Abstract
Background
Most quality of life (QoL) data in AF has been collected from clinical trial patients. We sought to characterize symptoms and QoL in a large inception cohort of unselected AF patients, and explore the impact of age, gender, and AF clinical course on QoL measures over time.
Methods
We collected symptom and QoL data on 963 patients with new onset AF enrolled in a multi-center observational registry. Patients were primarily managed with pharmacologic therapy and cardioversion. QoL instruments including the SF-12, University of Toronto AF Severity Scale, and the AF Symptom Checklist were completed at baseline and repeated over 2.5 years. Time-weighted QoL summary scores over the first year were calculated for each patient. Factors associated with those summary scores were explored in multivariable analyses.
Results
QoL was moderately impaired at baseline, but quickly approached population norms and remained stable thereafter. Following multivariable adjustment, female gender was strongly associated with higher symptom scores and lower QoL scores. Older (age>65 years) patients reported less prominent disease-specific impairment in QoL than younger patients. In part because 73% of patients appeared to maintain sinus rhythm for the first year, AF clinical course had a comparatively small impact on QoL during this timeframe.
Conclusions
QoL is impaired in newly diagnosed AF patients, but improves to normal levels with standard treatments. Within the first year after diagnosis, gender, age, and comorbid conditions are more strongly associated with QoL outcomes than the clinical course of AF itself.
Patients with atrial fibrillation (AF) exhibit an extremely broad range of symptoms and health-related quality of life (QoL), ranging from asymptomatic to severely impaired.1, 2 Because interventions for terminating or suppressing AF have not been shown to prevent strokes or reduce mortality,3, 4 the primary goal of rhythm control interventions should be to reduce symptoms and improve QoL.
A number of studies have demonstrated meaningful changes in quality of life following both pharmacologic and non-pharmacologic rhythm control interventions, 5–8 but such studies have generally enrolled highly symptomatic patients. In contrast, little is known about quality of life in unselected patients with AF. We therefore sought to characterize quality of life and its determinants in a prospective observational registry of new-onset AF patients. Based on prior studies, 9, 10 we hypothesized that, in addition to comorbid conditions and clinical course, certain demographic factors such as age and gender would substantially impact QoL outcomes and the reporting of symptoms.
Methods
Study population
The Fibrillation Registry Assessing Costs, Therapies, Adverse events and Lifestyle (FRACTAL) is an inception cohort study of patients with atrial fibrillation or flutter. Between May 1997 and June 2000, 1005 patients were enrolled at 17 centers in the United States and Canada following their first electrocardiographically confirmed episode. AF occurring within 7 days of cardiac surgery was excluded. Patient management remained at the discretion of local practitioners. The study protocol was approved by the institutional committee on human research at each site, and patients provided informed consent prior to study entry. Of the initial registry cohort (N=1005), 963 (96%) completed at least the baseline quality of life questionnaires and form the basis for this report.
Data Collection and Follow-up
At each enrolling center, trained research assistants collected baseline data at study entry by chart review and either personal or telephone interviews of study subjects. Baseline data collection forms captured standard demographic variables, in-depth cardiovascular and health history questions (including review of echocardiogram reports, when available), individual components of the Charlson comorbidity index,11 and historical items pertaining to the initial episode of AF such as associated illness and the presence or absence of six specific symptoms. At the time of study entry, additional data were gathered regarding all aspects of initial AF management, including cardioversion, antiarrhythmic drugs, cardiac procedures, anticoagulation, and physician specialty type.
Patients were contacted for follow-up at 3, 6, 12, 18, 24 and 30 months following enrollment. At each follow-up, AF recurrence, changes in the management plan, current medication usage, adverse events, cardiac procedures, and health care resource utilization were ascertained.
Quality of Life instruments
Three quality of life instruments were used in this study. The Medical Outcomes Study Short Form-12 (SF-12),12 derived from the widely used SF-36, was used as a generic health status measure. The SF-12 provides two summary scales: the physical (PCS) and mental component scores (MCS), each scaled to a population norm of 50, with higher scores indicating better QoL. Mean PCS and MCS scores for US residents aged 65–74 without AF are 43.3 and 52.7, respectively.13
Two disease-specific scales were also used: the Arrhythmia Symptom Frequency and Severity Checklist 14 and a version of the University of Toronto AF Severity Scale.5 The Symptom Checklist asks patients to quantify the frequency (graded 0–4), and severity (graded 0–3), of 16 individual AF symptoms. Frequency and severity scores therefore range from 0–64 and 0–48, with higher scores indicating greater symptom burden. Healthy subjects aged 54 ± 14 years reported mean frequency and severity scores of 10 and 8 in a previous study.2 The University of Toronto Scale that was used generates four summary scores for physical, psychological, functional, and exercise limitations, each set to a maximum value of 100 – indicating no impairment – with lower scores representing greater limitation. This scale is intended to capture AF-specific impairments in quality of life that extend beyond the domain of symptoms.
