Abstract
Reactive oxygen species (ROS) are generated as by-products of cellular metabolism, primarily in the mitochondria. Although ROS are essential participants in cell signaling and regulation, when their cellular production overwhelms the intrinsic antioxidant capacity, damage to cellular macromolecules such as DNA, proteins, and lipids ensues. Such a state of “oxidative stress” is thought to contribute to the pathogenesis of a number of neurodegenerative diseases. Growing evidence supports the involvement of oxidative stress as a common component of glaucomatous neurodegeneration in different subcellular compartments of retinal ganglion cells (RGCs). Besides the evidence of direct cytotoxic consequences leading to RGC death, it also seems highly possible that ROS are involved in signaling RGC death by acting as a second messenger and/or modulating protein function by redox modifications of downstream effectors through enzymatic oxidation of specific amino acid residues. Different studies provide cumulating evidence, which supports the association of ROS with different aspects of the neurodegenerative process. Oxidative protein modifications during glaucomatous neurodegeneration increase neuronal susceptibility to damage and also lead to glial dysfunction. Oxidative stress-induced dysfunction of glial cells may contribute to spreading neuronal damage by secondary degeneration. Oxidative stress also promotes the accumulation of advanced glycation end products in glaucomatous tissues. It is also evident that oxidative stress takes part in the activation of immune response during glaucomatous neurodegeneration, as ROS stimulate the antigen presenting ability of glial cells and also function as co-stimulatory molecules during antigen presentation. By discussing current evidence, this review provides a broad perspective on cellular mechanisms and potential consequences of oxidative stress in glaucoma.
1. Brief introduction to oxidative stress
Partially reduced metabolites of molecular oxygen (O2) are referred to as “reactive oxygen species” (ROS) due to their higher reactivities relative to molecular O2. ROS are generated intracellularly through a variety of processes, for example, as by-products of normal aerobic metabolism and as second messengers in various signal transduction pathways. ROS can also be derived from exogenous sources, either being taken up directly by cells from the extracellular milieu, or produced as a consequence of the cell's exposure to some environmental insult.
Any electron-transferring protein or enzymatic system can result in the formation of ROS as by-products of cellular electron transfer reactions. However, mitochondria constitute the greatest source of ROS since the mitochondrial electron transport consumes approximately 85% of the O2 that the cell uses. Aerobic energy metabolism is dependent on oxidative phosphorylation, a process by which the oxidoreduction energy of mitochondrial electron transport is converted to the high-energy phosphate bond of ATP. Production of mitochondrial ROS occurs primarily by impairment of the electron transfer chain at the flavin mononucleotide group of complex I (NADH-ubiquinone oxidoreductase) or at ubiquinone site of complex III (ubiquinone-cytochrome c oxidoreductase). When the electron transport chain is inhibited, the electrons accumulate in the early stages of the electron transport chain. O2 serves as the final electron acceptor for cytochrome c oxidase, the terminal enzymatic component of the mitochondrial enzymatic complex, which catalyzes the electron reduction of O2 to H2O. Partially reduced and highly reactive metabolites of O2 formed during these electron transfer reactions include superoxide anion (O2−·) and hydrogen peroxide (H2O2), formed by one- and two-electron reductions of O2, respectively. In the presence of transition metal ions, the even more reactive hydroxyl radical (OH·) can be formed (Finkel and Holbrook, 2000; Starkov et al., 2004). Once generated, ROS may further impair mitochondrial electron transport and enhance ROS production. Besides the mitochondrial source, ROS can also be generated by specialized plasma membrane oxidases in normal physiological signaling by growth factors and cytokines. In addition, interaction of the superoxide radical with nitric oxide that is produced by constitutive and inducible nitric-oxide synthases forms peroxynitrite, which is a potent secondary oxidant (Murphy, 1999).
Several of these species are produced at low levels during normal physiological conditions. Controlled production of ROS and regulated redox modifications of transcription factors or enzymes are an essential part of signal transduction pathways serving important regulatory functions. However, when present at high and/or sustained levels, ROS can cause severe damage to mitochondrial and cellular proteins, lipids, and nucleic acids. In addition to the detrimental effects to cellular integrity, ROS may inhibit complex enzymes in the electron transport chain of the mitochondria, resulting in a shutdown of mitochondrial energy production. Thus, the apparent paradox in the roles of ROS as essential biomolecules in the regulation of cellular functions or as toxic by-products of metabolism is related in part to differences in the concentrations of ROS produced. To protect against the potentially damaging effects of ROS, cells possess several antioxidant enzymes such as superoxide dismutase (which reduces O2−·· to H2O2), catalase, glutathione peroxidase (which reduces H2O2 to H2O), and small molecule substances such as vitamins C and E. Unfortunately, the antioxidant defense mechanisms are not always adequate to counteract the production of ROS. When ROS generation exceeds the cell's antioxidant capacity to prevent oxidative injury, they create a constant threat to cells leading to a condition termed “oxidative stress”, which has been implicated in a large number of human diseases (Finkel, 1998; Suzukawa et al., 2000).
Electrical excitability and the structural and synaptic complexity of neurons present unusual demands upon cellular systems that produce or respond to ATP and ROS. The central nervous system indeed exhibits a distinct susceptibility to oxidative stress due to its enhanced metabolic rate with high levels of O2 utilization and ATP synthesis, higher lipid content, reduced capacity for cellular regeneration, and chemical reactions involving dopamine oxidation and glutamate. Besides the increased risk factors for the generation of elevated levels of ROS, the brain may also suffer from an inadequate defense system against oxidative stress. Consequently, oxidative damage has been implicated in a wide variety of neurodegenerative diseases, as well as degenerative processes of aging (Andersen, 2004; Potashkin and Meredith, 2006). Importance of oxidative damage for glaucomatous neurodegeneration has also becoming increasingly apparent over the past few years. This review summarizes the current evidence supporting the association of oxidative stress with glaucomatous neurodegeneration by discussing known cellular mechanisms and potential consequences of ROS generation in glaucoma.
2. Oxidative injury during glaucomatous neurodegeneration
2.1. Tissue stress in the glaucomatous optic nerve head and retina
Progressive loss of optic nerve axons and retinal ganglion cells (RGCs) result in characteristic optic nerve atrophy and visual field defects in glaucoma patients. In many patients with glaucoma, intraocular pressure (IOP) is higher than the statistically normal limits; and extensive evidence supports that elevated IOP-initiated factors are important for the initiation and progression of neuronal damage in these patients. Current therapeutic management of glaucoma therefore aims to halt disease progression by reducing elevated IOP. However, although IOP lowering treatment can provide neuroprotection and retard the disease progression in many glaucoma patients, it is not always sufficient to fully prevent disease progression. This is just one reason that recent efforts have focused on the development of alternative treatment strategies for neuroprotection. In addition, IOP is not elevated in all of the eyes exhibiting characteristics of glaucomatous neurodegeneration. Similar clinical (Tezel et al., 1996) and histopathological findings (Wax et al., 1998a) in glaucomatous eyes either with elevated or normal IOP indicate that there is also an IOP-independent component of neuronal damage in glaucoma. Considerable evidence mostly from blood flow studies has illustrated that besides IOP, tissue hypoxia is also associated with the pathogenic mechanisms underlying glaucomatous neurodegeneration. Clinical evidence of vascular abnormalities in glaucoma patients include vasospasm, systemic hypotension, angiographic vascular perfusion defects, and alterations in blood flow parameters, which may result in reduced vascular perfusion in the optic nerve head and/or retina (Chung et al., 1999; Cioffi and Wang, 1999; Flammer, 1994; Flammer et al., 2002; Hayreh, 1978; Osborne et al., 1999). An hypoxic tissue stress in glaucomatous eyes, which is thought to develop secondary to or independent from the elevated IOP, may adversely affect neuronal survival by inducing cell death (Gross et al., 1999; Kitano et al., 1996; Osborne et al., 2004; Rosenbaum et al., 1997; Tezel and Wax, 1999; Tezel and Wax, 2000a).
Recently identified molecular mechanisms responsible for the expression of hypoxia-induced genes and proteins provide new opportunities to better understand the hypoxic component of the neurodegenerative process in glaucoma. For example, hypoxia-inducible factor-1alpha (HIF-1α), an O2-regulated transcriptional activator, has been identified to function as a master regulator of O2 homeostasis. The expression and activity of this protein is tightly regulated by cellular O2 concentration and exponentially increases as cells are exposed to decreasing O2 concentrations (Jiang et al., 1996). Under hypoxic conditions, HIF-1α activates the transcription of a broad variety of genes, including those encoding erythropoietin, glucose transporters, glycolytic enzymes, vascular endothelial growth factor, inducible nitric oxide synthase, heme oxygenase 1, and other genes whose protein products increase O2 delivery or facilitate the metabolic adaptation to hypoxia (Guillemin and Krasnow, 1997; Iyer et al., 1998; Semenza, 1999; Wang et al., 1995). To assess hypoxic tissue stress in the glaucomatous optic nerve head and retina, we have determined the localization of HIF-1α expression in human donor eyes using immunohistochemistry (Tezel and Wax, 2004a). This histopathological study has revealed an increased immunolabeling of the glaucomatous optic nerve head and retina for HIF-1α compared with age-matched controls. Findings of this study have also revealed that the retinal location of the increased HIF-1α immunolabeling in glaucomatous eyes is closely concordant with the location of visual field defects recorded in these donors. Since the regions of HIF-1α induction represent the areas of decreased blood flow and decreased O2 delivery, these findings provide evidence that hypoxic tissue stress is present in glaucomatous eyes and is a likely component of the pathogenic mechanisms of neurodegeneration.
The increased HIF-1α immunolabeling in a diverse sample of glaucomatous donor eyes (Tezel and Wax, 2004a) supports that a sustained hypoxic insult, or possibly recurrent episodes of tissue hypoxia, is present in these eyes. Obviously, the glaucomatous tissue stress, initiated by either elevated IOP and/or hypoxia, is widespread and includes not only the optic nerve head but also the retina of glaucomatous eyes. Another important feature of tissue stress in glaucoma is its chronic nature. Widespread and persisting upregulation of different stress proteins in glaucomatous human eyes (Tezel et al., 2000b), as also observed in cell cultures exposed to glaucomatous stimuli (Tezel and Yang, 2005), support that optic nerve head and retina are under continuing stress in glaucomatous eyes. This cellular stress response is also consistent with the observation that glial cells located in the optic nerve head or retina undergo a long-term activation process in glaucomatous eyes (Tezel et al., 2003). The activated state of glial cells is a prominent feature important for their ultimate role as neurosupportive or neurodestructive during glaucomatous neurodegeneration (Tezel and Wax, 2003; Wax and Tezel, 2002).
Even if the elevated IOP is effectively lowered, which is not possible in all cases (Tezel et al., 2001c), stress conditions likely persist in many glaucomatous eyes due to accompanying hypoxia, ROS generation, glial activity, immune system involvement, and/or other mechanisms yet to be identified. The continuing nature of the glaucomatous tissue stress appears to be important in determining neuronal cell fate. Experimental studies indicate an early adaptive response following brief exposure to glaucomatous stimuli, including hypoxia. This is consistent with a phenomenon referred to as preconditioning. For example, it is known that neuronal cells exposed to a sublethal period of hypoxia become resistant to a subsequent period of lethal hypoxia (Bruer et al., 1997; Chen and Simon, 1997; Roth et al., 1998). Our ex vivo experiments have similarly demonstrated that brief preconditioning hypoxia induces HIF-1α expression in the retina, which accompanies the expression of adaptive proteins and provides resistance to cell death; however, exposure to hypoxia for a longer period initiates the cell death program (Tezel and Wax, 2001). These experiments have revealed that following exposure of isolated retinas to hypoxia for 1 hour, an early increase in the expression of HIF-1α is accompanied by an activation of intrinsic survival signals, such as nuclear factor-kappa B (NF-κB) and heat shock proteins, including HSP27 and HSP72. However, in isolated retinas exposed to hypoxia for 6 hours, p53 has been upregulated and caspases have been activated, instead of adaptive changes in protein expression. Thus, despite early adaptive modifications, sustained tissue stress in glaucomatous eyes can promote delayed neuronal cell death.
2.2. Oxidative component of the glaucomatous tissue stress
Increasing evidence supports that the glaucomatous tissue stress initiated by elevated IOP and/or tissue hypoxia has also involve an oxidative component. Experimental elevation of IOP induces oxidative stress in the retina. ROS are generated in the retina not only in retinal ischemia models induced by acute IOP elevation (Bonne et al., 1998; Muller et al., 1997), but also in experimental models induced by moderate and chronic elevation of IOP. For example, experimental elevation of IOP in rats using intraocular hyaluronic acid injections has resulted in a significant decrease in retinal antioxidants parallel to a time-dependent increase in retinal lipid peroxidation (Moreno et al., 2004). Another study using the cauterization of episcleral veins to induce IOP elevation has similarly demonstrated amplified ROS generation and increased lipid peroxidation in the ocular hypertensive rat retina (Ko et al., 2005). Our findings using the rat glaucoma model induced by hypertonic saline injections into episcleral veins have also revealed a prominent increase in protein carbonyl immunoreactivity after IOP elevation, which reflects the oxidative modification of proteins (Tezel et al., 2005). Our study has also demonstrated that the increased protein carbonyl immunoreactivity in ocular hypertensive retinas compared with controls is detectable mainly in the inner retinal layers. Double immunolabeling studies have shown that the protein carbonyl-positive cells in the inner retinal layers include brn-3-positive RGCs. These support that particularly the inner retinal layers, including RGCs, are prominently exposed to oxidative stress in ocular hypertensive eyes.
Hypoxia is also a prominent noxious stimulus leading to oxidative stress. Hypoxia-induced neuronal cell death has been associated with mitochondria through energy depletion, altered ionic homeostasis, and O2-sensing molecules, such as HIF-1α and NF-κB, eventually resulting in ROS generation (Banasiak et al., 2000; Halterman et al., 1999). Obviously, cells respond to graded hypoxia by increasing the generation of ROS; and mitochondria may function as an O2 sensor to mediate hypoxia-induced gene transcription (Chandel et al., 2000; Duranteau et al., 1998).
Additional supportive evidence for oxidative stress during glaucomatous neurodegeneration comes from studies in glaucoma patients. For example, in addition to elevated serum autoantibodies against heat shock proteins (Tezel et al., 1998; Wax et al., 1998b), which have crucial chaperone function, we have also detected autoantibodies against glutathione S-transferase (GST) in glaucomatous patient sera (Yang et al., 2001b). The GST, which belongs to a major group of detoxification enzymes, is regulated in vivo by ROS; and its induction by ROS appears to represent an adaptive response, since this enzyme detoxifies the toxic metabolites produced by oxidative stress (Hayes and Strange, 1995). Elevated titers of serum autoantibodies against GST, as well as autoantibodies to heat shock proteins, may therefore represent an immune response to the upregulation and/or increased exposure of these proteins in response to oxidative tissue stress in glaucomatous eyes. In addition, findings of a more recent study have demonstrated upregulated expression of iron-regulating proteins in the retina in an experimental monkey model of glaucoma and in human glaucoma (Farkas et al., 2004). Upregulation of these proteins with antioxidant properties further supports retinal oxidative stress in ocular hypertensive eyes.
2.3. Proposed pathogenic mechanisms of glaucomatous neurodegeneration associated with oxidative injury
Although RGCs exhibit unique characteristics of antioxidant defense mechanisms (Kortuem et al., 2000), survival of axotomized RGCs is critically sensitive to the oxidative redox state; and decreasing ROS generation promotes the survival of RGCs after axotomy (Geiger et al., 2002). Growing evidence also supports that the oxidative stress evident in glaucomatous tissues is an important factor for the loss of these neurons during glaucomatous neurodegeneration. As summarized in this section most of the proposed pathogenic mechanisms of glaucomatous neurodegeneration are well-known to include an oxidative component.
Histopathological studies of glaucomatous human donor eyes (Kerrigan et al., 1997; Wax et al., 1998a) and experimental studies using different animal models of glaucoma have demonstrated that while optic nerve axons are progressively lost in glaucomatous eyes, RGCs die through apoptosis (Garcia-Valenzuela et al., 1995; Li et al., 1999; Quigley et al., 1995). As summarized in Figure 1, multiple pathogenic mechanisms triggered by elevated IOP and/or tissue hypoxia have been associated with optic nerve degeneration and RGC apoptosis in glaucoma. A number of genetic susceptibility factors also support the multifactorial nature of the disease (Libby et al., 2005; Sarfarazi and Rezaie, 2003).
Figure 1.

Multiple pathogenic mechanisms have been proposed for glaucomatous neurodegeneration. Most of these mechanisms appear to be associated with a common pathway of oxidative injury. Although the initial site of glaucomatous injury is unclear, RGC survival and axon health are dependent on each other; and primary injury to any of these subcellular compartments subsequently affects the others.
