Abstract
Oviposition traps set in rural to urban environments in three south Florida counties were colonized predominantly by Culex quinquefasciatus Say (35.1%), Aedes albopictus (Skuse) (34.5%), Aedes aegypti (L.) (23.8%), and Culex nigripalpus Theobald (6.6%) during 1 yr of monthly sampling. Significant differences were detected among counties for abundances of Cx. quinquefasciatus and for percentage composition of that species and Ae. albopictus. Aerial images of habitats around each collection site were digitized, and coverages by each of 16 habitat variables were recorded. Abundances of Ae. aegypti were positively related to habitat variables associated with urbanization and negatively correlated to those reflecting rural characteristics. Multiple regression models of habitat selection explained similar proportions of variances in abundance of Ae. aegypti and Ae. albopictus, but signs of significant variables were opposite for these two species. No consistent trends of habitat associations were observed among counties for the two Culex spp. Co-occurrences of the four species in individual traps depended on container type (tub versus cup), and, for Aedes spp. with Culex spp., county. The results underscore the importance of scale in evaluating habitat selection and the utility of quantifiable habitat characteristics of intermediate scale to identify site characteristics favored by the arboviral vectors Ae. aegypti and Ae. albopictus.
Keywords: Aedes, Culex, Florida, rural, urban
The geographic distributions of Aedes aegypti (L) and Aedes albopictus (Skuse) overlap in tropical Asia, North and South America, and a few African nations. Spatial segregation because of differences in habitat preferences by the two species has been proposed as one mechanism promoting geographical coexistence (Hawley 1988, O’Meara et al. 1995, Thavara et al. 2001). Typically, Ae. aegypti predominates in urban areas, and Ae. albopictus predominates in rural areas; however, our knowledge of habitat preferences of these species remains qualitative and subjective because the environmental determinants have not been identified or quantified. The latter information is essential for predicting Ae. aegypti and Ae. albopictus incidence and abundance, and for risk assessment of disease transmission.
Furthermore, although the level of urbanization seems to be an important factor influencing the relative abundance of the two species, we do not have information on the influence of intermediate scale (hundreds of meters) habitat characteristics on mosquito presence and abundance, and data on micro-habitat (less than a meter) discrimination by the two species is scant.
Ae. aegypti is the primary vector of dengue, and Ae. albopictus also may play a role in the transmission of dengue in its native and invaded range (Effler et al. 2005). Although many species of North American mosquitoes have been detected infected with West Nile virus or resolved as competent laboratory vectors, the available evidence suggests that Culex spp., especially Culex nigripalpus Theobald, Culex salinarius Co-quillett, Culex restuans Theobald, Culex pipiens L., and Culex quinquefasciatus Say, are likely involved in bird to human transmission of the virus (Sardelis et al. 2002, Rutledge et al. 2003, Turell et al. 2005). In many peri-domestic environments in the southeastern United States, Ae. albopictus accounts for a high proportion of mosquito–human contacts, and, because of its broad host range, cannot be ruled out as a West Nile virus vector.
This contribution investigates habitat factors that are associated with the spatial distributions of Ae. aegypti, Ae. albopictus, and Culex species in three populated counties of peninsular Florida: Miami-Dade, Palm Beach, and Manatee counties (Fig. 1). Relationships between habitat variables at several scales and mosquito presence and abundance are investigated, and co-occurrences are examined to detect spatial and trap influences on interspecific associations between Ae. aegypti and Ae. albopictus and between Aedes spp. and Culex spp.
Fig. 1.
Location of Miami-Dade, Palm Beach, and Manatee counties.
Materials and Methods
In each county, 15 stations were selected to include rural, urban, suburban, and industrial/commercial settings. Each station was sampled for mosquitoes once per month from March 2002 to February 2003. At each sampling, oviposition traps for Aedes and Culex mosquitoes (O’Meara et al. 1989, Reiter et al. 1991, Service 1993, Rawlings et al. 1998) were deployed in the shade (in Florida, eggs exposed to the sun exhibit very poor survival; L.P.L., unpublished data.).
The Aedes traps were black plastic cups (400 ml) with (2- by 10-cm) masonite paddles immersed in a dilute (10%) oak infusion (O’Meara et al. 1989). Each cup had holes near the lip to prevent overflowing. Three cups were set at each station during each sampling. The Culex traps consisted of plastic tubs (32 by 23 by 17 cm) half-filled with undiluted oak infusion. The tubs also had holes near the lip on the shorter dimension to prevent overflowing. The traps were left in the field for 1 wk, after which time all the contents were collected and returned to the laboratory, where all immature mosquitoes were hatched and/or reared, identified to species, and counted. When >100 larvae were collected, all larvae were counted, but a random subset of 100 was chosen for identification and the species composition extrapolated to the total number of larvae.