Each of the QoL instruments was completed at the time of study enrollment and repeated at all but the 18 month follow-up interval (i.e. 3, 6, 12, 24 and 30 months after enrollment). Quality of life questionnaires were self-administered by study subjects at local sites, and forwarded to the data coordinating center, where data entry, cleaning, and analysis were performed.
Analytic Methods
All analyses were performed using SAS version 8.2 (SAS Institute, Cary, NC) software. Group-wise comparisons of discrete and continuous variables were made using Fisher’s exact and two sample t-tests, respectively. Statistical significance was defined as a two-tailed p-value of <0.05. Multivariable linear regression models were constructed as described below.
Missing data methods
For the SF-12, physical and mental component summary scores were considered missing if >50% of individual items were missing for a given time point (frequency 0.8–2.1%). If <50% of individual items were missing on a completed questionnaire, those missing items were imputed as the mode response for that item at that time point in the entire study population. Summary scores were then calculated using standard scoring algorithms.15
For the University of Toronto scales, single item imputation was used only for the functional limitations scale, which had a high rate of non-response to a few individual items (e.g. gardening or sexual activity). For this scale only, if <50% of individual items were missing, the summary score was calculated using the weighted average of the items for which responses were given. If >50% of items were missing, the functional limitations score was considered missing. For the other University of Toronto sub-scales, the summary score was treated as missing if individual items were missing (~1% for each).
The AF Symptom Checklist was scored according to published methods.14 If the frequency of a symptom was recorded as “never”, the severity of that symptom was set to zero. If a person indicated that a symptom was present, but did not record a severity, the severity was imputed as the mean severity score of other patients who had that symptom at that time point.
Generation of summary scores for repeated measures
Because AF is a relapsing-remitting condition (particularly early in its course), we felt the most appropriate QoL summary measure would be a composite score over the time interval for which we had nearly complete data, which was 12 months. Therefore, we generated a single summary measure for each of the 8 summary scales (2 each for the SF-12 and AF symptom checklist; 4 for the University of Toronto) by linearly interpolating each consecutive pair of scores for each subject between 0, 3, 6, and 12 months and calculating the area under the resulting curve, then dividing by 365. This results in a time-weighted average daily score for each summary scale for each subject. For subjects with a single missing score during the first 12 months, linear interpolation was made across the missing time point. This is equivalent to imputing the missing value as the mean of the scores just before and just after the missing time point.
Regression Analysis on QoL summary measures
The influence of various patient characteristics, therapies, and clinical evolution of AF on the 12-month average QoL summary scores was explored using multivariable linear regression. Age was dichotomized at 65 in these models because it was close to the average at enrollment (66 ± 14 years) and because this age is sometimes used as a cut point for evaluating stroke risk in AF.16, 17
For each of the 8 summary QoL measures, linear regression models with stepwise selection were constructed with a p-value for entrance set at 0.15 and p-value for exit set a 0.10 in order to include all important measured confounders. Covariates entered into these models included demographics (age, gender, race), cardiac and non-cardiac comorbid illnesses, initial AF pattern (persistent, paroxysmal, or permanent), initial medical therapies, and the number of AF recurrences recorded during follow-up. Some of these covariates were chosen based on a relationship to QoL scores demonstrated in prior studies.5, 10, 18–20 Valvular disease was defined as moderate or severe valvular regurgitation or stenosis documented by echocardiography at study enrollment.
Results
Quality of life surveys were completed by 963 registry participants at enrollment. Baseline characteristics of this population, stratified by gender and by age above or below 65 years, are displayed in Table I.
Table I.
