Abstract
This study tested the hypothesis that the hippocampus has a relatively specific role in retaining information over delays. Thirty-seven subjects with probable Alzheimer’s disease were evaluated with a verbal memory task and structural MRI. Cortical gray matter but not hippocampal volume predicted immediate free recall. In contrast, hippocampal volume was the best predictor of how well information was retained over a delay, even after controlling for levels of immediate recall. Results suggest that the role of the hippocampus is relatively specific to the consolidation of new memories.
Keywords: Hippocampus, Memory, Consolidation, Alzheimer’s disease
INTRODUCTION
It is well established that the hippocampus is an important anatomic substrate of episodic memory (Squire, 1998). Numerous case studies and empirical investigations show that hippocampal damage can produce amnesic syndromes (Kramer & Delis, 1998). Alzheimer’s disease (AD) is a particularly important target population to study because pathological signs of the disease are first observed in the medial temporal lobe, and memory impairment is a hallmark feature (Braak & Braak, 1995). Hippocampal atrophy in AD patients has been shown to predict impaired memory performance (Jack et al., 1999; Mungas et al., 2001; Petersen et al., 2000). Furthermore, there is a fair degree of specificity in this relationship in AD patients, with memory scores selectively related to hippocampal volumes, and non-memory performances related to other brain regions (Fama et al., 1997).
As a construct, however, memory is both cognitively and neuroanatomically complex. Several cognitive processes such as attention, working memory, organization, and processing ability affect how well information is initially processed and encoded. These abilities may be mediated primarily by frontal and other cortical structures rather than the hippocampus (Fletcher & Henson, 2001; Squire, 1992; Stuss & Alexander, 2000). Memory performance can also be influenced by subcortical structures and white matter (Mungas et al., 2001).
Another important issue is the precise role the hippocampus plays in memory. The hippocampus is thought to be critical for the consolidation of new memories, that is, the capacity to retain information over delays (Squire, 1998). Typically, however, investigators have correlated hippocampal volumes with single measures of memory such as immediate recall (Petersen et al., 2000), delayed recall (Hackert et al., 2002), or delayed recognition (Kopelman et al., 2001). While correlations between hippocampal volumes tend to be most robust with measures of delayed memory (Kohler et al., 1998), it is necessary to control for immediate memory in order to directly evaluate the specific relationship between hippocampal volume and retention over time. This is particularly important when studying neuroanatomical substrates of memory performance in AD because the diffuse cognitive impairment in AD can interfere with pre-memory aspects of information processing. In addition, the presence of diffuse cortical atrophy in AD raises the possibility of multiple neuroanatomical correlates of memory performance (Fama et al., 1997; Kopelman et al., 2001).
The primary goal of this study was to determine whether hippocampal volumes predict retention of information over a delay in patients with AD. Retention is influenced by many factors, an important one being how well the information was initially acquired. We hypothesized that if the hippocampus participates in the retention of information over delays, then (1) hippocampal volumes should predict delayed recall performance even after controlling for initial acquisition of the material; and (2) hippocampal volumes should have a stronger relationship with delayed recall than with immediate recall. We further hypothesized that hippocampal volumes would predict memory retention, whereas other brain structures known to influence memory performance such as cortex and white matter disease would not.
METHODS
Research Participants
Participants were recruited from three academic dementia centers participating in a multi-center collaborative study of cognitive impairment and dementia. There were 54 subjects who met NINCDS-ADRDA criteria for probable Alzheimer’s disease (AD) and who received the memory evaluation at baseline. Of these 54, hippocampal volumes were available for 39. One subject was excluded because the MRI was not obtained within 6 months of the memory testing, and a second subject was excluded because of extensive subcortical lacunes (total lacune volume greater than 1 cc). The final sample consisted of 37 subjects (20 men and 17 women) with AD. Descriptive information for the sample is provided in Table 1. For comparative purposes, data are also presented for a sample of 43 normal controls subjects comparable to the AD group on age and education.