Georeferenced aerial imagery for each site was obtained either commercially (Miami-Dade and Palm Beach counties) or from county geographical information system (GIS) facilities (Manatee Co.). A 100-m-diameter buffer was constructed around each site by using ArcGIS, and cover within this 100-m buffer was hand digitized and assigned to one of 17 cover categories (Table 1). Compound cover categories, made up of simple combinations of the measured variables, also were computed for each site (Table 1). Digitizing was simplified because we were familiar with all of the sites due to frequent sampling visits to each site. Problems with features that were unidentifiable or whose boundaries were not clear from the images were resolved by direct inspection/measurement at the site. In a few cases, access to such questionable features was not possible, in which case the feature was assigned to the “unknown” category. The resulting data include information on areal coverage, frequency, and distribution of each cover type at each site. The coverage data were imported into Statistica (StatSoft, Tulsa OK) or SAS (SAS Institute, Cary, NC) statistical packages for analysis.
Table 1.
Habitat variables used in this study (first column) and composition of derived compound variables
| Compound categoriesa |
||||||
|---|---|---|---|---|---|---|
| Measured cover categories | Canopy | Ground | A&C | Dirt | Water | Other |
| Ground vegetation | 1 | |||||
| Canopy vegetation | 1 | |||||
| Mixed vegetation | 1/2 | 1/2 | ||||
| Bare | 1 | |||||
| Unpaved lot | 1 | |||||
| Paved lot | 1 | |||||
| Unpaved road | 1 | |||||
| Paved road | 1 | |||||
| Building | 1 | |||||
| Open building | 1 | |||||
| Ditch | 1 | |||||
| Pond | 1 | |||||
| Lake | 1 | |||||
| Other water | 1 | |||||
| Fence | 1 | |||||
| Unknown | 1 | |||||
Compound variables are a simple sums of the coverage in those habitat variables marked with a 1 and 50% of the coverage in those marked with 1/2 under their respective columns.
Statistical Analyses
One-way analyses of variance of the total mosquito catch of each species (log transformed to achieve normality) and percentage of composition (arcsine square root transformed) against county were computed using the General Linear Models module of Statistica. Tukey’s honestly significant difference (HSD) post hoc test was used to identify significant individual comparisons. Similar analyses also were performed for the compound cover variables by county. Comparison of measured habitat variables between counties was accomplished via Kruskal–Wallis tests followed by multiple comparisons of mean ranks for each pair of groups via normal z-values, with post hoc probabilities corrected for the number of comparisons as per Siegel and Castellan (1988). Nonparametric tests were used for these analyses because of violations of assumptions for parametric analysis of variance (ANOVA) by some of the variables that could not be eliminated with data transformations.
Forward stepwise multiple regressions of (log) total mosquito abundance against the measured habitat variables were performed for each county and for the three counties combined using the Multiple Regression module of Statistica. Variables contributing significantly to the regression models were determined by significant F statistics and significant t-tests for standardized regression coefficients (β, Iles 1993)
Principal component analyses (PCAs) based on covariances were computed (PCA and Classification module, Statistica) for the same groups mentioned above, and the relative abundance and percentage of composition of the major mosquito species at each station were then plotted against the station’s factor coordinates for every combination of the first three principal components. Multiple regressions of total numbers of Ae. aegypti and Ae. albopictus versus site coordinates for the first two principal components also were calculated.
For analyses of co-occurrences, monthly samples were regarded as independent because traps were removed and replaced with new traps each month. Individual containers also were treated independently. Because of major differences in trap size, presence/absence data were analyzed, instead of absolute abundances, as contingency tables with the CATMOD procedure in SAS, with container type (cup or tub) and county (n = 3) as independent variables in maximum likelihood analyses of variance.
Results
Mosquito Abundances
In total, 49,525 mosquitoes were collected in the traps during the study. More than 99% of these mosquitoes belonged to the following four species: Cx. quinquefasciatus (17,393; 35.1%), Ae. albopictus (17,077; 34.5%), Ae. aegypti (11,774; 23.8%), and Cx. nigripalpus (3,248; 6.6%). Incidental species also collected included Toxorhynchites rutilus (Coquillett), Culex biscaynensis Zavortink & O’Meara, Cx. salinarius, Culex territans Walker as well as midges in the genus Corethrella (Corethrellidae). Analyses of variance indicate significant differences in overall abundance of Cx. quinquefasciatus and of percentage of composition of Ae. albopictus and Cx. quinquefasciatus (Table 2) among counties (Fig. 2). A posteriori comparisons of these data indicated that Cx. quinquefasciatus abundance was highest in Miami-Dade Co. and lowest in Manatee Co., whereas the percentage of composition of Ae. albopictus was greater in Manatee Co. than in Miami-Dade Co., and the opposite was true for Cx. quinquefasciatus.
Table 2.
Results of analysis of variance for differences in abundance and percent composition of the major mosquito species by county
| df | F | P ≤ | Tukey HSDa | |
|---|---|---|---|---|
| Abundance | ||||
| Ae. aegypti | 2/41 | 0.905 | 0.413 | |
| Ae. albopictus | 2/41 | 0.397 | 0.675 | |
| Cx. quinquefasciatus | 2/41 | 5.739 | 0.006 | MD > PB > MNTb |
| Cx. nigripalpus | 2/41 | 0.821 | 0.447 | |
| % composition | ||||
| Ae. aegypti | 2/39 | 0.145 | 0.865 | |
| Ae. albopictus | 2/39 | 3.869 | 0.029 | MNT > MD |
| Cx. quinquefasciatus | 2/39 | 4.558 | 0.017 | MD > MNT |
| Cx. nigripalpus | 2/39 | 0.721 | 0.493 | |
Significant (P ≤ 0.05) individual differences determined a posteriori (Tukey’s HSD test).
