Summary
Previous reports showed that patients with Alzheimer disease (AD) frequently have coexisting vascular-related pathologies, such as cerebral infarcts and white matter lesions. The aim of this study was to determine the effects of subcortical lacunar infarcts on brain structure in patients with AD. Semi-automated tissue segmentation and volumetry of magnetic resonance imaging data were performed in 38 AD patients without lacunes (AD-L), 24 AD patients with subcortical lacunes (AD+L), and 40 age-matched cognitively healthy subjects without lacunes. The following tissue volumes were quantified, expressed as percentage of total intracranial volume: ventricular cerebrospinal fluid (CSF), sulcal CSF, cortical gray matter (GM), subcortical GM, white matter (WM), white matter signal hyperintensities (WMSH), lacunes, and hippocampus. There was no difference in the Mini-Mental State Examination between the two AD groups. AD+L patients compared with AD-L subjects had significantly greater volumes of WMSH and ventricular CSF spaces (as expected) but smaller sulcal CSF spaces and no significant increase in cortical GM atrophy (both unexpected). In the AD groups, ventricular CSF correlated inversely with cortical GM but not with WM; sulcal CSF correlated inversely with cortical GM and WM. Cognitive impairment was associated with sulcal CSF volume but not with volumes of WMSH or lacunes. In conclusion, the presence of subcortical lacunes in those with AD is associated with more WM lesions and ventriculomegaly but not with cortical atrophy.
Keywords: Alzheimer disease, Cerebral infarcts, Magnetic resonance imaging
Alzheimer disease (AD) is characterized by progressive accumulation of neuritic plaques and neurofibrillary tangles, as well as brain atrophy beginning in the medial temporal lobes and extending to isocortical regions (Geula, 1998). In addition, AD patients frequently show coexisting vascular pathology (Gold et al., 1998; Holmes et al., 1999). Incomplete white matter (WM) infarcts (Brun and Englund, 1986) are seen on magnetic resonance imaging (MRI) as white matter signal hyperintensities (WMSH); lacunar infarcts (Marder et al., 1995) and microinfarcts (Goulding et al., 1999) are also detected on MRI. The presence of ischemic lesions in AD has been attributed to concomitant vascular disease (e.g., cerebral amyloid angiopathy, arteriosclerosis, or atherosclerosis) (Janota et al., 1989; Fazekas et al., 1993; Goulding et al., 1999). Several investigators have noted that the severity of dementia in AD patients is increased with the presence of lacunar infarcts (Snowdon et al., 1997; Heyman et al., 1998).
There are many reports comparing quantitative MRI among patients with AD versus normal elderly control subjects or patients with other types of dementia (Kesslak et al., 1991; Schmidt, 1992; Charletta et al., 1995; Laakso et al., 1995; Kidron et al., 1997; Tanabe et al., 1997; Pantel et al., 1998). There are few quantitative MRI data of AD patients with cerebrovascular disease. Therefore, the aim of our study was to determine brain structure differences between AD patients with subcortical lacunes (AD+L) from those without lacunes (AD-L). The specific goals were (1) to determine if AD+L patients had more WM lesions and disproportionate enlargement of cerebrospinal fluid (CSF) spaces than AD-L patients; (2) to determine if WM lesions and CSF changes are associated; and (3) to determine which MRI changes were associated with cognitive impairment.
METHODS
Subjects
Forty healthy elderly subjects and 62 AD patients were included in this quantitative MRI study (see Table 1 for demographics). The two AD groups with and without subcortical lacunar infarcts were selected for matching in Mini-Mental State Examination (MMSE) (Folstein et al., 1975) scores and in age from a larger population of AD patients, who had been studied in this laboratory using MRI. The elderly control subjects were recruited from the community. Patients with AD were referred from university-affiliated memory clinics in northern California. A diagnosis of probable or possible AD was made according to the criteria established by the National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association (McKhann et al., 1984). Exclusion criteria for all participants were aged less than 60 years, current use of medication likely to affect cognitive function, history or evidence of cortical stroke or other major neurologic disorders, psychiatric disorders after age 50 years, head trauma with loss of consciousness exceeding 15 min, alcohol or substance abuse, seizures, and abnormal thyroid metabolism. General cognitive function was assessed using the MMSE. All participants or their legal guardians provided informed consent to this study, which was approved by the Committee of Human Research of the University of California, San Francisco.