One of the mechanisms proposed for RGC death in glaucomatous eyes is that elevated IOP-induced axonal injury at the optic nerve head results in the blockage of neurotrophin transport to RGC bodies (Anderson and Hendrickson, 1974; Minckler et al., 1976; Pease et al., 2000; Quigley and Addicks, 1980). Not only mechanical injury and neurotrophin deprivation, but also vascular insults at the optic nerve head (Flammer, 1994; Hayreh, 1985; Osborne et al., 2001) have been proposed to lead to RGC death in glaucoma. Glaucomatous insults at the optic nerve head have also been associated with other cellular events. For example, glial cells play a major role in tissue remodeling in the glaucomatous optic nerve head and affect the susceptibility of neuronal tissue to further damage. It has been suggested that glial events may create a harmful extracellular environment in the optic nerve head, thereby facilitating the mechanical and/or biochemical injury of axons as they exit the eye (Hernandez, 2000).
Although mechanical and/or ischemic injury to axons at the optic nerve head may explain selective loss of RGC bodies by retrograde degeneration, regional (Araie, 1995; Jonas et al., 1993; Tezel et al., 2000a) and cellular (Glovinsky et al., 1991) differences in the susceptibility of individual RGCs to damage are not well-understood. In addition, widespread and chronic tissue stress is evident not only in the optic nerve head but also in the retina (Tezel et al., 2000b; Tezel and Wax, 2004a; Tezel et al., 2005). Considerable evidence now strongly suggests that intraretinal events, including chronic retinal ischemia (Chung et al., 1999; Flammer, 1994; Hayreh, 1985; Osborne et al., 1999), glutamate excitotoxicity (Dreyer et al., 1996; Vorwerk et al., 1999), and an activated autoimmune response (Tezel and Wax, 2000b; Tezel and Wax, 2004b), may also facilitate primary and/or secondary degeneration of RGCs in glaucoma.
Consistent with the evidence of oxidative stress in glaucomatous eyes, growing evidence supports an oxidative component to the neurodegenerative process in glaucoma. Oxidative injury due to amplified ROS generation (Levin, 1999), as well as nitric oxide-induced damage (Liu and Neufeld, 2000; Neufeld et al., 1999) has been suggested to be a part of the pathway for RGC death following axonal injury. Oxidative neuronal injury in glaucoma may also be associated with excitotoxic amino acids, including glutamate, which are known to induce ROS generation; and oxidative stress is a leading mechanism of glutamate neurotoxicity (Atlante et al., 2001). In addition, retinal glutamate damage has been shown to be mediated in part through nitric oxide, a highly reactive oxidant (Nucci et al., 2005). Free radical scavenging when combined with trophic factors has resulted in increased survival of RGCs in ocular hypertensive rat eyes (Ko et al., 2000). Similarly, free radicalscavenging in combination with a nitric oxide synthase inhibitor has potentiated the neurotrophic effect of brain-derived neurotrophic factor on axotomized RGCs (Klocker et al., 1998). Thus, as discussed in this review, oxidative damage appears to be a final common component of glaucomatous neurodegeneration, as well as contributing to the anterior segment pathology (Babizhayev and Bunin, 1989; Chen and Kadlubar, 2003; Izzotti et al., 2003) and DNA damage in glaucoma patients (Sacca et al., 2005).
Another series of findings supporting an oxidative component of the neurodegenerative process in glaucoma come from our studies demonstrating oxidative injury through the involvement of TNF death receptor signaling. TNF-α, which is upregulated in the glaucomatous optic nerve head and retina (Tezel et al., 2001b; Yan et al., 2000; Yuan and Neufeld, 2000), has recently been identified to be a mediator of RGC death induced by a range of stimuli (Tezel and Wax, 2000a; Tezel and Yang, 2004; Tezel et al., 2004b). It is evident that in response to glaucomatous stress, increased glial production of TNF-α facilitates RGC death, in vitro (Tezel and Wax, 2000a). TNF-α can also mediate RGC death following optic nerve injury (Tezel et al., 2004b) or IOP elevation (Banerjee et al., 2005), in vivo. Despite diverse bioactivities of TNF-α, which can promote both cell death and survival signals in neurons (Fontaine et al., 2002; Shohami et al., 1999), amplification of cell death signaling and/or destruction of cellular survival factors during a prolonged exposure to the injurious condition leads to TNF-α-mediated RGC death. For example, the activity of c-jun N-terminal kinase (JNK) signaling has been found to be a turning point switching the life balance toward TNF-α-mediated RGC death following optic nerve injury (Tezel et al., 2004b). In fact, RGC death induced by this cytokine involves oxidative injury (Tezel and Yang, 2004); and ROS generation is a component of TNF-α signaling (Xu et al., 2002).
More recently, light has been suggested to be a risk factor for oxidative injury in glaucoma (Osborne et al., 2006). It has been proposed that light entering the eye interacts with mitochondria, thereby leading to increased ROS generation in RGCs and their axons, and that when these neurons are in an energetically low state, their antioxidant capacity is exceeded and their survival is compromised.
3. ROS in the execution of RGC death in glaucoma
3.1. Caspase-dependent and caspase-independent components of RGC death
Simulation of the noxious conditions of human glaucoma in experimental models results in the apoptotic death of RGCs in a caspase-dependent manner. For example, in vitro studies provide compelling evidence that the apoptosis of retinal neurons induced by different stimuli share a common caspase cascade (Tezel and Wax, 2000a; Tezel and Wax, 2000b), which can be inhibited using specific inhibitors of caspases (Tezel and Wax, 1999). In addition, RGC death induced by IOP elevation, in vivo, has been shown to involve caspase activation (McKinnon et al., 2002a); and caspase inhibiton has promoted neuronal survival in experimental rat models of glaucoma (McKinnon et al., 2002b). Since RGC apoptosis induced by a broad array of different stimuli share a common caspase cascade, inhibition of caspases has been suggested to provide a means to protect and/or rescue RGCs from apoptotic cell death, regardless of the causative event. However, in many models of apoptosis, cells saved by caspase inhibition may not be able to recover, but eventually go on to die ;due to mitochondrial dysfunction (Deshmukh et al., 2000; McCarthy et al., 1997; Ohta et al., 1997).
Indeed, there is growing evidence that supports the involvement of mitochondria in RGC death induced by different stimuli. For example, p53, a transcription factor activating bax required for cytochrome c release from the mitochondria, has been associated with neuronal apoptosis in glaucoma (Nickells, 1999). Mitochondrial dysfunction has also been implicated in neuronal apoptosis in an experimental rat glaucoma model (Mittag et al., 2000; Tatton et al., 2001). Our recent in vitro studies using primary cultures of RGCs have provided additional evidence that the mitochondrial dysfunction accompanies RGC death induced by glaucoma-related stimuli, TNF-α and hypoxia (Tezel and Yang, 2004). TNF-α and hypoxia are two different stimuli known to preferentially trigger the receptor-mediated or mitochondria-mediated cell death pathways, respectively. However, findings of a series of experiments have revealed that the loss of mitochondrial membrane potential and the release of mitochondrial cell death mediators, including cytochrome c and apoptosis-inducing factor, accompany RGC death induced by either TNF-α or hypoxia. Although caspase inhibitor treatment has temporarily decreased the rate of RGC apoptosis following exposure to these stimuli, inhibition of caspases has not been adequate to block RGC death if the mitochondrial membrane potential is lost and the mitochondrial cell death mediators are released. As detailed below, the caspase-independent component of the RGC death induced by glaucomatous stimuli has been found to involve oxidative injury.
Similar to hypoxia-induced RGC death, as well as glutamate or nitric oxide toxicity, receptor-mediated death of RGCs through TNF-α also involves caspase-dependent and caspase-independent components of the mitochondrial cell death pathway (Figure 2). This is consistent with previous studies demonstrating that caspase-8 cleaves a pro-apoptotic member of the bcl-2 family of proteins, bid; and the activated bid participates in the activation of the mitochondrial cell death pathway (Li et al., 1998; Luo et al., 1998). In addition, ceramide generated following TNF death receptor binding may initiate mitochondrial events (Pastorino et al., 1996). In response to glaucomatous stimuli, a crosstalk between death promoting signals (such as caspase activation and mitochondrial dysfunction) and survival promoting signals (such as neurotrophic signals, and the activity of NF-κB and heat shock proteins) determines whether a RGC should live or die (Tezel and Yang, 2005). Evidently, a critical balance between the survival promoting extracellular signal-regulated kinase (ERK) pathway and the death promoting JNK pathway (Tezel and Yang, 2005; Tezel et al., 2004b) is important for the regulation of RGC fate.
Figure 2.

RGC death induced by glaucomatous stimuli involves receptor-mediated caspase activation, and caspase-dependent and -independent components of the mitochondrial cell death pathway. Amplified ROS generation and oxidative damage are associated with both receptor-mediated and mitochondria-mediated pathways of RGC death. A critical balance between a variety of intracellular signaling pathways linked to cell survival or cell death determines whether a RGC dies or survives the glaucomatous stress (IOP, intraocular pressure; ROS, reactive oxygen species; bcl-xL and bax, anti-apoptotic and pro-apoptotic members of the bcl-2 family of proteins, respectively; aif and c, mitochondrial cell death mediators, apoptosis inducing factor and cytochrome c, respectively; TNF-α, tumor necrosis factor-alpha; TNF-R1, TNF death receptor; NO, nitric oxide; NF-κB, nuclear factor-kappaB, transcription factor; IκB, NF-κB inhibiting protein; JNK and ERK, mitogen-activated protein kinases, c-jun N-terminal kinase and extracellular signal-regulated kinase, respectively).
3.2. Oxidant injury to RGCs
Our in vitro studies using primary cultures of RGCs support an important role of ROS in RGC death during glaucomatous injury. A prominent increase in ROS accumulation has been detected in RGC cultures following their exposure to glaucomatous death stimuli. Despite the inhibition of caspase activity, survival rate in these cultures has been found to be less than 70% following 48-hour incubation with death stimuli. In addition, when combined with caspase inhibition, tempol, a free radical scavenger, has reduced the production of ROS and provided an additional 20% increase in RGC survival (Tezel and Yang, 2004). These findings indicate that reducing the oxidant generation provides additional protection to RGCs temporarily saved by caspase inhibition. Thus, not only does ROS generation induce the activation of caspases, but ROS are also directly cytotoxic to RGCs in a caspase-independent manner. Since RGC death induced by glaucomatous stimuli involves both receptor-mediated caspase cascade, and mitochondria-mediated caspase-dependent and caspase-independent components of cell death cascade, neuroprotective strategies targeting RGC rescue should include tools to block the proteolytic caspase activity and also improve the ability of these neurons to survive cytotoxic consequences of mitochondrial dysfunction, including the ROS attack.
In addition to ROS-induced oxidative damage, nitric oxide toxicity also plays a potential role in the oxidative injury to RGC soma in glaucoma. Evidence supporting the role nitric oxide-mediated damage to RGCs during glaucomatous neurodegeneration includes in vitro findings obtained using primary co-cultures of RGCs and glia. In vitro studies have revealed that in addition to TNF-α, exposure to glaucomatous stimuli also induces nitric oxide production by glial cells; and treatment of co-cultures with a selective inhibitor of inducible nitric oxide synthase, 1400W, results in a decreased rate of RGC death (Tezel and Wax, 2000a). Nitric oxide produced by glial cells has also been suggested to play a role in retrograde axonal degeneration and RGC death following axotomy (Koeberle and Ball, 1999) as well as IOP elevation (Liu and Neufeld, 2000; Neufeld et al., 1999).
4. Widespread damage to RGCs from the retina to the brain
4.1. Secondary degeneration of RGCs
Although the loss of optic nerve axons in glaucomatous eyes is accompanied by the apoptosis of RGCs, the exact nature and the primary site of injury remain unclear. However, it is widely accepted that primarily undamaged neurons in a neurodegenerative insult to the optic nerve can eventually undergo a secondary degeneration due to toxic substances released by injured neurons or stressed glia (Levkovitch-Verbin et al., 2001; Tezel et al., 2004b; Yoles and Schwartz, 1998). The likelihood of a similar secondary degenerative process in glaucoma makes differentiation of the primary injury site more uncertain.
Our findings have revealed that TNF death receptor signaling is involved in secondary degeneration of RGCs following optic nerve injury (Tezel et al., 2004b). It would be interesting to study whether oxidants released from stressed cells are involved in damaging primarily uncompromised RGCs in glaucoma, thereby spreading neuronal damage by secondary degeneration. On the other hand, recent findings discussed in section 8 provide evidence for oxidative stress-induced dysfunction of glial cells in glaucomatous eyes. This evidence supports that besides the direct neurotoxicity of ROS released by neighboring cells, oxidative stress-induced dysfunction of supportive glia may also facilitate secondary degeneration of RGCs in glaucoma (Figure 3). Since secondarily degenerating neurons are viable targets for neuroprotective interventions, improved understanding of pathogenic processes leading to secondary degeneration can offer effective strategies for neuroprotection.
Figure 3.

In addition to neurodegenerative injury induced by intracellular ROS, ROS released from stressed cells into the extracellular milieu may also facilitate secondary degeneration of RGCs. ROS released by neighboring cells may be directly cytotoxic to primarily undamaged RGCs. Alternatively, various consequences of oxidative stress-induced dysfunction of supportive glia may take part in spreading neuronal damage by secondary degeneration of RGCs. By leading to glial dysfunction, oxidative stress-induced glial activation, glial protein oxidation, and AGE/RAGE signaling may all contribute to decreased glial support of RGCs. By stimulating the antigen presenting ability of glial cells, ROS may also be involved in aberrant activation of the immune system, thereby facilitating the progression of glaucomatous neurodegeneration.
4.2. Evidence for compartmentalized processes of glaucomatous neurodegeneration
Despite the fact that the initial site of injury is unclear, glaucomatous neurodegeneration evidently exhibits widespread damage through a set of compartmentalized subcellular processes (Whitmore et al., 2005). Glaucomatous neurodegeneration eventually involves RGC soma and dendrites in the retina, axons in the optic nerve, and synapses in the brain. It is clear that stressed RGC soma and/or axons can signal to other subcellular compartments to initiate autodestructive processes, which may affect neuronal function early in disease. For example, there is evidence of early dendritic abnormalities in glaucoma (Morgan, 2002); and pressure-induced neurodegeneration involves structural abnormalities of the dendritic arbor of RGCs (Weber et al., 1998). Growing evidence also supports the involvement of a distal self-destruction program in optic nerve axons in glaucoma, which may be activated by axonal injury at the optic nerve head and/or the injury of RGC bodies in the retina. For example, axon counts in human glaucoma and in experimental models using cross-sections of the myelinated optic nerve, distal to the proposed injury site at the optic nerve head, show axonal loss. Evidence supporting a distal self-destruction program in glaucoma also includes the degeneration of RGC synapses in the brain. Studies using postmortem tissues from glaucomatous donors or studies using an experimental primate model of glaucoma have demonstrated that neurodegenerative changes are also observed in the lateral geniculate nucleus (the major relay center between the eye and the visual cortex), or even in the visual cortex in relation to the severity of optic nerve damage in ocular hypertensive eyes (Yucel et al., 2003). These further support that distal self-destruction of optic nerve axons in glaucoma may extend from the eye to vision centers in the brain. Axonal degeneration in many neurodegenerative diseases occurs in similar self-destruction processes, including Wallerian degeneration (Waller, 1850) and dying back (Cavanagh, 1979), in which axons of unhealthy neurons progressively degenerate through an active process to disconnect from their target cells, thereby conserving resources in response to chronic insults (Coleman and Ribchester, 2004; Khan, 2004). Recent evidence supports that this active and regulated program of axonal self-destruction is molecularly distinct from somal apoptosis (Finn et al., 2000; Raff et al., 2002; Whitmore et al., 2003).
In addition to target-derived trophic signals, health signals from soma to distal axon and synapse are critically important for neuronal survival. RGC survival and axon health are similarly dependent on each other. Therefore, independent from the primary injury site, neuroprotective treatment strategies should mutually target different subcellular compartments of RGCs as a prerequisite for functional gain in glaucoma patients. Development of successful treatment strategies targeting optic nerve axons (by blocking axonal pathology and inducing axon regeneration) and RGC soma (by RGC rescue through inhibiting cell death signaling and/or amplifying intrinsic survival signaling) awaits the identification of precise pathogenic processes of neurodegeneration. Growing evidence from ongoing studies suggests oxidative stress as a common component of the neurodegenerative injury in different subcellular compartments of RGCs from the retina to the brain.
4.3. Oxidative stress in widespread damage to RGCs in glaucoma
Compartmentalized neurodegenerative cascades in glaucoma likely involve a common oxidative component. First, as summarized in section 3, there is considerable evidence supporting that ROS constitute an important noxious stimulus for the execution of cell death in RGC soma during glaucomatous neurodegeneration (Tezel and Yang, 2004; Tezel et al., 2005). Second, as discussed below, it is highly reasonable to assume that oxidative stress is not only involved in somal death but also in neurodegenerative processes in other subcellular compartments of RGCs in glaucoma.
