR-90 cells were treated with/without TGF-β1 in the presence/absence of SB431542 (1.0 μM). Cell lysates were collected after 16 h and assessed for Y397-phosphorylated FAK and S473-phosphorylated AKT by SDS-PAGE and western immunoblotting. The blots were stripped and probed for total FAK and total AKT, respectively. Results are representative of three separate experiments.
Given the differential inhibition of SMAD3 and p38 MAPK by SB431542, we next examined the ability of SB431542 to modulate the delayed activation of FAK and AKT by TGF-β1 in IMR-90 fibroblasts. At 16 h post-TGF-β1 treatment, SB431542 (1 μM) completely inhibits the activation of FAK, while only partial inhibition of AKT phosphorylation/activation is observed (Fig. 1C). These findings suggest that ALK5/SMAD3 signalling is required for TGF-β1-induced activation of FAK, but that TGF-β1-induced activation of AKT is mediated, in large part, by a mechanism independent of early SMAD signalling.
To further study the role of SMAD3 signalling in these TGF-β1 responses, IMR-90 fibroblasts were transfected with siRNA targeting SMAD3 (as described in “Materials and methods”). Both phosphorylated and total SMAD3 levels were significantly “knocked-down” in cells transfected with SMAD3-siRNA compared to cells transfected with non-targeting control siRNA (Fig. 2A). However, cells with siRNA knockdown of SMAD3 were able to robustly activate p38 MAPK at early time points (45 min) following TGF-β1 stimulation (Fig. 2A), suggesting that SMAD3 is not required for the rapid of activation of p38 MAPK. At 16 h following TGF-β1 stimulation, a slight increase in constitutive FAK phosphorylation was observed in SMAD3-knockdown cells; however, TGF-β1 failed to further increase FAK phosphorylation in these cells (Fig. 2A, B). This indicates that early activation of SMAD3 is essential for the delayed activation of FAK induced by TGF-β1. In contrast, under the same conditions, TGF-β1 maintains its ability to induce AKT phosphorylation at both Ser-473 and Thr-308 residues (Fig. 2A, B), indicating that SMAD3 is not required for TGF-β1-induced AKT activation. These data demonstrate that, although FAK and AKT are co-ordinately activated by TGF-β1, they are independently regulated by SMAD3 and p38 MAPK, respectively.
Fig. 2.

siRNA knockdown of SMAD3 blocks TGF-β1-induced activation of FAK without inhibiting the inducible activation of AKT. (A) IMR-90 fibroblasts were transfected with siRNA targeting SMAD3 (SMAD3 siRNA) or non-targeting siRNA (control siRNA). Following 48 h of transfection, cells were treated with TGF-β1 (2 ng/ml) for 45 min or 16 h in the absence of serum. Cell lysates were collected and subjected to SDS-PAGE and western immunoblotting. The samples obtained at 45 min were assessed for early signalling events of phosphorylated SMAD3 and p38 MAPK, then stripped and probed for total SMAD3 and p38 MAPK followed by GAPDH. The samples obtained at 16 h following TGF-β1 treatment were assessed for S473-phosphorylated AKT, T308-phosphorylated AKT, and Y397-phosphorylated FAK, then stripped and probed for the corresponding total protein expression. (B) Band-densities were determined for western blots in (A) as described in “Materials and methods” and phospho-protein to total protein ratios were calculated for Y397phospho-FAK (open bars), S473phospho-AKT (closed bars) and T308phospho-AKT (crossed bars). Values are mean±SEM; n=3 independent experiments.
3.2. TGF-β1-induced FAK is independent of p38 MAPK
Our previous studies have shown that activation of the PI3K-AKT pathway is dependent on early activation of p38 MAPK [8]; however, the potential role of p38 MAPK in TGF-β1-induced activation of FAK is not known. To investigate this possibility, we first assessed the effect of SB203580 (6 μM; a dose that effectively blocks p38 MAPK activity in these cells [8]) on the induction of FAK autophosphorylation/activation by TGF-β1. Treatment with 6 μM SB203580 failed to inhibit TGF-β1-induced FAK autophosphorylation (Fig. 3A); this suggests that p38 MAPK is not required for activation of FAK. To confirm that p38 MAPK is indeed dispensable for TGF-β1-induced activation of FAK, we employed dominant-negative over-expression of a kinase-mutant p38 MAPK (p38-KM) which we have shown to inhibit p38 MAPK activity [8]. In support of the pharmacologic studies with SB203580, TGF-β1 is capable of inducing FAK activation in cells over-expressing p38-KM (Fig. 3B). These results indicate that, in contrast to the previously described role of p38 MAPK in TGF-β1-induced AKTactivation [8], the induction of FAK autophosphorylation/activation does not require the early activation of the p38 MAPK pathway.
Fig. 3.
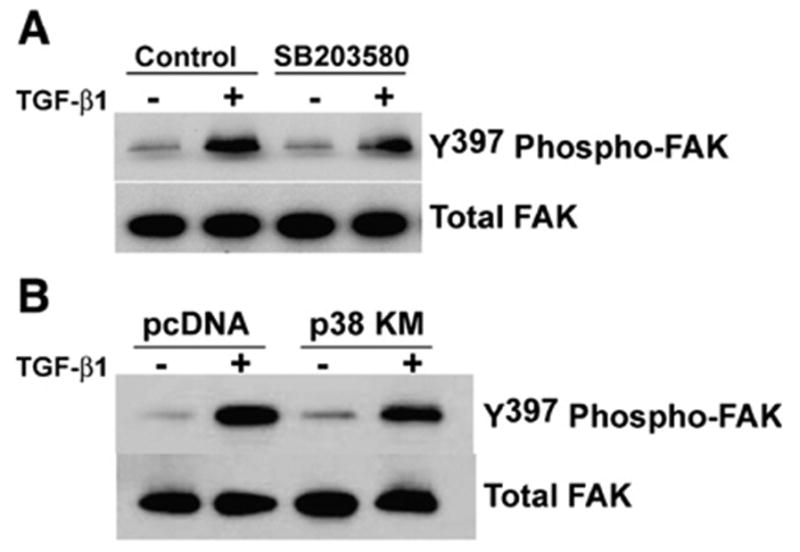
FAK activation by TGF-β1 is independent of p38 MAPK. (A) Quiescent IMR-90 cells were treated with TGF-β1 (2 ng/ml) in the presence/absence of the p38 MAPK inhibitor, SB203580 (6 μM). Cell lysates collected after 16 h were assessed for Y397-phosphorylated FAK by SDS-PAGE and western immunoblotting. Blots were stripped and probed for total FAK. (B) IMR-90 fibroblasts were stably transfected with dominant-negative, kinase-mutant p38 MAPK (p38-KM) or an empty vector control (pcDNA) and treated with TGF-β1 (2 ng/ ml) for 16 h. Whole cell lysates were assessed by western immunoblotting for Y397-phosphorylated FAK. Membranes were stripped and blotted for total FAK.