TABLE 1.
Demographics
| Controls | AD - L | AD + L | |
|---|---|---|---|
| Men/women | 16/24 | 13/25 | 10/14 |
| Age (years) | 78.1 ± 5.6 | 78.9 ± 4.4 | 79.2 ± 5.0 |
| Probable AD | — | 34 | 15 |
| Possible AD | — | 4 | 9 |
| MMSE score | 29.0 ± 0.9 | 19.0 ± 5.6 | 20.6 ± 5.9 |
AD - L, Alzheimer disease without lacunes; AD + L, Alzheimer disease with lacunes; MMSE, Mini-Mental State Examination. Probable and possible AD are defined according to National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association criteria. Age and MMSE scores are reported as mean ± standard deviation.
Patients with probable or possible AD were divided into two groups based on the absence of lacunes (AD-L, n = 38) or presence of lacunes (AD+L, n = 24), as seen on T1-, T2-, and proton density-weighted MRIs that were read by a neuroradiologist (D.N.). Lacunes and perivascular spaces were distinguished using the following operational criteria: lacunes were defined as small lesions hyperintense to CSF on the proton density-weighted images, located in the basal ganglia, thalamus, or WM, and with a diameter of 3–15 mm on the axial images. With one exception, lesions that were isointense to CSF on proton density images were categorized as perivascular spaces if <1 cm and as lacunes if >1 cm. Because perivascular spaces may be particularly large and prominent in the anterior commissure and the infraputaminal regions (Pullicino et al., 1995), all hyperintense lesions in these areas were arbitrarily categorized as perivascular spaces. The average number of supratentorial lacunes ± standard deviation (SD) was 3 ± 2, andthe average volume ± SD was 699 ± 886 mm3 in AD+L group.
Magnetic Resonance Imaging Acquisition
All imaging studies were performed on a 1.5-T Magnetom VISION system (Siemens Inc., Iselin, NJ) equipped with a standard quadrature head coil. The MRI protocol consisted of sagittal T1-weighted localizer images, oblique axial double spin echo (DSE) images parallel to the axis of optic nerves, and volumetric magnetization prepared rapid acquisition gradient echo (MP-RAGE) scans perpendicular to the DSE images, yielding T1-weighted coronal images approximately perpendicular to the long axis of the hippocampus. The measurement parameters of DSE images were TR/TE1/TE2 = 3000/20/80 ms, in-plane resolution = 1.0 × 1.25 mm2, and slice thickness = 3 mm, covering the whole brain from the vertex to the most inferior part of cerebellum. The measurement parameters of volumetric MP-RAGE scans were TR/TI/TE = 10/250/4 ms, flip angle = 15°, and spatial resolution = 1.4 × 1.0 × 1.0 mm3.
Magnetic Resonance Imaging Segmentation
Segmentation of the MRI data was performed using a semi-automated segmentation program developed in-house (Tanabe et al., 1997). This program uses coregistered T2-, proton density-, and T1-weighted MP-RAGE images to automatically assign each MRI pixel to a category of gray matter (GM), WM, or CSF, based on K-means cluster analysis methods. Subsequent interactive manual editing was performed by an experienced operator (D.L.A.) to distinguish ventricular from sulcal CSF and cortical from subcortical GM and to delineate WMSH and lacunes. Total intracranial volume was measured from the top of the brain to the slice where the cerebral peduncles appeared. Reliability test of within-operator measurements yielded 0.1–2% variability of the mean value and a correlation coefficient of 0.94. Volumes are expressed as the percentage of total intracranial volume.