In addition to the induction of direct cytotoxic events that lead to RGC death, ROS have also been suggested to function as signaling molecules for RGC death after axonal injury. Among other proposed mechanisms such as those initiated by neurotrophin deprivation (Pease et al., 2000) or TNF-α signaling (Tezel et al., 2004b), which is also associated with oxidative injury, RGC death after axonal injury has been proposed to be dependent on ROS generation in such a way that these oxidants act as intracellular signaling molecules responsible for transforming the information from the damaged axon into a RGC death signal. Based on the observation of increased superoxide anion in RGCs, likely proximal to caspase activation, ROS generation has been suggested to be a parallel system to neurotrophin deprivation for signaling RGC death after axonal injury (Lieven et al., 2006; Nguyen et al., 2003; Swanson et al., 2005). On the other hand, ROS production has been involved in growth factor signaling through the observations that pre-treatment with antioxidants or overexpression of a ROS-scavenging enzyme block tyrosine phosphorylation (Sundaresan et al., 1995; Suzukawa et al., 2000). However, an in vivo study has demonstrated that trophic factors and antioxidants provide synergistic effects on rescuing RGCs from death in eyes with experimentally elevated IOP (Ko et al., 2000). Thus, the role of ROS in signaling RGC death is yet unclear mostly due to a lack of understanding of the mechanisms by which ROS function in normal physiological and disease states. Nevertheless, it seems highly possible that ROS can act as a second messenger. Alternatively, redox modifications of downstream effectors, through direct enzymatic oxidation of specific amino acid residues, can modulate protein function during RGC signaling (Figure 4). Even small concentrations of ROS can produce changes in cellular redox state that can, in turn, affect the activity, protein-protein and DNA-protein interactions of enzymes and transcription factors (Finkel, 1998). In fact, many retinal proteins exhibit oxidative modifications during glaucomatous neurodegeneration (Tezel et al., 2005), which validates the possibility of ROS function in RGC death signaling. Although current understanding of the ROS-mediated signaling in glaucomatous neurodegeneration is limited, different studies provide cumulating information on the association of ROS with different aspects of the neurodegenerative process.
Figure 4.
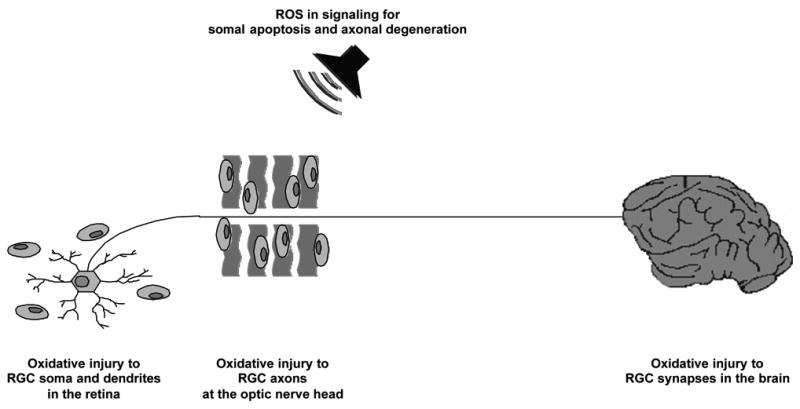
Glaucomatous neurodegeneration evidently exhibits widespread damage through a set of compartmentalized subcellular processes, which eventually involves RGC soma and dendrites in the retina, axons in the optic nerve, and synapses in the brain. Increasing evidence supports that widespread neurodegenerative cascades in glaucoma likely involve a common oxidative component. In addition to cytotoxic events induced by amplified ROS generation, ROS can also function as signaling molecules during glaucomatous neurodegeneration in different subcellular compartments of RGCs. ROS can act as a second messenger and/or modulate protein function by redox modifications of downstream effectors.
The potential role of oxidative stress in the maintenance of soma, dendrite, axon, or synapse health in glaucoma appears to be coupled with the mitochondrial dysfunction, which is importantly associated with ROS generation. There is evidence of mitochondrial dysfunction in glaucoma (Mittag et al., 2000; Tatton et al., 2001; Tezel and Yang, 2004); and as summarized in section 3, mitochondrial dysfunction critically participates in RGC death (Tezel and Yang, 2004). By demonstrating mitochondrial DNA abnormalities, a recent study also implicates mitochondrial dysfunction-associated oxidative stress as a risk factor for glaucoma patients (Abu-Amero et al., 2006). In addition to somal death, mitochondrial dysfunction likely contributes to compartmentalized neurodegenerative processes in glaucoma. Due to compartment-specific metabolic needs of neurons, mitochondria have to be appropriately distributed to serve the differential spatial and temporal demands of neurons; and the mitochondrial functional status is separately controlled in neurites and in the neuronal soma (Ikegami and Koike, 2003). Mitochondria in axons and presynaptic terminals provide sources of ATP to drive the ion pumps that are concentrated in these structures, to rapidly restore ion gradients following depolarization and neurotransmitter release. There is also a high level of dynamism in dendritic mitochondrial distribution, which is regulated by synaptic activity and correlated with synapse morphogenesis (Li et al., 2004). However, it should be recognized that important roles of mitochondria in the regulation of synaptic and dendritic function are not only associated with their ability to regulate ion levels but also ROS production. ROS generated in response to synaptic activity are known to contribute to the regulation of long-term structural and functional changes in neurons. The high-energy demands of synapses, together with their high levels of ROS production, place them at risk during conditions of increased stress as also seen during glaucomatous neurodegeneration. For example, it has been shown that exposure of the neuronal cells expressing mutations in presenilin-1 (early-onset inherited Alzheimer's disease) to amyloid beta-peptide 1–42 induces membrane lipid peroxidation in synapses and dendrites, which results in impaired ion and energy homoeostasis, thereby promoting synaptic degeneration (Guo et al., 1999; Mattson et al., 2001). Thus, although ROS have regulatory functions in normal physiological state, under stress conditions, increased oxidative damage to various synaptic proteins, along with energy depletion, can result in a local dysregulation of calcium homeostasis and synaptic degeneration. Mitochondrial abnormalities in synaptic terminals and distal regions of axons may in turn lead to a defect in axonal transport, thereby facilitating axonal degeneration (Ferreirinha et al., 2004).
Very little information is available on oxidative injury during synaptic degeneration in glaucoma, which comes from a study using an experimental monkey model of glaucoma. In this study, immunoreactivity for nitrotyrosine, a marker for peroxynitrite-mediated oxidative injury, has been found to be increased in the lateral geniculate nucleus of ocular hypertensive animals compared with controls, suggesting a role of oxidative injury in synaptic degeneration in glaucoma (Luthra et al., 2005). Thus, it is reasonable to suggest that oxidative stress in association with mitochondrial dysfunction may be involved in neurodegenerative processes in different compartments of RGCs in glaucoma. As discussed later, ROS play an important role in the activation of a systemic immune response in glaucoma patients, which may also be associated with neurodegenerative processes within different subcellular compartments of RGCs.
Currently available information on the mechanisms of the localized axonal degeneration involves the ubiquitin-proteasome (UPS) pathway. The UPS system plays major roles in regulating many cellular processes, including protein degradation by the proteasome. The UPS is highly compartmentalized in neurons and may affect complex and sometimes conflicting processes in different subcellular compartments. UPS-mediated protein destruction has been proposed to be involved in the localized self-destruction of axons, including RGC axons (Coleman and Ribchester, 2004). In addition to protein degradation during axonal degeneration, the proteasome has also been suggested to function in the regulation of signaling pathways that control axonal survival and degeneration under stress (MacInnis and Campenot, 2005). The potential importance of the UPS system in oxidative mechanisms of neurodegeneration appears to be associated with its involvement in the regulated degradation of oxidatively modified proteins (Poppek and Grune, 2006). Protein oxidation and generation of associated cytotoxic compounds are evident during glaucomatous neurodegeneration as summarized in sections 5.1 and 6.2. Increased generation of ROS, when accompanied by a decline in proteasome activity, results in the progressive accumulation of oxidatively damaged protein aggregates that can eventually contribute to cellular dysfunction and neurodegeneration. The UPS system is particularly important for the stabilization of the neuronal cytoskeleton and microtubules, while dysfunction of the UPS system leads to defects in axonal transport. Consistently, the intracellular accumulation of insoluble aggregates of structural proteins is a hallmark of many chronic neurodegenerative diseases; and the failure in their degradation by the proteasome can disrupt the normal physiology of the neuron. In particular, the disruption of axonal transport can trigger the mechanisms leading to active degeneration of axons (Coleman and Perry, 2002). UPS dysfunction appears to be a common feature of different neurodegenerative injuries and may also be associated with glaucomatous neurodegeneration. In case of UPS failure, even physiological levels of ROS may effect neuronal survival, while an oxidative insult can in turn cause UPS failure (Elkon et al., 2004).
Ongoing studies also provide additional promising clues to pursue. As an initial proteomic approach to identify signaling molecules involved in glaucomatous neurodegeneration, we have recently determined differential protein phosphorylation in the retina following experimental elevation of IOP in rats. Many signaling molecules have exhibited increased phosphorylation in ocular hypertensive retinas, one being collapsin response mediator protein-2 (CRMP-2) (Yang et al., 2006). The best-known function of CRMP-2 is the regulation of axonal outgrowth and guidance through mediating the semaphorin-induced growth cone collapse, a process that requires local rearrangement of the actin cytoskeleton (Goshima et al., 1995). CRMP-2 also regulates microtubule assembly and polymerization (Fukata et al., 2002). This protein belongs to the semaphorins family (Goshima et al., 1995), which are key proteins modulating the cell fate of axotomized RGCs (Shirvan et al., 2002). In addition, hyperphosphorylation of brain CRMPs in Alzheimer’s disease has been associated with the neurodegenerative pathology (Cole et al., 2004; Yoshida et al., 1998). What is most motivating is that ROS play an important role in semaphorin signaling (Ventura and Pelicci, 2002), which may also be involved in signaling RGC death during glaucomatous neurodegeneration.
5. Oxidative protein damage in glaucoma
5.1. Proteomic identification of oxidatively modified proteins in ocular hypertensive retinas
Recent evidence supports that besides direct cytotoxic consequences, ROS can also modulate protein function through oxidative modifications during glaucomatous neurodegeneration. Oxidant attack to proteins results in site-specific amino acid modifications, fragmentation of the peptide chain, aggregation of cross-linked reaction products, altered electrical charge, increased susceptibility to proteolysis, and function loss. Protein carbonyl formation is a widely utilized marker for protein oxidation. Several studies have demonstrated that the carbonyl content of proteins increases as a function of aging (Smith et al., 1991; Stadtman, 1992). In addition, this chemical modification of proteins has been implicated in the progression of neurodegeneration. It is well-established that oxidation is one of the most important causes of brain protein damage and dysfunction in several age-related neurodegenerative disorders (Berlett and Stadtman, 1997; Stadtman, 1992), including Alzheimer’s disease (Castegna et al., 2002a; Castegna et al., 2002b; Smith et al., 1991).
Our recent in vivo findings using a proteomic approach have revealed that one of the harmful consequences of ROS generation in glaucoma is the oxidative modification of many important retinal proteins (Tezel et al., 2005). Based on the evidence of amplified ROS generation during glaucomatous neurodegeneration (Tezel and Yang, 2004), this in vivo study has aimed to determine whether retinal proteins are oxidatively modified during glaucomatous neurodegeneration in ocular hypertensive eyes; and if so, what the targets are for protein oxidation in these eyes. Protein oxidation levels have been compared in ocular hypertensive and control retinas using immunochemical detection of protein carbonyls by two-dimensional oxyblot analysis coupled with protein identification through mass spectrometry. These analyses have demonstrated that oxidative protein modifications by ROS occur to a greater extent in ocular hypertensive retinas compared with controls (Figure 5). Increased protein oxidation in ocular hypertensive eyes supports the association of oxidative damage with neurodegeneration in glaucoma. Following oxidative modifications of retinal proteins in glaucomatous eyes, reduced ability of cells to cope with the glaucomatous tissue stress may result in impaired cellular homeostasis eventually contributing to neurodegeneration.
Figure 5.

Proteomic analysis was performed to determine whether retinal proteins are oxidatively modified during glaucomatous neurodegeneration in ocular hypertensive eyes following hypertonic saline injections into episcleral veins. Protein expression was determined by two dimensional (2D)-polyacrylamide gel electrophoresis of equally loaded protein samples obtained from ocular hypertensive and control retinas (PI, isoelectric point). Protein oxidation was determined by identifying the retinal proteins containing carbonyl groups using 2D-oxyblot analysis. Results of this in vivo study revealed that the protein modification by ROS occurs to a greater extent in ocular hypertensive eyes compared with the controls. Comparison of 2D-oxyblots with Coomassie Blue-stained 2D-gels showed that approximately 60 protein spots (out of over 400 spots) obtained using retinal protein lysates from ocular hypertensive retinas exhibited protein carbonyl immunoreactivity, which reflects oxidatively modified proteins. Following normalization of spots to their protein content measured by the intensity of Coomassie Blue staining, a significant increase was detected in carbonyl immunoreactivity of individual protein spots obtained using retinal protein lysates from ocular hypertensive eyes compared with the controls. The oxidized proteins identified through mass spectrometry included glyceraldehyde-3-phosphate dehydrogenase (GAPDH), a glycolytic enzyme; HSP72, a stress protein; and glutamine synthetase, an excitotoxicity-related protein (Modified with permission from (Tezel et al., 2005).
Selective recognition and degradation of the oxidatively modified proteins could be possible; however, oxidative stress may also induce protease-resistant protein cross-linking, thereby preventing this means of removing such proteins. By inhibiting the proteasome function, these aggregated, cross-linked, and oxidized protein products of oxidative stress cause a vicious cycle of progressively worsening accumulation of cytotoxic protein oxidation products (Davies, 2001; Poppek and Grune, 2006). Accumulation of the proteolysis-resistant ineffective protein aggregates leads to a loss in specific protein function, depletion of the cellular redox balance, and ultimately cell death (Berlett and Stadtman, 1997; Dean et al., 1997). Thus, effective function of protein degradation and repair systems (including the ubiquitin-proteosome system and the chaperone system), as well as other intrinsic antioxidant defense mechanisms, is important in determining cellular susceptibility to oxidative stress.
Peptide mass fingerprinting and peptide sequencing using mass spectrometry have identified specific targets of protein oxidation in the retina of ocular hypertensive eyes (Figure 5), which include glyceraldehyde-3-phosphate dehydrogenase (GAPDH), a glycolytic enzyme; HSP72, a stress protein; and glutamine synthetase, an excitotoxicity-related protein (Tezel et al., 2005).. Since GAPDH, HSP72, and glutamine synthetase are known to play important roles for cell survival and/or function in the retina, their oxidative modification appears to be associated with the neurodegenerative process in ocular hypertensive eyes. GAPDH, HSP72, and glutamine synthetase are proteins expressed by many cell types through the retina. While RGCs are preferentially susceptible to oxidative injury, other cell types, including glia, also prominently respond to oxidative stress in glaucomatous eyes, though in a different way. This is consistent with the detection of relatively widespread protein carbonyls in the inner retinal layers of ocular hypertensive eyes, including both RGCs and glia. However, protein oxidation should be particularly important for RGCs. This is because RGCs are predominantly injured in ocular hypertensive eyes and their energetic requirements are increased to recover from glaucomatous injury and maintain neuronal survival and function.
5.2. Potential consequences of retinal protein oxidation in glaucoma
It seems highly possible that oxidative protein modifications detected in the glaucomatous retina may lead to loss in specific protein function with important consequences facilitating RGC death, since the identified targets of retinal protein oxidation are known to be particularly sensitive to inactivation by oxidants. For example, oxidative inactivation of GAPDH, a major glycolytic enzyme, may lead to impaired glucose utilization in the retina of ocular hypertensive eyes. Since the survival and function of RGCs are highly energy dependent, and glucose is the major substrate for energy metabolism in the retina (Winkler et al., 2004), oxidative modification of GAPDH should be critically important for RGC survival in glaucoma. On the other hand, there is now clear evidence that the significance of GAPDH is not restricted to its pivotal glycolytic function. Besides its metabolic role, GAPDH has been shown to be involved in many other cellular processes, including DNA/RNA binding and regulation of protein expression (Sirover, 1999). Most importantly, nuclear function of this enzyme plays a role as an apoptosis signaling protein (Chen et al., 1999; Dastoor and Dreyer, 2001; Sawa et al., 1997) and has been associated with neuronal apoptosis in several neurodegenerative diseases, such as Alzheimer’s, Huntington’s, or Parkinson’s disease (Chuang and Ishitani, 1996; Mazzola and Sirover, 2001; Tatton et al., 2003). Consistently, our findings support the nuclear translocation of GAPDH in the glaucomatous retina (Tezel et al., 2005). Therefore, in addition to its consequences leading to impaired energy metabolism, oxidation of GAPDH could also be a signal for binding with nucleic acids and changing function. Thus, in addition to impaired glucose utilization, oxidative modification of this multifunctional protein in ocular hypertensive eyes may also be important for the regulation of apoptosis signaling during glaucomatous neurodegeneration (Tezel et al., 2005).