Baseline characteristics stratified by gender and age group
| Variables | Women (n= 382 ) | Men (n= 581) | P value | Age ≤65 (n=619) | Age>65 (n=344) | P value |
|---|---|---|---|---|---|---|
| Age (yrs) | 69 ± 13 | 64 ± 15 | <0.001 | -- | -- | -- |
| Female gender | -- | -- | -- | 32% | 44% | 0.002 |
| Hypertension | 55% | 45% | 0.001 | 34% | 57% | 0.001 |
| Valvular disease | 21% | 15% | 0.03 | 14% | 19% | 0.03 |
| CAD | 17% | 30% | <0.001 | 13% | 31% | <0.001 |
| Diabetes mellitus | 12% | 12% | NS | 4.4% | 12% | 0.001 |
| Heart failure | 17% | 19% | NS | 12% | 22% | 0.001 |
| Prior stroke/TIA | 8.0% | 6.9% | 0.05 | 2.9% | 9.5% | 0.001 |
The initial episode of AF either spontaneously converted to sinus rhythm or was successfully treated with cardioversion in 926 (96%) patients. AF was accepted as a permanent state from the outset in the other 37 patients. During the first year of follow-up, 10 more patients progressed to permanent AF, and 209 additional patients (22%) had ≥ 1 recurrences of AF (documented by EKG in 74%, ambulatory monitor or pacemaker telemetry in 3.5%, and physician exam or patient self-report in 22.5%). Therefore 707 patients (73%) had no known AF recurrence over the first year. Among patients with documented recurrences, 90% had ≤2 (range: 0–9).
Table II presents the presence or absence of 6 common symptoms typically associated with AF at the time of the first-documented episode, stratified by gender and age. These were assessed in the case report forms, rather than as part of the AF symptom checklist, which has a longer recall period. As shown, women with AF were more likely to be symptomatic than men, and were more likely to report palpitations as a symptom. Compared with younger patients, those >65 years in age were significantly more likely to report dyspnea and fatigue, but less likely to report dizziness or palpitations.
Table II.
Baseline symptoms* and treatments by gender and age group
| Women (n= 382) | Men (n= 581) | P value | Age ≤65 (n=644) | Age>65 (n=361) | P value | |
|---|---|---|---|---|---|---|
| Symptoms | ||||||
| Asymptomatic | 11% | 17% | 0.01 | 15% | 15% | NS |
| Dizziness | 28% | 23% | NS | 31% | 22% | 0.005 |
| Palpitations | 57% | 46% | 0.001 | 65% | 42% | <0.001 |
| Syncope | 2.5% | 3.2% | NS | 3.6% | 2.5% | NS |
| Chest pain | 23% | 23% | NS | 23% | 23% | NS |
| Dyspnea | 61% | 61% | NS | 56% | 64% | 0.03 |
| Fatigue | 48% | 50% | NS | 44% | 52% | 0.02 |
| Initial Therapies | ||||||
| Cardioversion | 43% | 47% | 0.30 | 42% | 47% | 0.10 |
| AAD | 51% | 48% | 0.40 | 55% | 48% | 0.03 |
| Warfarin | 63% | 66% | 0.90 | 56% | 69% | <0.001 |
Assessed on baseline case report forms; patients could have multiple symptoms, so columns do not sum to 100%. AAD= anti-arrhythmic drug
Unadjusted quality of life scores are displayed in Figure 1. For all three scales, patients reported a moderate level of symptoms and impaired QoL at baseline that improved to approximate population norms2, 13 over the first 6 months, with little change thereafter.
Figure 1.
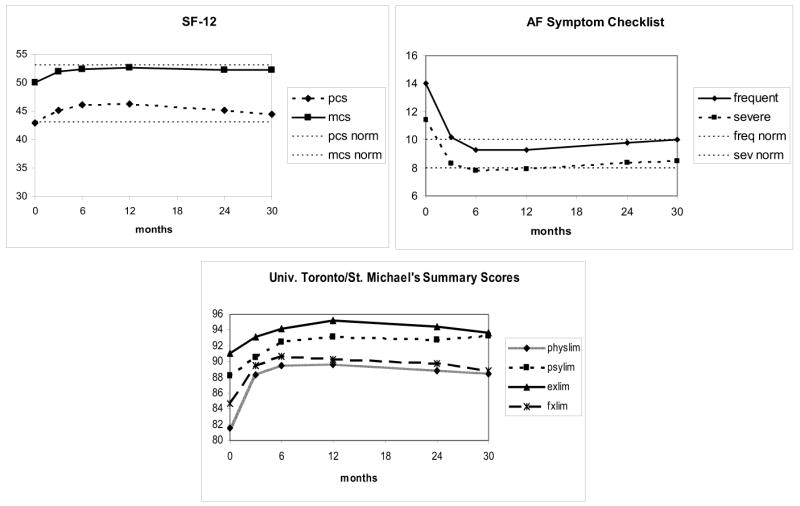
Unadjusted QoL summary scores for the FRACTAL population (N=963), from enrollment through 30 months. PCS= physical component summary score; MCS= mental component summary score; physlim= physical limitations scale; psylim= psychological limitations scale; fxlim= functional limitations scale; exlim= exercise limitations scale. Population norms for the SF-1213 and AF Symptom checklist2 are indicated by dashed horizontal lines.