Table 1.
Characteristics of the study sample and a comparison sample of 43 normal elderly controls
| AD
|
Controls
|
|||
|---|---|---|---|---|
| Variable | M | (SD) | M | (SD) |
| Age | 75.6 | (7.3) | 74.5 | (7.5) |
| Education | 15.1 | (3.5) | 15.6 | (3.1) |
| MMSE | 21.9 | (4.2)** | 29.1 | (1.5) |
| MAS Total Immediate Recall | 27.0 | (8.5)** | 56.3 | (10.0) |
| MAS Trial 6 | 5.3 | (1.9)** | 11.3 | (1.2) |
| MAS Delayed Recall | 1.7 | (2.3)** | 10.5 | (1.6) |
| Total cortical gray matter (cc) | 465.3 | (35.8)** | 528.3 | (31.0) |
| WMSH (cc) | 13.7 | (15.2)* | 6.6 | (8.9) |
| Hippocampal volume (cc) | 3.5 | (0.9)** | 4.7 | (0.5) |
p < .01.
p< .001.
MMSE = Mini-Mental State Exam; MAS = Memory Assessment Scale; WMSH = white matter signal hyperintensity.
Procedures
All subjects received a comprehensive clinical assessment including detailed medical history, neurologic exam, dementia oriented laboratory tests, standardized cognitive assessment, and a brain MRI. All subjects gave informed consent under protocols approved by the human subjects protection programs at their respective institutions.
Subjects were administered the MMSE and the Memory Assessment Scale List Learning Test (Williams, 1991) as part of a larger neuropsychological test battery. The list learning task is a 12-item list administered over six learning trials. A delayed free recall trial occurs after a 10-min interval filled with primarily nonverbal tasks. The primary measures of interest for the present study were immediate free recall (i.e., the number of items recalled over the six learning trials) and retention of information over the 10-min delay (i.e., the number of items recalled after the 10-min delay relative to the number of items recalled on the final learning trial). The total number of words recalled summed over the six learning trials was selected as the index of immediate recall because it captures the improvement in recall over the learning trials due to episodic memory, is more reliable than any individual trial or the slope, has the largest loading on general memory factors in factor analytic studies, and is widely used in neuropsychology as a memory measure (Delis et al., 1988).
MRI variables of interest were total hippocampal volume, cortical gray matter volume, and volume of white matter signal hyperintensities. Subjects were scanned on two different machines carefully calibrated to yield T1-weighted volumetric images with identical resolution and similar tissue contrast. Based on an analysis from 94 normal subjects, who were scanned on the different machines, differences between the scanners in measuring hippocampal volumes were not significant when contrasted against age and gender [F(3,90) = 0.42, p >.5]. Repeating this analysis for hippocampus normalized to total intracranial volume yielded a similar result [F(3,90) = 0.31, p> .5], indicating that volume measurements in this study were not skewed by systematic errors from the different scanners.
Image acquisition and segmentation methods have been previously described (Fein et al., 2000; Mungas et al., 2001). A computer algorithm was used to classify brain MRI pixels first into principal tissue types of gray matter, white matter, and cerebrospinal fluid. Subsequently, an operator-guided computer algorithm was applied to further subdivide gray matter into cortical and subcortical gray matter, and white matter into white matter lesions and normal appearing white matter. In addition, total intracranial volume was computed by summing over all pixels within the intracranial vault. Region of interest volumes were normalized to total intracranial volume to control for variation in head size. Normalization of regions of interest volumes was accomplished by multiplying each volume by the ratio of the average control group total intracranial volume (1,337 cc) to that particular subject’s total intracranial volume.
White matter signal hyperintensities appear as hyper-intense areas on T-2 weighted MRI in the periventricular white matter and centrum semiovale. These lesions may represent variable degrees of demyelination, edema, or infarction. White matter signal hyperintensity volumes were log-transformed to normalize the distribution.