HSP72 has been identified as another target of retinal protein oxidation in ocular hypertensive eyes (Tezel et al., 2005). Heat shock proteins, which constitute an important component of intrinsic defense mechanisms, are upregulated in glaucomatous human eyes (Tezel et al., 2000b) and improve RGC survival after glaucomatous injury (Caprioli et al., 1996; Ishii et al., 2003; Park et al., 2001). Because appropriate expression of heat shock proteins is critical for their function in cellular protection, it is likely that alterations in HSP72 activity due to oxidative protein modification in ocular hypertensive eyes can ultimately result in impaired cellular response to tissue stress in these eyes.
And finally, oxidative damage to glutamine synthetase may be associated with glaucomatous injury to RGCs (Tezel et al., 2005). Glutamine synthetase is a key enzyme that helps maintain the physiological levels of extracellular glutamate through the glutamate-glutamine cycle (Butterfield et al., 1997; Castegna et al., 2002a). Retinal glutamine synthetase, which is located mainly in Müller cells (Linser et al., 1984; Newman and Reichenbach, 1996), plays a crucial role in modulating the neurotoxic effect of glutamate on RGCs. Therefore, following oxidative modification of glutamine synthetase, the resultant failure in the effective conversion of glutamate to glutamine may be associated with the glutamate excitotoxicity to RGCs implicated in the neurodegenerative process of glaucoma, while glutamate is also known to induce ROS production in neuronal cells. Previous evidence also associates redox-related events with the modulation of glutamate transport in the retina (Muller et al., 1998). In respect to decreased levels of retinal glutamate transporters in human as well as in experimental glaucoma (Martin et al., 2002; Naskar et al., 2000), oxidative injury to glial glutamate transport should be particularly important. Recent observations of intravitreal glutamate concentrations in glaucomatous eyes are conflicting; and RGCs, in vitro and in situ, have been suggested to be relatively invulnerable to excitotoxicity (Ullian et al., 2004). Nevertheless, the contribution of glutamate excitotoxicity to RGC death under overwhelming stress conditions in glaucomatous eyes, in vivo, can not be excluded. Oxidative alterations in the effective buffering of extracellular glutamate in the glaucomatous retina therefore appear to be important in controlling potential neurotoxicity to RGCs.
6. Advanced glycation end products (AGEs) in the glaucomatous optic nerve head and retina
6.1. Association of ROS with generation of AGEs
Oxidative stress increases with age in the brain; and the ability of neurons to respond to oxidative stress declines with age mostly due toan imbalance between increasing oxidant production and decreasing antioxidant capacity. Similar to many other age-dependent neurodegenerative diseases of the brain, age-dependent pathogenic processes associated with oxidative stress are not unexpected in glaucoma, since this disease is also more common in the elderly (Quigley and Vitale, 1997).
Extended exposure of proteins to reducing sugars and/or their end-compounds leads to non-enzymatic glycation of amino groups by the Maillard reaction, which alters the biological activity and degradation processes of proteins. In the early stage of this post-translational protein modification, synthesis of intermediates leads to the formation of Amadori compounds. In the late stage, AGEs are irreversibly formed with cross-linking after a complex cascade of chemical rearrangements, including oxidation. Studies of the contribution of protein glycation to diseases have been primarily focused on its relationship to diabetes and diabetes-related complications. However, it has then become clear that AGEs also accumulate in various tissues in the course of physiological aging. In addition, due to their synergism with oxidative stress, AGEs have more recently been implicated in neurodegenerative diseases. AGEs are commonly thought to exacerbate disease progression through two general mechanisms. First, the modified molecules, which form detergent-insoluble and protease-resistant non-degradable aggregates, impair normal cellular/tissue functions. Second, the AGEs modulate cellular function through binding to specific receptors; and depending on the cell type and concurrent signaling, receptor-mediated events result in cell activation/proliferation, chemotaxis, angiogenesis, generation of ROS, and/or apoptotic cell death (Neeper et al., 1992; Schmidt et al., 2000; Thornalley, 1998; Yan et al., 1996). Thus, AGEs lead to ROS generation, while AGE production is promoted by oxidative stress.
6.2. AGE accumulation in glaucomatous tissues
Based on the known synergism of AGEs with oxidative stress, we have recently determined the association of AGEs and their receptor (receptor for AGEs, RAGE) with glaucoma, which is also an age-dependent disease. Using immunohistochemical analyses, the extent and cellular localization of AGEs and RAGE have been determined in the optic nerve head and retina of human donor eyes with glaucoma compared with the controls from age-matched donors (Luo et al., 2006). Findings of this study have revealed that compared with the age-matched controls, an advanced accumulation of AGEs and upregulation of RAGE are detectable in the glaucomatous optic nerve head and retina. Some glial cells, RGCs, and axons in glaucomatous eyes have exhibited granular intracellular immunolabeling for AGEs. However, increased AGE immunolabeling in these eyes has been found predominantly extracellular. The most prominent extracellular immunolabeling for AGEs has been detected in laminar cribriform plates and optic nerve head blood vessels. Some RAGE immunolabeling has been located on scattered RGCs; however, increased RAGE immunolabeling in glaucomatous eyes has been predominantly detected on glial cells, mainly including Müller cells in the retina. Since the generation of AGEs is an age-dependent event, advanced accumulation of AGEs in the glaucomatous optic nerve head and retina supports that an accelerated aging process accompanies neurodegeneration in glaucomatous eyes, which involves oxidative injury.
6.3. Potential consequences of AGEs in the glaucomatous optic nerve head and retina
AGEs detected in glaucomatous tissues (Luo et al., 2006) may be directly cytotoxic and/or initiate a receptor-mediated signaling, both of which can promote cell death and/or dysfunction during glaucomatous neurodegeneration. Intracellular and extracellular accumulation of the AGEs detected in glaucomatous tissues may have different cytotoxic consequences facilitating the progression of neurodegeneration (Takeuchi et al., 2000a). For example, intracellular aggregates of AGEs detected in RGCs, their axons, and glia in glaucomatous eyes may interfere with normal cellular functions, including axonal transport and intracellular protein traffic (Cullum et al., 1991). However, accelerated accumulation of AGEs in glaucomatous tissues has been detected predominantly in the extracellular matrix of the optic nerve head and retina. Since AGEs accumulate with age on many long-lived macromolecules like collagen, it is unsurprising that these products are most prominently detectable within the extracellular matrix. Accumulation of AGEs in the extracellular matrix may elicit several alterations, which include decreased solubility, decreased susceptibility to enzymes, and changes in thermal stability, mechanical strength, and stiffness (Bruel and Oxlund, 1996; Vlassara et al., 1994). Such alterations in physicochemical properties of the extracellular matrix may be particularly important in the glaucomatous optic nerve head. This is because extracellular matrix sheets of the lamina cribrosa, which provide mechanical support for optic nerve axons, exhibit increased rigidity in glaucomatous eyes (Burgoyne et al., 2005; Hernandez, 2000; Johnson et al., 1996). Advanced accumulation of AGEs in laminar cribriform plates and blood vessels of the glaucomatous optic nerve head may therefore facilitate the mechanical injury of axons caused by elevated IOP and/or impair the microcirculation.
RAGE has also been upregulated on RGCs and glia of glaucomatous eyes, predominantly Müller cells in the retina. Presence of RAGE on RGCs and glia makes them susceptible for AGE-mediated events through receptor-mediated signaling. Upregulation of RAGE in glaucomatous eyes suggests that in addition to direct cytotoxic effects of intracellular or extracellular AGEs, these reactive products may also initiate a specific receptor-mediated signaling. Based on known outcomes of RAGE-mediated signaling (Thornalley, 1998), major cellular events associated with glaucomatous neurodegeneration, such as glial activation and dysfunction (including activated glial migration, increased glial production of TNF-α and nitric oxide synthase, and activated immune-regulatory function), inappropriate activation of signaling molecules (for example, mitogen-activated protein kinases, MAPKs, or NF-κB), activated immune response, and neuronal apoptosis, may all have important links to advanced glycation processes coupled by oxidative stress in glaucoma.
Our more recent in vivo study using a proteomic approach has demonstrated aberrant protein glycation in ocular hypertensive, as well as diabetic retinas, and identified common targets of this post-translational protein modification in two different disease models (Atmaca-Sonmez et al., 2006). Increased retinal protein glycation in ocular hypertensive eyes is consistent with the evidence of AGE accumulation in ocular tissues of non-diabetic glaucoma patients, as well as AGE accumulation by physiological aging through life-long exposure to normoglycemia. Protein glycation is also evident in other neurodegenerative diseases of the brain like Alzheimer’s disease (Kanninen et al., 2004). Based on the known process of AGE generation, not only glucose, but also various other sugars and their end-compounds can participate in the aberrant glycation of proteins (Glomb and Monnier, 1995; Takeuchi et al., 2000b). Existing evidence supports that aberrant protein glycation may be accelerated by oxidative stress end-products in glaucomatous eyes. For example, as summarized in section 5, a potential decline in GAPDH activity due to protein oxidation during glaucomatous neurodegeneration (Tezel et al., 2005) may lead to alternative metabolic routes and glyceraldehyde generation. Such end-compounds have been shown to contribute to increased protein glycation and AGE generation even under normoglycemic conditions (Choei et al., 2004).
7. Association of oxidative stress with tissue remodeling in glaucoma
In addition to tissue alterations secondary to extracellular AGE accumulation, oxidative stress in the glaucomatous optic nerve head and retina may also be associated with other cellular events involved in tissue remodeling. The characteristic clinical appearance of neurodegenerative alterations in the glaucomatous optic nerve head is called optic disc cupping and corresponds to backward bowing and disorganization of lamina cribrosa along with neuronal loss (Tezel et al., 2004a). Glaucomatous tissue remodeling involves prominent glial responses leading to alterations in extracellular matrix composition and distribution (Hernandez, 2000). Consistent with optic disc cupping and cavernous degeneration in glaucomatous eyes (Wax et al., 1998a), and as also supported by in vitro observations (Tezel et al., 2001a), tissue remodeling events in the glaucomatous optic nerve head preferentially result in tissue degradation, rather than scar tissue formation as detected in other types of optic nerve injuries. Such tissue degradation, despite glial activation and increased glial production of extracellular matrix molecules, has been associated with a parallel increase in the secretion of matrix metalloproteinases in glaucomatous eyes (Agapova et al., 2001; Yan et al., 2000).
In addition to AGE generation, redox modulation of matrix degradation also links the oxidative stress to glaucomatous tissue remodeling. Evidence implicates ROS as key regulators of matrix metalloproteinase expression and activity during tissue remodeling in a number of pathologies (Nelson and Melendez, 2004). Matrix metalloproteinase activity in association with ROS production has been linked to various tissue remodeling events, which may also have important implications in glaucomatous neurodegeneration. For example, ROS-regulated matrix metalloproteinase activity has been associated with the disruption of blood-brain barrier through digestion of the endothelial basal lamina (Kim et al., 2003). Increased matrix metalloproteinases in the perivascular area of glaucomatous eyes (Yan et al., 2000) may similarly influence the blood-eye barrier in these eyes. By facilitating the access of immune-related cells in and out of the optic nerve head and retina, a defect in blood-eye barrier may be associated with the involvement of immune system in glaucoma (Tezel and Wax, 2004b). In addition, matrix metalloproteinases play a regulatory role in axon pathfinding (Nordstrom et al., 1995), and remodeling and integrity of synapses (Bilousova et al., 2006; Kim et al., 2005; Leone et al., 2005) and dendritic architecture (Szklarczyk et al., 2002) after neuronal injury. However, whether oxidative stress-regulated matrix metalloproteinases participate in determining the functional status of the optic nerve during glaucomatous neurodegeneration is currently unknown.
In addition to remodeling of the extracellular environment of neurons, oxidative stress-regulated matrix metalloproteinase activity may also be associated with neurotoxicity. For example, nitric oxide-induced activation of matrix metalloproteinases in RGCs has been contributed to glutamate-induced RGC death (Manabe et al., 2005). Retinal matrix metalloproteinase activity has also been related to RGC death after optic nerve ligation (Chintala et al., 2002) or IOP elevation (Guo et al., 2005). Although no direct evidence currently supports the causative importance of matrix metalloproteinases in RGC death, these proteases may modulate neurodegenerative events in glaucoma. For example, the release of TNF-α (a cell death mediator involved in glaucomatous neurodegeneration) from its membrane-bound precursor is a matrix metalloproteinase-dependent process (Chandler et al., 1997; Gearing et al., 1995). Thus, not only resultant structural alterations, but also cellular components of tissue remodeling events, including oxidative stress-regulated matrix metalloproteinase activity, may have important links to glaucomatous neurodegeneration.
8. Oxidative stress-induced dysfunction of glial cells in glaucoma
8.1. Diverse cellular responses to tissue stress in glaucomatous eyes
As supported by glial activation, tissue stress in glaucomatous eyes does not only affect RGCs. In fact, widespread stress response, including stress protein upregulation and hypoxic and oxidative stress, is also evident in glial cells in the glaucomatous optic nerve head and retina. Based on double immunolabeling studies, increased expression of different heat shock proteins (Tezel et al., 2000b) or HIF-1α (Tezel and Wax, 2004a) in glaucomatous human eyes has been localized to both RGCs and glial cells. Immunohistochemical observations are also consistent with relatively widespread oxidative stress in the inner retinal layers of ocular hypertensive eyes (Tezel et al., 2005). Protein carbonyl immunoreactivity, which reflects protein oxidation, is not only localized to RGCs but also to glia in these eyes. Thus, glaucomatous tissue stress affects a wide range of cells, including glia.
However, RGCs and glia are known to differ in their susceptibility to glaucomatous injury. For example, in vitro studies using primary co-cultures of RGCs and glia have demonstrated that RGCs undergo apoptosis following exposure to glaucomatous stimuli, but glial cells survive the same stress conditions (Tezel and Wax, 2000a). This is also in agreement with the common thought originated from in vivo experiments and clinical observations that glaucoma is a selective disease, which results in the degeneration of retinal neurons, specifically the RGCs and their axons. Obviously, despite preferential susceptibility of RGCs to primary and/or secondary degeneration in glaucoma, glial cells are relatively protected against glaucomatous injury, but rather they exhibit an activated phenotype, in vitro (Tezel et al., 2001a) and in vivo (Tanihara et al., 1997; Tezel et al., 2003; Varela and Hernandez, 1997; Wang et al., 2002; Wang et al., 2000). This differential response of glial cells to tissue stress by activation persists during the chronic course of glaucomatous neurodegeneration (Tezel et al., 2003).
8.2. Oxidative stress and glial dysfunction in glaucoma
Like many other stressors, ROS elicit a wide spectrum of cellular responses ranging from proliferation to growth arrest, senescence, and cell death. Cellular signaling pathways are generally subject to dual redox regulation, in which redox may have opposite effects on upstream signaling systems and downstream transcription factors. Cell death or survival is determined depending on such a dual redox regulation and a cross-talk between the cellular signaling system and the cellular redox state. The particular outcome observed can vary significantly from one cell type to the next, as well as with respect to the severity and duration of oxidative stress. However, whatever the effect seen, it largely reflects the balance between a variety of intracellular signaling pathways linked to cell survival or death that are activated in response to the oxidative insult (Kamata and Hirata, 1999). The major signaling pathways known to be involved in regulating the cellular response to oxidative stress include MAPKs, PI3-kinase/Akt pathway, heat shock protein expression, p53 signaling, and NF-κB signaling (Martindale and Holbrook, 2002). Current evidence supports that these important cellular pathways are differentially regulated between RGCs and glia exposed to glaucomatous stimuli (Tezel et al., 2003; Tezel and Yang, 2005).
As an attempt to better understand diverse cellular responses to glaucomatous tissue stress, such as the death of neurons but the sparing and activation of glia, we have recently performed in vitro experiments using primary cultures of RGCs and glia. Our comparative experiments have provided evidence that the differential susceptibility of these cell types to glaucomatous stimuli is associated, in part, with differential regulation and activity of various signaling molecules which are thought to determine the ultimate cell fate. These signaling molecules include those associated with MAPKs (which include ERK, JNK, and p38 kinase) and NF-κB (Tezel and Yang, 2005). These in vitro findings are consistent with the differential activity of MAPKs between RGCs and glial cells in glaucomatous human eyes (Tezel et al., 2003) and with in vivo findings demonstrating an important role of MAPKs in determining the RGC fate following optic nerve injury (Tezel et al., 2004b). Evidently, a dynamic balance between the survival promoting ERK pathway and the death promoting JNK-p38 pathway is important in determining the ultimate cell fate (Xia et al., 1995). Similarly, NF-κB signaling is a regulator of cellular survival and death programs (O'Neill and Kaltschmidt, 1997). Therefore, besides many other factors, differential activation of signaling molecules between RGCs and glia is likely associated with the relative protection of glial cells against glaucomatous injury.
Glial cells are under oxidative stress in glaucomatous eyes; and intrinsic ROS produced by glia, or exogenous ROS present in the extracellular milieu have the potential to harm these cells. However, in addition to an upregulated activity of survival-promoting signaling, glial cells are also well-equipped with efficient antioxidant defense mechanisms for self-protection against oxidative damage. Brain glia exhibit substantial activities of antioxidants, including superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase, as well as NADPH-regenerating enzymes (Bambrick et al., 2004). Consistent with observations in brain astrocytes, optic nerve head astrocytes also exhibit a prominent antioxidant response to oxidative stress (Malone and Hernandez, 2006).