3.3. Induction of AKT activation by TGF-β1 is independent of FAK activation
Activation of AKT has been shown to be regulated by FAK, primarily in the context of cell adhesion signalling [16,21,25]. We next determined if FAK activation by TGF-β1 was necessary for the induction of AKT activation in response to this growth factor. To investigate this possibility, we employed a dominant-negative approach to disrupt FAK autophosphorylation by stably transfecting IMR-90 fibroblasts with a plasmid encoding a mutant FAK (Y397F-FAK; tyrosine-397 residue substituted with phenylalanine) or with an empty vector plasmid (pcDNA). A significant reduction in baseline and TGF-β1-stimulated FAK phosphorylation was confirmed in cells expressing dominant-negative FAK (Fig. 4A, top panels; 4B, open bars). However, AKT phosphorylation was robustly induced in response to TGF-β1 stimulation in both control and Y397F-FAK mutant cells (Fig. 4A, bottom panels; 4B, closed bars). These results indicate that the induction of AKT phosphorylation/ activation in response to TGF-β1 is not dependent on FAK autophosphorylation, further supporting the concept that TGF-β1 independently regulates the FAK and AKT protein kinase pathways.
Fig. 4.
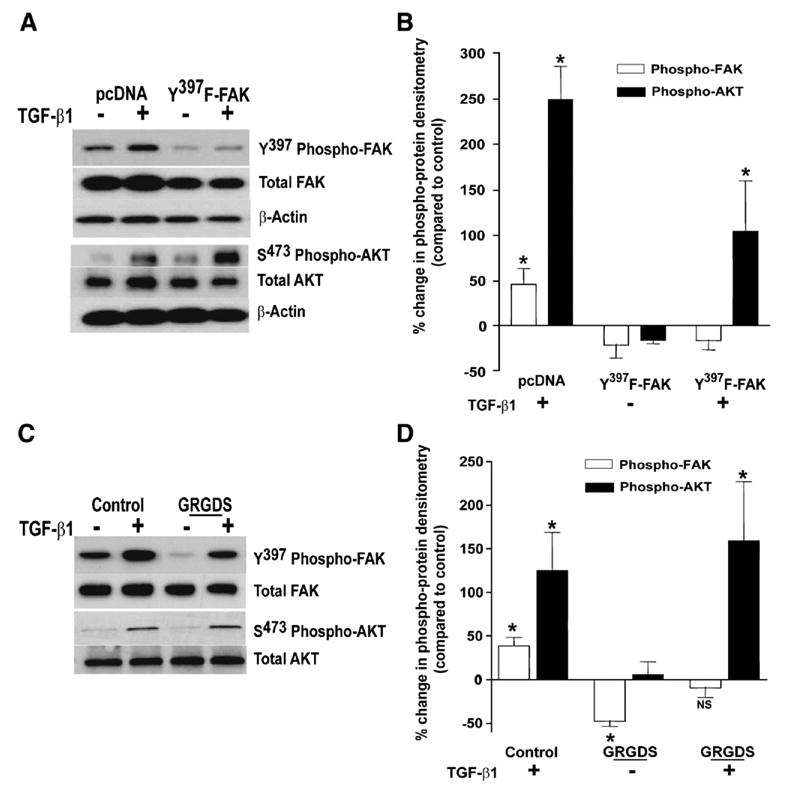
AKT activation by TGF-β1 is independent of FAK signalling. (A) IMR-90 cells stably overexpressing dominant-negative, mutant FAK (Y397F-FAK) or an empty vector control (pcDNA) were treated with/without TGF-β1 (2 ng/ml) for 16 h. Whole cell lysates were assessed for Y397-phosphorylated FAK or for S473-phosphorylated AKT with western immunoblotting. Blots were stripped and blotted for the corresponding total protein and then for β-actin to confirm equal loading. A representative blot from three independent experiments is shown. (B) Densitometric analysis was done to determine changes in phospho-FAK (open bars) and phospho-AKT (closed bars) relative to the expression of each phospho-kinase in empty-vector controls. Data represent relative densitometric changes from five independent experiments and were analyzed by ANOVA with Bonferroni post-test. *p<0.01 compared to untreated controls. (C) IMR-90 fibroblasts were treated for 16 h with/without TGF-β1 (2 ng/ml) in the presence or absence of soluble peptides containing the RGD amino acid sequence (GRGDS, 0.5 mg/ml). Whole cell lysates were analyzed by SDS-PAGE and western immunoblotting for Y397-phosphorylated FAK or for S473-phosphorylated AKT. Blots were stripped and probed for the corresponding total protein. A representative blot from five independent experiments is shown. (D) Relative densitometry of blots in (C), were analyzed as described (B) above. Changes in phospho-FAK (open bars) and phospho-AKT (closed bars) were calculated relative to the expression of the respective phospho-kinase in untreated controls. * p<0.01 compared to expression of the respective phospho-kinase in untreated controls.
