Abstract
Multiple predator species can interact as well as strongly affect lower trophic levels, resulting in complex, nonadditive effects on prey populations and community structure. Studies of aquatic systems have shown that interactive effects of predators on prey are not necessarily predictable from the direct effects of each species alone. To test for complex interactions, the individual and combined effects of a top and intermediate predator on larvae of native and invasive mosquito prey were examined in artificial analogues of water-filled treeholes. The combined effects of the two predators were accurately predicted from single predator treatments by a multiplicative risk model, indicating additivity. Overall survivorship of both prey species decreased greatly in the presence of the top predator Toxorhynchites rutilus. By itself, the intermediate predator Corethrella appendiculata increased survivorship of the native prey species Ochlerotatus triseriatus and decreased survivorship of the invasive prey species Aedes albopictus relative to treatments without predators. Intraguild predation did not occur until alternative prey numbers had been reduced by approximately one-half. Owing to changes in size structure accompanying its growth, T. rutilus consumed more prey as time progressed, whereas C. appendiculata consumed less. The intermediate predator, C. appendiculata, changed species composition by preferentially consuming A. albopictus, while the top predator, T. rutilus, reduced prey density, regardless of species. Although species interactions were in most cases predicted from pairwise interactions, risk reduction from predator interference occurred when C. appendiculata densities were increased and when the predators were similarly sized.
Keywords: intraguild predation, invasive species, mosquitoes, multiple predators, predator identity, predator-mediated coexistence
Introduction
In nature, predation is an important factor in structuring communities (Sih et al. 1998). Direct and indirect effects of predators can influence behavior and demography of prey. Predators can exhibit trait-mediated indirect effects (Werner 1992) that influence both prey habitat use (Diehl and Eklov 1995) and activity levels (Juliano and Reminger 1992), and in turn may change length of larval period and size at emergence to adulthood for animals with complex life cycles (Wilbur 1987). Many studies have examined single predator, single prey systems (e.g., Focks et al. 1980, Morin 1983, Russo 1983), but relatively few have considered interactions among multiple predators and multiple prey (Sih and Krupa 1996, Nystrom et al. 2001). Although analyses of the effects of a single predator are informative, they may not accurately predict community structure when more than one predator species is present. Outcomes of single-species predator–prey encounters mimic nature if there is only one predator species in the natural system or if the effects of a second predator species are additive, such that the predator species do not interact. Because even simple communities may have multiple predator species (e.g., Fincke 1999), experiments with multiple predators are valuable for making predictions about community structure.
Predators affect prey community structure through indirect and direct effects including direct interference, such as intraguild predation (IGP) (Polis et al. 1989), changes in predator behavior, and changes in prey behavior (Peckarsky and McIntosh 1998). When two predator species are present in a delimited community such as a treehole or other container, they are likely to interact, which may affect community structure (Sih and Krupa 1996). In the simplest case, combined effects of the two predator species can be accurately predicted from single predator treatments, resulting in additive effects on prey (e.g., Van Buskirk 1988). Although additivity does not preclude indirect effects, predictions about community structure should be less complex than when predators have nonadditive effects. Predators may also interact, resulting in less than (risk reduction) or greater than expected (risk enhancement) predation rates (Sih et al. 1998). Risk enhancement can occur when one predator causes the prey species to react differently, putting the prey at a higher risk of predation from the second predator. Risk reduction typically results from interactions between predator species, resulting in IGP or behavioral avoidance. Risk enhancement may be detrimental to the fitness of the prey, whereas risk reduction may be detrimental to the fitness of the predator by reducing the number of prey consumed or by consumption of predators via IGP. Predicting the impact of multiple predators becomes even more complex as both predator and prey change in size over time, potentially changing the directions of interactions between them (Wilbur 1988).
The objectives of this study were to quantify predation and to test for additivity in treehole systems by the top predator Toxorhynchites rutilus (Coquillett), an intermediate predator Corethrella appendiculata (Grabham), and their interactions on survival and performance of competing larval mosquito prey Aedes albopictus (Skuse) and Ochlerotatus triseriatus (Say).
Study system
In water-filled treeholes and artificial containers such as shaded, discarded tires, the larval dipterans C. appendiculata and T. rutilus are the two most common aquatic predator species in Florida (Lounibos 1983, Bradshaw and Holzapfel 1984). C. appendiculata is an ambush predator with four larval instars, of which only the third and fourth are large enough to consume the first- and second-instar larvae of mosquitoes (Grabham 1906, Lounibos 1985). First-instar larvae of T. rutilus are approximately the same size as C. appendiculata fourth-instar larvae (Lounibos 1985). Toxorhynchites spp. larvae are primarily ambush predators (Steffan and Evenhuis 1981) and consume both subsurface aquatic prey and terrestrial insects from the water surface (Campos and Lounibos 2000a; see Plate 1).
Plate 1.

A fourth-instar Toxohynchites rutilus larva consuming a fourth-instar Ochlerotatus triseriatus larva. Photo credit: L. P. Lounibos.
The prey mosquito larvae, A. albopictus and O. triseriatus, are similar in behavior and biology (Jenkins and Carpenter 1946, Hawley 1988). However, O. triseriatus may reduce risky behavior in the presence of T. rutilus, while A. albopictus does not (Kesavaraju and Juliano 2004). O. triseriatus is a native species found throughout the eastern U.S. (Jenkins and Carpenter 1946), and A. albopictus was accidentally introduced into the U.S. in the mid 1980s and has since become established in much of the southeast. (O’Meara et al. 1995). Larvae of both species feed by filtering particles and by browsing on leaves and the submerged walls of treeholes or artificial containers (Walker and Merritt 1991, Leonard and Juliano 1995). In the absence of predators, A. albopictus outcompetes O. triseriatus via exploitative competition (Livdahl and Willey 1991, Barrera 1996).