MD, Miami-Dade; MNT, Manatee; PB, Palm Beach.
Fig. 2.
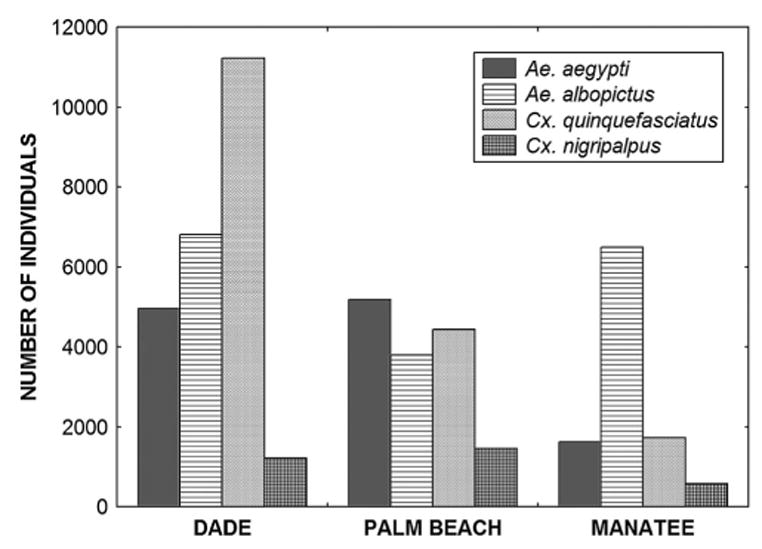
Total numbers of mosquitoes collected in oviposition traps per county.
Habitat Variables
There were few significant overall differences among counties in the measured habitat variables. Kruskal–Wallis tests revealed significant variation in the variables mixed vegetation (H2 ≤ 8.164, P = 0.02, n = 44), open building (H2 = 23.379, P ≤ 0.001, n = 44), and unknown (H2 = 12,314, P ≤ 0.005, n = 44). Multiple comparison of these variables indicate that for mixed vegetation, Miami-Dade Co. had significantly higher cover than Manatee Co.; for open building, the rankings were Miami-Dade > Manatee > Palm Beach and for unknown Palm Beach > Manatee. The only significant difference between counties in coverage by the different compound variables was in the “other” category, where Miami-Dade had significantly higher cover than Palm Beach (Table 3).
Table 3.
Results of analysis of variance for differences in coverage by county
| df | F | P ≤ | Tukey HSDa | |
|---|---|---|---|---|
| Canopy | 2/41 | 0.871 | 0.427 | |
| Ground | 2/41 | 0.631 | 0.537 | |
| A&C | 2/41 | 0.880 | 0.424 | |
| Dirt | 2/41 | 0.935 | 0.401 | |
| Water | 2/41 | 0.208 | 0.812 | |
| Other | 2/41 | 7.250 | 0.002 | MD > MNT = PBb |
Significant (P ≤ 0.05) individual differences determined a posteriori (Tukey’s HSD test).
MD, Miami-Dade; MNT, Manatee; PB, Palm Beach.
Habitat and Mosquitoes
For Ae. aegypti, only an urbanization-related variable (building) contributed positively to the mosquito abundance regression equations (Table 4), whereas variables related to more rural or open settings (bare, canopy vegetation, mixed vegetation, and unpaved road) made significant negative contributions. The only exception was the variable open building, which was (negatively) significant in the equation for Manatee Co. The converse was true for Ae. albopictus, with significant urbanization-related variables (building, raved road, and open building) exhibiting negative coefficients, and ground vegetation, unpaved road, and bare exhibiting positive coefficients (Table 4). Unpaved lot, however, exhibited a significant negative coefficient in Manatee Co. For Ae. aegypti, regression equations explain (R2) from 80.7% of the variation in mosquito abundance in Miami-Dade and Manatee counties to 51.2% for all counties combined. The amount of variation explained by the first variable entering the equations range from 56.8% (building in Palm Beach Co.) to 33.3% (open building in Manatee Co.; Table 4). For Ae. albopictus, variation explained by the regressions range from 83.2% in Palm Beach Co. to 37.4% in Miami-Dade Co. First variables explain from 56.7% (building, Palm Beach Co.) to 31.7% (building, all counties combined).
Table 4.