The exogenous addition of soluble peptides containing the arginine–glycine–aspartate (RGD) amino acid sequence has been shown to disrupt cell adhesion signalling [26,27]. We first determined if introduction of an RGD-containing peptide modulates FAK autophosphorylation in IMR-90 fibroblasts. Treatment of quiescent IMR-90 fibroblasts with the soluble RGD-containing peptide, GRGDS (0.5 mg/ml), decreased basal levels of FAK autophosphorylation by approximately 50% compared to controls (Fig. 4C, top panel; Fig. 4D, open bars). Stimulation with TGF-β1 was able to augment FAK in cells treated with GRGDS, but only to levels of the untreated control (Fig. 4C, D). In contrast, treatment with soluble RGD-containing peptides had no significant effect on either basal or TGF-β1-stimulated levels of phosphorylated AKT (Fig. 4C, bottom panel; Fig. 4D, closed bars). Treatment of IMR-90 cells with a control peptide (GRADSP, 0.5 mg/ml) had no effect on basal or TGF-β1-stimulated activation of FAK or AKT (data not shown). These studies demonstrate the ability of soluble RGD-containing peptides to suppress constitutive FAK autophosphorylation without significantly altering either constitutive or inducible AKT phosphorylation. Thus, multiple strategies/interventions that either block or suppress FAK signalling fail to suppress the inducible activation of AKT by TGF-β1.
3.4. Loss of cell adhesion or exogenous RGD-containing peptides induces fibroblast anoikis
Anoikis is a form of programmed cell death induced by the loss of cell adhesion or adhesion-dependent signalling [28]. We utilized two experimental models to induce anoikis of human lung fibroblasts: (1) by mechanical/enzymatic detachment of cells and maintenance in suspension for specified periods of time; (2) by competitive interruption of integrin-ECM interactions by addition of soluble RGD-containing peptides [29,30].
Cell suspension studies were performed on quiescent (“growth-arrested”) IMR-90 fibroblast cultures at 80–90% confluence and the measured end-points included apoptosis (by ssDNA ELISA) and cell survival (ability of suspended cells to re-adhere to tissue culture plastic — assessed by Coulter counting) as described in “Material and methods”. Cells in suspension demonstrated a time-dependent increase in apoptotic rates compared to adherent (“control”) cells, with detectable apoptosis observed within 1 h of suspension and further increases noted at 2 and 6 h of suspension (Fig. 5A). Similarly, the number of cells able to survive and re-adhere to tissue culture plastic decreased as time in suspension was increased (Fig. 5B). These studies indicate that lung fibroblasts subjected to loss of adhesion undergo apoptosis/anoikis and loss of cell viability that is dependent on the time-period for which they are maintained in a non-adherent (suspended) state.
Fig. 5.
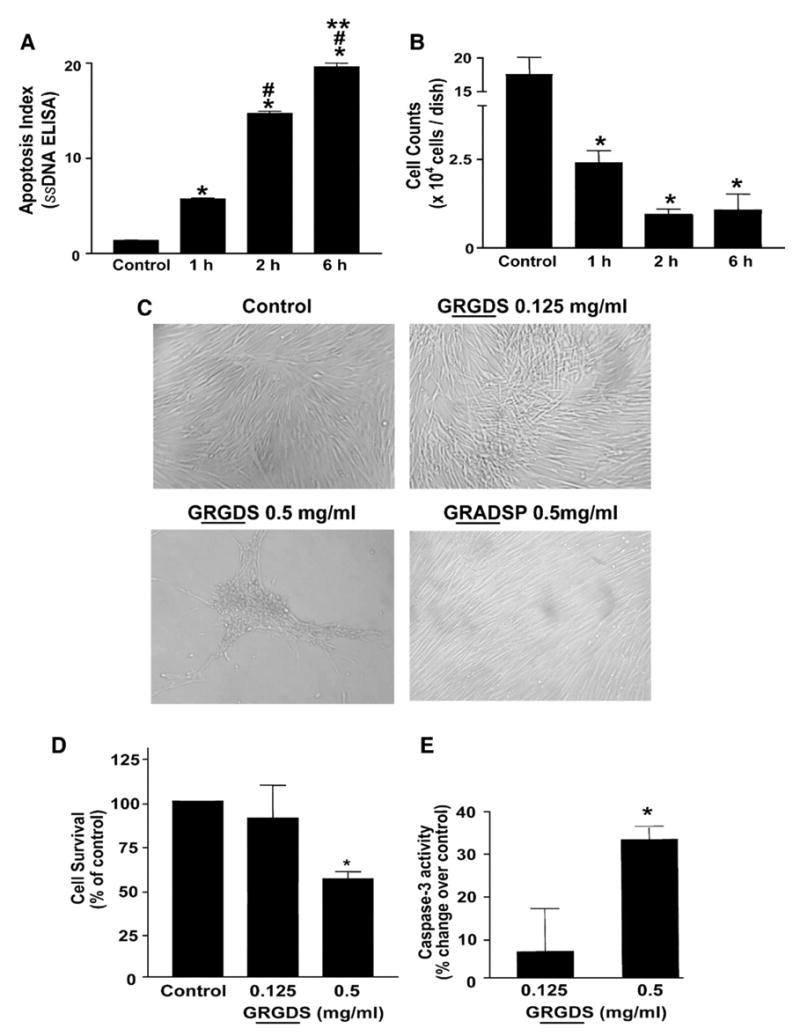
Induction of anoikis in IMR-90 fibroblasts. Quiescent IMR-90 fibroblasts were kept in suspension for the indicated time periods as described in “Material and methods”. At the end of the suspension period, cells were: (A) aliquoted into a 96-well plate for analysis of apoptosis with an ELISA for ssDNA; or, (B) allowed to re-adhere to tissue culture dishes for 3 h and numbers of attached cells were assessed by Coulter counting. Values are mean±SEM; n=3 for each time point. * p<0.001 compared to control, # p<0.001 compared to 1 h, ** p<0.001 vs. 2 h. (C) Quiescent IMR-90 fibroblasts were treated with soluble RGD-containing peptides (GRGDS, 0.125 mg/ml or 0.5 mg/ml) or a non-RGD containing peptide (GRADSP, 0.5 mg/ml) for 24 h and photographed under light microscopy. (D) Quiescent IMR-90 fibroblasts were treated as in (C), and cell viability was assessed at 24 h as described in Materials and methods. Values are mean±SEM; n=6 per condition and the experiment was done in triplicate. * p<0.05 compared to control. (E) IMR-90 cells were treated with soluble RGD-containing fibronectin peptides (GRGDS, 0.125 mg/ml or 0.5 mg/ml) for 24 h. Whole cell lysates were collected and equal amounts of protein were analyzed for caspase-3 activity as described in “Materials and methods”. Values are mean±SEM; n=3 per condition and the experiment was repeated 3 times with similar results. * p<0.01 compared to control.