Hippocampal Volumetry
Quantitative voluming of both hippocampi was obtained using coronal T1-weighted MP-RAGE images. Images were re-sliced perpendicular to the long axis of the hippocampus for manual tracing. Boundaries of the hippocampus were drawn following the guidelines of Watson et al. (1992), including hippocampal proper, dentate gyrus, subiculum, fimbria, and alveus. The areas of all regions of interest were then automatically calculated and normalized to the total intracranial volume. Voluming of the hippocampus was performed by the same radiologist (A.T.D.). Within-rater reliability was 1.8%.
Statistics
Magnetic resonance imaging volumes normalized to individual total intracranial volume were compared across the three groups (normal control subjects, AD-L, and AD+L) using multivariate analysis of variance (MANOVA), with age and sex as covariates. Significant effects were followed by post hoc Scheffe tests. To account for multiple comparisons, the level of significance for tests of differences in brain tissue types between groups was increased to 0.05/6 = 0.008 (for the six tissue types: cortical and subcortical GM, sulcal and ventricular CSF, WM, and WM lesions). Relationships between measures were tested using Pearson correlation coefficients and linear regression. The level of significance was p < 0.05.
RESULTS
The demographics data of the subjects are listed in Table 1. There was no significant difference in age (p = 0.2) and severity of dementia (p = 0.4) as measured by MMSE between the AD-L and AD+L groups. Table 2 lists the results from the brain segmentation and hippocampal voluming. Overall, changes in ventricular CSF spaces were significantly associated with group (F[2,99] = 11.7; p < 0.0001]. Elderly control subjects had ventricular CSF spaces of 3.74 ± 1.13%, smaller than in both AD+L (6.06 ± 2.18%; p < 0.0001) and AD-L (4.78 ± 1.34%, p = 0.0009) patients. Further, the difference in ventricular CSF spaces between AD+L and AD-L patients was significant (p < 0.001). Sulcal CSF spaces were also significantly associated with group (F[2,99] = 7.7; p < 0.001). Sulcal CSF was 20.45 ± 3.12% in control subjects and was smaller than in AD-L patients (25.15 ± 3.59%; p < 0.0001) but not significantly different from AD+L patients (22.57 ± 4.29%; p = 0.03). In contrast, the difference between AD-L and AD+L patients in sulcal CSF was significant (p < 0.0008). Both WM lesion burden (F[2,99] = 16.1; p < 0.0001) and normal WM volumes (F[2,99] = 7.8; p < 0.0007] were significantly associated with group. AD+L patients had more WM lesions (p < 0.0001) than control subjects, but WM lesions were not significantly different between AD-L patients and control subjects. WM was significantly reduced in AD-L (p = 0.0004) and AD+L groups (p = 0.007) when compared with control subjects. However, WM was not significantly different between AD-L and AD+L patients. Changes in cortical GM volume were significantly associated with group (F[2,99] = 36.1; p < 0.001]. Cortical GM in control subjects was 38.72 ± 1.86% and decreased to 35.26 ± 2.75% in AD-L patients (p < 0.0001) and to 35.10 ± 2.29% in AD+L patients (p < 0.0001). However, the difference in cortical GM between AD-L and AD+L was not significant. Subcortical GM was significantly reduced in AD-L patients (p = 0.002) and in AD+L patients (p = 0.03) when compared with control subjects, but the difference in subcortical GM between AD+L and AD-L patients was not significant. Hippocampal volumes were significantly reduced in AD-L (p < 0.0001) and AD+L (p < 0.0001) groups compared with control subjects; however, hippocampal volume was not significantly different (p = 0.9) between AD-L and AD+L patients. There was no significant difference in total intracranial volume among control subjects, AD-L, and AD+L patients (F[2,99] = 0.7; p = 0.5).
TABLE 2.