Figures 2 and 3 display unadjusted results for the physical component (PCS) and mental component (MCS) scales of the SF-12 and the AF Symptom Checklist, stratified by gender. For 7 of the 8 summary scales assessed, women reported significantly greater impairment (lower QoL scores and higher symptom scores) than men at baseline, and these baseline differences between genders persisted throughout follow-up.
Figure 2.
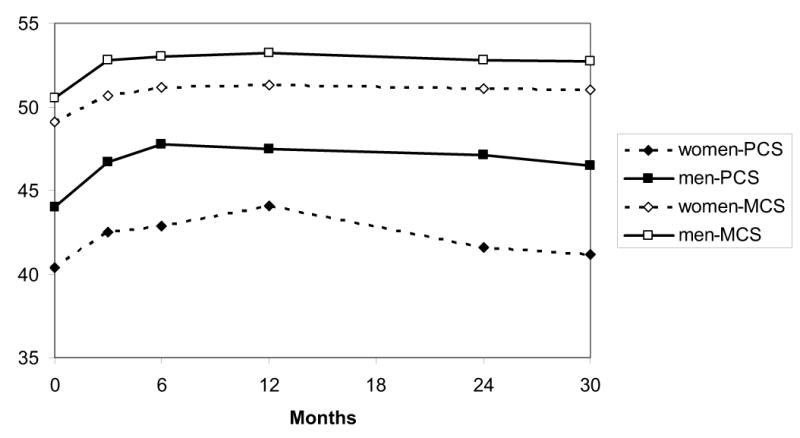
Unadjusted SF-12 summary scores, stratified by gender, from enrollment through 30 months. PCS= physical component summary score; MCS= mental component summary score.
Figure 3.
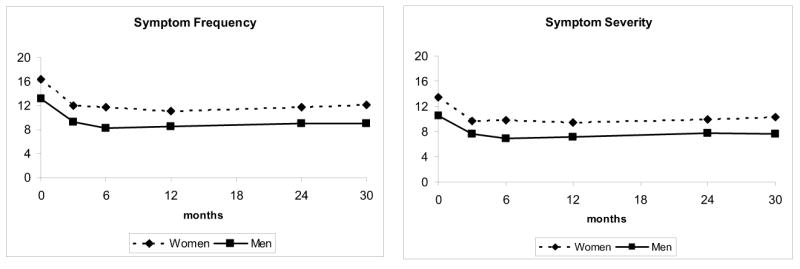
Unadjusted symptom frequency and symptoms severity scores, stratified by gender, from enrollment through 30 months.
Of the 963 subjects who completed baseline QoL surveys, 813 (84%) completed surveys with all QoL instruments during at least 2 of the 3 follow-up periods (3, 6, and 12 months) and were included in the calculation of time-weighted summary scores and regression analyses. There were no significant differences in the baseline characteristics of those 813 subjects compared with the 150 who completed all surveys at <2 of the first 3 follow-up periods.
The results of the multivariable models constructed to assess the 0–12 month summary score for each of the 8 quality of life scales are shown in Table III. As expected, non-cardiac comorbid illnesses mainly correlated with diminished scores on scales reflecting general health and functional status, while cardiac conditions correlated with both reduced general physical function and higher AF symptom scores.
Table III.
Regression coefficients from multivariable models of 8 QoL time-weighted summary scores (baseline through 12-months) demonstrating the relationships between various patient characteristics and treatments, and QoL scores.