Semi-automated hippocampal volumetry was carried out using a commercially available high dimensional brain mapping tool (Medtronic Surgical Navigation Technologies, Louisville, CO), that has been recently validated and compared to manual tracing of the hippocampus (Hsu et al., 2002). Measurement of hippocampal volume is achieved first by placing manually 22 control points as local landmarks for the hippocampus on the individual brain MRIs: one landmark at the hippocampal head, one at the tail, and four per image (i.e., at the superior, inferior, medial and lateral boundaries) on five equally spaced images perpendicular to the long axis of the hippocampus. Second, fluid image transformation was used to match the individual brains to a template brain, and pixels corresponding to hippocampus were labeled and counted to obtain volumes (Christensen et al., 1997). This method of hippocampal voluming has well documented reliability, with intraclass coefficients of .94 (Hsu et al., 2002).
Statistical Analyses
Multiple regression was used to determine which MRI variables best predicted immediate and delayed memory. For immediate memory, imaging variables were entered simultaneously into the regression in a single step. For delayed memory, a multi-stage multiple regression was used to control for initial acquisition. The first stage of the model was a baseline model in which recall on the last list learning trial was entered. In the second stage, MRI variables were entered simultaneously.
RESULTS
The first set of analyses examined MRI predictors of immediate free recall (see Table 2). Multiple regression indicated that cortical gray matter was the only significant predictor of immediate free recall, with 29.1% of the variance explained ( β= .52; t = 3.47, p = .001). Hippocampal volumes did not add any predictive value to the model. The contribution of cortical gray matter to immediate free recall remained significant even when MMSE was forced into the regression model prior to entering cortical gray matter.
Table 2a.
MRI predictors of immediate recall
| β | t | p value | |
|---|---|---|---|
| Hippocampus | .078 | .531 | .599 |
| Cortical gray | .520 | 3.471 | .001 |
| WMSH | −.025 | −.167 | .868 |
A different pattern emerged when delayed recall was the dependent variable. Although 41.3% of the variance in delayed recall was explained by the number of words recalled during the final learning trial, hippocampal volume explained an additional 7.6% of the variance ( β= .28; t = 2.24, p < .05). Cortical gray matter and white matter signal hyperintensities did not make a significant contribution to the variance. The contribution of hippocampal volume to delayed recall remained significant even when MMSE was forced into the regression model prior to entering hippocampal volume.
Twenty of the AD subjects had zero free recall after the delay, producing a skewed distribution that could potentially bias the results of the multiple regression. Consequently, we repeated the regression analyses using only those subjects that recalled one or more item during the delayed recall trial. Despite the reduction in statistical power, the results were unchanged. After the final learning trial accounted for 42.8% of the variance, hippocampal volume explained an additional 20.8% ( β= .47; t = 2.83, p< .05). The relationship between hippocampal volume and delayed recall (after partialling out the variance associated with the final learning trial) is shown in Figure 1. Delayed recall was correlated both with left hippocampus (r = .54, p< .05) and with right hippocampus (r = .61, p< .05).
Fig. 1.
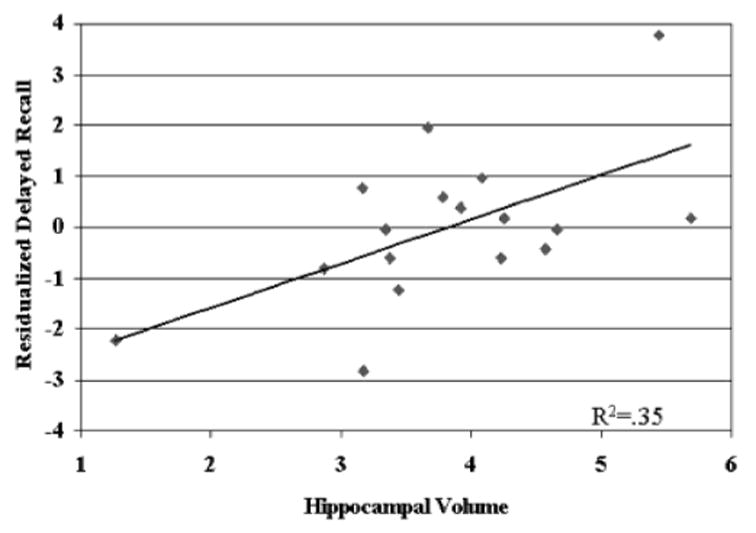
Scatterplot of the relationship between hippocampal volume and delayed recall (residualized after controlling for the last acquisition trial).