Methods
Field experiment
Effects of multiple predators on prey survival and growth were examined in a field experiment using plastic cups as treehole analogues. Water was collected from discarded tires in Gainesville, Florida, USA and sieved through a 180-μ m filter to remove macroinvertebrates, including their eggs. Water from multiple tires was pooled and mixed before being allotted to treatments. Twenty-eight 500-mL black plastic cups were used as experimental microcosms for the predator and prey treatments. Each container received 350 mL of tire water and 2 g of chopped oak leaves (Quercus virginiana) that were dried at 65 ° C for 48 hours. The containers were covered with screen (mesh ~ 0.76 mm) to prevent any immigration into the system, but allowing rainfall to enter. A single hole was punched above the water line on the side of each container and covered with nylon mesh ( ~ 210 μ m), to allow for overflow while reducing loss of small larvae. Deionized water was added as needed to maintain 350 mL of water in each container. The containers were placed in a forested site within the Natural Area Teaching Lab of the University of Florida campus for three days before the beginning of the experiment to allow microorganisms to accumulate on the leaf litter and sides of the containers. The containers were mounted on stakes approximately 1 m above the ground to limit disruptions by foraging animals. An HOBO data logger (Onset, Bourne, Massachusetts, USA) was placed at the site to monitor daily temperature, which averaged 25.5 ° C ± 2.79 °C (mean ± SD) from 6 June 2003 to 30 July 2003.
Each container received 50 first-instar A. albopictus and 50 first-instar O. triseriatus from locally derived F1 and F2 colonies of these species (Lounibos et al. 2001). These densities are within the range of natural densities (0.05–0.47 larvae/mL) of O. triseriatus measured after spring rainfall in south Florida treeholes (Lounibos 1983). Among censuses conducted after the establishment of the invasive species in Florida, densities of A. albopictus and O. triseriatus cohabiting the same tree-holes were similar (Lounibos et al. 2001). The predators, T. rutilus and C. appendiculata, were obtained from laboratory colonies that originated from Florida. Field-collected larvae of both predators were added at irregular intervals to supplement colonies of these species. Treatments consisted of no predator, one first-instar T. rutilus, two fourth-instar C. appendiculata, or a combination of one first-instar T. rutilus and two fourth-instar C. appendiculata. Although this does not allow for direct testing of substitutable effects (Sih et al. 1998), in pilot experiments, cannibalism occurred between two first-instar T. rutilus larvae within hours, even in the presence of alternative prey, suggesting no difference between one or two larvae. Further, life table analyses of this species in natural treeholes revealed the highest risk of death in the first instar, primarily attributable to cannibalism (Campos and Lounibos 2000b). Two C. appendiculata were used because treeholes often contain 5–15 third- and fourth-instar larvae (Lounibos 1983). Forty-eight hours before the experiment, fourth-instar C. appendiculata larvae that had molted from the third instar within the previous 24 hours were fed aquatic nematodes ad libitum and then starved for 24 hours before the experiment began. T. rutilus eggs were obtained from laboratory colonies, and larvae of this species were less than 10 hours old and unfed at the start of the experiment.
Containers were brought back to the lab daily, censused, and subsequently replaced in the field. At each census period, prey were identified to species using a dissecting microscope and the number and instar of surviving larvae of each species were recorded, the latter determined by comparative head capsule widths. Prey immatures were left in their containers until they emerged as adults, which were removed by aspiration and frozen. After the final emergence, thawed adults were dried at 65 ° C for 48 hours and subsequently weighed to the nearest 0.001 mg on a microbalance.
Laboratory experiment
This experiment was designed to determine the effects of multiple predators with changes in prey density and a higher density of the intermediate predator. Conditions were similar to the field experiment, except conducted in a laboratory. The cups were maintained during experiments at 25 ± 1 ° C with a 14:10 light:dark cycle and relative humidity (RH) of ~ 70%. Each container received equal numbers of first-instar A. albopictus and first-instar O. triseriatus from locally derived F1 and F2 colonies of these species (Lounibos et al. 2001) with total densities of 100, 200, or 400 per container. Treatments consisted of no predator, one first-instar T. rutilus, 10 fourth-instar C. appendiculata, or a combination of one first-instar T. rutilus and 10 fourth-instar C. appendiculata. After 24 hours, predators were removed and the numbers of prey surviving were determined.
Statistical analysis
Daily mean proportion of total prey (both species combined) surviving was calculated for the field experiment, taking into account the number of adults emerging each day. These data were used to describe temporal variations in prey consumption. Mean development time, survivorship (proportion surviving), and dry mass of males and females per replicate were analyzed by one-way ANOVA using PROC GLM in SAS (SAS Institute 1989) for each prey species. Analysis of mass was separated by sex due to differential responses of males and females to predation and competition (Lounibos et al. 1993, Behhomme et al. 2003, Alto et al. 2005). Females of most mosquito species emerge larger and later than their male counterparts because of increased nutritional requirements to produce eggs. However, development time for males and females was combined since they responded similarly to all treatments and pooling them increased the number of replicates with adults. Some data did not meet assumptions of normality or homogeneity of variance and were thus transformed. A. albopictus and O. triseriatus survivorship were arcsine transformed. A. albopictus development time was reciprocal transformed. When significant effects were found, all pairwise comparisons were made, correcting for experimentwise α = 0.05, by the Tukey-Kramer method (SAS Institute 1989).