Although glial cells survive the glaucomatous tissue stress, they consequently exhibit alterations in many of their cellular functions. As summarized in Figure 3, oxidative stress-induced dysfunction of glial cells may be associated with spreading neuronal damage by secondary degeneration of RGCs in glaucoma. An exciting alteration in glial function under glaucomatous stress is associated with their neurosupportive/protective ability. Despite the well-known role of glial cells in supporting RGCs, considerable evidence suggests that under glaucomatous stress, their neuroprotective ability may lessen and glial cells may even become neurodestructive by releasing increased amounts of neurotoxic substances, including TNF-α and nitric oxide, both associated with oxidative injury. It is clear that when released by glia, perhaps as a nonspecific means of destroying damaged cells, such neurotoxic agents can facilitate neurodegeneration (Tezel and Wax, 2000a; Tezel and Yang, 2004). Oxidative stress, also in association with a chronic AGEs/RAGE signaling (Luo et al., 2006), appears to be an important component of cellular events persistently keeping glial cells in the activated state in glaucomatous eye, thereby leading to decreased glial ability to protect RGCs from injury.
Thus, despite relative protection of glial cells against oxidative cell death, oxidants likely impair important glial functions during glaucomatous neurodegeneration. In addition to other oxidative stress-associated dysfunctions, alterations in the glial uptake and metabolism of glutamate represent another glial dysfunction leading to neurotoxicity. In fact, control of extracellular environment is one of the numerous neurosupportive functions of glial cells. Müller cells are known to be the primary cell type responsible for the glial removal of excess glutamate from the extracellular space in the retina (Harada et al., 1998; Kawasaki et al., 2000; Reichelt et al., 1997). However, not only do ROS decrease the retinal glutamate transport (Muller et al., 1998), but also glutamine synthetase (modulates glutamate-glutamine cycle, as discussed in section 5.2,) is oxidatively modified in ocular hypertensive eyes (Tezel et al., 2005).
Another oxidative stress-induced glial dysfunction during glaucomatous neurodegeneration is associated with their role in the activation of an aberrant immune response (Tezel and Wax, 2003). As discussed in the next section, the immune-regulatory function of optic nerve head and retinal glia is also altered in glaucomatous eyes. Thus, oxidative stress-induced alterations in glial function appear to be important in determining their diverse roles as being supportive or damaging to RGCs (Figure 3).
9. The role of ROS in the activation of immune response in glaucoma patients
Increasing evidence from clinical and experimental studies over the past decade strongly supports the involvement of the immune system in the neurodegenerative process of glaucoma (Tezel and Wax, 2004b). In addition to serum and tissue findings supporting aberrant immune system activity in glaucoma patients (Tezel et al., 1999; Tezel et al., 1998; Wax et al., 1998a; Wax et al., 2001; Wax et al., 1998b), glial cells in glaucomatous human eyes also exhibit an activation in their antigen presenting ability (Tezel et al., 2003; Yang et al., 2001c). Seemingly conflicting aspects of the immune system involvement in glaucoma, as being neuroprotective or neurodestructive, are associated with the regulation of T cell activity. While an aberrant immune process through the activation of pathogenic T cells may lead to an autoimmune neurodegenerative disease, boosting natural protective autoimmunity through regulatory T cells has been suggested as a neuroprotective strategy (Schwartz, 2003a; Schwartz, 2003b). Immune system function in glaucoma patients may partly be associated with immune surveillance (Raivich et al., 1998), which allows early contact of the immune system with the injury site for the removal of stressed or damaged cells and cellular debris. However, although T cell-mediated immune responses may initially be beneficial to limit neurodegeneration, a failure to properly control aberrant, stress-induced immune response likely converts the protective immunity into an autoimmune neurodegenerative process. It is evident that such an autoimmune process accompanies the progression of neurodegeneration in some, if not all, glaucoma patients.
Tissue stress is probably a major force that drives a resting immune system over the threshold of antigen-specific activation, since several stress-associated co-stimulatory factors are required for the activation of resting antigen presenting cells. Recent evidence supports that oxidative stress-induced alterations in glial immune-regulatory function play an important role in the regulation of T cell activity and aberrant activation of the immune system in glaucoma patients. To explore the effects of ROS on the phenotype and antigen presenting function of glial cells, we have recently performed in vitro experiments using glial cell cultures pre-treated with ROS generating compounds and then co-incubated with syngeneic CD4+ T cells (Tezel et al., 2006). No morphological changes have been detected in optic nerve head or retinal glia when treated with different chemical generators of superoxide anion, nitric oxide, and hydroxyl radical. However, these glial cells have vigorously responded to ROS with a variety of immunologically relevant functions, including stimulated antigen presentation to T cells. First, following pre-treatment with ROS generating compounds, MHC class II molecules have been upregulated on glial cells. Second, compared with the control glia, glial cells in ROS generating systems have been found to be more potent inducers of T cell activation in a cell density- and time-dependent manner as assessed by increased proliferation (approximately three-fold) and increased TNF-α secretion (approximately six-fold) of T cells. These findings support that ROS upregulate the glial MHC class II expression and stimulate the antigen presenting ability of optic nerve head and retinal glia. This is consistent with findings in glaucomatous human eyes that glial activation in these eyes is accompanied by HLA-DR (a human MHC class II molecule) upregulation (Yang et al., 2001c). Not only microglial cells (Neufeld, 1999), but also glial fibrillary acidic protein-positive astrocytes exhibit increased immunolabeling for HLA-DR in glaucomatous human eyes (Yang et al., 2001c). Another important finding of our in vitro study is that when a ROS scavenging treatment was applied, stimulation of T cell activation has been decreased, despite the persistence of MHC up-regulation on glial cells (Tezel et al., 2006). This finding indicates an additional co-stimulatory function of ROS during antigen presentation, which is also required to elicit an activated immune response.
Thus, besides other factors involved in activating an aberrant immune response during glaucomatous neurodegeneration (such as increased expression and/or exposure of retinal antigens due to tissue damage), ROS also facilitate communication of glial cells with T cells. The regulatory role of ROS in antigen presentation by glia links the oxidative tissue stress to the initiation and modulation of an adaptive immune response during glaucomatous neurodegeneration, which evidently includes the T cell-mediated cellular response (Yang et al., 2001a) consequently leading to the B cell-mediated humoral response (Wax et al., 2002). Thus, in addition to many other harmful consequences, ROS also take part in the regulation of immune response which likely contributes to the glaucomatous neurodegenerative process (Tezel and Wax, 2000b; Tezel and Wax, 2004b; Wax et al., 2006).
10. Conclusions
Growing evidence supports that RGCs in glaucomatous eyes are under oxidative stress, which is associated with pathogenic mechanisms leading to glaucomatous neurodegeneration. Increased ROS generation leads to RGC degeneration, glial dysfunction, and activation of immune response in glaucoma. However, many aspects of the relationship between oxidative stress and the neurodegenerative process are not entirely clear. During glaucomatous neurodegeneration, ROS may be directly neurotoxic to RGCs, may contribute to secondary degeneration by inducing glial dysfunction, and may also function as signaling molecules by acting as a second messenger and/or by regulating redox modifications of downstream effectors. Exact molecular mechanisms and the real impact of oxidative stress on the development and progression of glaucomatous neurodegeneration need to be further elucidated. An improved understanding of the cellular mechanisms leading to oxidative injury in RGCs during glaucomatous neurodegeneration, and modulation of pathways involved in mediating cellular responses to oxidative stress, may offer unique opportunities for neuroprotective interventions aimed at the effective treatment of glaucoma, a leading cause of blindness.
Acknowledgments
Dr. Tezel is supported by National Eye Institute (R01 EY013813, R24 EY015636), Bethesda, MD; and an unrestricted grant to University of Louisville Department of Ophthalmology & Visual Sciences from Research to Prevent Blindness Inc., New York, NY. Dr. Tezel is also a recipient of the Research to Prevent Blindness Sybil B. Harrington Special Scholar Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Abu-Amero KK, Morales J, Bosley TM. Mitochondrial abnormalities in patients with primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2006;47:2533–2541. doi: 10.1167/iovs.05-1639. [DOI] [PubMed] [Google Scholar]
- Agapova OA, Ricard CS, Salvador-Silva M, Hernandez MR. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human optic nerve head astrocytes. Glia. 2001;33:205–216. doi: 10.1002/1098-1136(200103)33:3<205::aid-glia1019>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Andersen JK. Oxidative stress in neurodegeneration: cause or consequence? Nat Med. 2004;10:S18–25. doi: 10.1038/nrn1434. Suppl. [DOI] [PubMed] [Google Scholar]
- Anderson DR, Hendrickson A. Effect of intraocular pressure on rapid axoplasmic transport in monkey optic nerve. Invest Ophthalmol. 1974;13:771–783. [PubMed] [Google Scholar]
- Araie M. Pattern of visual field defects in normal-tension and high-tension glaucoma. Curr Opin Ophthalmol. 1995;6:36–45. doi: 10.1097/00055735-199504000-00007. [DOI] [PubMed] [Google Scholar]
- Atlante A, Calissano P, Bobba A, Giannattasio S, Marra E, Passarella S. Glutamate neurotoxicity, oxidative stress and mitochondria. FEBS Lett. 2001;497:1–5. doi: 10.1016/s0014-5793(01)02437-1. [DOI] [PubMed] [Google Scholar]
- Atmaca-Sonmez P, Yang X, Cai J, Eskan MA, Yolcu ES, Tezel G. Proteomic identification of glycated proteins shared in ocular hypertensive and diabetic rat retinas. Invest Ophthalmol Vis Sci. 2006;47:E-Abstract 197. Available at www.iovs.org.
- Babizhayev MA, Bunin A. Lipid peroxidation in open-angle glaucoma. Acta Ophthalmol (Copenh) 1989;67:371–377. doi: 10.1111/j.1755-3768.1989.tb01617.x. [DOI] [PubMed] [Google Scholar]
- Bambrick L, Kristian T, Fiskum G. Astrocyte mitochondrial mechanisms of ischemic brain injury and neuroprotection. Neurochem Res. 2004;29:601–608. doi: 10.1023/b:nere.0000014830.06376.e6. [DOI] [PubMed] [Google Scholar]
- Banasiak KJ, Xia Y, Haddad GG. Mechanisms underlying hypoxia-induced neuronal apoptosis. Prog Neurobiol. 2000;62:215–249. doi: 10.1016/s0301-0082(00)00011-3. [DOI] [PubMed] [Google Scholar]
- Banerjee K, Yang X, Tezel G. Up-regulation of TNF-alpha signaling in ocular hypertensive rat eyes. Invest Ophthalmol Vis Sci. 2005;46:E-Abstract 3772. Available at www.iovs.org.
- Berlett BS, Stadtman ER. Protein oxidation in aging, disease, and oxidative stress. J Biol Chem. 1997;272:20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- Bilousova TV, Rusakov DA, Ethell DW, Ethell IM. Matrix metalloproteinase-7 disrupts dendritic spines in hippocampal neurons through NMDA receptor activation. J Neurochem. 2006;97:44–56. doi: 10.1111/j.1471-4159.2006.03701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonne C, Muller A, Villain M. Free radicals in retinal ischemia. Gen Pharmacol. 1998;30:275–280. doi: 10.1016/s0306-3623(97)00357-1. [DOI] [PubMed] [Google Scholar]
- Bruel A, Oxlund H. Changes in biomechanical properties, composition of collagen and elastin, and advanced glycation endproducts of the rat aorta in relation to age. Atherosclerosis. 1996;127:155–165. doi: 10.1016/s0021-9150(96)05947-3. [DOI] [PubMed] [Google Scholar]
- Bruer U, Weih MK, Isaev NK, Meisel A, Ruscher K, Bergk A, Trendelenburg G, Wiegand F, Victorov IV, Dirnagl U. Induction of tolerance in rat cortical neurons: hypoxic preconditioning. FEBS Lett. 1997;414:117–121. doi: 10.1016/s0014-5793(97)00954-x. [DOI] [PubMed] [Google Scholar]
- Burgoyne CF, Downs JC, Bellezza AJ, Suh JK, Hart RT. The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retin Eye Res. 2005;24:39–73. doi: 10.1016/j.preteyeres.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Hensley K, Cole P, Subramaniam R, Aksenov M, Aksenova M, Bummer PM, Haley BE, Carney JM. Oxidatively induced structural alteration of glutamine synthetase assessed by analysis of spin label incorporation kinetics: relevance to Alzheimer's disease. J Neurochem. 1997;68:2451–2457. doi: 10.1046/j.1471-4159.1997.68062451.x. [DOI] [PubMed] [Google Scholar]
- Caprioli J, Kitano S, Morgan JE. Hyperthermia and hypoxia increase tolerance of retinal ganglion cells to anoxia and excitotoxicity. Invest Ophthalmol Vis Sci. 1996;37:2376–2381. [PubMed] [Google Scholar]
- Castegna A, Aksenov M, Aksenova M, Thongboonkerd V, Klein JB, Pierce WM, Booze R, Markesbery WR, Butterfield DA. Proteomic identification of oxidatively modified proteins in Alzheimer's disease brain. Part I: creatine kinase BB, glutamine synthase, and ubiquitin carboxy-terminal hydrolase L-1. Free Radic Biol Med. 2002a;33:562–571. doi: 10.1016/s0891-5849(02)00914-0. [DOI] [PubMed] [Google Scholar]
- Castegna A, Aksenov M, Thongboonkerd V, Klein JB, Pierce WM, Booze R, Markesbery WR, Butterfield DA. Proteomic identification of oxidatively modified proteins in Alzheimer's disease brain. Part II: dihydropyrimidinase-related protein 2, alpha-enolase and heat shock cognate 71. J Neurochem. 2002b;82:1524–1532. doi: 10.1046/j.1471-4159.2002.01103.x. [DOI] [PubMed] [Google Scholar]
- Cavanagh JB. The 'dying back' process. A common denominator in many naturally occurring and toxic neuropathies. Arch Pathol Lab Med. 1979;103:659–664. [PubMed] [Google Scholar]
- Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000;275:25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- Chandler S, Miller KM, Clements JM, Lury J, Corkill D, Anthony DC, Adams SE, Gearing AJ. Matrix metalloproteinases, tumor necrosis factor and multiple sclerosis: an overview. J Neuroimmunol. 1997;72:155–161. doi: 10.1016/s0165-5728(96)00179-8. [DOI] [PubMed] [Google Scholar]
- Chen J, Simon R. Ischemic tolerance in the brain. Neurology. 1997;48:306–311. doi: 10.1212/wnl.48.2.306. [DOI] [PubMed] [Google Scholar]