| PCS | MCS | Symptom Frequency | Symptom Severity | Physical Limitations | Functional Limitations | Exercise Limitations | Psychological Limitations | |
|---|---|---|---|---|---|---|---|---|
| Mean ± s.d. | 45.3±9.3 | 52.1±7.1 | 10.3±7.3 | 8.6±6.1 | 88.4±9.6 | 87.7±15.3 | 93.7±9.7 | 91.5±10.9 |
| Median (IQR) | 47.5(38–53) | 54 (48–57) | 8.5 (5–15) | 7 (4–12) | 90 (83–96) | 94 (83–98) | 98 (91–100) | 96 (86–100) |
| NYHA >1 | −3.4* | – | +2.0* | +1.4† | −3.2* | −4.9* | – | −2.5† |
| Female gender | −4.5* | −2.0* | +3.6* | +3.0* | −3.3* | −5.7* | – | – |
| Age >65 years | −1.4‡ | +1.4† | −2.3* | −1.8* | – | −2.6‡ | +1.5‡ | +2.3† |
| Nonwhite race | – | – | – | – | – | – | −4.2† | −3.6‡ |
| Valve disease | −3.7* | −1.9† | +2.3* | +1.6† | −3.6* | −6.3* | −3.6* | −2.9† |
| Prior MI | −2.3† | – | – | – | – | – | −4.4* | – |
| Cardiomyopathy | – | – | – | – | – | – | −3.4‡ | – |
| Hypertension | −1.7† | – | – | – | – | −2.8† | – | – |
| Recurrences | – | – | +0.6‡ | +0.7† | −0.9† | – | −1.0† | −1.7* |
| Permanent AF | – | – | – | – | −2.7‡ | – | – | – |
| Persistent AF | – | – | – | – | – | – | – | – |
| Initial AAD | −1.6† | −1.3† | +1.1‡ | +1.1† | – | −2.9† | – | −1.5‡ |
| Initial warfarin | −1.7† | – | – | – | −1.4‡ | – | −1.6‡ | – |
| COPD | −4.2* | −2.0‡ | +4.1* | +3.1* | −4.5* | −9.8* | – | – |
| Asthma | −3.2† | – | – | – | −3.3‡ | – | – | – |
| Renal disease | – | – | – | – | −5.6‡ | – | – | – |
| Diabetes mellitus | −2.4‡ | – | – | – | – | −4.7† | – | – |
| Hemiplegia | −12.6† | – | – | – | – | −17.4† | – | – |
| CTD | −6.5† | – | – | – | – | −10.3† | – | – |
Only coefficients with multivariate p values <0.05 are shown. Those with multivariate p values >0.05 and <0.10 were also retained in the models. PCS= physical component summary score; MCS= mental component summary score; IQR= interquartile range; NYHA= New York Heart Association Class; Initial AAD= antiarrhythmic drug prescribed at enrollment; Recurrences= number of AF recurrences documented during follow-up; Permanent AF= acceptance of permanent AF at any time during follow-up; persistent AF= first episode not self-terminating; COPD= chronic obstructive pulmonary disease; CTD= connective tissue disease.
p<0.001,
p<0.01,
p<0.05
After multivariate adjustment, women reported significantly lower QoL and higher symptoms scores than men, as seen on 6 of the 8 summary scores assessed. These differences were highly statistically significant and clinically meaningful, including a ~10% difference on the SF-12 PCS score and nearly 40% differences for the symptom frequency and severity scores.
The effect of age on QoL scores was less clear. Patients >65 years in age, compared with younger patients, had slightly lower adjusted scores on scales relating to general health and physical functioning, but slightly higher scores on scales pertaining to psychological health. Older patients reported significantly lower mean symptom frequency and severity scores than younger patients, suggesting that their AF resulted in smaller average disease-specific impairment in QoL.
Persistent (defined by the performance of cardioversion to terminate the first episdoe) and permanent AF had almost no significant effect on any QoL measure (Table III), however, few patients (N=47, or 4.9%) progressed to permanent AF during the first year. The model coefficients suggest that single episodes of recurrent AF were associated with small decrements in QoL. Patients with numerous recurrences would be expected to experience larger reductions in QoL – but only ~3% of registry participants had >2 recurrences documented. Patients who were prescribed antiarrhythmic drugs at baseline had modestly lower QoL scores and higher symptoms scores than those who were not, suggesting these drugs tended to be prescribed for more symptomatic patients.
We examined the potential effect of missing data on our multivariable models by comparing the results shown in Table III with “complete case” analyses by including only subjects with no missing data from 0–12 months in the models. This resulted in only small changes in the magnitude, but not the direction, of the regression coefficients.
Discussion
In this large inception cohort of newly diagnosed AF patients, we found moderate symptom burden and reductions in both generic and disease-specific quality of life at baseline, which normalized quickly after initial treatment and remained largely stable thereafter. Symptomatic recurrences of AF were relatively infrequent during early follow-up (22% at one year) in this population. QoL and symptom scores were therefore influenced less by the clinical course of AF than by comorbid illnesses and demographic factors such as age and, prominently, gender.