Sizes of hippocampus and other brain structures
| Controls | AD - L | AD + L | |
|---|---|---|---|
| Ventricular CSF (%) | 3.74 ± 1.13 | 4.78 ± 1.34* | 6.06 ± 2.18*† |
| Sulcal CSF (%) | 20.45 ± 3.12 | 25.15 ± 3.59* | 22.57 ± 4.29† |
| WMSH (%) | 0.44 ± 0.35 | 0.76 ± 0.55 | 1.89 ± 1.65*† |
| WM (%) | 35.29 ± 2.54 | 32.90 ± 2.94* | 33.88 ± 2.49* |
| Cortical GM (%) | 38.72 ± 1.86 | 35.26 ± 2.75* | 35.10 ± 2.29* |
| Subcortical GM (%) | 1.36 ± 0.24 | 1.16 ± 0.34* | 1.21 ± 0.28* |
| Hippocampus (%) | 0.43 ± 0.06 | 0.30 ± 0.07* | 0.31 ± 0.06* |
| TIV (cm3) | 1254 ± 129 | 1273 ± 117 | 1290 ± 145 |
AD - L, Alzheimer disease without lacunes; AD + L, Alzheimer disease with lacunes; CSF, cerebrospinal fluid; WMSH, white matter signal hyperintensities; WM, white matter; GM, gray matter; TIV, total intracranial volume. Data are reported as percentage of TIV in terms of mean ± standard deviation.
p < 0.008 compared with controls
p < 0.008 AD + L compared with AD - L.
Considering AD patients as one group, ventricular CSF correlated inversely with cortical GM (r = 0.35; p = 0.006; Fig. 1A) but not with total WM. Contrary to ventricular CSF, sulcal CSF correlated inversely with cortical GM (r = 0.47; p = 0.0001) and with total WM (r = 0.75; p <0.0001) in AD patients (Fig. 1B, C). No significant correlation was found between volume of lacunes and WMSH, ventricular CSF, or sulcal CSF.
FIG. 1.
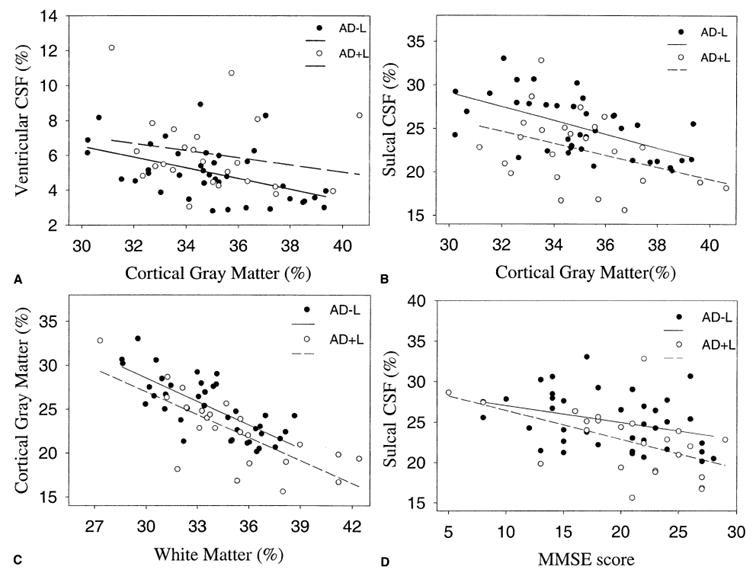
Scatter-plots illustrating the relationship between sizes of different brain structures and Mini-Mental State Examination (MMSE) scores in Alzheimer disease (AD) patients with (AD+L) and without (AD-L) subcortical lacunar infarctions. (A) Ventricular cerebrospinal fluid (CSF) versus cortical gray matter. (B) Sulcal CSF versus cortical gray matter. (C) Sulcal CSF versus total white matter. (D) MMSE score versus sulcal CSF. Tissue volumes are expressed as percentage of individual total intracranial volume.
We also explored the correlation between segmentation measures and cognitive impairment, as measured by MMSE. Among patients with AD, the only significant determinant of MMSE scores was the volume of sulcal CSF (r = 0.43; p = 0.0004) (Fig. 1D). Volumes of lacunes, WMSH, or cortical GM had no significant association with MMSE scores.
DISCUSSION
The major findings in this study were (1) AD+L patients compared with AD-L patients had significantly more WMSH and larger ventricular CSF volumes (as expected) but no significant cortical GM losses and even smaller sulcal CSF volumes (both not expected); (2) the volume of ventricular CSF correlated inversely with cortical GM; (3) sulcal CSF spaces correlated inversely with volumes of cortical GM and total WM; and (4) dementia severity as measured by MMSE correlated inversely with sulcal CSF.