Next, we assessed the ability of soluble RGD-containing peptides to induce anoikis/apoptosis of IMR-90 fibroblasts. Treatment with the RGD-containing peptide, GRGDS, induced morphological changes, characterized by cell “crowding” and formation of stellate-shaped conglomerates, at 18–24 h in IMR-90 fibroblast cultures; this effect was most marked with the 0.5 mg/ml (vs. 0.125 mg/ml) concentration of the GRGDS peptide (Fig. 5C). These morphological changes were not observed in cells treated with a non-RGD containing control peptide, GRADSP (Fig. 5C). To determine if treatment with the RGD-containing peptide induces loss of cell viability, the ability of trypsinized cells to re-adhere to tissue culture plastic was again assessed. IMR-90 fibroblasts with 0.5 mg/ml of the GRGDS peptide demonstrated significant decreases in cell number, suggesting loss of cell viability (Fig. 5D). To confirm that this loss of cell viability was related to induction of apoptosis/anoikis, GRGDS-treated cells were assayed for expression of activated caspase-3 (by ELISA). A significant increase in caspase-3 activity was observed in cells treated with 0.5 mg/ml of the GRGDS peptide (Fig. 5E). Together, these results indicate that fibroblast apoptosis/anoikis can be reproducibly induced (and detected) in a time- and dose-dependent manner by the disruption of cell adhesion or adhesion-dependent signalling.
3.5. TGF-β1 protects human lung myofibroblasts from anoikis
Previous studies have shown that TGF-β1 protects lung myofibroblasts apoptosis induced by IL-1 [7] or serum deprivation [8]. Whether TGF-β1-differentiated myofibroblasts are resistant to anoikis is not known; moreover, mechanisms for apoptosis/anoikis protection have not been elucidated. First, to determine whether TGF-β1-differentiated myofibroblasts are protected from anoikis induced by soluble RGD peptides, IMR-90 cells were pre-treated with/without TGF-β1 for 16 h and then exposed to two different concentrations of the GRGDS peptide for 24 h prior to assessments of apoptosis. Myofibroblasts induced to differentiate in response to TGF-β1 were resistant to anoikis, as evidenced by lower rates of apoptosis, as assessed by ssDNA ELISA (Fig. 6A), phosphatidylserine labelling (PS-FITC; Fig. 6B, C) and by the expression of activated caspase-3 by Western blot analysis (Fig. 6D). No effects on apoptosis were detected in cells treated with the non-RGD containing peptide (GRADS; data not shown). Together, these studies demonstrate that TGF-β1, simultaneous with the induction of myofibroblast differentiation, confers significant protection from anoikis-induced cell death.
Fig. 6.
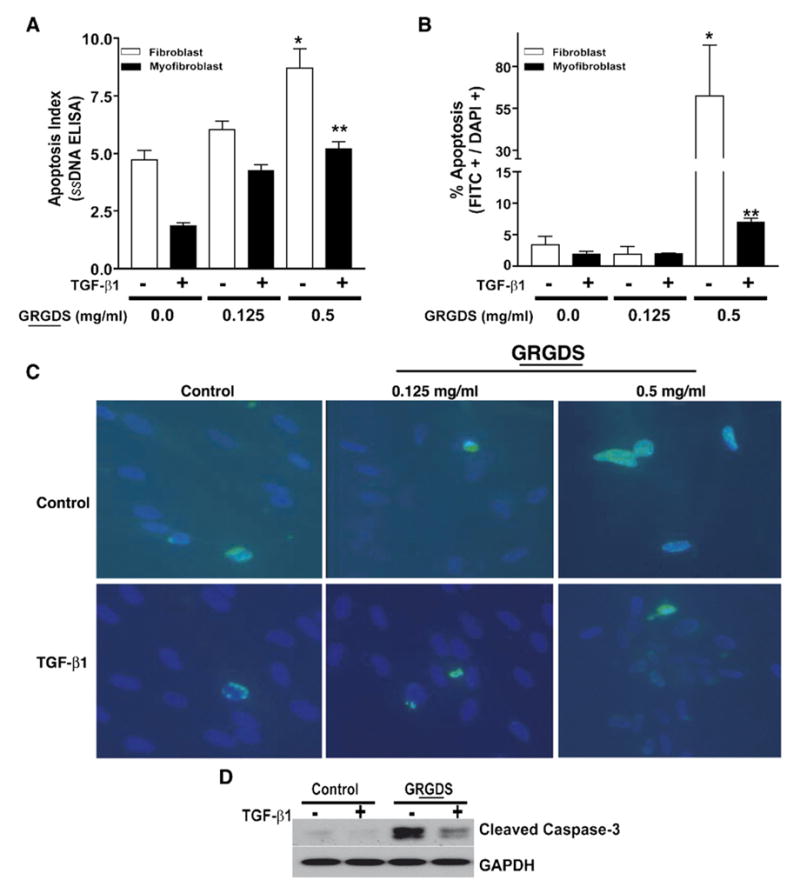
Myofibroblasts are protected from anoikis induced by soluble RGD-containing peptides. (A) Quiescent IMR-90 fibroblasts were treated±TGF-β1 (2 ng/ml) for 16 h. The media was then changed to DMEM with 0.01% FBS containing 0, 0.125, or 0.5 mg/ml of soluble RGD-containing peptides for 24 h and apoptosis was assessed with an ELISA for ssDNA. Values are mean±SEM; n=4 per condition and the experiment was repeated 3 times with similar results. * p<0.001 compared to untreated control. ** p<0.01 compared to 0.5 mg/ml GRGDS treatment without TGF-β1. (B) Cells treated as in (A) were assessed for apoptosis with immunofluorescence staining for phosphatidylserine (FITC, green staining) expression. Quantification of apoptosis was determined by examination of 3 random high power fields from each of three different dishes. % apoptosis represents the total number of FITC+ cells divided by the total number of DAPI+ cells. Results are representative of three independent experiments with similar results. Values are mean±SEM; * p<0.05 compared to untreated control. ** p<0.01 compared to 0.5 mg/ml GRGDS treatment without TGF-β1. (C) Representative photographs from one high-power field of the studies in (B) are shown. Nuclear counterstaining was done with DAPI. (D) IMR-90 fibroblasts were treated with TGF-β1 for 16 h prior to treatment with/without soluble peptides containing RGD (0.5 mg/ml) for 24 h. Cell lysates were collected, subjected to SDS-PAGE and western immunoblotting for activated (cleaved) caspase-3 was performed. The blot was then stripped and probed for GAPDH.