Significant (P ≤ 0.05) contributions to forward stepwise multiple regressions of Aedes spp. and Culex spp. abundance with compound habitat categories by county
| County | Factor | Slope | R2a | P ≤ | Factor | Slope | R2 | P ≤ |
|---|---|---|---|---|---|---|---|---|
| Ae. aegypti | Ae. albopictus | |||||||
| Miami-Dade | Building | + | 0.480 | 0.004 | Ground vegetation | + | 0.374 | 0.015 |
| Bare | − | 0.675 | 0.020 | |||||
| Canopy | − | 0.807 | 0.019 | |||||
| Palm Beach | Building | + | 0.568 | 0.002 | Building | − | 0.567 | 0.002 |
| Mixed vegetation | − | 0.730 | 0.026 | Unpaved road | + | 0.691 | 0.047 | |
| Paved road | − | 0.771 | 0.047 | |||||
| Bare | + | 0.832 | 0.049 | |||||
| Manatee | Open building | − | 0.330 | 0.025 | Unpaved lot | − | 0.407 | 0.011 |
| Unpaved road | − | 0.546 | 0.034 | Building | − | 0.681 | 0.008 | |
| Mixed vegetation | − | 0.807 | 0.003 | |||||
| All | Building | + | 0.395 | 0.001 | Building | − | 0.317 | 0.001 |
| Bare | − | 0.454 | 0.041 | Ground vegetation | + | 0.405 | 0.018 | |
| Canopy | − | 0.512 | 0.046 | Bare | + | 0.482 | 0.019 | |
| Open building | − | 0.530 | 0.050 | |||||
| Cx. nigripalpus | Cx. quinquefasciatus | |||||||
| Miami-Dade | Paved lot | − | 0.412 | 0.001 | ||||
| Mixed vegetation | − | 0.625 | 0.023 | |||||
| Bare | − | 0.721 | 0.024 | |||||
| Pond | + | 0.820 | 0.030 | |||||
| Unpaved road | − | 0.905 | 0.044 | |||||
| Building | + | 0.978 | 0.049 | |||||
| Palm Beach | Ground vegetation | − | 0.501 | 0.005 | Mixed vegetation | − | 0.560 | 0.003 |
| Paved lot | − | 0.661 | 0.043 | |||||
| Mixed vegetation | − | 0.873 | 0.001 | |||||
| Paved road | + | 0.959 | 0.003 | |||||
| Water | + | 0.984 | 0.012 | |||||
| Open building | + | 0.993 | 0.041 | |||||
| Manatee | Building | + | 0.269 | 0.048 | Paved lot | + | 0.121 | 0.020 |
| Unpaved road | + | 0.593 | 0.009 | Bare | + | 0.249 | 0.012 | |
| Pond | + | 0.843 | 0.002 | |||||
| All | Paved road | + | 0.412 | 0.010 | Paved lot | + | 0.119 | 0.022 |
| Bare | + | 0.240 | 0.013 |
Variables to include in the model were determined by significant F statistics and significant t-tests for standardized regression coefficients.
Cumulative R2.
The regressions for Cx. nigripalpus and Cx. quinquefasciatus (Table 4) did not exhibit consistent patterns. For Cx. nigripalpus, more variables contributed significantly to the regression equations than for the other three species (six in Miami-Dade and Palm Beach counties, three in Manatee Co., and one for all sites combined). Cx. quinquefasciatus, however, had the fewest significant variables (none in Miami-Dade Co., one in Palm Beach Co., and two each in Manatee Co. and all sites combined). The only consistent pattern for the Culex species with respect to urban- and rural-related variables was that canopy vegetation did not enter significantly in any of the regressions. For Cx. nigripalpus, regression equations explain from 99.3% of the variation in mosquito abundance in Palm Beach Co. to 41.2% for all counties combined. The amount of variation explained by the first variable entering the equations range from 50.1% (ground vegetation in Palm Beach Co.) to 26.9% (building in Manatee Co.; Table 4). For Cx. quinquefasciatus, variation explained by the regressions range from 56.0% in Palm Beach Co. to 0% in Miami-Dade Co. First variables explain from 56.0% (mixed vegetation, Palm Beach) to 11.9% (paved lot, all counties combined).
Habitat Components
The percentage of variance in measured habitat variables by county explained by the first principal component ranged from 74.2% in Palm Beach Co. to 47.2% in Miami-Dade Co. Cumulative variance explained by the first three components ranged from 96.6% in Palm Beach Co. to 99.1% in Miami-Dade Co. (Table 5). For all sites combined, the first component explained 57.3% of the variance, and the first three components 97.4%. Four compound variables–canopy, A&C, dirt, and ground vegetation–accounted for most of the variance in the sites across the three counties.
Table 5.