The most surprising finding was that the AD+L group had similar amounts of cortical GM and even fewer sulcal CSF spaces than AD-L patients, despite significantly more WM lesions and ventricular CSF spaces. This finding suggests that cortical atrophy in these patients is dominantly a result of AD pathology and not significantly related to cerebral vascular factors. It has been shown that AD patients had more WMSH than elderly control subjects, both in neuroimaging (Schmidt, 1992; Tanabe et al., 1997) and histopathologic studies (Scheltens et al., 1995). Coexistence of lacunes and WMSH has also been demonstrated in those with AD, either on MRIs (Scheltens et al., 1992; Charletta et al., 1995) or by neuropathologic studies (Brun and Englund, 1986; Scheltens et al., 1992). Our study demonstrated that AD+L patients also had significantly more WMSH than AD-L patients with similar MMSE scores. This finding implies that patients with lacunes have a greater burden of small vessel disease, reflecting in the increased volume of WMSH, another manifestation of microangiopathy (Sabri et al., 1999).
Significantly enlarged ventricular and sulcal volumes in AD patients were consistently reported in the literature (Kidron et al., 1997; Tanabe et al., 1997; Pantel et al., 1998) and are confirmed by this study. Further, there were significant differences in CSF enlargement between AD-L and AD+L patients: AD+L group had more ventriculomegaly and less sulcal widening than the AD-L group. Because the magnitudes of cortical atrophy and hippocampal volume losses were similar between AD-L and AD+L patients, the difference in CSF volumes was most likely the result of the additional loading of lacunes and WMSH in AD+L patients. Quantitative MRI studies demonstrated significantly larger ventricles in dementia patients with lacunar infarcts and WM lesions compared with control subjects (Loeb et al., 1992; Giubilei et al., 1997). In addition, enlargement of the sulcal spaces tends to be greater than that of the ventricles in early AD, whereas greater progression of ventriculomegaly occurs during the later period (Wippold et al., 1991). These findings support our proposition that, compared with AD-L group, the greater ventriculomegaly of AD+L patients in late stages results from combinational effects of AD and vascular pathologies. Contrarily, the influence of vascular insults on sulcal spaces of AD patients is elusive. Presence (Loeb et al., 1992) and absence (Giubilei et al., 1997) of differences in the volume of sulcal CSF between patients with vascular dementia and normal subjects have been reported. Pantel et al. (1998) did not find a difference between AD and subcortical vascular dementia in the whole volume of CSF. But Charletta et al. (1995) found that more frequently there was widening of the cerebral sulci in AD than in vascular dementia, and there was similar enlargement of the lateral ventricles between AD and vascular dementia. The finding of less sulcal widening in the AD+L group than in the AD-L group in the current study implies that AD and vascular pathologies have different effects on sulcal CSF, consistent with Charletta’s report (1995). Further, our finding suggested that the coexistent vascular insults may counteract the sulcal widening effect caused by AD pathology. However, future studies are necessary to confirm these preliminary results and to elucidate the interaction between AD pathologies and vascular insults on sulcal CSF spaces.
Our second major finding was that ventriculomegaly was significantly associated with decreased cortical GM but not with changes of total WM, which is consistent with previous reports (Tanabe et al., 1997; Symonds et al., 1999). The significant correlation between ventricular CSF and cortical GM in the combined group of AD-L and AD+L patients suggested that increased ventriculomegaly in AD+L group mainly resulted from greater cortical GM loss caused by additional cortical damage from ischemic insults. It has been shown that there were ischemic changes in WMSH (Yamaji et al., 1997; Tohgi et al., 1998) and lacune-associated hypoperfusion in the GM and WM of AD patients (Obara et al., 1994). In addition to neurodegeneration of the limbic system and cortical GM caused by AD pathology (i.e., senile plaques and fibrillary tangles), AD+L patients also had insults from ischemia and infarction, which cause neuronal loss. Therefore, AD+L patients are expected to have more central atrophy as a result of superimposed vascular insults in the subcortical GM and WM. In the current study, however, there was no significant difference in the volumes of subcortical GM or normal-appearing WM between the AD-L and AD+L groups. The expected central atrophy in the subcortical regions may either be absent or masked by the more severe and diffuse volume decreases of cortical GM.