3.6. Activation of the AKT pathway contributes to anoikis-resistance in myofibroblasts
Adhesion-dependent FAK activation is a critical pathway that protects cells from anoikis [13]; this is supported by our own observations in fibroblasts treated with the RGD-containing peptide that both inhibited FAK autophosphorylation and induced apoptosis. Based on our findings that that AKT activation by TGF-β1 is independent of the SMAD3/FAK pathway, we investigated the potential additive role of AKT in protecting myofibroblasts from anoikis-induced cell death. IMR-90 fibroblasts were treated with TGF-β1±LY294002 (10 μM), a pharmacologic inhibitor of the PI3K-AKT pathway, with/without GRGDS (0.5 mg/ml) prior to assessment of apoptosis. Blockade of PI3K-AKT significantly attenuated the protective effect of TGF-β1 on myofibroblast anoikis and cell survival (Fig. 7). Thus, myofibroblasts induced to differentiate in response to TGF-β1 stimulation consistently demonstrate an anti-anoikis phenotype and enhanced viability/survival, an effect that is significantly inhibited by blockade of the PI3K/ AKT pathway. However, blockade of PI3K/AKT activation with LY294002 (10 μM) treatment did not inhibit TGF-β1-induced FAK activation or myofibroblast differentiation (data not shown). These results suggest that, in addition to the activation of adhesion-dependent FAK, the PI3K-AKT pathway contributes to the resistance against anoikis acquired by TGF-β1-differentiated myofibroblasts.
Fig. 7.
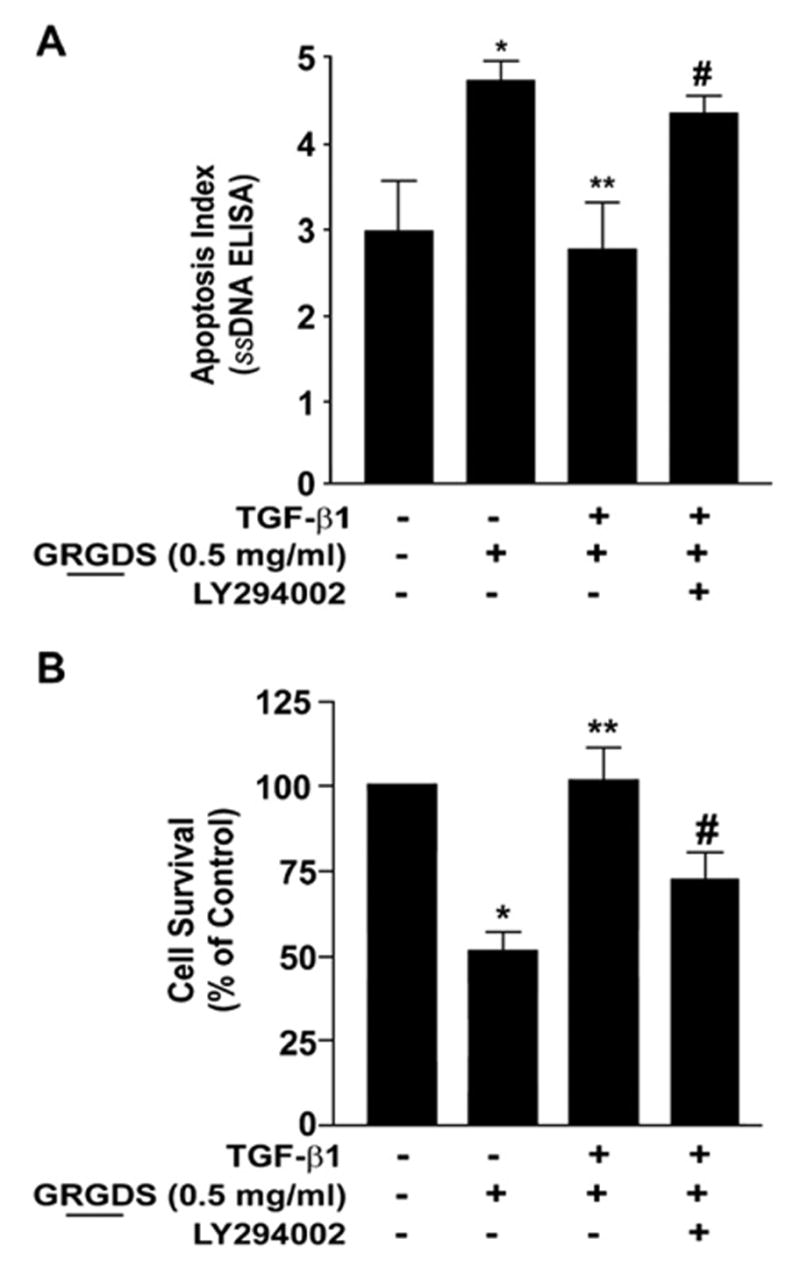
Myofibroblast viability and anoikis-resistance is dependent on PI3K-AKT. (A) Quiescent IMR-90 fibroblasts were stimulated with/without TGF-β1 in the presence/absence of LY294002 (10 μM) for 16 h prior to treatment with soluble RGD-containing peptides (GRGDS, 0.5 mg/ml) for 24 h and assessed for viability as described in “Materials and methods”. Values are mean±SEM; n=3 and experiments was repeated 3 times with similar results. * p<0.01 compared to untreated controls. ** p<0.001 compared to GRGDS 0.5 mg/ml alone. #=not significant compared to 0.5 mg/ml GRGDS alone. (B) IMR-90 cells were cultured in a 96 well plate, growth-arrested and treated±TGF-β1 with/without the PI3K inhibitor LY294002 (10 μM) for 16 h prior to the addition of soluble RGD containing fibronectin peptide (GRGDS 0.5 mg/ml) for 24 h. Apoptosis was assessed with ELISA for ssDNA. Values are mean±SEM; n=4 per condition, and the experiment was repeated three times with similar results. * p<0.05 compared to untreated control. ** p<0.05 compared to 0.5 mg/ml GRGDS treatment alone. #=not significant compared to 0.5 mg/ml GRGDS alone.