A multiplicative risk model, described in Soluk and Collins (1988), was used to predict the combined survivorship of prey from single predator treatments. By log transforming survivorship data, the multiplicative model can be tested using ANOVA (Sih et al. 1998). Log-transformed survivorship data from the field experiment were analyzed by prey species for the first six days using a two-way repeated measures analysis of variance (PROC GLM). After six days, prey began to pupate and it became difficult to determine which species was consumed. Therefore, data for both prey species were combined and analyzed using the same method from the beginning of the experiment to day 14. Data from the laboratory experiment were analyzed using a two-way ANOVA (PROC GLM) on log-transformed data for each prey species. Nonadditivity was assessed by the significance of interactions. The number of each species consumed was analyzed using ANOVA (PROC GLM) to determine density effects.
Results
Field experiment
Prey responses
Combined predation over the duration of the experiment was predicted by the multiplicative model for prey species together (Fig. 1) as well as A. albopictus (Fig. 2A) and O. triseriatus (Fig. 2B) separately. Repeated-measures ANOVA did not show an interaction between the predators on prey survivorship for A. albopictus (F1,23 = 0.24, P = 0.629) or O. triseriatus (F1,23 = 0.29, P = 0.597) over the duration of the experiment. However, combined predation for A. albopictus on day 3 indicated a trend towards risk reduction (Fig. 2A).
Fig. 1.
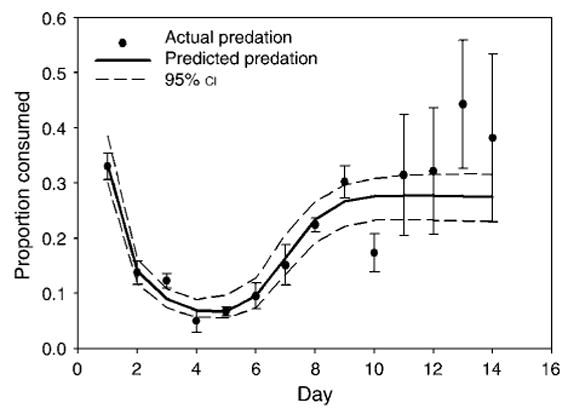
Predicted and actual (mean ± SE) per capita mortality of prey (Ochlerotatus triseriatus and Aedes albopictus, both species combined) in combined predator treatments. Predicted values were calculated from the single-predator treatments using the multiplicative risk model (Soluk and Collins 1988).
Fig. 2.
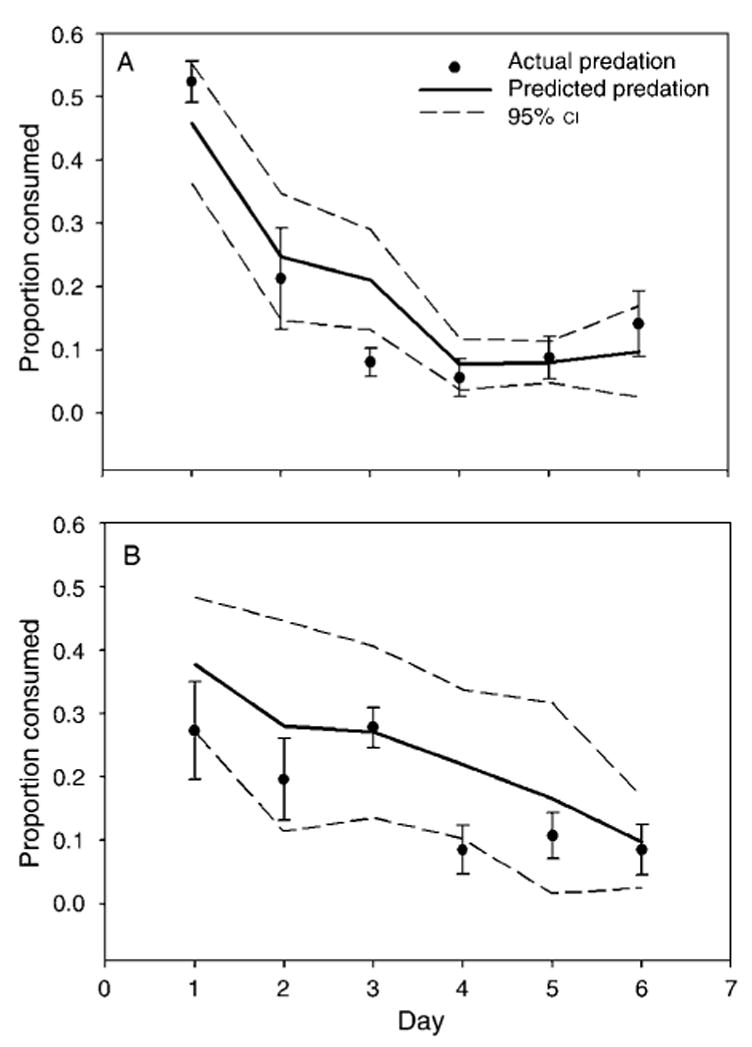
Predicted and actual (mean ± SE) per capita mortality of prey in combined predator treatments for (A) A. albopictus and (B) O. triseriatus in the field. Predicted values were calculated from the single-predator treatments using the multiplicative risk model (Soluk and Collins 1988).
Daily mean proportion of prey surviving indicates differential effects of predator species over time (Fig. 3). There was a significant effect of predation on A.albopictus mean survivorship (F3,23 = 19.16, P < 0.0001; Fig. 4A). Mean survivorship was significantly greater for the treatment without predators compared to those with predators. Mean survivorship was significantly greater for A. albopictus exposed only to C. appendiculata compared to those exposed to T. rutilus. There was no significant difference between mean survivorship when exposed to T. rutilus alone and T. rutilus with C. appendiculata (Fig. 4A).
Fig. 3.