Factor loadings for the first three PCs of the compound cover variables
| Canopy | Ground | A&C | Dirt | Water | Other | |
|---|---|---|---|---|---|---|
| Miami-Dade (99.1) | ||||||
| PC1 (47.2) | −0.9455 | −0.0756 | 0.6682 | 0.4340 | 0.1511 | 0.0836 |
| PC2 (29.9) | 0.1521 | 0.0006 | 0.6981 | 30.8314 | −0.0437 | 0.0926 |
| PC3 (22.1) | 0.2813 | 30.9908 | 0.2482 | 0.3360 | −0.2008 | −0.2172 |
| Palm Beach (96.6) | ||||||
| PC1 (74.2) | 30.9418 | −0.0067 | 0.9627 | −0.4247 | −0.1250 | 0.4748 |
| PC2 (18.2) | −0.2995 | 0.9815 | −0.2544 | −0.5313 | 0.1202 | −0.0057 |
| PC3 (4.2) | 0.1245 | 0.1485 | 0.0723 | −0.3694 | 30.8520 | 0.1256 |
| Manatee (97.6) | ||||||
| PC1 (57.3) | 30.9227 | −0.0124 | 0.9231 | 0.1787 | −0.1334 | −0.2474 |
| PC2 (24.4) | 0.2902 | 30.9924 | 0.2427 | 0.2836 | −0.0893 | −0.6539 |
| PC3 (16.0) | 0.2391 | 0.0690 | 0.2906 | 30.9342 | 0.0201 | −0.0547 |
| All (97.4) | ||||||
| PC1 (57.3) | 30.8433 | −0.0767 | 0.8691 | 0.1135 | −0.2313 | 0.5876 |
| PC2 (21.1) | −0.0724 | 0.8335 | −0.0274 | −0.7884 | 0.0261 | 0.2080 |
| PC3 (19.0) | 0.4448 | −0.2624 | 0.0545 | −0.2760 | −0.7886 | 0.2663 |
Numbers in parentheses indicate the percentage of the variance explained by the factor or by the first three factors if next to a county name. Loadings > 0.8 are shown in bold.
Plots of factor coordinates of each station versus principal components (PC) 1 and 2 for all stations combined with mosquito relative abundance reveal that stations where Ae. albopictus was abundant tended to fall on the negative side of the PC1 axis, and their PC2 coordinates increased almost linearly with increases in the PC1 coordinates (Fig. 3). Sites with abundant Ae. aegypti, however, tended to have positive PC1 coordinates and showed a decrease in PC2 coordinates with an increase in PC1 coordinates. Regressions of Ae. aegypti and Ae. albopictus abundances at each station with station coordinates for PC1 and PC2 were highly significant (Ae. aegypti: F1, 41 = 9.065, P ≤ 0.0006; and Ae. albopictus: F1, 41 = 12.140, P ≤ 0.0001).
Fig. 3.
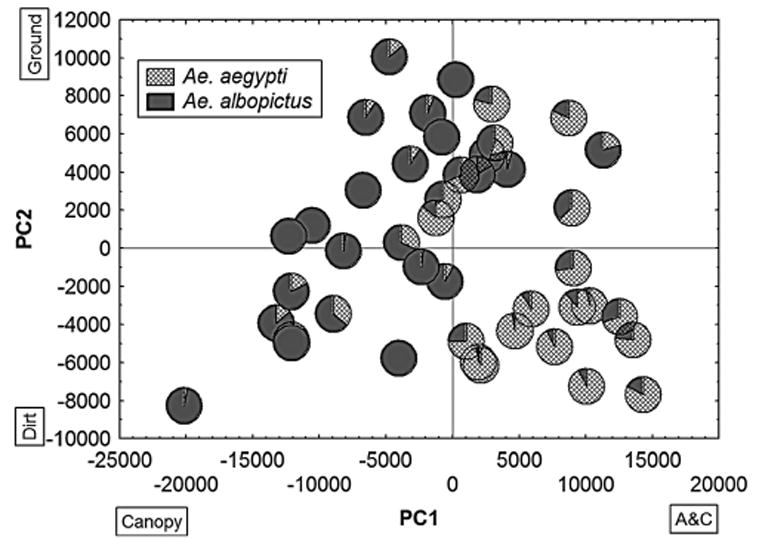
Factor coordinates of all stations plotted against PC1 and 2 of the compound habitat variable and Aedes mosquito relative abundance at each station. Boxes identify variables with high loadings in the corresponding sides of the axes.
No distinct patterns, however, were evident for Culex spp. (Fig. 4). At the county level, patterns were similar, but reflected the loadings at each county. For example, at Palm Beach Co., PC1 and PC2 loadings were similar to the loadings for all counties combined, and the site coordinates reflected mosquito relative abundances similarly. In Manatee Co., however, ground vegetation had a high negative loading for PC2 (positive for all counties and Palm Beach).
Fig. 4.
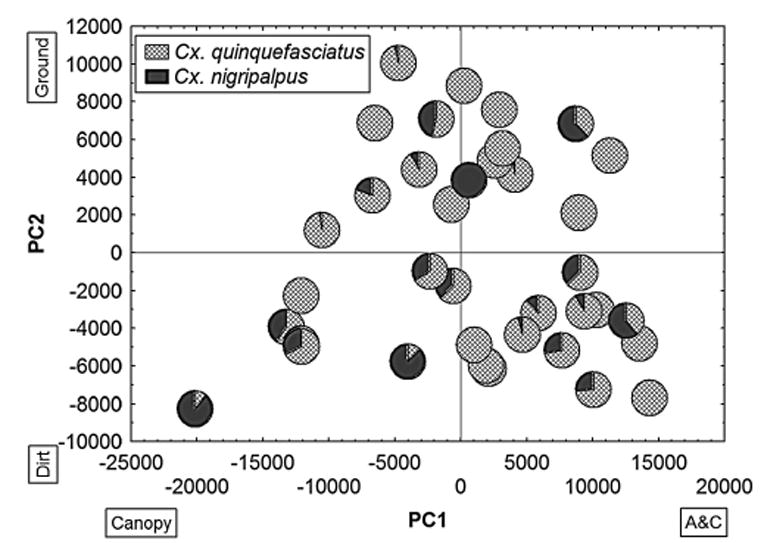
Factor coordinates of all stations plotted against PC1 and 2 of the compound habitat variable and Culex mosquito relative abundance at each station. Boxes identify variables with high loadings in the corresponding sides of the axes.