The third finding of this study was that sulcal CSF spaces correlated inversely with cortical GM or total WM in the combined AD group. This finding was in keeping with the neuroimaging study by Symonds et al. (1999), who found sulcal CSF was a strong predictor of the volume of cortical GM or total WM in AD patients. These findings suggested that the inverse correlation between sulcal CSF and total WM was because the majority of WM underlies cortical GM and is composed of corticocortical connections among cortical areas (Symonds et al., 1999).
Finally, MMSE scores correlated weakly but significantly with the volumes of sulcal CSF in patients with AD: the greater the sulcal spaces, the lower the MMSE scores. This finding was consistent with the finding of Tanabe et al. (1997), who proposed sulcal CSF enlargement closely reflected neuronal loss, resulting in cognitive impairment in AD patients. No other MRI measures had significant correlation with MMSE scores in the current study. Even though there was no significant correlation between MMSE and total WM or cortical GM, which are both determinants of sulcal CSF volumes, a correlation between MMSE scores and sulcal spaces probably reflected the results of combination effects of GM and WM atrophy on cognitive function. It has been shown that the presence of cerebrovascular disease was associated with greater overall severity of cognitive dysfunction in AD patients (Snowdon et al., 1997; Heyman et al., 1998). However, our data could not identify significant correlation of MMSE scores with the volume of WMSH or lacunes. Recently, Esiri et al. (1999) demonstrated that the adverse effect of cerebrovascular pathology on cognitive performance in AD predominantly occurred in the earliest stage. Absence of correlation between MMSE scores and WM lesions or lacunes in the present study is most likely because most patients in our study already had marked dementia and were in the late stage of AD process. Therefore, the superimposed influence caused by additional vascular changes in the AD+L group could not be disclosed in this study.
There are several limitations to the current study. First, we did not account for differences of cerebrovascular and cardiovascular risk factors among the groups, which may distort our findings because of the potential bias when recruiting subjects. Therefore, a relationship between vascular risk factors and lacunes or WM lesions could not be established. Other clinical data, such as apolipoprotein E status, family history, age of onset, and more specific cognitive measures than MMSE that may have distinguished AD+L from the AD-L subjects, were not available, and their contribution to MRI changes in AD+L and AD-L groups could not be established. Second, because our objective was to evaluate the volume of total WM in the presence or absence of lacunes in AD patients, we defined total WM as “normal WM’’ plus WMSH. It would have been impossible to separate normal WM fibers within an area of hyperintensities. Third, this study measured global volume changes as opposed to local changes in different regions of the brain. However, cerebral structures may be vulnerable differently to AD pathologies and vascular insults. Evaluation of CSF spaces in terms of different anatomic locations may possess the ability to explore further the relationship between focal changes of CSF spaces and specific cerebral structures.
This quantitative MRI study showed that there were significant differences in the volumes of CSF spaces and WM lesions between AD-L and AD+L groups. Increased ventriculomegaly in patients with AD+L is largely the result of enhanced cortical GM loss, presumably reflecting subcortical vascular insults. However, in the absence of more sensitive cognitive tests or longitudinal follow-up evaluation, the clinical significance of ventricular differences along AD remained unknown. Further studies with a larger sample size and histopathologic correlation are necessary to substantiate these preliminary findings.
Footnotes
Presented in part at the 8th Annual Meeting of International Society for Magnetic Resonance in Medicine, April 2000, Denver, Colorado, U.S.A.
