Abstract
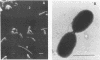
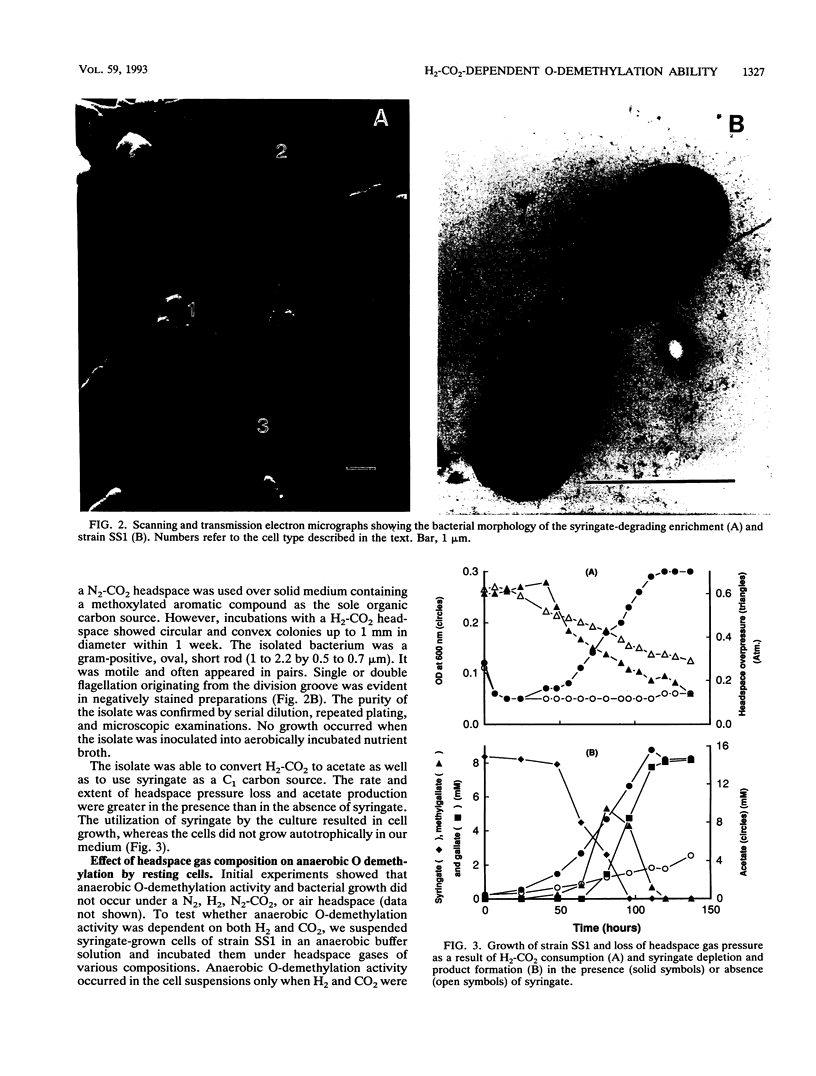
The ability of microorganisms in sediments from the Atlantic Coastal Plain to biodegrade methoxylated aromatic compounds was examined. O-demethylation activity was detected in deep (121- and 406-m) sediments, as well as in the surface soil. A syringate-demethylating consortium, containing at least three types of bacteria, was enriched from a deep-sediment sample in a medium containing syringate as the sole organic carbon source and with a N2-CO2 atmosphere. An isolate which demethylated syringate was obtained from the enrichment on an agar medium incubated under a H2-CO2 but not a N2-CO2 or N2 atmosphere. O demethylation of syringate of this isolate was dependent on the presence of both H2 and CO2 in the gas phase. The metabolism of syringate occurred in a sequential manner: methylgallate accumulated transiently before it was converted to gallate. Mass balance analysis suggests that the stoichiometry of the reaction in this isolate proceeds in accordance with the following generalized equation: C7H3O3(OCH3)n- + nHCO3- + nH2 → C7H3O3(OH)n- + nCH3COO- + nH2O.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brauman A., Kane M. D., Labat M., Breznak J. A. Genesis of acetate and methane by gut bacteria of nutritionally diverse termites. Science. 1992 Sep 4;257(5075):1384–1387. doi: 10.1126/science.257.5075.1384. [DOI] [PubMed] [Google Scholar]
- Braun K., Gottschalk G. Effect of molecular hydrogen and carbon dioxide on chemo-organotrophic growth of Acetobacterium woodii and Clostridium aceticum. Arch Microbiol. 1981 Jan;128(3):294–298. doi: 10.1007/BF00422533. [DOI] [PubMed] [Google Scholar]
- Daniel S. L., Keith E. S., Yang H., Lin Y. S., Drake H. L. Utilization of methoxylated aromatic compounds by the acetogen Clostridium thermoaceticum: expression and specificity of the co-dependent O-demethylating activity. Biochem Biophys Res Commun. 1991 Oct 15;180(1):416–422. doi: 10.1016/s0006-291x(05)81309-9. [DOI] [PubMed] [Google Scholar]
- DeWeerd K. A., Concannon F., Suflita J. M. Relationship between hydrogen consumption, dehalogenation, and the reduction of sulfur oxyanions by Desulfomonile tiedjei. Appl Environ Microbiol. 1991 Jul;57(7):1929–1934. doi: 10.1128/aem.57.7.1929-1934.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWeerd K. A., Saxena A., Nagle D. P., Jr, Suflita J. M. Metabolism of the 18O-methoxy substituent of 3-methoxybenzoic acid and other unlabeled methoxybenzoic acids by anaerobic bacteria. Appl Environ Microbiol. 