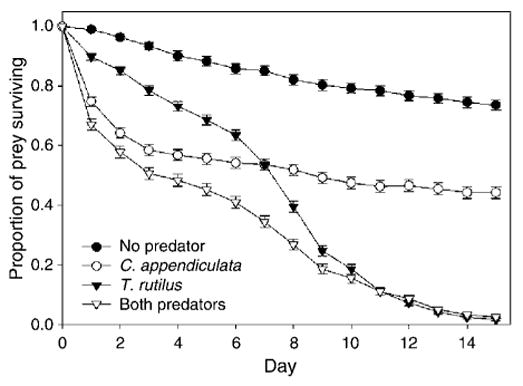
Daily proportion of total prey (A. albopictus and O. triseriatus combined) surviving (mean ± SE) in three predator treatments (Toxorhynchites rutilus, Corethrella appendiculata, and both predators) and in the absence of predators. Curves end when all prey have been consumed, have died, or have emerged as adults.
Fig. 4.
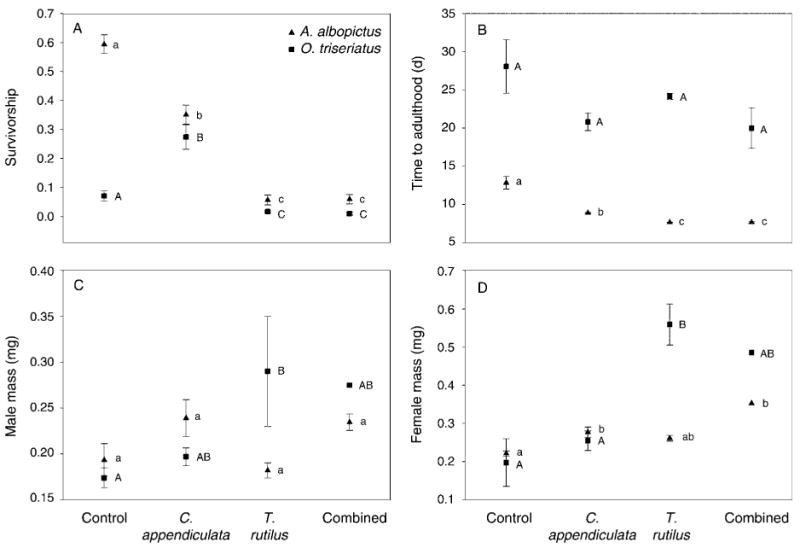
Mean (A) survivorship (proportion of the original number of larvae surviving to adulthood), (B) time to adulthood, and (C, D) dry mass of A. albopictus and O. triseriatus (C) males and (D) females. The absence of a common lowercase (A. albopictus) or uppercase (O. triseriatus) letter beside a mean indicates significant differences between predator treatments resulting from pairwise comparisons (P < 0.01) for A. albopictus and O. triseriatus, respectively.
There was a significant effect of predation on O. triseriatus mean survivorship (F3,23 = 18.50, P < 0.001). Mean survivorship was significantly greater when exposed to C. appendiculata alone than any other treatment, including the treatment with no predator. Mean survivorship with C. appendiculata was twice as high as when no predator was present. There was no significant difference in mean survivorship between exposure to T. rutilus alone and exposure to T. rutilus and C. appendiculata together (Fig. 4A).
There were no significant effects of predator treatment on time to adulthood for O. triseriatus (F3,19 = 2.41, P = 0.098; Fig. 4B), but the predator effect on mean time to adulthood was significant for A. albopictus (F3,22 = 56.27, P < 0.0001). All of the predator treatments resulted in significantly less mean time to adulthood than the treatments without predators. Prey that survived treatments with T. rutilus took significantly less time to complete development than those with C. appendiculata (Fig. 4B).
Predators had a marginally significant effect on mean mass of A. albopictus adult males (F3,21 = 3.05, P = 0.051). In general, males emerging from treatments with only T. rutilus were smaller than those from any other treatment (Fig. 4C). There were significant effects of predators on mean mass of A. albopictus adult females (F3,14 = 9.92, P < 0.001) (Fig. 4D). Females emerging from treatments with C. appendiculata were significantly larger in mean mass than those in treatments without predators. There were significant effects of predators on mean mass of O. triseriatus adult males (F3,12 = 6.98, P < 0.006) and females (F3,10 = 14.48, P < 0.001). Males and females in treatments with T. rutilus emerged significantly larger than those in treatments without predators (Fig. 4C, 4D). Average instar was similar among all treatments until day 8 when prey in treatments with T. rutilus (3.69 ± 0.03 [mean ± SE], n = 98) were generally smaller than those with only C. appendiculata (4.02 ± 0.007, n = 49).
Predator response
In treatments with only C. appendiculata, 86% of the C. appendiculata larvae survived to adulthood (two died as pupae), while in treatments with C. appendiculata and T. rutilus, none of the C. appendiculata larvae survived to adulthood. Thus, mean survivorship of C. appendiculata was significantly greater in treatments without T. rutilus (t6 = 9.3, P < 0.0001). When T. rutilus was present, C. appendiculata survived an average of 5.17 ± 0.87 d (mean ± SE, n = 12). At this point the average instar of T. rutilus was 2.75 ± 0.28 (n = 6) and, ~ 40% of the mosquito prey remained. Although none of the T. rutilus larvae died, a few did not pupate because the number of prey available was insufficient. In treatments without C. appendiculata, six out of the seven T. rutilus larvae pupated, while in the treatments with C. appendiculata four out of the six T. rutilus larvae pupated. T. rutilus in treatments without C. appendiculata took an average of 18.67 ± 0.49 d to pupate, while those in treatments with C. appendiculata took an average of 20.25 ± 0.63 d to pupate, which times were not significantly different (t6 = 1.98, P = 0.095).