Co-occurrences
Among all cups in which one or more Aedes spp. were recovered, Ae. aegypti and Ae. albopictus were found together in 22.7% (Table 6). In tubs, the two species together represented 33.3% of all Aedes-positive occurrences, which accounted for a significant container effect on Aedes spp. co-occurrences (Table 7). Although no significant county effect was detected, a significant county × container interaction (Table 7) was associated with an unexpectedly low rate of co-occurrences of the two Aedes spp. in Manatee tubs (18.4%).
Table 6.
Numbers of co-occurrences and occurrences alone in traps
|
Ae. aegypti–Ae albopictus |
Culex spp.–Aedes spp.
|
||||
|---|---|---|---|---|---|
| Site/trap | n | Alone | Co-occur | Alone | Co-occur |
| Manatee/cup | 495 | 108 | 32 | 125 | 2 |
| Manatee/tub | 165 | 40 | 9 | 36 | 17 |
| Miami-Dade/cup | 495 | 109 | 34 | 132 | 15 |
| Miami-Dade/tub | 165 | 34 | 19 | 25 | 37 |
| Palm Beach/cup | 495 | 100 | 27 | 127 | 6 |
| Palm Beach/tub | 165 | 26 | 22 | 33 | 24 |
| Total cup | 1,485 | 317 | 93 | 384 | 23 |
| Total tub | 495 | 100 | 50 | 94 | 78 |
Table 7.
Maximum likelihood ANOVA for co-occurrences of Ae. aegypti and Ae. albopictus and Aedes spp. and Culex spp. in relation to trap type and county
|
Ae. albopictus–Ae. aegypti |
Aedes spp.–Culex spp.
|
||||
|---|---|---|---|---|---|
| Source | df | χ2 | P ≤ | χ2 | P ≤ |
| Intercept | 1 | 82.2 | 0.0001 | 102.6 | 0.0001 |
| County | 2 | 5.3 | 0.7200 | 15.8 | 0.0004 |
| Container | 1 | 4.9 | 0.0267 | 76.8 | 0.0001 |
| County × container | 2 | 6.5 | 0.0379 | 0.9 | 0.6345 |
| Likelihood ratio | 0 | ||||
Co-occurrences of Aedes spp. with Culex spp. represented only 5.7% of all cups that harbored one or more species from these two genera, but they accounted for 45.4% of all Culex-positive plus Aedes-positive tubs, which produced a highly significant container effect (Table 7). The significant county effect in maximum likelihood ANOVA (Table 7) was associated with much higher levels of co-occurrences in both container types in Miami-Dade Co. (Table 6).
Discussion
Results from this study indicate that factors at several scales, ranging from county to trap type, affected the abundance of Culex and Aedes mosquitoes at our sites. For example, Cx. quinquefasciatus is a predominantly urban mosquito whose larvae can be found in water containing a high degree of organic pollution and close to human habitation and whose females bite mammals in preference to birds (Cornel et al. 2003). The abundance of this species in the three counties (Miami-Dade > Palm Beach > Manatee; Table 2) probably reflects the relative abundance of highly urbanized habitats in each (average housing units per square kilometer at Miami-Dade, Palm Beach, and Manatee counties are 174.3, 112.7, and 76.2, respectively; U.S. Census statistics, 2000 and 2002). Although all three counties have significant urban coverage, urban sprawl in Miami-Dade Co. has left only pockets of green habitat throughout the county. Palm Beach Co. is similar to Miami-Dade Co., but it still has significant undeveloped acreages in the western portions of the county, whereas in Manatee Co. there is significant undeveloped coverage with interspersed highly urbanized areas. These differences occurred in spite of the fact that the only consequential difference in coverage between counties at our particular study sites was the opposite of what you would expect from the above county wide patterns (mixed vegetation cover was greater at our sites in Miami-Dade Co. than at our sites in Manatee Co.; see Results and Table 3). Although a high percentage of the variation in Cx. nigripalpus abundance was explained by the stepwise regressions for Dade and Palm Beach counties (to be expected, given the large number of significant variables), the Culex species did not exhibit consistent patterns among counties in terms of which habitat variables entered into the regressions equations and their signs (Table 4).
Aedes mosquitoes, however, seemed to respond more to intermediate scale factors, such as measures of habitat cover within a 100-m-diameter of each sampling site (Table 1). Thus, results of the multiple regressions of mosquito abundance with the measured habitat variables indicated a positive association by Ae. aegypti with urbanization-related variables such as building coverage and a negative association with variables more commonly associated with rural areas such as canopy and mixed vegetation coverage, whereas the opposite was true for Ae. albopictus (Table 4).