References
- Brun A, Englund E. A white matter disorder in dementia of the Alzheimer type: a pathoanatomic study. Ann Neurol. 1986;19:253–62. doi: 10.1002/ana.410190306. [DOI] [PubMed] [Google Scholar]
- Charletta D, Gorelick PB, Dollear TJ, et al. CT and MRI findings among African-Americans with Alzheimer’s disease, vascular dementia, and stroke without dementia. Neurology. 1995;45:1456–61. doi: 10.1212/wnl.45.8.1456. [DOI] [PubMed] [Google Scholar]
- Esiri MM, Nagy Z, Smith MZ, et al. Cerebrovascular disease and threshold for dementia in the early stages of Alzheimer’s disease. Lancet. 1999;354:919–20. doi: 10.1016/S0140-6736(99)02355-7. [DOI] [PubMed] [Google Scholar]
- Fazekas F, Kleinert R, Offenbacher H, et al. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993;43:1683–9. doi: 10.1212/wnl.43.9.1683. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PRJ. Mini-Mental State. A practical method for grading the cognitive state of patients for the clinicians. Psychol Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Geula C. Abnormalities of neural circuitry in Alzheimer’s disease: hippocampus and cortical cholinergic innervation. Neurology. 1998;51(suppl 1):S18–29. doi: 10.1212/wnl.51.1_suppl_1.s18. [DOI] [PubMed] [Google Scholar]
- Giubilei F, Bastianello S, Paolillo A, et al. Quantitative magnetic resonance analysis in vascular dementia. J Neurol. 1997;244:246–51. doi: 10.1007/s004150050079. [DOI] [PubMed] [Google Scholar]
- Gold G, Giannakopoulos P, Bouras C. Reevaluating the role of vascular changes in the differential diagnosis of Alzheimer’s disease and vascular dementia. Eur Neurol. 1998;40:121–9. doi: 10.1159/000007968. [DOI] [PubMed] [Google Scholar]
- Goulding JMR, Signorini DF, Chatterjee S, et al. Inverse relation between Braak stage and cerebrovascular pathology in Alzheimer predominant dementia. J Neurol Neurosurg Psychiatry. 1999;67:654–7. doi: 10.1136/jnnp.67.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman A, Fillenbaum GG, Welsh-Bohmer KA, et al. Cerebral infarcts in patients with autopsy-proven Alzheimer’s disease: CERAD, part XVIII. Neurology. 1998;51:159–62. doi: 10.1212/wnl.51.1.159. [DOI] [PubMed] [Google Scholar]
- Holmes C, Cairns N, Lantos P, et al. Validity of current clinical criteria for Alzheimer’s disease, vascular dementia and dementia with Lewy bodies. Br J Psychiatry. 1999;174:45–50. doi: 10.1192/bjp.174.1.45. [DOI] [PubMed] [Google Scholar]
- Janota I, Mirsen TR, Hachinski VC, et al. Neuropathologic correlates of leuko-araiosis. Arch Neurol. 1989;46:1124–8. doi: 10.1001/archneur.1989.00520460118023. [DOI] [PubMed] [Google Scholar]
- Kesslak JP, Nalcioglu O, Cotman CW. Quantification of magnetic resonance scans for hippocampal and parahippocampal atrophy in Alzheimer’s disease. Neurology. 1991;41:51–4. doi: 10.1212/wnl.41.1.51. [DOI] [PubMed] [Google Scholar]
- Kidron D, Black SE, Stanchev P, et al. Quantitative MR volumetry in Alzheimer’s disease: topographic markers and the effects of sex and education. Neurology. 1997;49:1504–12. doi: 10.1212/wnl.49.6.1504. [DOI] [PubMed] [Google Scholar]
- Laakso MP, Soininen H, Partanen K, et al. Volumes of hippocampus, amygadala and frontal lobes in the MRI-based diagnosis of early Alzheimer’s disease: correlation with memory functions. J Neural Transm. 1995;9:73–86. doi: 10.1007/BF02252964. [DOI] [PubMed] [Google Scholar]
- Loeb C, Gandolfo C, Croce R, et al. Dementia associated with lacunar infarction. Stroke. 1992;23:1225–9. doi: 10.1161/01.str.23.9.1225. [DOI] [PubMed] [Google Scholar]