Laboratory experiment
Both C. appendiculata (F1,43 = 738.49, P < 0.0001) and T. rutilus (F1,43 = 15.10, P = 0.0003) significantly reduced survivorship of A. albopictus. Furthermore, the combined effects of predators resulted in risk reduction for A. albopictus (F1,43 = 13.95, P = 0.0005, Fig. 5A), primarily at low prey densities. C. appendiculata significantly reduced survivorship of O. triseriatus (F1,43 = 195.57, P < 0.0001), but T. rutilus did not (F1,43 = 0.27, P = 0.6049). The combined effects of predators were predicted by a multiplicative model for O. triseriatus (F1,43 = 1.52, P = 0.225, Fig. 5B).
Fig. 5.
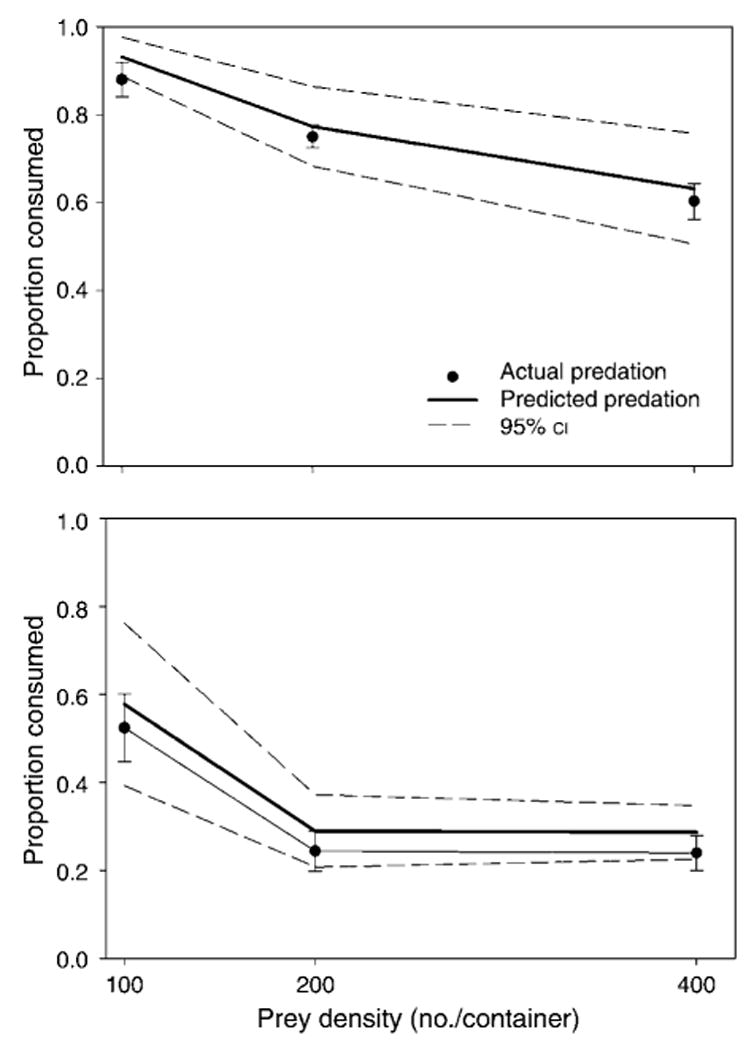
Predicted and actual (mean ± SE) per capita mortality of prey in combined predator treatments at three prey densities for (A) A. albopictus and (B) O. triseriatus in the laboratory. Predicted values were calculated from the single-predator treatments using the multiplicative risk model (Soluk and Collins 1988).
Two-way ANOVA showed that C. appendiculata consumed a greater number of A. albopictus (F2,43 = 74.59, P < 0.0001) and O. triseriatus (F2,43 = 9.04, P = 0.0005) as prey density increased. T. rutilus consumed similar numbers of A. albopictus (F2,43 = 0.04, P = 0.9572) and O. triseriatus (F2,43 = 0.37, P = 0.6942) as densities increased. However, interactions among predators did not change with density for A. albopictus (F2,43 = 2.42, P = 0.1013) and O. triseriatus (F2,43 = 0.31, P = 0.7345).
Discussion
Complex interactions in food webs are expected to result in nonlinearities in species interactions (Werner and Peacor 2003). Both predator and prey may modify behavior in the presence of an additional species, changing the magnitude or direction of the interaction (interaction modification sensu Wootton 1994). Thus, predicting interactions in food webs from pairwise interactions may not be possible or may yield misleading results (Yodzis 1988). However, our study suggests the ability to predict the combined effect of two predators from their independent effects, even in the presence of IGP, changes in size structure, and exploitative competition. Although behavior was not quantified, O. triseriatus is known to reduce activity levels in the presence of T. rutilus (Kesavaraju and Juliano 2004), creating additional complexity. Adaptive prey behavior should also change the effect of predators on prey, resulting in nonlinear interactions (Schmitz and Booth 1997). However, Sokol-Hessner and Schmitz (2002) found additive effects in a terrestrial system with complex interactions including adaptive behavior and habitat partitioning of predators. Thus, mechanisms leading to additive effects will need to be determined when negative and positive direct/indirect interactions confound the complexity of the system.