When the arrangement of sites along principal component axes is combined with the relative abundance of the four major species at each site, it is evident that Aedes abundances reflect the site’s characteristics as represented by their factor coordinates (Fig. 3). Sites where Ae. aegypti was abundant clustered on the positive side of PC1, which had a high positive loading for A&C, a variable associated with urbanization, and on the negative side of PC2, which had a high negative loading for dirt, which reflects a rural setting. Conversely, sites where Ae. albopictus was abundant clustered along the negative side of PC1, where canopy vegetation (normally associated with rural-forested areas) had a high loading. The first principal component alone, with high loadings for canopy at one end and A&C at the other end can separate the majority of sites where either Ae. aegypti or Ae. albopictus predominate.
The PC2 axis has high negative loadings for dirt and high positive loadings for ground vegetation. It is interesting to note the patterns of changes in PC2 coordinates with changes in PC1 coordinates for both Ae. aegypti- and Ae. albopictus-rich sites. For the former species, PC2 coordinates tend to increase with decreases in PC1 coordinates, whereas for the latter, PC2 coordinates increase with increases in PC1. Regressions of the abundance of both species with PC1 and PC2 coordinates are highly significant (see Results). Ground vegetation can reflect a suburban setting (lawns and gardens) or a rural setting (low growing crops or pastures), but ground vegetation cover associated with asphalt and concrete (A&C) (Ae. aegypti) is usually mostly lawns, whereas ground vegetation associated with canopy vegetation (Ae. albopictus) normally indicates pastures and low-growing crops.
Culex species, however, did not show such distinct patterns (Fig. 4). Stations with high relative abundance of Cx quinquefasciatus showed considerable spread along both axes, whereas Cx. nigripalpus predominated in only a few stations with no consistent pattern.
The overall frequency of co-occurrence of Ae. aegypti and Ae. albopictus in cups in three counties (22.7%) was less than reported for West Palm Beach (48%) but more than Boca Raton (13%), based on much briefer surveys of these cities (Braks et al. 2003). The significantly higher frequency of co-occurrence of these species in tubs may be related to the much larger volume of the latter trap type. Tubs in Manatee Co. registered fewer co-occurrences than the other two counties, because of the relative scarcity of Ae. aegypti in that county (Fig. 2), leading to the significant county × container interaction. The co-occurrences of these species suggest that interspecific larval competition, thought to account for the range reduction of Ae. aegypti by Ae. albopictus in Florida and elsewhere (Juliano 1998, Braks et al. 2004), is probable in nature.
The high frequency of co-occurrences of Aedes spp. with Culex spp. in tubs, but not in cups, is facilitated by the predilection of Florida Culex (Culex) spp., particularly Cx. nigripalpus, to oviposit in the larger container type (O’Meara et al. 1989). Based on results of the competitive superiority of Ae. albopictus larvae co-occurring with Cx. pipiens (Costanzo et al. 2005), Culex spp. in tubs in Florida may suffer competitively in the presence of Aedes spp. larvae. Among our study areas, such competitive interactions were most likely in Miami-Dade Co., where the frequencies of inter-generic co-occurrence were highest.
It should be emphasized that the current study addresses only oviposition habitats of these mosquito species, which may differ from habitats used for other activities, such as host seeking. These data do show the general preference of Ae aegypti and Ae albopictus for different habitats and container types.
In the New World, most oviposition by Ae. aegypti occurs outdoors (Braks et al. 2003, Honório et al. 2003), whereas more domesticated populations of this species usually oviposit indoors in parts of Asia (Thavara et al. 2001) and Africa (Lounibos 2003).
Although habitat segregation between Ae. aegypti and Ae. albopictus is well established in the Old World (Fontenille and Rodhain 1989), some of the habitat variables used in the current study, such as paved surfaces, will not be present in undeveloped sites. Thus, a further challenge may be elucidation of the underlying cues used by these species for habitat selection and segregation (Juliano et al. 2004).
Acknowledgments
We thank Marlon Nelms, Miami-Dade County Public Works Department, Mosquito Control Division; Ed Bradford, Palm Beach County Department of Environmental Resource Management, Mosquito Control Division; and Mark Latham, Manatee County Mosquito Control District for invaluable help with this project. Steve Juliano, George O’Meara, and two anonymous reviewers provided valuable comments on an earlier drafts of the manuscript. We also thank the citizens who allowed us to set up collecting stations in their property, This work was partially funded through National Institutes of Health Grant AI-044793 and Florida Department of Agriculture and Consumer Services Contract 6849.