4. Discussion
TGF-β1 is a multifunctional growth factor that plays a central role in tissue homeostasis, reparative responses following tissue injury and in the stromal reaction associated with tumour progression and metastasis [2,11,31]. Effects of TGF-β1 on target cells are highly cell-specific and contextual [1]. Despite the recognition that TGF-β1 functions as an anti-apoptotic signal for fibroblasts/myofibroblasts [7,8,32], mechanisms for this effect have not been well defined. TGF-β superfamily ligands signal via heterotetrameric complexes of type II and type I receptors (TβR-II and TβR-I, respectively) leading to activation of SMAD proteins which represent the central mediators of downstream signalling [1]. There is emerging evidence for non-SMAD signals that mediate specific biological actions of TGF-β [33,34]. Our studies in human lung fibroblasts have demonstrated that early and rapid activation of p38 MAPK by TGF-β1 is essential for the production of an autocrine growth factor that mediates activation of the PI3K-AKT pathway [8]. Additionally, our prior studies have demonstrated delayed, but sustained, activation of FAK that is required for stable induction of myofibroblast differentiation by TGF-β1 [12]. A specific requirement for SMAD3-dependent signalling in the activation of FAK and AKT, potential crosstalk between SMAD-dependent and -independent signalling, or their role(s) in the anoikis-resistance of myofibroblasts have not been previously reported. The current studies provide support for a model in which TGF-β1 activates SMAD3 to regulate downstream activation of FAK, while p38 MAPK simultaneously mediates the downstream activation of AKT (schematic in Fig. 8). Interestingly, TGF-β1-induced activation of FAK is independent of p38 MAPK, while TGF-β1-induced activation of AKT is independent of SMAD3. Importantly, TGF-β1-induced activation of PI3K-AKT contributes to the observed anoikis-resistance of myofibroblasts. This is the first study to demonstrate that myofibroblasts induced to differentiate in response to TGF-β1 are resistant to anoikis-mediated cell death and that the acquisition of an anoikis-resistant myofibroblast phenotype is mediated by the activation of two independent, and parallel, protein kinase pathways involving FAK and AKT.
Fig. 8.

Model of combinatorial signalling by TGF-β1 of the inducible activation of FAK and AKT in anoikis-resistant myofibroblasts. TGF-β1 activates PKB/ AKT by a p38 MAPK-dependent, SMAD3 independent mechanism; while, activation of FAK is mediated via a SMAD3-dependent, p38 MAPK-independent pathway in human lung fibroblasts. The activation of these pathways occurs concurrently with the induction of myofibroblast differentiation and acquisition of an anoikis-resistant phenotype. Inducible activation of the PI3K-AKT pathway by TGF-β1 contributes to anoikis-resistance in myofibroblasts.
Our studies conclusively demonstrate that the induction of FAK activation by TGF-β1 is dependent on SMAD3 signalling, but is independent of p38 MAPK. A pharmacologic inhibitor of TβR-I/ALK5, SB431542, that is known to inhibit TGF-β1-induced SMAD3 [35], effectively blocks TGF-β1-induced FAK activation. That SMAD3 signalling was required for this effect was confirmed by siRNA targeting of SMAD3. In contrast, pharmacologic and genetic strategies that disrupt p38 MAPK activation and block activation of the PI3K-AKT pathways [8], were incapable of inhibiting FAK activation induced by TGF-β1. Although studies by our group [12] and others [36] have shown the capacity of TGF-β1 to activate FAK in certain cell types, a role for SMAD3 in mediating this effect has not been previously demonstrated. The requirement for SMAD3 in FAK activation is consistent with a role for SMAD3 in myofibroblast differentiation both in-vitro [37,38] and in-vivo [39–41].
Several lines of evidence support the conclusion that TGF-β1 induces activation of AKT by a mechanism independent of the SMAD3-FAK pathway. First, at doses of SB431542, a TβR-I/ ALK5 inhibitor, that completely blocks the activation of SMAD3 and FAK, TGF-β1 was able to significantly induce AKT activation. The relatively small decrease in TGF-β1-induced AKT activation with 1 μM SB431542 may be accounted for by the observed ~40% inhibition of p38 MAPK at this dose. Second, siRNA knockdown of SMAD3 failed to attenuate AKT activation by TGF-β1, while it effectively blocks TGF-β1-induced FAK activation. Third, disruption of FAK autophosphorylation with expression of mutant FAK does not alter TGF-β1-induced AKT activation. Finally, introduction of an RGD-containing peptide that suppresses FAK autophosphorylation and induces anoikis does not inhibit activation of AKT in response to TGF-β1 stimulation. A SMAD-independent mechanism for activation of PI3K-AKT by TGF-β1 has been previously demonstrated in a subset of fibroblast cell lines, although this effect occurred more rapidly and, presumably, in the absence of an autocrine mechanism [42].
This is the first study, to our knowledge, that demonstrates a novel effect of TGF-β1 to induce anoikis-resistance in any cell type. Importantly, the activation of PI3K-AKT by TGF-β1 was found to be a significant contributor to this anoikis-resistant phenotype of myofibroblasts. There is evidence for a role of FAK and/or ILK signalling in the constitutive activation of AKT in adherent cells [16,22]; however, studies on the role of adhesion-dependent FAK and ILK in inducible AKT activation by growth factors are lacking. Our studies clearly indicate that FAK is not required for the inducible activation of AKT in response to TGF-β1 stimulation; this may be related to a mechanism whereby soluble autocrine factor(s) secreted in a p38 MAPK-dependent and SMAD3-independent manner are capable of activating the PI3K-AKT pathway [8]. Interestingly, blockade of the PI3K-AKT pathway did not completely inhibit anoikis-resistance conferred by TGF-β1, suggesting that the participation of non-AKT and non-FAK pathways may also contribute to the anoikis-resistant phenotype [43]; alternatively, the additional protection may be attributed to the relatively small, but significant, increases in FAK activity observed in cells treated with TGF-β1 despite the presence of RGD-containing peptides.