Size structure may have played a key role in the occurrence of additive effects. T. rutilus larvae were small at the onset of the field experiment and could not consume large numbers of prey. However, C. appendiculata consumed most of the prey at the start of this experiment but later on were too small to consume the larger prey. Thus, at any point in time, the combined effect of predation may have depended primarily on one predator species. Additive effects are thought to occur only when both predators have significant impacts on the prey at the same point in time (Sih et al. 1998). Further, both predators forage using a sit and wait technique, which indicates similar functional roles in the community, potentially leading to additive effects (Sih et al. 1998). However, the occurrence of IGP and preference for A. albopictus (Griswold and Lounibos 2005) suggests interference among the predators, which may have been a function of prey density and container size. Although additive effects occurred over the length of the field experiment, risk-reduction occurred on day 3 (Fig. 2A), day 10 (Fig. 1), and in the laboratory experiment at increased densities of C. appendiculata (Fig. 5A) for the preferred prey species. It should be noted that the appearance of risk-reduction and risk-enhancement (Fig. 1) after day 8 are likely to be artifacts of variations in individual predators and low prey densities as the intermediate predator had been consumed by this time. Thus, the lowered densities in treatments with C. appendiculata compared to those without may have altered encounter rates between the top predator and prey. The presence of risk-reduction in the two other cases suggests predators were competing for the preferred resource as interference primarily occurred at lower prey densities. Soluk (1993) also demonstrated interference between sculpins and stone-flies for a preferred prey species, attributable to changes in habitat selection and behavior, since stoneflies serve as prey for sculpin. Further, Finke and Denno (2004) found that increases in predator diversity dampen predation and trophic cascades through IGP, increasing prey density and decreasing basal resources. Asymmetric IGP is known to result in risk reduction (Wissinger and McGrady 1993), especially when IG prey change behavior in response to IG predators. Risk reduction also occurs when the total number of predators is reduced due to IGP, lessening predation on the prey (Relyea and Yurewicz 2002). However, Wissinger (1999) suggested that the IG prey may not modify their behavior in the presence of IG predators if they are pressured to develop fast as occupants of ephemeral treeholes. Furthermore, an increase in predator density typically leads to decreased prey density, but may not increase the behavioral response of the prey since prey are already responding to the presence of a predator (Werner and Peacor 2003). This is especially true in closed aquatic systems where many prey detect predators by chemical cues (e.g., Kesavaraju and Juliano 2004).
Although combined predation rates were predicted based on the independent effects of predators, changes in community structure varied with predator species. C. appendiculata changed species composition by preferentially consuming A. albopictus, while T. rutilus reduced prey density, irrespective of species. Similar outcomes have been observed in freshwater systems with amphibians, where the top predator regulated abundance and the intermediate predator influenced species composition (Morin 1983, Fauth and Resetarits 1991). However, when both predators were together, prey survival rates were similar to treatments with only T. rutilus, indicating that the presence of the intermediate predator does not affect the ability of the top predator to depress prey abundance. Thus, elimination of the intermediate predator by the top predator did not benefit the basal prey species, possibly due to initial low densities of the intermediate predator. Although the two predators had separate effects on the prey, both may have decreased interspecific competition between A. albopictus and O. triseriatus by reducing the numbers of prey, as indicated by the decreased development time of A. albopictus in the presence of predators and larger O. triseriatus adults emerging from treatments with only T. rutilus. Contrary to some communities whose functionally redundant species may play similar ecological roles (e.g., Morin 1995), our treehole community harbors predator species with very different roles in structuring prey communities. Strong interactions between predators are expected to relax the overall effects of predators on their prey (Finke and Denno 2002). This was not the case in our study as the IG predator consumed the IG prey, but did not change the overall prey survivorship.
Although our study utilized container volumes within the range found in nature, some of the inherent habitat complexity found in treeholes may have been excluded. Treeholes are lined with crevices that may provide refuge for prey and typically have leaf litter penetrating a large portion of the water column. Our containers were smooth and only had detritus covering the bottom, which may better resemble artificial containers found in the field (e.g., discarded tires), also colonized by this same community. The mean developmental times of O. triseriatus in our field experiment ( ~ 20–30 d) fell within the ranges of other seminatural experiments on this species in treeholes (29–71 d; Leonard and Juliano 1995) and discarded tires (12–16 d; Lounibos et al. 1993).
The effects of habitat complexity on multiple predator effects are variable. Facilitation between bluegills and dragonflies only occurred at low levels of habitat complexity (Swisher et al. 1998). Furthermore Harvey et al. (2004) found that increasing the amount of cover 10-fold did not affect predation rates. Other studies have found that habitat complexity interferes with foraging and changes the encounter rate, and may be especially important when predators differentially select habitat patches (Finke and Denno 2002, Corkum and Cronin 2004). However, changes in habitat structure in the form of artificial leaves did not affect predation by C. appendiculata and T. rutilus (Alto et al. 2005). Thus, it is likely additive effects would have occurred even in the presence of additional complexity.
Although studies focusing on multiple predators are now more common in the literature (Sih et al. 1998), only a few have been conducted in container habitats (Lounibos 1983, 1985, Fincke 1999). Since predator guilds in these systems typically consist of only a few species, community manipulations are rather simple compared to other large-scale aquatic systems. Although this study did not include other prey species found in treeholes (e.g., midge larvae), it focused on the two primary insect predators and the two most common nonbenthic prey species in Florida, as well as a natural basal resource within a scale found in nature. Models of community dynamics attempt to use the least amount of information on species interactions to adequately portray the system as a whole (Levin 1992). Our study suggests that even when a multiplicative model adequately accounts for the effects of multiple predator species, more complex interactions such as IGP may underlie the patterns. Thus, data fitting multiplicative models should be interpreted with caution unless the magnitude and direction of indirect effects between predator and prey are well known. Previous studies of multiple predators in aquatic systems suggest that it may be difficult to generalize about predator–predator interactions among habitats and predator and prey types because of the variable nature of interference and additive and synergistic interactions among predators (Sih et al. 1998). However, our results corroborate others showing that invertebrate ambush predators in temporary freshwater habitats have additive effects on prey (e.g., Van Buskirk 1988). The ability to generalize predator-predator interactions may be facilitated by studies of communities with few predators as a building block for more complex systems.