DISCUSSION
One important finding from the present study is that the relationship between MRI brain volumes and memory in AD varies depending on which component of episodic memory is assessed. Initial acquisition of verbal material over six learning trials was best predicted by cortical gray matter volume. Cortical gray matter volume reflects the degree of cortical atrophy, with smaller cortical gray matter volume indicating greater cortical atrophy. In AD, cortical gray matter correlates significantly with overall cognitive functioning and degree of dementia, and thus can be viewed as a marker of dementia severity (Fein et al., 2000; Mungas et al., 2001). When cortical gray matter, hippocampal volumes and white matter signal hyperintensities were simultaneously entered into a regression model predicting immediate recall, only cortical gray matter volume explained a significant proportion of the variance. This finding highlights the importance of including non-hippocampal structures in correlational studies of memory (Cahn et al., 1998; Kopelman et al., 2001) and is consistent with other studies that have shown specificity in the relationship between memory and medial temporal structures (Cahn et al., 1998; Fama et al., 1997).
The lack of a significant relationship between immediate memory and hippocampal volumes is consistent with some other studies that have reported that hippocampal volume was more strongly related to delayed recall than immediate recall (Kohler et al., 1998; O’Driscoll et al., 2001). The fact that hippocampal volumes did not predict immediate memory in this study should not be misconstrued to imply that there is no relationship between hippocampus and immediate memory. Significant correlations between hippocampal volume and immediate recall have been reported in several studies (Hackert et al., 2002; Kopelman et al., 2001; Laakso et al., 1995; Petersen et al., 2000). The likely reason for the discrepant findings is that the current study simultaneously considered the contribution of other brain structures that explained much of the variance in immediate recall.
In contrast to the immediate recall results, hippocampal volume was the best predictor of how well information was retained over a delay, even after controlling for levels of initial acquisition. This was true even when the sample was restricted to only those subjects who demonstrated delayed recall greater than zero. Cortical gray matter did not remain in the regression model. These results conform to the view that the role of the hippocampus in memory is relatively specific to the consolidation of new memories. This specificity has also been described in rats. Winocur et al. (2001) reported that rats with large lesions to the hippocampal formation showed normal acquisition of a food preference but had a faster rate of forgetting. Sass et al. (1992) reported that in patients with temporal lobe epilepsy undergoing surgical removal of mesial temporal structures, there was a significant correlation between percent retention scores on Wechsler Memory Scale Logical Memory and hippocampal neuron loss in CA3. Kopelman et al. (2001) also examined rate of forgetting using measures of picture, word, and design recognition memory over delays ranging between one and thirty minutes. However, their correlations between regional MR volumes, including hippocampus, did not reach statistical significance. The reasons for the discrepant findings are not clear, but may be due to different samples. They studied memory-impaired subjects with mixed etiologies, including Korsakoff’s, brain tumors, herpes encephalitis, hypoxic insult, and frontal lesions. In addition, they only reported on univariate analyses between single measures of memory and single MR regions of interest.
Although the current study controlled for the effect of immediate recall on delayed recall, there was insufficient statistical power to also control for demographic variables like age, sex, and education that might also predict delayed recall. The effects of age would be particularly important to evaluate, since both memory and hippocampal volumes decline with age. In addition, the relationship between delayed recall and hippocampal volumes was observed for patients with AD. Further studies are needed to determine whether this relationship is found in other groups, including normal aging and non-AD neurodegenerative conditions.