Although the precise mechanism(s) of myofibroblast apoptosis in the resolution of wound healing and tissue repair are not well defined, anoikis is likely a relevant model to study apoptotic mechanisms since cell adhesion and biomechanical tension unloading appear to play important roles in this physiological context [44,45]. Our observations that the PI3K-AKT and integrin-FAK pathways are activated in a combinatorial manner by TGF-β1 to promote myofibroblast survival have important implications for broader understanding of dysregulated tissue repair and fibrotic diseases. Recent studies from our laboratory have shown that both FAK and AKT activation are increased in areas of active fibrogenesis following acute lung injury in mice [46]. Treatment with a protein tyrosine kinase inhibitor that blocks TGF-β1-activation of FAK and AKT in lung fibroblasts is protective against the development of fibrosis in this animal model [46]. Additionally, we have shown that alveolar mesenchymal cells isolated from patients with non-resolving (fibrotic/persistent) acute respiratory distress syndrome (ARDS) demonstrate high levels of AKT activation and resistance to apoptosis compared to mesenchymal cells from patients with resolving ARDS [47]. These studies support an in-vivo role for an apoptosis-resistant mesenchymal cell/myofibroblast phenotype in fibrotic lung diseases. Thus, a better understanding of the signalling pathways that regulate this myofibroblast phenotype may provide novel targets for therapy of these, otherwise, treatment-unresponsive disorders.
5. Conclusions
In this study, we demonstrate: (1) a novel action of TGF-β1 to protect differentiated myofibroblasts from anoikis-mediated cell death; (2) an important role for the inducible activation of PI3K-AKT and FAK by TGF-β1 in the acquisition of an anoikis-resistant myofibroblast phenotype; and (3) independent and coordinate regulation of FAK and AKT activation by TGF-β1 via SMAD-dependent and -independent mechanisms, respectively. The significance of these findings are that they provide mechanistic insights into TGF-β1 regulation of myofibroblast survival and persistence, a key feature of progressive human fibrotic disorders [9] and of the stromal reaction that supports tumour progression/metastasis [11]. Moreover, these studies provide a novel paradigm for TGF-β1 signalling of cell survival in mesenchymal cells, in contrast to its well recognized anti-proliferative/pro-apoptotic effects on epithelial cells.
Acknowledgments
This work was supported by the National Institutes of Health grants, K08 HL081059 (to J.C.H.), American Lung Association Dalsemer Award (to J.C.H.), K08 HL070990 (to E.S.W.), R01 HL067967 (to V.J.T.) and P50 HL074024 (to V.J.T.). We thank Dr. Kenneth Yamada, National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD, for graciously providing the FAK mutant and control plasmids used in these studies.
References
- 1.Massague J. Nat Rev, Mol Cell Biol. 2000;1(3):169. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 2.Blobe GC, Schiemann WP, Lodish HF. N Engl J Med. 2000;342(18):1350. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 3.Moses HL, Coffey RJ, Jr, Leof EB, Lyons RM, Keski-Oja J. J Cell Physiol, Suppl. 1987;(Suppl 5):1. doi: 10.1002/jcp.1041330403. [DOI] [PubMed] [Google Scholar]
- 4.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Nat Rev Mol Cell Biol. 2002;3(5):349. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 5.Chodon T, Sugihara T, Igawa HH, Funayama E, Furukawa H. Am J Pathol. 2000;157(5):1661. doi: 10.1016/s0002-9440(10)64803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thannickal VJ, Aldweib KD, Rajan T, Fanburg BL. Biochem Biophys Res Commun. 1998;251(2):437. doi: 10.1006/bbrc.1998.9443. [DOI] [PubMed] [Google Scholar]
- 7.Zhang HY, Phan SH. Am J Respir Cell Mol Biol. 1999;21(6):658. doi: 10.1165/ajrcmb.21.6.3720. [DOI] [PubMed] [Google Scholar]
- 8.Horowitz JC, Lee DY, Waghray M, Keshamouni VG, Thomas PE, Zhang H, Cui Z, Thannickal VJ. J Biol Chem. 2004;279(2):1359. doi: 10.1074/jbc.M306248200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thannickal VJ, Toews GB, White ES, Lynch JP, III, Martinez FJ. Annu Rev Med. 2004;55:395. doi: 10.1146/annurev.med.55.091902.103810. [DOI] [PubMed] [Google Scholar]
- 10.Desmouliere A, Guyot C, Gabbiani G. Int J Dev Biol. 2004;48(5–6):509. doi: 10.1387/ijdb.041802ad. [DOI] [PubMed] [Google Scholar]
- 11.De Wever O, Mareel M. J Pathol. 2003;200(4):429. doi: 10.1002/path.1398. [DOI] [PubMed] [Google Scholar]
- 12.Thannickal VJ, Lee DY, White ES, Cui Z, Larios JM, Chacon R, Horowitz JC, Day RM, Thomas PE. J Biol Chem. 2003;278(14):12384. doi: 10.1074/jbc.M208544200. [DOI] [PubMed] [Google Scholar]
- 13.Frisch SM, Vuori K, Ruoslahti E, Chan-Hui PY. J Cell Biol. 1996;134(3):793. doi: 10.1083/jcb.134.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao YF, Gotwals PJ, Koteliansky VE, Sheppard D, Van De Water L. J Biol Chem. 2002;277(17):14467. doi: 10.1074/jbc.M201100200. [DOI] [PubMed] [Google Scholar]