- Chen JZ, Kadlubar FF. A new clue to glaucoma pathogenesis. Am J Med. 2003;114:697–698. doi: 10.1016/s0002-9343(03)00199-2. [DOI] [PubMed] [Google Scholar]
- Chen RW, Saunders PA, Wei H, Li Z, Seth P, Chuang DM. Involvement of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and p53 in neuronal apoptosis: evidence that GAPDH is upregulated by p53. J Neurosci. 1999;19:9654–9662. doi: 10.1523/JNEUROSCI.19-21-09654.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintala SK, Zhang X, Austin JS, Fini ME. Deficiency in matrix metalloproteinase gelatinase B (MMP-9) protects against retinal ganglion cell death after optic nerve ligation. J Biol Chem. 2002;277:47461–47468. doi: 10.1074/jbc.M204824200. [DOI] [PubMed] [Google Scholar]
- Choei H, Sasaki N, Takeuchi M, Yoshida T, Ukai W, Yamagishi S, Kikuchi S, Saito T. Glyceraldehyde-derived advanced glycation end products in Alzheimer's disease. Acta Neuropathol (Berl) 2004;108:189–193. doi: 10.1007/s00401-004-0871-x. [DOI] [PubMed] [Google Scholar]
- Chuang DM, Ishitani R. A role for GAPDH in apoptosis and neurodegeneration. Nat Med. 1996;2:609–610. doi: 10.1038/nm0696-609. [DOI] [PubMed] [Google Scholar]
- Chung HS, Harris A, Evans DW, Kagemann L, Garzozi HJ, Martin B. Vascular aspects in the pathophysiology of glaucomatous optic neuropathy. Surv Ophthalmol. 1999;43:S43–50. doi: 10.1016/s0039-6257(99)00050-8. Suppl 1. [DOI] [PubMed] [Google Scholar]
- Cioffi GA, Wang L. Optic nerve blood flow in glaucoma. Semin Ophthalmol. 1999;14:164–170. doi: 10.3109/08820539909061470. [DOI] [PubMed] [Google Scholar]
- Cole AR, Knebel A, Morrice NA, Robertson LA, Irving AJ, Connolly CN, Sutherland C. GSK-3 phosphorylation of the Alzheimer epitope within collapsin response mediator proteins regulates axon elongation in primary neurons. J Biol Chem. 2004;279:50176–50180. doi: 10.1074/jbc.C400412200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman MP, Perry VH. Axon pathology in neurological disease: a neglected therapeutic target. Trends Neurosci. 2002;25:532–537. doi: 10.1016/s0166-2236(02)02255-5. [DOI] [PubMed] [Google Scholar]
- Coleman MP, Ribchester RR. Programmed axon death, synaptic dysfunction and the ubiquitin proteasome system. Curr Drug Targets CNS Neurol Disord. 2004;3:227–238. doi: 10.2174/1568007043337436. [DOI] [PubMed] [Google Scholar]
- Cullum NA, Mahon J, Stringer K, McLean WG. Glycation of rat sciatic nerve tubulin in experimental diabetes mellitus. Diabetologia. 1991;34:387–389. doi: 10.1007/BF00403175. [DOI] [PubMed] [Google Scholar]
- Dastoor Z, Dreyer JL. Potential role of nuclear translocation of glyceraldehyde-3-phosphate dehydrogenase in apoptosis and oxidative stress. J Cell Sci. 2001;114:1643–1653. doi: 10.1242/jcs.114.9.1643. [DOI] [PubMed] [Google Scholar]
- Davies KJ. Degradation of oxidized proteins by the 20S proteasome. Biochimie. 2001;83:301–310. doi: 10.1016/s0300-9084(01)01250-0. [DOI] [PubMed] [Google Scholar]
- Dean RT, Fu S, Stocker R, Davies MJ. Biochemistry and pathology of radical-mediated protein oxidation. Biochem J. 1997;324:1–18. doi: 10.1042/bj3240001. Pt 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh M, Kuida K, Johnson EM., Jr Caspase inhibition extends the commitment to neuronal death beyond cytochrome c release to the point of mitochondrial depolarization. J Cell Biol. 2000;150:131–143. doi: 10.1083/jcb.150.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer EB, Zurakowski D, Schumer RA, Podos SM, Lipton SA. Elevated glutamate levels in the vitreous body of humans and monkeys with glaucoma. Arch Ophthalmol. 1996;114:299–305. doi: 10.1001/archopht.1996.01100130295012. [DOI] [PubMed] [Google Scholar]
- Duranteau J, Chandel NS, Kulisz A, Shao Z, Schumacker PT. Intracellular signaling by reactive oxygen species during hypoxia in cardiomyocytes. J Biol Chem. 1998;273:11619–11624. doi: 10.1074/jbc.273.19.11619. [DOI] [PubMed] [Google Scholar]
- Elkon H, Melamed E, Offen D. Oxidative stress, induced by 6-hydroxydopamine, reduces proteasome activities in PC12 cells: implications for the pathogenesis of Parkinson's disease. J Mol Neurosci. 2004;24:387–400. doi: 10.1385/JMN:24:3:387. [DOI] [PubMed] [Google Scholar]
- Farkas RH, Chowers I, Hackam AS, Kageyama M, Nickells RW, Otteson DC, Duh EJ, Wang C, Valenta DF, Gunatilaka TL, Pease ME, Quigley HA, Zack DJ. Increased expression of iron-regulating genes in monkey and human glaucoma. Invest Ophthalmol Vis Sci. 2004;45:1410–1417. doi: 10.1167/iovs.03-0872. [DOI] [PubMed] [Google Scholar]
- Ferreirinha F, Quattrini A, Pirozzi M, Valsecchi V, Dina G, Broccoli V, Auricchio A, Piemonte F, Tozzi G, Gaeta L, Casari G, Ballabio A, Rugarli EI. Axonal degeneration in paraplegin-deficient mice is associated with abnormal mitochondria and impairment of axonal transport. J Clin Invest. 2004;113:231–242. doi: 10.1172/JCI20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T. Oxygen radicals and signaling. Curr Opin Cell Biol. 1998;10:248–253. doi: 10.1016/s0955-0674(98)80147-6. [DOI] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Finn JT, Weil M, Archer F, Siman R, Srinivasan A, Raff MC. Evidence that Wallerian degeneration and localized axon degeneration induced by local neurotrophin deprivation do not involve caspases. J Neurosci. 2000;20:1333–1341. doi: 10.1523/JNEUROSCI.20-04-01333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flammer J. The vascular concept of glaucoma. Surv Ophthalmol. 1994;38:S3–6. doi: 10.1016/0039-6257(94)90041-8. Suppl. [DOI] [PubMed] [Google Scholar]
- Flammer J, Orgul S, Costa VP, Orzalesi N, Krieglstein GK, Serra LM, Renard JP, Stefansson E. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002;21:359–393. doi: 10.1016/s1350-9462(02)00008-3. [DOI] [PubMed] [Google Scholar]
- Fontaine V, Mohand-Said S, Hanoteau N, Fuchs C, Pfizenmaier K, Eisel U. Neurodegenerative and neuroprotective effects of tumor necrosis factor (TNF) in retinal ischemia: Opposite roles of TNF receptor 1 and TNF receptor 2. J Neurosci. 2002;22:RC216. doi: 10.1523/JNEUROSCI.22-07-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata Y, Itoh TJ, Kimura T, Menager C, Nishimura T, Shiromizu T, Watanabe H, Inagaki N, Iwamatsu A, Hotani H, Kaibuchi K. CRMP-2 binds to tubulin heterodimers to promote microtubule assembly. Nat Cell Biol. 2002;4:583–591. doi: 10.1038/ncb825. [DOI] [PubMed] [Google Scholar]
- Garcia-Valenzuela E, Shareef S, Walsh J, Sharma SC. Programmed cell death of retinal ganglion cells during experimental glaucoma. Exp Eye Res. 1995;61:33–44. doi: 10.1016/s0014-4835(95)80056-5. [DOI] [PubMed] [Google Scholar]
- Gearing AJ, Beckett P, Christodoulou M, Churchill M, Clements JM, Crimmin M, Davidson AH, Drummond AH, Galloway WA, Gilbert R, et al. Matrix metalloproteinases and processing of pro-TNF-alpha. J Leukoc Biol. 1995;57:774–777. doi: 10.1002/jlb.57.5.774. [DOI] [PubMed] [Google Scholar]
- Geiger LK, Kortuem KR, Alexejun C, Levin LA. Reduced redox state allows prolonged survival of axotomized neonatal retinal ganglion cells. Neuroscience. 2002;109:635–642. doi: 10.1016/s0306-4522(01)00493-6. [DOI] [PubMed] [Google Scholar]
- Glomb MA, Monnier VM. Mechanism of protein modification by glyoxal and glycolaldehyde, reactive intermediates of the Maillard reaction. J Biol Chem. 1995;270:10017–10026. doi: 10.1074/jbc.270.17.10017. [DOI] [PubMed] [Google Scholar]
- Glovinsky Y, Quigley HA, Dunkelberger GR. Retinal ganglion cell loss is size dependent in experimental glaucoma. Invest Ophthalmol Vis Sci. 1991;32:484–491. [PubMed] [Google Scholar]
- Goshima Y, Nakamura F, Strittmatter P, Strittmatter SM. Collapsin-induced growth cone collapse mediated by an intracellular protein related to UNC-33. Nature. 1995;376:509–514. doi: 10.1038/376509a0. [DOI] [PubMed] [Google Scholar]
- Gross RL, Hensley SH, Gao F, Wu SM. Retinal ganglion cell dysfunction induced by hypoxia and glutamate: potential neuroprotective effects of beta-blockers. Surv Ophthalmol. 1999;43:S162–170. doi: 10.1016/s0039-6257(99)00054-5. Suppl 1. [DOI] [PubMed] [Google Scholar]
- Guillemin K, Krasnow MA. The hypoxic response: huffing and HIFing. Cell. 1997;89:9–12. doi: 10.1016/s0092-8674(00)80176-2. [DOI] [PubMed] [Google Scholar]
- Guo L, Moss SE, Alexander RA, Ali RR, Fitzke FW, Cordeiro MF. Retinal ganglion cell apoptosis in glaucoma is related to intraocular pressure and IOP-induced effects on extracellular matrix. Invest Ophthalmol Vis Sci. 2005;46:175–182. doi: 10.1167/iovs.04-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Fu W, Holtsberg FW, Steiner SM, Mattson MP. Superoxide mediates the cell-death-enhancing action of presenilin-1 mutations. J Neurosci Res. 1999;56:457–470. doi: 10.1002/(SICI)1097-4547(19990601)56:5<457::AID-JNR2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Halterman MW, Miller CC, Federoff HJ. Hypoxia-inducible factor-1alpha mediates hypoxia-induced delayed neuronal death that involves p53. J Neurosci. 1999;19:6818–6824. doi: 10.1523/JNEUROSCI.19-16-06818.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada T, Harada C, Watanabe M, Inoue Y, Sakagawa T, Nakayama N, Sasaki S, Okuyama S, Watase K, Wada K, Tanaka K. Functions of the two glutamate transporters GLAST and GLT-1 in the retina. Proc Natl Acad Sci U S A. 1998;95:4663–4666. doi: 10.1073/pnas.95.8.4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JD, Strange RC. Potential contribution of the glutathione S-transferase supergene family to resistance to oxidative stress. Free Radic Res. 1995;22:193–207. doi: 10.3109/10715769509147539. [DOI] [PubMed] [Google Scholar]
- Hayreh SS. Pathogenesis of optic nerve damage and visual field defects. In: Heilman K, Richardson KT, editors. Glaucoma, conceptions of a disease. WB Saunders; Philadelphia: 1978. pp. 104–180. [Google Scholar]
- Hayreh SS. Inter-individual variation in blood supply of the optic nerve head. Its importance in various ischemic disorders of the optic nerve head, and glaucoma, low-tension glaucoma and allied disorders. Doc Ophthalmol. 1985;59:217–246. doi: 10.1007/BF00159262. [DOI] [PubMed] [Google Scholar]
- Hernandez MR. The optic nerve head in glaucoma: role of astrocytes in tissue remodeling. Prog Retin Eye Res. 2000;19:297–321. doi: 10.1016/s1350-9462(99)00017-8. [DOI] [PubMed] [Google Scholar]
- Ikegami K, Koike T. Non-apoptotic neurite degeneration in apoptotic neuronal death: pivotal role of mitochondrial function in neurites. Neuroscience. 2003;122:617–626. doi: 10.1016/j.neuroscience.2003.08.057. [DOI] [PubMed] [Google Scholar]
- Ishii Y, Kwong JM, Caprioli J. Retinal ganglion cell protection with geranylgeranylacetone, a heat shock protein inducer, in a rat glaucoma model. Invest Ophthalmol Vis Sci. 2003;44:1982–1992. [PubMed] [Google Scholar]
- Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Cellular and developmental control of O2 homeostasis by hypoxia- inducible factor 1 alpha. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzotti A, Sacca SC, Cartiglia C, De Flora S. Oxidative deoxyribonucleic acid damage in the eyes of glaucoma patients. Am J Med. 2003;114:638–646. doi: 10.1016/s0002-9343(03)00114-1. [DOI] [PubMed] [Google Scholar]
- Jiang BH, Semenza GL, Bauer C, Marti HH. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol. 1996;271:C1172–1180. doi: 10.1152/ajpcell.1996.271.4.C1172. [DOI] [PubMed] [Google Scholar]
- Johnson EC, Morrison JC, Farrell S, Deppmeier L, Moore CG, McGinty MR. The effect of chronically elevated intraocular pressure on the rat optic nerve head extracellular matrix. Exp Eye Res. 1996;62:663–674. doi: 10.1006/exer.1996.0077. [DOI] [PubMed] [Google Scholar]
- Jonas JB, Fernandez MC, Sturmer J. Pattern of glaucomatous neuroretinal rim loss. Ophthalmology. 1993;100:63–68. doi: 10.1016/s0161-6420(13)31694-7. [DOI] [PubMed] [Google Scholar]
- Kamata H, Hirata H. Redox regulation of cellular signalling. Cell Signal. 1999;11:1–14. doi: 10.1016/s0898-6568(98)00037-0. [DOI] [PubMed] [Google Scholar]
- Kanninen K, Goldsteins G, Auriola S, Alafuzoff I, Koistinaho J. Glycosylation changes in Alzheimer's disease as revealed by a proteomic approach. Neurosci Lett. 2004;367:235–240. doi: 10.1016/j.neulet.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Kawasaki A, Otori Y, Barnstable CJ. Muller cell protection of rat retinal ganglion cells from glutamate and nitric oxide neurotoxicity. Invest Ophthalmol Vis Sci. 2000;41:3444–3450. [PubMed] [Google Scholar]
- Kerrigan LA, Zack DJ, Quigley HA, Smith SD, Pease ME. TUNEL-positive ganglion cells in human primary open-angle glaucoma. Arch Ophthalmol. 1997;115:1031–1035. doi: 10.1001/archopht.1997.01100160201010. [DOI] [PubMed] [Google Scholar]
- Khan AA. Wallerian degeneration in the optic nerve of the rabbit. Cells Tissues Organs. 2004;177:104–109. doi: 10.1159/000079185. [DOI] [PubMed] [Google Scholar]
- Kim GW, Gasche Y, Grzeschik S, Copin JC, Maier CM, Chan PH. Neurodegeneration in striatum induced by the mitochondrial toxin 3-nitropropionic acid: role of matrix metalloproteinase-9 in early blood-brain barrier disruption? J Neurosci. 2003;23:8733–8742. doi: 10.1523/JNEUROSCI.23-25-08733.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Fillmore HL, Reeves TM, Phillips LL. Elevation of hippocampal MMP-3 expression and activity during trauma-induced synaptogenesis. Exp Neurol. 2005;192:60–72. doi: 10.1016/j.expneurol.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Kitano S, Morgan J, Caprioli J. Hypoxic and excitotoxic damage to cultured rat retinal ganglion cells. Exp Eye Res. 1996;63:105–112. doi: 10.1006/exer.1996.0096. [DOI] [PubMed] [Google Scholar]
- Klocker N, Cellerino A, Bahr M. Free radical scavenging and inhibition of nitric oxide synthase potentiates the neurotrophic effects of brain-derived neurotrophic factor on axotomized retinal ganglion cells In vivo. J Neurosci. 1998;18:1038–1046. doi: 10.1523/JNEUROSCI.18-03-01038.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko ML, Hu DN, Ritch R, Sharma SC. The combined effect of brain-derived neurotrophic factor and a free radical scavenger in experimental glaucoma. Invest Ophthalmol Vis Sci. 2000;41:2967–2971. [PubMed] [Google Scholar]
- Ko ML, Peng PH, Ma MC, Ritch R, Chen CF. Dynamic changes in reactive oxygen species and antioxidant levels in retinas in experimental glaucoma. Free Radic Biol Med. 2005;39:365–373. doi: 10.1016/j.freeradbiomed.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Koeberle PD, Ball AK. Nitric oxide synthase inhibition delays axonal degeneration and promotes the survival of axotomized retinal ganglion cells. Exp Neurol. 1999;158:366–381. doi: 10.1006/exnr.1999.7113. [DOI] [PubMed] [Google Scholar]
- Kortuem K, Geiger LK, Levin LA. Differential susceptibility of retinal ganglion cells to reactive oxygen species. Invest Ophthalmol Vis Sci. 2000;41:3176–3182. [PubMed] [Google Scholar]
- Leone L, De Stefano ME, Del Signore A, Petrucci TC, Paggi P. Axotomy of sympathetic neurons activates the metalloproteinase-2 enzymatic pathway. J Neuropathol Exp Neurol. 2005;64:1007–1017. doi: 10.1097/01.jnen.0000187053.59018.3c. [DOI] [PubMed] [Google Scholar]
- Levin LA. Direct and indirect approaches to neuroprotective therapy of glaucomatous optic neuropathy. Surv Ophthalmol. 1999;43:S98–101. doi: 10.1016/s0039-6257(99)00027-2. Suppl 1. [DOI] [PubMed] [Google Scholar]