References Cited
- Braks MAH, Honório NA, Lourenço-de-Oliveira R, Juliano SA, Lounibos LP. Convergent habitat segregation of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in southeastern Brazil and Florida. J Med Entomol. 2003;40:785–794. doi: 10.1603/0022-2585-40.6.785. [DOI] [PubMed] [Google Scholar]
- Braks MAH, Honório NA, Lounibos LP, Lourenço-de-Oliveira R, Juliano SA. Interspecific competition between two invasive species of container mosquitoes in Brazil. Ann Entomol Soc Am. 2004;97:130–139. [Google Scholar]
- Cornel AJ, McAbee RD, Rasgon J, Stanich MA, Scott TW, Coetzee M. Differences in extent of genetic introgression between sympatric Culex pipiens and Culex quinquefasciatus (Diptera: Culicidae) in California and South Africa. J Med Entomol. 2003;40:36–51. doi: 10.1603/0022-2585-40.1.36. [DOI] [PubMed] [Google Scholar]
- Costanzo KS, Mormann K, Juliano SA. Asymmetrical competition and patterns of abundance of Aedes albopictus and Culex pipiens (Diptera: Culicidae) J Med Entomol. 2005;42:559–570. doi: 10.1093/jmedent/42.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effler PV, Pang L, Kitsutani P, Vorndam V, Nakata M, Ayers T, Elm J, Tom T, Reiter P, Rigau-Perez JG, et al. Dengue fever, Hawaii, 2001–2003. Emerg Infect Dis. 2005;11:742–749. doi: 10.3201/eid1105.041063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenille D, Rodhain F. Biology and distribution of Aedes albopictus and Aedes aegypti in Madagascar. J Am Mosq Control Assoc. 1989;5:219–225. [PubMed] [Google Scholar]
- Hawley WA. The biology of Aedes albopictus. J Am Mosq Control Assoc Suppl. 1988;1:1–39. [PubMed] [Google Scholar]
- Honório NA, da Costa Silva W, Leite PJ, Monteiro Gonçalves J, Lounibos LP, Lourenço-de-Oliveira R. Dispersal of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in an urban endemic dengue area in the State of Rio de Janeiro, Brazil. Mem Inst Oswaldo Cruz. 2003;98:191–198. doi: 10.1590/s0074-02762003000200005. [DOI] [PubMed] [Google Scholar]
- Iles TC. Multiple regression. In: Fry JC, editor. Biological data analysis. Oxford University Press; Oxford, United Kingdom: 1993. pp. 127–172. [Google Scholar]
- Juliano SA. Species introduction and replacement among mosquitoes: interspecific resource competition or apparent competition? Ecology. 1998;79:255–268. [Google Scholar]
- Juliano SA, Lounibos LP, O’Meara GF. A field test for competitive effects of Aedes albopictus on Aedes aegypti in south Florida: differences between sites of coexistence and exclusion. Oecologia (Berl) 2004;39:583–593. doi: 10.1007/s00442-004-1532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounibos LP. Genetic control trials and the ecology of Aedes aegypti at the Kenya coast. In: Takken W, Scott TW, editors. Ecological aspects for application of genetically modified mosquitoes. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2003. pp. 33–43. [Google Scholar]
- O’Meara GF, Vose FE, Carlson DB. Environmental factors influencing oviposition by Culex (Culex) (Diptera: Culicidae) in two types of traps. J Med Entomol. 1989;26:528–534. doi: 10.1093/jmedent/26.6.528. [DOI] [PubMed] [Google Scholar]
- O’Meara GF, Evans LF, Jr, Gettman AD, Cuda JP. Spread of Aedes albopictus and decline of Ae. aegypti(Diptera: Culicidae) in Florida. J Med Entomol. 1995;32:554–562. doi: 10.1093/jmedent/32.4.554. [DOI] [PubMed] [Google Scholar]
- Rawlings SC, Martinez R, Wiltshire S, Legall G. A comparison for surveillance systems for the dengue vector Aedes aegypti in Port of Spain, Trinidad. J Am Mosq Control Assoc. 1998;14:131–136. [PubMed] [Google Scholar]
- Reiter P, Amador MA, Nelson C. Enhancement of the CDC ovitrap with hay infusions for daily monitoring of Aedes aegypti populations. J Am Mosq Control Assoc. 1991;7:52–55. [PubMed] [Google Scholar]
- Rutledge CR, Day JF, Lord CC, Stark LM, Tabachnick WJ. West Nile virus infection rates in Culex nigripalpus (Diptera: Culicidae) do not reflect transmission rates in Florida. J Med Entomol. 2003;40:253–258. doi: 10.1603/0022-2585-40.3.253. [DOI] [PubMed] [Google Scholar]
- Sardelis MR, Turell MJ, O’Guinn ML, Andre RG, Roberts DR. Vector competence of three North American strains of Aedes albopictus for West Nile virus. J Am Mosq Control Assoc. 2002;18:284–289. [PubMed] [Google Scholar]
- Service MW. Mosquito ecology: field sampling methods. 2. Applied Science Publishers Ltd; London, United Kingdom: 1993. [Google Scholar]
- Siegel S, Castellan NJ. Nonparametric statistics for the behavioral sciences. 2. McGraw-Hill; New York: 1988. [Google Scholar]
- Thavara U, Tawatsin A, Chansang C, Kong-Ngamsuk W, Paosriwong S, Boon-Long J, Rongsriyam Y, Komalamisra N. Larval occurrence, oviposition behavior and biting activity of potential mosquito vectors of dengue on Samui Island, Thailand. J Vector Ecol. 2001;26:172–180. [PubMed] [Google Scholar]
- Turell MJ, Dohm DJ, Sardelis MR, Guinn O, Andreadis TG, Blow JA. An update on the potential of North American mosquitoes (Diptera: Culicidae) to transmit West Nile virus. J Med Entomol. 2005;42:57–62. doi: 10.1093/jmedent/42.1.57. [DOI] [PubMed] [Google Scholar]