In summary, we describe the relationship between hippocampal volumes and retention of verbal information over delays. Importantly, these relatively specific contributions of hippocampal atrophy could be detected in a disorder that causes widespread destruction of cortical structures critical to a variety of cognitive functions. This suggests that failure of delayed memory may serve as a reasonably specific marker of hippocampal dysfunction, whereas impaired immediate recall may reflect injury to other brain regions involved in attention, organization, and modality-specific processing. This study also highlights the importance of fractionating episodic memory into its component parts, and simultaneously considering multiple brain structures when studying brain–behavior relationships.
Table 2b.
MRI predictors of delayed recall after controlling for immediate recall
| β | t | p value | |
|---|---|---|---|
| Trial 6 | .580 | 4.242 | .000 |
| Hippocampus | .277 | 2.170 | .038 |
| Cortical gray | .075 | .557 | .582 |
| WMSH | .058 | .448 | .657 |
Acknowledgments
This work was supported by National Institute on Aging 1P01 AG12435 (PI: Helen Chui, M.D.), California Department of Health Services 03-75271 (PI: Bruce Miller, M.D.). The authors would like to thank Kevin Delucchi, Ph.D., for his assistance with the biostatistics.
References
- Braak H, Braak E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiology of Aging. 1995;16:271–284. doi: 10.1016/0197-4580(95)00021-6. [DOI] [PubMed] [Google Scholar]
- Cahn DA, Sullivan EV, Shear PK, Marsh L, Fama R, Lim KO, Yesavage JA, Tinklenberg JR, Pfefferbaum A. Structural MRI correlates of recognition memory in Alzheimer’s disease. Journal of the International Neuropsychological Society. 1998;4:106–114. doi: 10.1017/s1355617798001064. [DOI] [PubMed] [Google Scholar]
- Christensen GE, Joshi SC, Miller MI. Volumetric transformation of brain anatomy. IEEE Transactions Medical Imaging. 1997;16:864–877. doi: 10.1109/42.650882. [DOI] [PubMed] [Google Scholar]
- Delis DC, Freeland J, Kramer JH, Kaplan E. Integrating clinical assessment with cognitive neuroscience: construct validation of the California Verbal Learning Test. Journal of Consulting and Clinical Psychology. 1988;56:123–130. doi: 10.1037//0022-006x.56.1.123. [DOI] [PubMed] [Google Scholar]
- Fama R, Sullivan EV, Shear PK, Marsh L, Yesavage JA, Tinklenberg JR, Lim KO, Pfefferbaum A. Selective cortical and hippocampal volume correlates of Mattis Dementia Rating Scale in Alzheimer disease. Archives of Neurology. 1997;54:719–728. doi: 10.1001/archneur.1997.00550180039010. [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Tanabe J, Cardenas V, Weiner MW, Jagust WJ, Reed BR, Norman D, Schuff N, Kusdra L, Greenfield T, Chui H. Hippocampal and cortical atrophy predict dementia in subcortical ischemic vascular disease. Neurology. 2000;55:1626–1635. doi: 10.1212/wnl.55.11.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC, Henson RN. Frontal lobes and human memory: Insights from functional neuroimaging. Brain. 2001;124:849–881. doi: 10.1093/brain/124.5.849. [DOI] [PubMed] [Google Scholar]
- Hackert VH, den Heijer T, Oudkerk M, Koudstaal PJ, Hofman A, Breteler MM. Hippocampal head size associated with verbal memory performance in nondemented elderly. Neuroimage. 2002;17:1365–1372. doi: 10.1006/nimg.2002.1248. [DOI] [PubMed] [Google Scholar]