- Levkovitch-Verbin H, Quigley HA, Kerrigan-Baumrind LA, D'Anna SA, Kerrigan D, Pease ME. Optic nerve transection in monkeys may result in secondary degeneration of retinal ganglion cells. Invest Ophthalmol Vis Sci. 2001;42:975–982. [PubMed] [Google Scholar]
- Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- Li Y, Schlamp CL, Nickells RW. Experimental induction of retinal ganglion cell death in adult mice. Invest Ophthalmol Vis Sci. 1999;40:1004–1008. [PubMed] [Google Scholar]
- Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Libby RT, Gould DB, Anderson MG, John SW. Complex genetics of glaucoma susceptibility. Annu Rev Genomics Hum Genet. 2005;6:15–44. doi: 10.1146/annurev.genom.6.080604.162209. [DOI] [PubMed] [Google Scholar]
- Lieven CJ, Hoegger MJ, Schlieve CR, Levin LA. Retinal ganglion cell axotomy induces an increase in intracellular superoxide anion. Invest Ophthalmol Vis Sci. 2006;47:1477–1485. doi: 10.1167/iovs.05-0921. [DOI] [PubMed] [Google Scholar]
- Linser PJ, Sorrentino M, Moscona AA. Cellular compartmentalization of carbonic anhydrase-C and glutamine synthetase in developing and mature mouse neural retina. Brain Res. 1984;315:65–71. doi: 10.1016/0165-3806(84)90077-4. [DOI] [PubMed] [Google Scholar]
- Liu B, Neufeld AH. Expression of nitric oxide synthase-2 (NOS-2) in reactive astrocytes of the human glaucomatous optic nerve head. Glia. 2000;30:178–186. doi: 10.1002/(sici)1098-1136(200004)30:2<178::aid-glia7>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Luo C, Yang X, Tezel G. Accelerated aging in glaucoma: Immunohistochemical assessment of advanced glycation end-products in the human retina and optic nerve head. Invest Ophthalmol Vis Sci. 2006;47:E-Abstract 398. doi: 10.1167/iovs.06-0737. Available at www.iovs.org. [DOI] [PMC free article] [PubMed]
- Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- Luthra A, Gupta N, Kaufman PL, Weinreb RN, Yucel YH. Oxidative injury by peroxynitrite in neural and vascular tissue of the lateral geniculate nucleus in experimental glaucoma. Exp Eye Res. 2005;80:43–49. doi: 10.1016/j.exer.2004.08.016. [DOI] [PubMed] [Google Scholar]
- MacInnis BL, Campenot RB. Regulation of Wallerian degeneration and nerve growth factor withdrawal-induced pruning of axons of sympathetic neurons by the proteasome and the MEK/Erk pathway. Mol Cell Neurosci. 2005;28:430–439. doi: 10.1016/j.mcn.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Malone P, Hernandez MR. 4-hydroxynonenal, a product of oxidative stress, leads to an antioxidant response in optic nerve head astrocytes. Invest Ophthalmol Vis Sci. 2006;47:E-Abstract 1256. doi: 10.1016/j.exer.2006.10.020. Available at www.iovs.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe S, Gu Z, Lipton SA. Activation of matrix metalloproteinase-9 via neuronal nitric oxide synthase contributes to NMDA-induced retinal ganglion cell death. Invest Ophthalmol Vis Sci. 2005;46:4747–4753. doi: 10.1167/iovs.05-0128. [DOI] [PubMed] [Google Scholar]
- Martin KR, Levkovitch-Verbin H, Valenta D, Baumrind L, Pease ME, Quigley HA. Retinal glutamate transporter changes in experimental glaucoma and after optic nerve transection in the rat. Invest Ophthalmol Vis Sci. 2002;43:2236–2243. [PubMed] [Google Scholar]
- Martindale JL, Holbrook NJ. Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol. 2002;192:1–15. doi: 10.1002/jcp.10119. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Gary DS, Chan SL, Duan W. Perturbed endoplasmic reticulum function, synaptic apoptosis and the pathogenesis of Alzheimer's disease. Biochem Soc Symp. 2001:151–162. doi: 10.1042/bss0670151. [DOI] [PubMed] [Google Scholar]
- Mazzola JL, Sirover MA. Reduction of glyceraldehyde-3-phosphate dehydrogenase activity in Alzheimer's disease and in Huntington's disease fibroblasts. J Neurochem. 2001;76:442–449. doi: 10.1046/j.1471-4159.2001.00033.x. [DOI] [PubMed] [Google Scholar]
- McCarthy NJ, Whyte MK, Gilbert CS, Evan GI. Inhibition of Ced-3/ICE-related proteases does not prevent cell death induced by oncogenes, DNA damage, or the Bcl-2 homologue Bak. J Cell Biol. 1997;136:215–227. doi: 10.1083/jcb.136.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon SJ, Lehman DM, Kerrigan-Baumrind LA, Merges CA, Pease ME, Kerrigan DF, Ransom NL, Tahzib NG, Reitsamer HA, Levkovitch-Verbin H, Quigley HA, Zack DJ. Caspase activation and amyloid precursor protein cleavage in rat ocular hypertension. Invest Ophthalmol Vis Sci. 2002a;43:1077–1087. [PubMed] [Google Scholar]
- McKinnon SJ, Lehman DM, Tahzib NG, Ransom NL, Reitsamer HA, Liston P, LaCasse E, Li Q, Korneluk RG, Hauswirth WW. Baculoviral IAP repeat-containing-4 protects optic nerve axons in a rat glaucoma model. Mol Ther. 2002b;5:780–787. doi: 10.1006/mthe.2002.0608. [DOI] [PubMed] [Google Scholar]
- Minckler DS, Tso MO, Zimmerman LE. A light microscopic, autoradiographic study of axoplasmic transport in the optic nerve head during ocular hypotony, increased intraocular pressure, and papilledema. Am J Ophthalmol. 1976;82:741–757. doi: 10.1016/0002-9394(76)90012-x. [DOI] [PubMed] [Google Scholar]
- Mittag TW, Danias J, Pohorenec G, Yuan HM, Burakgazi E, Chalmers-Redman R, Podos SM, Tatton WG. Retinal damage after 3 to 4 months of elevated intraocular pressure in a rat glaucoma model. Invest Ophthalmol Vis Sci. 2000;41:3451–3459. [PubMed] [Google Scholar]
- Moreno MC, Campanelli J, Sande P, Sanez DA, Keller Sarmiento MI, Rosenstein RE. Retinal oxidative stress induced by high intraocular pressure. Free Radic Biol Med. 2004;37:803–812. doi: 10.1016/j.freeradbiomed.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Morgan JE. Retinal ganglion cell shrinkage in glaucoma. J Glaucoma. 2002;11:365–370. doi: 10.1097/00061198-200208000-00015. [DOI] [PubMed] [Google Scholar]
- Muller A, Maurin L, Bonne C. Free radicals and glutamate uptake in the retina. Gen Pharmacol. 1998;30:315–318. doi: 10.1016/s0306-3623(97)00362-5. [DOI] [PubMed] [Google Scholar]
- Muller A, Pietri S, Villain M, Frejaville C, Bonne C, Culcas M. Free radicals in rabbit retina under ocular hyperpressure and functional consequences. Exp Eye Res. 1997;64:637–643. doi: 10.1006/exer.1996.0277. [DOI] [PubMed] [Google Scholar]
- Murphy MP. Nitric oxide and cell death. Biochim Biophys Acta. 1999;1411:401–414. doi: 10.1016/s0005-2728(99)00029-8. [DOI] [PubMed] [Google Scholar]
- Naskar R, Vorwerk CK, Dreyer EB. Concurrent downregulation of a glutamate transporter and receptor in glaucoma. Invest Ophthalmol Vis Sci. 2000;41:1940–1944. [PubMed] [Google Scholar]
- Neeper M, Schmidt AM, Brett J, Yan SD, Wang F, Pan YC, Elliston K, Stern D, Shaw A. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem. 1992;267:14998–15004. [PubMed] [Google Scholar]
- Nelson KK, Melendez JA. Mitochondrial redox control of matrix metalloproteinases. Free Radic Biol Med. 2004;37:768–784. doi: 10.1016/j.freeradbiomed.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Neufeld AH. Microglia in the optic nerve head and the region of parapapillary chorioretinal atrophy in glaucoma. Arch Ophthalmol. 1999;117:1050–1056. doi: 10.1001/archopht.117.8.1050. [DOI] [PubMed] [Google Scholar]
- Neufeld AH, Sawada A, Becker B. Inhibition of nitric-oxide synthase 2 by aminoguanidine provides neuroprotection of retinal ganglion cells in a rat model of chronic glaucoma. Proc Natl Acad Sci U S A. 1999;96:9944–9948. doi: 10.1073/pnas.96.17.9944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman E, Reichenbach A. The Muller cell: a functional element of the retina. Trends Neurosci. 1996;19:307–312. doi: 10.1016/0166-2236(96)10040-0. [DOI] [PubMed] [Google Scholar]
- Nguyen SM, Alexejun CN, Levin LA. Amplification of a reactive oxygen species signal in axotomized retinal ganglion cells. Antioxid Redox Signal. 2003;5:629–634. doi: 10.1089/152308603770310293. [DOI] [PubMed] [Google Scholar]
- Nickells RW. Apoptosis of retinal ganglion cells in glaucoma: an update of the molecular pathways involved in cell death. Surv Ophthalmol. 1999;43:S151–161. doi: 10.1016/s0039-6257(99)00029-6. Suppl 1. [DOI] [PubMed] [Google Scholar]
- Nordstrom LA, Lochner J, Yeung W, Ciment G. The metalloproteinase stromelysin-1 (transin) mediates PC12 cell growth cone invasiveness through basal laminae. Mol Cell Neurosci. 1995;6:56–68. doi: 10.1006/mcne.1995.1006. [DOI] [PubMed] [Google Scholar]
- Nucci C, Tartaglione R, Rombola L, Morrone LA, Fazzi E, Bagetta G. Neurochemical evidence to implicate elevated glutamate in the mechanisms of high intraocular pressure (IOP)-induced retinal ganglion cell death in rat. Neurotoxicology. 2005;26:935–941. doi: 10.1016/j.neuro.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Ohta T, Kinoshita T, Naito M, Nozaki T, Masutani M, Tsuruo T, Miyajima A. Requirement of the caspase-3/CPP32 protease cascade for apoptotic death following cytokine deprivation in hematopoietic cells. J Biol Chem. 1997;272:23111–23116. doi: 10.1074/jbc.272.37.23111. [DOI] [PubMed] [Google Scholar]
- O'Neill LA, Kaltschmidt C. NF-kappa B: a crucial transcription factor for glial and neuronal cell function [see comments] Trends Neurosci. 1997;20:252–258. doi: 10.1016/s0166-2236(96)01035-1. [DOI] [PubMed] [Google Scholar]
- Osborne NN, Casson RJ, Wood JP, Chidlow G, Graham M, Melena J. Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog Retin Eye Res. 2004;23:91–147. doi: 10.1016/j.preteyeres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Osborne NN, Lascaratos G, Bron AJ, Chidlow G, Wood JP. A hypothesis to suggest that light is a risk factor in glaucoma and the mitochondrial optic neuropathies. Br J Ophthalmol. 2006;90:237–241. doi: 10.1136/bjo.2005.082230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne NN, Melena J, Chidlow G, Wood JP. A hypothesis to explain ganglion cell death caused by vascular insults at the optic nerve head: possible implication for the treatment of glaucoma. Br J Ophthalmol. 2001;85:1252–1259. doi: 10.1136/bjo.85.10.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne NN, Ugarte M, Chao M, Chidlow G, Bae JH, Wood JP, Nash MS. Neuroprotection in relation to retinal ischemia and relevance to glaucoma. Surv Ophthalmol. 1999;43:S102–128. doi: 10.1016/s0039-6257(99)00044-2. Suppl 1. [DOI] [PubMed] [Google Scholar]
- Park KH, Cozier F, Ong OC, Caprioli J. Induction of heat shock protein 72 protects retinal ganglion cells in a rat glaucoma model. Invest Ophthalmol Vis Sci. 2001;42:1522–1530. [PubMed] [Google Scholar]
- Pastorino JG, Simbula G, Yamamoto K, Glascott PA, Jr, Rothman RJ, Farber JL. The cytotoxicity of tumor necrosis factor depends on induction of the mitochondrial permeability transition. J Biol Chem. 1996;271:29792–29798. doi: 10.1074/jbc.271.47.29792. [DOI] [PubMed] [Google Scholar]
- Pease ME, McKinnon SJ, Quigley HA, Kerrigan-Baumrind LA, Zack DJ. Obstructed axonal transport of BDNF and its receptor TrkB in experimental glaucoma. Invest Ophthalmol Vis Sci. 2000;41:764–774. [PubMed] [Google Scholar]
- Poppek D, Grune T. Proteasomal defense of oxidative protein modifications. Antioxid Redox Signal. 2006;8:173–184. doi: 10.1089/ars.2006.8.173. [DOI] [PubMed] [Google Scholar]
- Potashkin JA, Meredith GE. The role of oxidative stress in the dysregulation of gene expression and protein metabolism in neurodegenerative disease. Antioxid Redox Signal. 2006;8:144–151. doi: 10.1089/ars.2006.8.144. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Addicks EM. Chronic experimental glaucoma in primates. II. Effect of extended intraocular pressure elevation on optic nerve head and axonal transport. Invest Ophthalmol Vis Sci. 1980;19:137–152. [PubMed] [Google Scholar]
- Quigley HA, Nickells RW, Kerrigan LA, Pease ME, Thibault DJ, Zack DJ. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Invest Ophthalmol Vis Sci. 1995;36:774–786. [PubMed] [Google Scholar]
- Quigley HA, Vitale S. Models of open-angle glaucoma prevalence and incidence in the United States. Invest Ophthalmol Vis Sci. 1997;38:83–91. [PubMed] [Google Scholar]
- Raff MC, Whitmore AV, Finn JT. Axonal self-destruction and neurodegeneration. Science. 2002;296:868–871. doi: 10.1126/science.1068613. [DOI] [PubMed] [Google Scholar]
- Raivich G, Jones LL, Kloss CU, Werner A, Neumann H, Kreutzberg GW. Immune surveillance in the injured nervous system: T-lymphocytes invade the axotomized mouse facial motor nucleus and aggregate around sites of neuronal degeneration. J Neurosci. 1998;18:5804–5816. doi: 10.1523/JNEUROSCI.18-15-05804.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichelt W, Pannicke T, Biedermann B, Francke M, Faude F. Comparison between functional characteristics of healthy and pathological human retinal Muller glial cells. Surv Ophthalmol. 1997;42:S105–117. doi: 10.1016/s0039-6257(97)80033-1. Suppl 1. [DOI] [PubMed] [Google Scholar]
- Rosenbaum DM, Rosenbaum PS, Gupta A, Michaelson MD, Hall DH, Kessler JA. Retinal ischemia leads to apoptosis which is ameliorated by aurintricarboxylic acid. Vision Res. 1997;37:3445–3451. doi: 10.1016/S0042-6989(96)00328-8. [DOI] [PubMed] [Google Scholar]
- Roth S, Li B, Rosenbaum PS, Gupta H, Goldstein IM, Maxwell KM, Gidday JM. Preconditioning provides complete protection against retinal ischemic injury in rats. Invest Ophthalmol Vis Sci. 1998;39:777–785. [PubMed] [Google Scholar]
- Sacca SC, Pascotto A, Camicione P, Capris P, Izzotti A. Oxidative DNA damage in the human trabecular meshwork: clinical correlation in patients with primary open-angle glaucoma. Arch Ophthalmol. 2005;123:458–463. doi: 10.1001/archopht.123.4.458. [DOI] [PubMed] [Google Scholar]
- Sarfarazi M, Rezaie T. Optineurin in primary open angle glaucoma. Ophthalmol Clin North Am. 2003;16:529–541. doi: 10.1016/s0896-1549(03)00061-0. [DOI] [PubMed] [Google Scholar]
- Sawa A, Khan AA, Hester LD, Snyder SH. Glyceraldehyde-3-phosphate dehydrogenase: nuclear translocation participates in neuronal and nonneuronal cell death. Proc Natl Acad Sci U S A. 1997;94:11669–11674. doi: 10.1073/pnas.94.21.11669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt AM, Yan SD, Yan SF, Stern DM. The biology of the receptor for advanced glycation end products and its ligands. Biochim Biophys Acta. 2000;1498:99–111. doi: 10.1016/s0167-4889(00)00087-2. [DOI] [PubMed] [Google Scholar]
- Schwartz M. Neurodegeneration and neuroprotection in glaucoma: development of a therapeutic neuroprotective vaccine: the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2003a;44:1407–1411. doi: 10.1167/iovs.02-0594. [DOI] [PubMed] [Google Scholar]
- Schwartz M. Neuroprotection as a treatment for glaucoma: pharmacological and immunological approaches. Eur J Ophthalmol. 2003b;13:S27–31. doi: 10.1177/112067210301303s05. Suppl 3. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Perspectives on oxygen sensing. Cell. 1999;98:281–284. doi: 10.1016/s0092-8674(00)81957-1. [DOI] [PubMed] [Google Scholar]
- Shirvan A, Kimron M, Holdengreber V, Ziv I, Ben-Shaul Y, Melamed S, Melamed E, Barzilai A, Solomon AS. Anti-semaphorin 3A antibodies rescue retinal ganglion cells from cell death following optic nerve axotomy. J Biol Chem. 2002;277:49799–49807. doi: 10.1074/jbc.M204793200. [DOI] [PubMed] [Google Scholar]