- 15.Wen LP, Fahrni JA, Troie S, Guan JL, Orth K, Rosen GD. J Biol Chem. 1997;272(41):26056. doi: 10.1074/jbc.272.41.26056. [DOI] [PubMed] [Google Scholar]
- 16.Xia H, Nho RS, Kahm J, Kleidon J, Henke CA. J Biol Chem. 2004;279(31):33024. doi: 10.1074/jbc.M313265200. [DOI] [PubMed] [Google Scholar]
- 17.Zhan M, Zhao H, Han ZC. Histol Histopathol. 2004;19(3):973. doi: 10.14670/HH-19.973. [DOI] [PubMed] [Google Scholar]
- 18.Vivanco I, Sawyers CL. Nat Rev Cancer. 2002;2(7):489. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 19.Datta SR, Brunet A, Greenberg ME. Genes Dev. 1999;13(22):2905. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 20.Dan HC, Sun M, Kaneko S, Feldman RI, Nicosia SV, Wang HG, Tsang BK, Cheng JQ. J Biol Chem. 2004;279(7):5405. doi: 10.1074/jbc.M312044200. [DOI] [PubMed] [Google Scholar]
- 21.Sonoda Y, Watanabe S, Matsumoto Y, Aizu-Yokota E, Kasahara T. J Biol Chem. 1999;274(15):10566. doi: 10.1074/jbc.274.15.10566. [DOI] [PubMed] [Google Scholar]
- 22.Persad S, Attwell S, Gray V, Mawji N, Deng JT, Leung D, Yan J, Sanghera J, Walsh MP, Dedhar S. J Biol Chem. 2001;276(29):27462. doi: 10.1074/jbc.M102940200. [DOI] [PubMed] [Google Scholar]
- 23.Frankfurt OS, Krishan A. J Immunol Methods. 2001;253(1–2):133. doi: 10.1016/s0022-1759(01)00387-8. [DOI] [PubMed] [Google Scholar]
- 24.Roberts AB, Tian F, Byfield SD, Stuelten C, Ooshima A, Saika S, Flanders KC. Cytokine Growth Factor Rev. 2006;17(1–2):19. doi: 10.1016/j.cytogfr.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Schaller MD. Biochim Biophys Acta. 2001;1540(1):1. doi: 10.1016/s0167-4889(01)00123-9. [DOI] [PubMed] [Google Scholar]
- 26.Akiyama SK, Yamada KM. J Biol Chem. 1985;260(19):10402. [PubMed] [Google Scholar]
- 27.Hadden HL, Henke CA. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1553. doi: 10.1164/ajrccm.162.4.2001015. [DOI] [PubMed] [Google Scholar]
- 28.Frisch SM, Screaton RA. Curr Opin Cell Biol. 2001;13(5):555. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- 29.Michel JB. Arterioscler Thromb Vasc Biol. 2003;23(12):2146. doi: 10.1161/01.ATV.0000099882.52647.E4. [DOI] [PubMed] [Google Scholar]
- 30.Maubant S, Saint-Dizier D, Boutillon M, Perron-Sierra F, Casara PJ, Hickman JA, Tucker GC, Van Obberghen-Schilling E. Blood. 2006;108(9):3035. doi: 10.1182/blood-2006-05-023580. [DOI] [PubMed] [Google Scholar]
- 31.Leask A, Abraham DJ. FASEB J. 2004;18(7):816. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 32.Jelaska A, Korn JH. Arthritis Rheum. 2000;43(10):2230. doi: 10.1002/1529-0131(200010)43:10<2230::AID-ANR10>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 33.Derynck R, Zhang YE. Nature. 2003;425(6958):577. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 34.Moustakas A, Heldin CH. J Cell Sci. 2005;118(Pt 16):3573. doi: 10.1242/jcs.02554. [DOI] [PubMed] [Google Scholar]
- 35.Laping NJ, Grygielko E, Mathur A, Butter S, Bomberger J, Tweed C, Martin W, Fornwald J, Lehr R, Harling J, Gaster L, Callahan JF, Olson BA. Mol Pharmacol. 2002;62(1):58. doi: 10.1124/mol.62.1.58. [DOI] [PubMed] [Google Scholar]
- 36.Wang H, Radjendirane V, Wary KK, Chakrabarty S. Oncogene. 2004;23(32):5558. doi: 10.1038/sj.onc.1207701. [DOI] [PubMed] [Google Scholar]
- 37.Hu B, Wu Z, Phan SH. Am J Respir Cell Mol Biol. 2003;29(3 Pt 1):397. doi: 10.1165/rcmb.2003-0063OC. [DOI] [PubMed] [Google Scholar]
- 38.Uemura M, Swenson ES, Gaca MD, Giordano FJ, Reiss M, Wells RG. Mol Biol Cell. 2005;16(9):4214. doi: 10.1091/mbc.E05-02-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bonniaud P, Kolb M, Galt T, Robertson J, Robbins C, Stampfli M, Lavery C, Margetts PJ, Roberts AB, Gauldie J. J Immunol. 2004;173(3):2099. doi: 10.4049/jimmunol.173.3.2099. [DOI] [PubMed] [Google Scholar]
- 40.Lakos G, Takagawa S, Chen SJ, Ferreira AM, Han G, Masuda K, Wang XJ, DiPietro LA, Varga J. Am J Pathol. 2004;165(1):203. doi: 10.1016/s0002-9440(10)63289-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramirez AM, Takagawa S, Sekosan M, Jaffe HA, Varga J, Roman J. Am J Pathol. 2004;165(4):1223. doi: 10.1016/S0002-9440(10)63382-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilkes MC, Mitchell H, Penheiter SG, Dore JJ, Suzuki K, Edens M, Sharma DK, Pagano RE, Leof EB. Cancer Res. 2005;65(22):10431. doi: 10.1158/0008-5472.CAN-05-1522. [DOI] [PubMed] [Google Scholar]
- 43.Verderio EA, Telci D, Okoye A, Melino G, Griffin M. J Biol Chem. 2003;278(43):42604. doi: 10.1074/jbc.M303303200. [DOI] [PubMed] [Google Scholar]
- 44.Grinnell F, Zhu M, Carlson MA, Abrams JM. Exp Cell Res. 1999;248(2):608. doi: 10.1006/excr.1999.4440. [DOI] [PubMed] [Google Scholar]
- 45.Desmouliere A, Redard M, Darby I, Gabbiani G. Am J Pathol. 1995;146(1):56. [PMC free article] [PubMed] [Google Scholar]
- 46.Vittal R, Horowitz JC, Moore BB, Zhang H, Martinez FJ, Toews GB, Standiford TJ, Thannickal VJ. Am J Pathol. 2005;166(2):367. doi: 10.1016/S0002-9440(10)62260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Horowitz JC, Cui Z, Moore TA, Meier TR, Reddy RC, Toews GB, Standiford TJ, Thannickal VJ. Am J Physiol Lung Cell Mol Physiol. 2006;290(3):L415. doi: 10.1152/ajplung.00276.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