- Hsu YY, Schuff N, Du AT, Mark K, Zhu X, Hardin D, Weiner MW. Comparison of automated and manual MRI volumetry of hippocampus in normal aging and dementia. Journal of Magnetic Resonance Imaging. 2002;16:305–310. doi: 10.1002/jmri.10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Petersen RC, Xu YC, O’Brien PC, Smith GE, Ivnik RJ, Boeve BF, Waring SC, Tangalos EG, Kokmen E. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler S, Black SE, Sinden M, Szekely C, Kidron D, Parker JL, Foster JK, Moscovitch M, Winocour G, Szalai JP, Bronskill MJ, Winocur G. Memory impairments associated with hippocampal versus parahippocampal-gyrus atrophy: An MR volumetry study in Alzheimer’s disease. Neuropsychologia. 1998;36:901–914. doi: 10.1016/s0028-3932(98)00017-7. [DOI] [PubMed] [Google Scholar]
- Kopelman MD, Lasserson D, Kingsley D, Bello F, Rush C, Stanhope N, Stevens T, Goodman G, Heilpern G, Kendall B, Colchester A. Structural MRI volumetric analysis in patients with organic amnesia, 2: Correlations with anterograde memory and executive tests in 40 patients. Journal of Neurology, Neurosurgery and Psychiatry. 2001;71:23–28. doi: 10.1136/jnnp.71.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer JH, Delis DC. Neuropsychological assessment of memory. In: Goldstein G, Nussbaum PD, editors. Neuropsychology. Human brain function: Assessment and rehabilitation. New York: Plenum Press; 1998. pp. 333–356. [Google Scholar]
- Laakso MP, Soininen H, Partanen K, Helkala EL, Hartikainen P, Vainio P, Hallikainen M, Hänninen T, Riekkinen PJ., Sr Volumes of hippocampus, amygdala and frontal lobes in the MRI-based diagnosis of early Alzheimer’s disease: Correlation with memory functions. Journal of Neural Transmission. Parkinsons Disease and Dementia Section. 1995;9:73–86. doi: 10.1007/BF02252964. [DOI] [PubMed] [Google Scholar]
- Mungas D, Jagust WJ, Reed BR, Kramer JH, Weiner MW, Schuff N, Norman D, Mack WJ, Willis L, Chui HC. MRI predictors of cognition in subcortical ischemic vascular disease and Alzheimer’s disease. Neurology. 2001;57:2229–2235. doi: 10.1212/wnl.57.12.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Driscoll GA, Florencio PS, Gagnon D, Wolff AV, Benkelfat C, Mikula L, Lal S, Evans AC. Amygdala-hippocampal volume and verbal memory in first-degree relatives of schizophrenic patients. Psychiatry Research. 2001;107:75–85. doi: 10.1016/s0925-4927(01)00095-6. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Jack CR, Jr, Xu YC, Waring SC, O’Brien PC, Smith GE, Ivnik RJ, Tangalos EG, Boeve BF, Kokmen E. Memory and MRI-based hippocampal volumes in aging and AD. Neurology. 2000;54:581–587. doi: 10.1212/wnl.54.3.581. [DOI] [PubMed] [Google Scholar]
- Sass KJ, Sass A, Westerveld M, Lencz T, Novelly RA, Kim JH, Spencer DD. Specificity in the correlation of verbal memory and hippocampal neuron loss: Dissociation of memory, language, and verbal intellectual ability. Journal of Clinical and Experimental Neuropsychology. 1992;14:662– 672. doi: 10.1080/01688639208402854. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychological Review. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory systems. Comptes Rendus de L Academie des Sciences. Serie III, Sciences de la Vie. 1998;321:153–156. doi: 10.1016/s0764-4469(97)89814-9. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP. Executive functions and the frontal lobes: A conceptual view. Psychological Research. 2000;63:289–298. doi: 10.1007/s004269900007. [DOI] [PubMed] [Google Scholar]
- Williams JM. Memory Assessment Scales. Odessa: Psychological Assessment Resources; 1991. [Google Scholar]
- Winocur G, McDonald RM, Moscovitch M. Antero-grade and retrograde amnesia in rats with large hippocampal lesions. Hippocampus. 2001;11:18–26. doi: 10.1002/1098-1063(2001)11:1<18::AID-HIPO1016>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]