1988 May;54(5):1237–1242. doi: 10.1128/aem.54.5.1237-1242.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane M. D., Breznak J. A. Acetonema longum gen. nov. sp. nov., an H2/CO2 acetogenic bacterium from the termite, Pterotermes occidentis. Arch Microbiol. 1991;156(2):91–98. doi: 10.1007/BF00290979. [DOI] [PubMed] [Google Scholar]
- Kerby R., Zeikus J. G. Anaerobic catabolism of formate to acetate and CO2 by Butyribacterium methylotrophicum. J Bacteriol. 1987 May;169(5):2063–2068. doi: 10.1128/jb.169.5.2063-2068.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumholz L. R., Crawford R. L., Hemling M. E., Bryant M. P. Metabolism of gallate and phloroglucinol in Eubacterium oxidoreducens via 3-hydroxy-5-oxohexanoate. J Bacteriol. 1987 May;169(5):1886–1890. doi: 10.1128/jb.169.5.1886-1890.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajoie S. F., Bank S., Miller T. L., Wolin M. J. Acetate production from hydrogen and [13C]carbon dioxide by the microflora of human feces. Appl Environ Microbiol. 1988 Nov;54(11):2723–2727. doi: 10.1128/aem.54.11.2723-2727.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matin A. Organic nutrition of chemolithotrophic bacteria. Annu Rev Microbiol. 1978;32:433–468. doi: 10.1146/annurev.mi.32.100178.002245. [DOI] [PubMed] [Google Scholar]
- Shelton D. R., Tiedje J. M. Isolation and partial characterization of bacteria in an anaerobic consortium that mineralizes 3-chlorobenzoic Acid. Appl Environ Microbiol. 1984 Oct;48(4):840–848. doi: 10.1128/aem.48.4.840-848.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaki M., Kamagata Y., Nakamura K., Mikami E. Utilization of methoxylated benzoates and formation of intermediates by Desulfotomaculum thermobenzoicum in the presence or absence of sulfate. Arch Microbiol. 1992;157(3):209–212. doi: 10.1007/BF00245151. [DOI] [PubMed] [Google Scholar]
- Taylor B. F. Aerobic and Anaerobic Catabolism of Vanillic Acid and Some Other Methoxy-Aromatic Compounds by Pseudomonas sp. Strain PN-1. Appl Environ Microbiol. 1983 Dec;46(6):1286–1292. doi: 10.1128/aem.46.6.1286-1292.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z. R., Daniel S. L., Drake H. L. Characterization of a CO-dependent O-demethylating enzyme system from the acetogen Clostridium thermoaceticum. J Bacteriol. 1988 Dec;170(12):5747–5750. doi: 10.1128/jb.170.12.5747-5750.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]