Previous studies have shown that AF patients have impaired QoL compared with healthy controls 2, 18 and that QoL among AF patients improves with a variety of pharmacological and non-pharmacological rhythm control and rate control interventions.5–8, 21–23 Rate control vs. rhythm control trials13, 18 have reported that QoL generally improves over time in AF patients to a similar degree for either strategy, although the RACE trial found that the presence of sinus rhythm at study termination was associated with larger QoL improvements.18
As might be expected, the new-onset AF patients enrolled in FRACTAL reported lower baseline symptom scores and higher baseline SF-12 summary scores than patients enrolled in typical clinical trials.2, 5, 7, 13 Moreover, in this unselected population, symptom frequency and severity scores by 6 months were similar to those found in healthy subjects (mean 10 and 8, respectively) 2 and SF-12 physical and mental component summary scores were roughly equal to a U.S. reference population age 65–74 without AF (mean 43 and 53, respectively).13 These findings suggest that most patients early in the “natural history” of their AF will enjoy a preserved QoL with the type of management seen in this registry – including rate controlling agents in ~70% of patients and membrane-active antiarrhythmic drugs in ~50%. More aggressive rhythm control interventions, such as pulmonary vein isolation, may be most beneficial for highly symptomatic patients, or for those in whom important symptoms persist despite initial pharmacological therapy, rather than considered as first-line therapy.24 A study randomizing patients with new onset AF to first line ablation versus medical therapy would be required to evaluate this question.
Our analysis also points to a very prominent association between gender and both symptoms and QoL in AF. The fact that women with AF report greater symptom burden and lower QoL has been reported previously.10, 19 However, prior investigations have not adjusted QoL scores for known clinical and demographic differences between men and women with AF.9 After doing this, we found that the effect of female gender on QoL scores was similar to or greater than that of NYHA class ≥2 heart failure (compared with 0-1), moderate to severe valvular disease, or COPD in our study cohort.
It remains unclear whether this large gender difference in QoL among AF patients is due to biophysical or psychosocial factors – or both. Prior authors have speculated that this effect may be related to depression,10, 19 which is known to be more common among women than men, and has been shown to strongly influence QoL measures in other cardiovascular conditions.25, 26 Whatever the cause, it is evident that women with AF differ from men in their demographic and clinical characteristics, their subjective experience of the condition, and possibly in how they are managed and how they respond to therapy.9, 19, 27 Better understanding of the factors that underlie these differences will be needed in order to optimize outcomes for women with AF.
To a lesser extent, prior studies have also pointed to a relationship between age and QoL in AF. One group, in fact, has questioned whether AF reduces QoL in elderly patients at all.28 It makes intuitive sense that younger, more active patients may find AF significantly more disruptive than older patients, particularly those with additional health problems. The results of our study, in which older age was associated with lower AF symptom frequency and severity scores, support this notion, and are consistent with analysis from the RACE trial, which reported that age <69 was associated with a greater likelihood of improved QoL during study follow-up.18 It follows that younger patients may be likely to benefit more from aggressive rhythm control interventions than the elderly.
We also found a distinct difference in the pattern of symptoms reported by older, compared with younger patients. The greater prevalence of dizziness and palpitations in younger patients could be related to effects of aging on the cardiac conduction system, with younger patients typically having more rapid ventricular rates during AF. On the other hand, the greater degree of dyspnea and fatigue in older patients could result from age-related changes in diastolic function and a relatively greater reliance on the atrial contribution to diastolic filling. Understanding of these age-related differences may have clinical value in assessing patient’s initial symptoms and response to treatment.
Our analysis is limited by several factors. First, because of the observational nature of the registry, and the low rate of early documented AF recurrence and progression to permanent AF, we cannot draw firm conclusions regarding the relative merits of a rhythm control strategy or the benefit of actual sinus rhythm maintenance on QoL in this population. These issues have been addressed in other studies.8, 18 Our surveillance methods for AF recurrence, which relied significantly on symptoms and patient self-report, probably underestimated AF episodes. However, asymptomatic AF, by nature, would not be expected to have a large impact on our QoL measures. The period of follow-up was relatively short, therefore we cannot draw conclusions about QoL for AF patients with longer-standing disease. Finally, the data in this study were collected prior to the publication of the AFFIRM trial, at a time when ablative techniques for AF management were not widely used. AF management in today’s clinical environment may therefore differ from that observed in our registry.