- Marder K, Richards M, Bello J, et al. Clinical correlates of Alzheimer’s disease with and without silent radiographic abnormalities. Arch Neurol. 1995;52:146–51. doi: 10.1001/archneur.1995.00540260050015. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Service Task Force on Alzheimer’s disease. Neurology. 1984;34:934–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Obara K, Meyer JS, Muramatsu K, et al. Lacune-associated cerebral hypoperfusion correlates with cognitive testing. J Stroke Cerebrovasc Dis. 1994;4:121–9. doi: 10.1016/S1052-3057(10)80120-X. [DOI] [PubMed] [Google Scholar]
- Pantel J, Schroder J, Essig M, et al. In vivo quantification of brain volumes in subcortical vascular dementia and Alzheimer’s disease: an MRI-based study. Dement Geriatr Cogn Disord. 1998;9:309–16. doi: 10.1159/000017082. [DOI] [PubMed] [Google Scholar]
- Pullicino PM, Miller LL, Alexandrov A, et al. Infraputaminal lacunes: clinical and pathologic correlations. Stroke. 1995;26:1598–602. doi: 10.1161/01.str.26.9.1598. [DOI] [PubMed] [Google Scholar]
- Sabri O, Ringelstein E-B, Hellwig D, et al. Neuropsychologic impairment correlates with hypoperfusion and hypometabolism but not with severity of white matter lesions on MRI in patients with cerebral microangiopathy. Stroke. 1999;30:556–66. doi: 10.1161/01.str.30.3.556. [DOI] [PubMed] [Google Scholar]
- Scheltens P, Barkhof F, Valk J, et al. White matter lesions on magnetic resonance imaging in clinically diagnosed Alzheimer’s disease: evidence for heterogeneity. Brain. 1992;115:735–48. doi: 10.1093/brain/115.3.735. [DOI] [PubMed] [Google Scholar]
- Scheltens P, Barkhof F, Leys D, et al. Histopathologic correlates of white matter changes on MRI in Alzheimer’s disease and normal aging. Neurology. 1995;45:883–8. doi: 10.1212/wnl.45.5.883. [DOI] [PubMed] [Google Scholar]
- Schmidt R. Comparison of magnetic resonance imaging in Alzheimer’s disease, vascular dementia and normal aging. Eur Neurol. 1992;32:164–9. doi: 10.1159/000116816. [DOI] [PubMed] [Google Scholar]
- Snowdon DA, Greiner LH, Mortimer JA, et al. Brain infarction and the clinical expression of Alzheimer’s disease: the nun study. JAMA. 1997;277:813–7. [PubMed] [Google Scholar]
- Symonds IL, Zisook S, Archibald SL, et al. Does an increase in sulcal or ventricular fluid predict where brain tissue is lost? J Neuroimaging. 1999;9:201–9. doi: 10.1111/jon199994201. [DOI] [PubMed] [Google Scholar]
- Tanabe JL, Amend D, Schuff N, et al. Tissue segmentation of the brain in Alzheimer disease. AJNR Am J Neuroradiol. 1997;18:115–23. [PMC free article] [PubMed] [Google Scholar]
- Tohgi H, Yonezawa H, Takahashi S, et al. Cerebral blood flow and oxygen metabolism in senile dementia of Alzheimer’s type and vascular dementia with deep white matter changes. Neuroradiology. 1998;40:131–7. doi: 10.1007/s002340050553. [DOI] [PubMed] [Google Scholar]
- Watson C, Andermann F, Gloor P, et al. Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic resonance imaging. Neurology. 1992;42:1743–50. doi: 10.1212/wnl.42.9.1743. [DOI] [PubMed] [Google Scholar]
- Wippold FJ, II, Gado MH, Morris JC, et al. Senile dementia and healthy aging: a longitudinal CT study. Radiology. 1991;179:215–9. doi: 10.1148/radiology.179.1.2006279. [DOI] [PubMed] [Google Scholar]
- Yamaji S, Ishii K, Masahiro S, et al. Changes in cerebral blood flow and oxygen metabolism related to magnetic resonance imaging white matter hyperintensities in Alzheimer’s disease. J Nucl Med. 1997;38:1471–4. [PubMed] [Google Scholar]