Acknowledgments
Thanks to R. Escher and J. Butler for providing prey larvae and insectary space, respectively. Previous drafts of the manuscript were improved by comments from B. Alto, A. Ellis, S. Juliano, E. Preisser, and J. Rey. Research supported in part by NIH grant R01 AI-44793. This is University of Florida Experiment Station Journal Series No. R-10859.
Literature Cited
- Alto BW, Griswold MW, Lounibos LP. Habitat complexity and sex-dependent predation of mosquito larvae in containers. Oecologia. 2005;146:300–310. doi: 10.1007/s00442-005-0198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera R. Competition and resistance to starvation in larvae of container-inhabiting Aedes mosquitoes. Ecological Entomology. 1996;21:117–127. [Google Scholar]
- Behhomme S, Agnew P, Sidobre C, Michalakis Y. Sex-specific reaction norms to intraspecific larval competition in the mosquito Aedes aegypti. Journal of Evolutionary Biology. 2003;16:721–730. doi: 10.1046/j.1420-9101.2003.00576.x. [DOI] [PubMed] [Google Scholar]
- Bradshaw WE, Holzapfel CM. Seasonal development of tree-hole mosquitoes (Diptera: Culicidae) and Chaoborids in relation to weather and predation. Journal of Medical Entomology. 1984;21:366–378. [Google Scholar]
- Campos RE, Lounibos LP. Natural prey and digestion times of Toxorhynchites rutilus (Diptera: Culicidae) in southern Florida. Annals of the Entomological Society of America. 2000a;93:1280–1287. [Google Scholar]
- Campos RE, Lounibos LP. Life tables of Toxorhynchites rutilus (Diptera: Culicidae) in nature in southern Florida. Journal of Medical Entomology. 2000b;37:385–392. doi: 10.1093/jmedent/37.3.385. [DOI] [PubMed] [Google Scholar]
- Corkum LD, Cronin DJ. Habitat complexity reduces aggression and enhances consumption in crayfish. Journal of Ethology. 2004;22:23–27. [Google Scholar]
- Diehl S, Eklov P. Effects of piscivore-mediated habitat use on resources, diet, and growth of perch. Ecology. 1995;76:1712–1726. [Google Scholar]
- Fauth JE, Resetarits WJ. Interactions between the salamander Siren intermedia and the keystone predator Notophthalmus viridescens. Ecology. 1991;72:827–838. [Google Scholar]
- Fincke OM. Organization of predator assemblages in Neotropical tree holes: effects of abiotic factors and priority. Ecological Entomology. 1999;24:13–23. [Google Scholar]
- Finke DL, Denno RF. Intraguild predation diminished in complex-structured vegetation: implications for prey suppression. Ecology. 2002;83:643–652. [Google Scholar]
- Finke DL, Denno RF. Predator diversity dampens trophic cascades. Nature. 2004;429:407–410. doi: 10.1038/nature02554. [DOI] [PubMed] [Google Scholar]
- Focks DA, Dame DA, Cameron AL, Boston MD. Predator–prey interaction between insular populations of Toxorhynchites rutilus rutilus (Diptera, Culicidae) and Aedes aegypti (Diptera, Culicidae) Environmental Entomology. 1980;9:37–42. [Google Scholar]
- Grabham M. A new Corethrella from Jamaica. Entomological News. 1906;17:343–345. [Google Scholar]
- Griswold MW, Lounibos LP. Does differential predation allow invasive and native mosquito larvae to coexist in Florida? Ecological Entomology 30:122–127. 2005 doi: 10.1111/j.0307-6946.2005.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey BC, White JL, Nakamato RJ. An emergent multiple predator effect may enhance biotic resistance in a stream fish assemblage. Ecology. 2004;85:127–133. [Google Scholar]
- Hawley WA. The biology of Aedes albopictus. Journal of the American Mosquito Control Association. 1988;4(supplement):1–39. [PubMed] [Google Scholar]
- Jenkins DW, Carpenter SJ. Ecology of the treehole breeding mosquitoes of nearctic North America. Ecological Monographs. 1946;16:33–47. [Google Scholar]
- Juliano SA, Reminger L. The relationship between vulnerability to predation and behavior of larval treehole mosquitoes—geographic and ontogenetic differences. Oikos. 1992;63:465–476. [Google Scholar]
- Kesavaraju B, Juliano SA. Differential behavioral responses to water-borne cues to predation in two container dwelling mosquitoes. Annals of the Entomological Society of America. 2004;97:194–201. doi: 10.1603/0013-8746(2004)097[0194:dbrtwc]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léonard PM, Juliano SA. Effect of leaf-litter and density on fitness and population performance of the treehole mosquito Aedes triseriatus. Ecological Entomology. 1995;20:125–136. [Google Scholar]
- Levin SA. The problem of pattern and scale in ecology. Ecology. 1992;73:1943–1967. [Google Scholar]
- Livdahl TP, Willey MS. Prospects for an invasion—competition between Aedes albopictus and native Aedes triseriatus. Science. 1991;253:189–191. doi: 10.1126/science.1853204. [DOI] [PubMed] [Google Scholar]
- Lounibos LP. The mosquito community of treeholes in subtropical Florida. In: Frank JH, Lounibos LP, editors. Phytotelmata: terrestrial plants as hosts for aquatic insect communities. Plexus, Medford; New Jersey, USA: 1983. pp. 223–246. [Google Scholar]
- Lounibos LP. Interactions influencing production of treehole mosquitoes in south Florida. In: Lounibos LP, Rey JR, Frank JH, editors. Ecology of mosquitoes: proceedings of a workshop. Florida Medical Entomology Laboratory; Vero Beach, Florida, USA: 1985. pp. 65–77. [Google Scholar]