Acknowledgments
FRACTAL registry participating centers (and local investigators), include the following: Beth Israel Deaconess Medical Center, Boston, MA (P. Zimetbaum, M.E. Josephson); Rhode Island Hospital, Providence, RI (R. Lemery, A.E. Buxton); Mayo Clinic, Rochester, MN (P. Friedman, M.S. Stanton); Montreal General Hospital, Montreal, Quebec (T. Hadjis); Sentara Virginia Beach General Hospital, Virginia Beach, VA (J.J. Griffin); Victoria Heart Institute, Victoria, B.C. (R.A. Leather); University of Pittsburgh Medical Center, Pittsburgh, PA (D. Schwartzmann); Good Samaritan Hospital, Los Angeles, CA (D. Cannom); Loyola University Medical Center, Chicago, IL (B. Olshansky); Kaiser Permanente Medical Center, Los Angeles, CA (A. Kotlewski); Duke University Medical Center, Durham, NC (T.D. Bahnson); Framingham/MetroWest Medical Center, Framingham, MA (D. Love); St. Paul Heart Clinic, St. Paul, MN (D.N. Dunbar); University of Maryland Medical Center, Baltimore, MD (M.R.Gold); Mount Auburn Hospital, Cambridge, MA (P. Voukydis); University of Rochester Medical Center, Rochester, NY (D. Huang); Hospital of the University of Pennsylvania, Philadelphia, PA (D. Callans).
Footnotes
Financial support: the FRACTAL registry has received financial support from Medtronic, Inc (Minneapolis, MN) and AstraZeneca (Wilmington, DE). Dr Reynolds is the recipient of grant K23HL077171 from the National Heart, Lung, and Blood Institute (NHLBI). Dr. Essebag is the recipient of a Clinician Scientist Award from the Canadian Institutes of Health Research (CIHR).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Callans D. Asymptomatic atrial fibrillation in symptomatic patients. J Cardiovasc Electrophysiol. 2004;15:925–926. doi: 10.1046/j.1540-8167.2004.04345.x. [DOI] [PubMed] [Google Scholar]
- 2.Dorian P, Jung W, Newman D, et al. The impairment of health-related quality of life in patients with intermittent atrial fibrillation: implications for the assessment of investigational therapy. J Am Coll Cardiol. 2000;36(4):1303–9. doi: 10.1016/s0735-1097(00)00886-x. [DOI] [PubMed] [Google Scholar]
- 3.Van Gelder IC, Hagens VE, Bosker HA, et al. A Comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347(23):1834–1840. doi: 10.1056/NEJMoa021375. [DOI] [PubMed] [Google Scholar]
- 4.Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347(23):1825–33. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 5.Dorian P, Paquette M, Newman D, et al. Quality of life improves with treatment in the Canadian Trial of Atrial Fibrillation. Am Heart J. 2002;143(6):984–90. doi: 10.1067/mhj.2002.122518. [DOI] [PubMed] [Google Scholar]
- 6.Lonnerholm S, Blomstrom P, Nilsson L, et al. Effects of the maze operation on health-related quality of life in patients with atrial fibrillation. Circulation. 2000;101(22):2607–11. doi: 10.1161/01.cir.101.22.2607. [DOI] [PubMed] [Google Scholar]
- 7.Pappone C, Rosanio S, Augello G, et al. Mortality, morbidity, and quality of life after circumferential pulmonary vein ablation for atrial fibrillation: outcomes from a controlled nonrandomized long-term study. J Am Coll Cardiol. 2003;42(2):185–97. doi: 10.1016/s0735-1097(03)00577-1. [DOI] [PubMed] [Google Scholar]
- 8.Singh BN, Singh SN, Reda DJ, et al. Amiodarone versus sotalol for atrial fibrillation. N Engl J Med. 2005;352:1861–1872. doi: 10.1056/NEJMoa041705. [DOI] [PubMed] [Google Scholar]
- 9.Humphries KH, Kerr CR, Connolly SJ, et al. New onset atrial fibrillation: sex differences in presentation, treatment, and outcome. Circulation. 2001;103:2365–2370. doi: 10.1161/01.cir.103.19.2365. [DOI] [PubMed] [Google Scholar]
- 10.Paquette M, Roy D, Talajic M, et al. Role of gender and personality on quality-of-life impairment in intermittent atrial fibrillation. Am J Cardiol. 2000;86(7):764–8. doi: 10.1016/s0002-9149(00)01077-8. [DOI] [PubMed] [Google Scholar]
- 11.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 12.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 13.The AFFIRM Investigators. Quality of life in atrial fibrillation: the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. Am Heart J. 2005;149:112–120. doi: 10.1016/j.ahj.2004.03.065. [DOI] [PubMed] [Google Scholar]