- Shohami E, Ginis I, Hallenbeck JM. Dual role of tumor necrosis factor alpha in brain injury. Cytokine Growth Factor Rev. 1999;10:119–130. doi: 10.1016/s1359-6101(99)00008-8. [DOI] [PubMed] [Google Scholar]
- Sirover MA. New insights into an old protein: the functional diversity of mammalian glyceraldehyde-3-phosphate dehydrogenase. Biochim Biophys Acta. 1999;1432:159–184. doi: 10.1016/s0167-4838(99)00119-3. [DOI] [PubMed] [Google Scholar]
- Smith CD, Carney JM, Starke-Reed PE, Oliver CN, Stadtman ER, Floyd RA, Markesbery WR. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proc Natl Acad Sci U S A. 1991;88:10540–10543. doi: 10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman ER. Protein oxidation and aging. Science. 1992;257:1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- Starkov AA, Fiskum G, Chinopoulos C, Lorenzo BJ, Browne SE, Patel MS, Beal MF. Mitochondrial alpha-ketoglutarate dehydrogenase complex generates reactive oxygen species. J Neurosci. 2004;24:7779–7788. doi: 10.1523/JNEUROSCI.1899-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- Suzukawa K, Miura K, Mitsushita J, Resau J, Hirose K, Crystal R, Kamata T. Nerve growth factor-induced neuronal differentiation requires generation of Rac1-regulated reactive oxygen species. J Biol Chem. 2000;275:13175–13178. doi: 10.1074/jbc.275.18.13175. [DOI] [PubMed] [Google Scholar]
- Swanson KI, Schlieve CR, Lieven CJ, Levin LA. Neuroprotective effect of sulfhydryl reduction in a rat optic nerve crush model. Invest Ophthalmol Vis Sci. 2005;46:3737–3741. doi: 10.1167/iovs.05-0155. [DOI] [PubMed] [Google Scholar]
- Szklarczyk A, Lapinska J, Rylski M, McKay RD, Kaczmarek L. Matrix metalloproteinase-9 undergoes expression and activation during dendritic remodeling in adult hippocampus. J Neurosci. 2002;22:920–930. doi: 10.1523/JNEUROSCI.22-03-00920.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi M, Bucala R, Suzuki T, Ohkubo T, Yamazaki M, Koike T, Kameda Y, Makita Z. Neurotoxicity of advanced glycation end-products for cultured cortical neurons. J Neuropathol Exp Neurol. 2000a;59:1094–1105. doi: 10.1093/jnen/59.12.1094. [DOI] [PubMed] [Google Scholar]
- Takeuchi M, Makita Z, Bucala R, Suzuki T, Koike T, Kameda Y. Immunological evidence that non-carboxymethyllysine advanced glycation end-products are produced from short chain sugars and dicarbonyl compounds in vivo. Mol Med. 2000b;6:114–125. [PMC free article] [PubMed] [Google Scholar]
- Tanihara H, Hangai M, Sawaguchi S, Abe H, Kageyama M, Nakazawa F, Shirasawa E, Honda Y. Up-regulation of glial fibrillary acidic protein in the retina of primate eyes with experimental glaucoma. Arch Ophthalmol. 1997;115:752–756. doi: 10.1001/archopht.1997.01100150754011. [DOI] [PubMed] [Google Scholar]
- Tatton WG, Chalmers-Redman R, Brown D, Tatton N. Apoptosis in Parkinson's disease: signals for neuronal degradation. Ann Neurol. 2003;53:S61–70. doi: 10.1002/ana.10489. Suppl 3. discussion S70–62. [DOI] [PubMed] [Google Scholar]
- Tatton WG, Chalmers-Redman RM, Sud A, Podos SM, Mittag TW. Maintaining mitochondrial membrane impermeability. an opportunity for new therapy in glaucoma? Surv Ophthalmol. 2001;45:S277–283. doi: 10.1016/s0039-6257(01)00207-7. Suppl 3 discussuin S295–276. [DOI] [PubMed] [Google Scholar]
- Tezel G, Chauhan BC, LeBlanc RP, Wax MB. Immunohistochemical assessment of the glial mitogen-activated protein kinase activation in glaucoma. Invest Ophthalmol Vis Sci. 2003;44:3025–3033. doi: 10.1167/iovs.02-1136. [DOI] [PubMed] [Google Scholar]
- Tezel G, Dorr D, Kolker AE, Wax MB, Kass MA. Concordance of parapapillary chorioretinal atrophy in ocular hypertension with visual field defects that accompany glaucoma development. Ophthalmology. 2000a;107:1194–1199. doi: 10.1016/s0161-6420(00)00114-7. [DOI] [PubMed] [Google Scholar]
- Tezel G, Edward DP, Wax MB. Serum autoantibodies to optic nerve head glycosaminoglycans in patients with glaucoma. Arch Ophthalmol. 1999;117:917–924. doi: 10.1001/archopht.117.7.917. [DOI] [PubMed] [Google Scholar]
- Tezel G, Hernandez MR, Wax MB. Immunostaining of heat shock proteins in the retina and optic nerve head of normal and glaucomatous eyes. Arch Ophthalmol. 2000b;118:511–518. doi: 10.1001/archopht.118.4.511. [DOI] [PubMed] [Google Scholar]
- Tezel G, Hernandez MR, Wax MB. In vitro evaluation of reactive astrocyte migration, a component of tissue remodeling in glaucomatous optic nerve head. Glia. 2001a;34:178–189. doi: 10.1002/glia.1052. [DOI] [PubMed] [Google Scholar]
- Tezel G, Kass MA, Kolker AE, Wax MB. Comparative optic disc analysis in normal pressure glaucoma, primary open-angle glaucoma, and ocular hypertension. Ophthalmology. 1996;103:2105–2113. doi: 10.1016/s0161-6420(96)30382-5. [DOI] [PubMed] [Google Scholar]
- Tezel G, Li LY, Patil RV, Wax MB. Tumor necrosis factor-alpha and its receptor-1 in the retina of normal and glaucomatous eyes. Invest Ophthalmol Vis Sci. 2001b;42:1787–1794. [PubMed] [Google Scholar]
- Tezel G, Seigel GM, Wax MB. Autoantibodies to small heat shock proteins in glaucoma. Invest Ophthalmol Vis Sci. 1998;39:2277–2287. [PubMed] [Google Scholar]
- Tezel G, Siegmund KD, Trinkaus K, Wax MB, Kass MA, Kolker AE. Clinical factors associated with progression of glaucomatous optic disc damage in treated patients. Arch Ophthalmol. 2001c:813–818. doi: 10.1001/archopht.119.6.813. [DOI] [PubMed] [Google Scholar]
- Tezel G, Trinkaus K, Wax MB. Alterations in the morphology of lamina cribrosa pores in glaucomatous eyes. Br J Ophthalmol. 2004a;88:251–256. doi: 10.1136/bjo.2003.019281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G, Wax MB. Inhibition of caspase activity in retinal cell apoptosis induced by various stimuli in vitro. Invest Ophthalmol Vis Sci. 1999;40:2660–2667. [PubMed] [Google Scholar]
- Tezel G, Wax MB. Increased production of tumor necrosis factor-alpha by glial cells exposed to simulated ischemia or elevated hydrostatic pressure induces apoptosis in cocultured retinal ganglion cells. J Neurosci. 2000a;20:8693–8700. doi: 10.1523/JNEUROSCI.20-23-08693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G, Wax MB. The mechanisms of hsp27 antibody-mediated apoptosis in retinal neuronal cells. J Neurosci. 2000b;20:3552–3562. doi: 10.1523/JNEUROSCI.20-10-03552.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G, Wax MB. Increased retinal expression of hypoxia inducible factor-1alpha: Correlation with functional damage in postmortem glaucomatous eyes with the expression of adaptive and pathogenic proteins in isolated retina exposed to hypoxia. Invest Ophthalmol Vis Sci (Supplement) 2001:S410. (Abstract). [Google Scholar]
- Tezel G, Wax MB. Glial modulation of retinal ganglion cell death in glaucoma. J Glaucoma. 2003;12:63–68. doi: 10.1097/00061198-200302000-00014. [DOI] [PubMed] [Google Scholar]
- Tezel G, Wax MB. Hypoxia-inducible factor 1alpha in the glaucomatous retina and optic nerve head. Arch Ophthalmol. 2004a;122:1348–1356. doi: 10.1001/archopht.122.9.1348. [DOI] [PubMed] [Google Scholar]
- Tezel G, Wax MB. The immune system and glaucoma. Curr Opin Ophthalmol. 2004b;15:80–84. doi: 10.1097/00055735-200404000-00003. [DOI] [PubMed] [Google Scholar]
- Tezel G, Yang X. Caspase-independent component of retinal ganglion cell death, in vitro. Invest Ophthalmol Vis Sci. 2004;45:4049–4059. doi: 10.1167/iovs.04-0490. [DOI] [PubMed] [Google Scholar]
- Tezel G, Yang X. Comparative gene array analysis of TNF-alpha-induced MAPK and NF--kappaB signaling pathways between retinal ganglion cells and glial cells. Exp Eye Res. 2005;81:207–217. doi: 10.1016/j.exer.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Tezel G, Yang X, Cai J. Proteomic identification of oxidatively modified retinal proteins in a chronic pressure-induced rat model of glaucoma. Invest Ophthalmol Vis Sci. 2005;46:3177–3187. doi: 10.1167/iovs.05-0208. [DOI] [PubMed] [Google Scholar]
- Tezel G, Yang X, Luo C, Peng Y, Sun SL, Sun D. Mechanisms of immune system activation in glaucoma: Oxidative stress stimulates antigen presentation by the retina and optic nerve head glia. Invest Ophthalmol Vis Sci. 2006;47:E-Abstract 1265. doi: 10.1167/iovs.06-0810. Available at www.iovs.org. [DOI] [PMC free article] [PubMed]
- Tezel G, Yang X, Yang J, Wax MB. Role of tumor necrosis factor receptor-1 in the death of retinal ganglion cells following optic nerve crush injury in mice. Brain Res. 2004b;996:202–212. doi: 10.1016/j.brainres.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Thornalley PJ. Cell activation by glycated proteins. AGE receptors, receptor recognition factors and functional classification of AGEs. Cell Mol Biol (Noisy-legrand) 1998;44:1013–1023. [PubMed] [Google Scholar]
- Ullian EM, Barkis WB, Chen S, Diamond JS, Barres BA. Invulnerability of retinal ganglion cells to NMDA excitotoxicity. Mol Cell Neurosci. 2004;26:544–557. doi: 10.1016/j.mcn.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Varela HJ, Hernandez MR. Astrocyte responses in human optic nerve head with primary open-angle glaucoma. J Glaucoma. 1997;6:303–313. [PubMed] [Google Scholar]
- Ventura A, Pelicci PG. Semaphorins: green light for redox signaling? Sci STKE 2002. 2002:PE44. doi: 10.1126/stke.2002.155.pe44. [DOI] [PubMed] [Google Scholar]
- Vlassara H, Bucala R, Striker L. Pathogenic effects of advanced glycosylation: biochemical, biologic, and clinical implications for diabetes and aging. Lab Invest. 1994;70:138–151. [PubMed] [Google Scholar]
- Vorwerk CK, Gorla MS, Dreyer EB. An experimental basis for implicating excitotoxicity in glaucomatous optic neuropathy. Surv Ophthalmol. 1999;43:S142–150. doi: 10.1016/s0039-6257(99)00017-x. Suppl 1. [DOI] [PubMed] [Google Scholar]
- Waller AV. Experiments on the setion of glossopharyngeal and hypoglossal nerves of the frog, and observations on the alterations produced thereby in the structure of their primitive fibres. Philos Trans R Soc Lond B Biol Sci. 1850;140:423–429. [Google Scholar]
- Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Cioffi GA, Cull G, Dong J, Fortune B. Immunohistologic evidence for retinal glial cell changes in human glaucoma. Invest Ophthalmol Vis Sci. 2002;43:1088–1094. [PubMed] [Google Scholar]
- Wang X, Tay SS, Ng YK. An immunohistochemical study of neuronal and glial cell reactions in retinae of rats with experimental glaucoma. Exp Brain Res. 2000;132:476–484. doi: 10.1007/s002210000360. [DOI] [PubMed] [Google Scholar]
- Wax M, Yang J, Tezel G. Autoantibodies in glaucoma. Curr Eye Res. 2002;25:113–116. doi: 10.1076/ceyr.25.2.113.10157. [DOI] [PubMed] [Google Scholar]
- Wax MB, Tezel G. Neurobiology of glaucomatous optic neuropathy: diverse cellular events in neurodegeneration and neuroprotection. Mol Neurobiol. 2002;26:45–55. doi: 10.1385/MN:26:1:045. [DOI] [PubMed] [Google Scholar]
- Wax MB, Tezel G, Edward PD. Clinical and ocular histopathological findings in a patient with normal-pressure glaucoma [see comments] Arch Ophthalmol. 1998a;116:993–1001. doi: 10.1001/archopht.116.8.993. [DOI] [PubMed] [Google Scholar]
- Wax MB, Tezel G, Kawase K, Kitazawa Y. Serum autoantibodies to heat shock proteins in glaucoma patients from Japan and the United States. Ophthalmology. 2001;108:296–302. doi: 10.1016/s0161-6420(00)00525-x. [DOI] [PubMed] [Google Scholar]
- Wax MB, Tezel G, Saito I, Gupta RS, Harley JB, Li Z, Romano C. Anti-Ro/SS-A positivity and heat shock protein antibodies in patients with normal-pressure glaucoma [see comments] Am J Ophthalmol. 1998b;125:145–157. doi: 10.1016/s0002-9394(99)80084-1. [DOI] [PubMed] [Google Scholar]
- Wax MB, Tezel G, Yang J, Peng G, Patil RV, Agarwal N, Sappington RM, Calkins DJ. Induced autoimmunity to heat shock proteins elicits glaucomatous loss of retinal ganglion cells via a FAS/FAS-Ligand pathway. Invest Ophthalmol Vis Sci. 2006;47:E-Abstract 1828. Available at www.iovs.org.
- Weber AJ, Kaufman PL, Hubbard WC. Morphology of single ganglion cells in the glaucomatous primate retina. Invest Ophthalmol Vis Sci. 1998;39:2304–2320. [PubMed] [Google Scholar]
- Whitmore AV, Libby RT, John SW. Glaucoma: Thinking in new ways-a role for autonomous axonal self-destruction and other compartmentalised processes? Prog Retin Eye Res. 2005 doi: 10.1016/j.preteyeres.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Whitmore AV, Lindsten T, Raff MC, Thompson CB. The proapoptotic proteins Bax and Bak are not involved in Wallerian degeneration. Cell Death Differ. 2003;10:260–261. doi: 10.1038/sj.cdd.4401147. [DOI] [PubMed] [Google Scholar]
- Winkler BS, Starnes CA, Sauer MW, Firouzgan Z, Chen SC. Cultured retinal neuronal cells and Muller cells both show net production of lactate. Neurochem Int. 2004;45:311–320. doi: 10.1016/j.neuint.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Xu YC, Wu RF, Gu Y, Yang YS, Yang MC, Nwariaku FE, Terada LS. Involvement of TRAF4 in oxidative activation of c-Jun N-terminal kinase. J Biol Chem. 2002;277:28051–28057. doi: 10.1074/jbc.M202665200. [DOI] [PubMed] [Google Scholar]
- Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J, Migheli A, Nawroth P, Stern D, Schmidt AM. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer's disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- Yan X, Tezel G, Wax MB, Edward DP. Matrix metalloproteinases and tumor necrosis factor alpha in glaucomatous optic nerve head. Arch Ophthalmol. 2000;118:666–673. doi: 10.1001/archopht.118.5.666. [DOI] [PubMed] [Google Scholar]
- Yang J, Patil RV, Yu H, Gordon M, Wax MB. T cell subsets and sIL-2R/IL-2 levels in patients with glaucoma. Am J Ophthalmol. 2001a;131:421–426. doi: 10.1016/s0002-9394(00)00862-x. [DOI] [PubMed] [Google Scholar]
- Yang J, Tezel G, Patil RV, Romano C, Wax MB. Serum autoantibody against glutathione S-transferase in patients with glaucoma. Invest Ophthalmol Vis Sci. 2001b;42:1273–1276. [PubMed] [Google Scholar]
- Yang J, Yang P, Tezel G, Patil RV, Hernandez MR, Wax MB. Induction of HLA-DR expression in human lamina cribrosa astrocytes by cytokines and simulated ischemia. Invest Ophthalmol Vis Sci. 2001c;42:365–371. [PubMed] [Google Scholar]
- Yang X, Luo C, Cai J, Tezel G. Proteomic identification of phosphorylated proteins in a chronic pressure-induced rat model of glaucoma. Invest Ophthalmol Vis Sci. 2006;47:E-Abstract 198. doi: 10.1167/iovs.05-0208. Available at www.iovs.org. [DOI] [PubMed]
- Yoles E, Schwartz M. Degeneration of spared axons following partial white matter lesion: implications for optic nerve neuropathies. Exp Neurol. 1998;153:1–7. doi: 10.1006/exnr.1998.6811. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Watanabe A, Ihara Y. Collapsin response mediator protein-2 is associated with neurofibrillary tangles in Alzheimer's disease. J Biol Chem. 1998;273:9761–9768. doi: 10.1074/jbc.273.16.9761. [DOI] [PubMed] [Google Scholar]
- Yuan L, Neufeld AH. Tumor necrosis factor-alpha: A potentially neurodestructive cytokine produced by glia in the human glaucomatous optic nerve head. Glia. 2000;32:42–50. [PubMed] [Google Scholar]
- Yucel YH, Zhang Q, Weinreb RN, Kaufman PL, Gupta N. Effects of retinal ganglion cell loss on magno-, parvo-, koniocellular pathways in the lateral geniculate nucleus and visual cortex in glaucoma. Prog Retin Eye Res. 2003;22:465–481. doi: 10.1016/s1350-9462(03)00026-0. [DOI] [PubMed] [Google Scholar]