- Lounibos LP, Nishimura N, Escher RL. Fitness of a treehole mosquito—influences of food type and predation. Oikos . 1993;66:114–118. [Google Scholar]
- Lounibos LP, O’Meara GF, Escher RL, Nishimura N, Cutwa M, Nelson T, Campos RE, Juliano SA. Testing predictions of displacement of native Aedes by the invasive Asian Tiger Mosquito Aedes albopictus in Florida, USA. Biological Invasions. 2001;3:151–166. [Google Scholar]
- Morin PJ. Predation, competition, and the composition of larval anuran guilds. Ecological Monographs. 1983;53:119–138. [Google Scholar]
- Morin PJ. Functional redundancy, non-additive interactions, and supply-side dynamics in experimental pond communities. Ecology. 1995;76:133–149. [Google Scholar]
- Nystrom P, Svensson O, Lardner B, Bronmark C, Graneli W. The influence of multiple introduced predators on a littoral pond community. Ecology. 2001;82:1023–1039. [Google Scholar]
- O’Meara GF, Evans LF, Gettman AD, Cuda JP. Spread of Aedes albopictus and decline of Aedes aegypti (Diptera, Culicidae) in Florida. Journal of Medical Entomology. 1995;32:554–562. doi: 10.1093/jmedent/32.4.554. [DOI] [PubMed] [Google Scholar]
- Peckarsky BL, McIntosh AR. Fitness and community consequences of avoiding multiple predators. Oecologia. 1998;113:565–576. doi: 10.1007/s004420050410. [DOI] [PubMed] [Google Scholar]
- Polis GA, Myers CA, Holt RD. The ecology and evolution of intraguild predation—potential competitors that eat each other. Annual Review of Ecology and Systematics. 1989;20:297–330. [Google Scholar]
- Relyea RA, Yurewicz KL. Predicting community outcomes from pairwise interactions: Integrating density-and trait-mediated effects. Oecologia. 2002;131:569–579. doi: 10.1007/s00442-002-0910-z. [DOI] [PubMed] [Google Scholar]
- Russo RJ. The functional-response of Toxorhynchites rutilus rutilus (Diptera, Culicidae), a predator on container-breeding mosquitoes. Journal of Medical Entomology. 1983;20:585–590. doi: 10.1093/jmedent/20.4.383. [DOI] [PubMed] [Google Scholar]
- SAS Institute. SAS/STAT user’s guide. SAS Institute; Cary, North Carolina, USA: 1989. [Google Scholar]
- Schmitz OJ, Booth G. Modelling food web complexity: the consequences of individual-based, spatially explicit behavioural ecology on trophic interactions. Evolutionary Ecology. 1997;11:379–398. [Google Scholar]
- Sih A, Krupa JJ. Direct and indirect effects of multiple enemies on water strider mating dynamics. Oecologia. 1996;105:179–188. doi: 10.1007/BF00328544. [DOI] [PubMed] [Google Scholar]
- Sih A, Englund G, Wooster D. Emergent impacts of multiple predators on prey. Trends in Ecology and Evolution. 1998;13:350–355. doi: 10.1016/s0169-5347(98)01437-2. [DOI] [PubMed] [Google Scholar]
- Sokol-Hessner L, Schmitz OJ. Aggregate effects of multiple predator species on a shared prey. Ecology. 2002;83:2367–2372. [Google Scholar]
- Soluk DA. Multiple predator effects: predicting combined functional response of stream fish and invertebrate predators. Ecology. 1993;74:219–225. [Google Scholar]
- Soluk DA, Collins NC. Synergistic interactions between fish and stoneflies—facilitation and interference among stream predators. Oikos. 1988;52:94–100. [Google Scholar]
- Steffan WA, Evenhuis NL. Biology of Toxorhynchites. Annual Review of Entomology. 1981;26:159–181. [Google Scholar]
- Swisher BJ, Soluk DA, Wahl DH. Nonadditive predation in littoral habitats: influences of habitat complexity. Oikos. 1998;81:31–37. [Google Scholar]
- Van Buskirk J. Interactive effects of dragonfly predation in experimental pond communities. Ecology. 1988;69:857–867. [Google Scholar]
- Walker ED, Merritt RW. Behavior of larval Aedes triseriatus (Diptera: Culicidae) Journal of Medical Entomology. 1991;28:581–589. doi: 10.1093/jmedent/28.5.581. [DOI] [PubMed] [Google Scholar]
- Werner EE. Individual behavior and higher-order species interactions. American Naturalist. 1992;140:S5–S32. [Google Scholar]
- Werner EE, Peacor SD. A review of trait-mediated indirect interactions. Ecology. 2003;84:1083–1100. [Google Scholar]
- Wilbur HM. Regulation of structure in complex systems: experimental temporary pond communities. Ecology. 1987;68:1437–1452. [Google Scholar]
- Wilbur HM. Interactions between growing predators and growing prey. In: Ebenman B, Persson L, editors. Size-structured populations. Springer-Verlag; Berlin, Germany, USA: 1988. pp. 157–172. [Google Scholar]
- Wissinger SA. Ecology of wetland invertebrates: synthesis and application for conservation and management. In: Batzer DP, Rader RB, Wissinger SA, editors. Invertebrates in freshwater wetlands of North America: ecology and management. Wiley and Sons; New York, New York, USA: 1999. pp. 1043–1086. [Google Scholar]
- Wissinger SA, McGrady J. Intraguild predation and interference competition between larval dragonflies: direct and indirect effects on shared prey. Ecology. 1993;74:207–218. [Google Scholar]
- Wootton JT. The nature and consequences of indirect effects. Annual Review of Ecology and Systematics. 1994;25:443–466. [Google Scholar]
- Yodzis P. The indeterminacy of ecological interactions as perceived through perturbation experiments. Ecology. 1988;69:508–515. [Google Scholar]