- 14.Bubien RS, Knotts-Dolson SM, Plumb VJ, et al. Effect of radiofrequency catheter ablation on health-related quality of life and activities of daily living in patients with recurrent arrhythmias. Circulation. 1996;94(7):1585–1591. doi: 10.1161/01.cir.94.7.1585. [DOI] [PubMed] [Google Scholar]
- 15.Ware JEJ. SF-36 health survey: manual and interpretation guide. Boston: The Health Institute, New England Medical Center; 1993. [Google Scholar]
- 16.Atrial Fibrillation Investigators. Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation: analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994;154:1449–1457. [PubMed] [Google Scholar]
- 17.Singer DE, Albers GW, Dalen JE, et al. Antithrombotic therapy in atrial fibrillation: the Seventh ACCP Conference on antithrombotic and thrombolytic therapy. Chest. 2004;126(3 Suppl):429S–456S. doi: 10.1378/chest.126.3_suppl.429S. [DOI] [PubMed] [Google Scholar]
- 18.Hagens VE, Ranchor AV, Van Sonderen E, et al. Effect of rate or rhythm control on quality of life in persistent atrial fibrillation. Results from the Rate Control versus Electrical Cardioversion (RACE) Study. J Am Coll Cardiol. 2004;43:241–247. doi: 10.1016/j.jacc.2003.08.037. [DOI] [PubMed] [Google Scholar]
- 19.Rienstra M, Van Veldhuisen DJ, Hagens VE, et al. Gender-related differences in rhythm control treatment in persistent atrial fibrillation. Data of the Rate Control versus Electrical Cardioversion (RACE) study. J Am Coll Cardiol. 2005;46:1298–1306. doi: 10.1016/j.jacc.2005.05.078. [DOI] [PubMed] [Google Scholar]
- 20.van den Berg MP, Hassink RJ, Tuinenburg AE, et al. Quality of life in patients with paroxysmal atrial fibrillation and its predictors: importance of the autonomic nervous system. Eur Heart J. 2001;22(3):247–53. doi: 10.1053/euhj.2001.2180. [DOI] [PubMed] [Google Scholar]
- 21.Kay GN, Ellenbogen KA, Giudici M, et al. The Ablate and Pace Trial: a prospective study of catheter ablation of the AV conduction system and permanent pacemaker implantation for treatment of atrial fibrillation. J Interv Card Electrophysiol. 1998;2(2):121–35. doi: 10.1023/a:1009795330454. [DOI] [PubMed] [Google Scholar]
- 22.Brignole M, Gianfranchi L, Menozzi C, et al. Assessment of atrioventricular junction ablation and DDDR mode-switching pacemaker versus pharmacological treatment in patients with severely symptomatic paroxysmal atrial fibrillation: a randomized controlled study. Circulation. 1997;96(8):2617–2624. doi: 10.1161/01.cir.96.8.2617. [DOI] [PubMed] [Google Scholar]
- 23.Newman DM, Dorian P, Paquette M, et al. Effect of an implantable cardioverter defibrillator with atrial detection and shock therapies on patient-perceived, health-related quality of life. Am Heart J. 2003;145(5):841–6. doi: 10.1016/S0002-8703(02)94817-9. [DOI] [PubMed] [Google Scholar]
- 24.Wazni OM, Marrouche NF, Martin DO, et al. Radiofrequency ablation vs. antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation. JAMA. 2005;293:2634–2640. doi: 10.1001/jama.293.21.2634. [DOI] [PubMed] [Google Scholar]
- 25.Gottlieb SS, Khatta M, Friedmann E, et al. The influence of age, gender and race on the prevalence of depression in heart failure patients. J Am Coll Cardiol. 2004;43:1542–1549. doi: 10.1016/j.jacc.2003.10.064. [DOI] [PubMed] [Google Scholar]
- 26.Ruo B, Rumsfeld JS, Hlatky MA, et al. Depressive symptoms and health-related quality of life: the Heart and Soul Study. JAMA. 2003;290:215–221. doi: 10.1001/jama.290.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dagres N, Clague JR, Breithardt G, et al. Significant gender-related differences in radiofrequency catheter ablation therapy. J Am Coll Cardiol. 2003;42:1103–1107. doi: 10.1016/s0735-1097(03)00925-2. [DOI] [PubMed] [Google Scholar]
- 28.Howes CJ, Reid MC, Brandt C, et al. Exercise tolerance and quality of life in elderly patients with chronic atrial fibrillation. J Cardiovasc Pharmacol Ther. 2001;6(1):23–9. doi: 10.1177/107424840100600103. [DOI] [PubMed] [Google Scholar]


