Abstract
Neurodegenerative diseases are associated with profound changes in social and emotional function. The emergence of increasingly sophisticated methods for measuring brain volume has facilitated correlation of local changes in tissue content with cognitive and behavioural changes in neurodegenerative disease. The current study examined neuroanatomical correlates of behavioural abnormalities, as measured by the Neuropsychiatric Inventory, in 148 patients with dementia using voxel-based morphometry. Of 12 behaviours examined, 4 correlated with tissue loss: apathy, disinhibition, eating disorders and aberrant motor behaviour. Increasing severity across these four behaviours was associated with tissue loss in the ventral portion of the right anterior cingulate cortex (vACC) and adjacent ventromedial superior frontal gyrus (vmSFG), the right ventromedial prefrontal cortex (VMPC) more posteriorly, the right lateral middle frontal gyrus, the right caudate head, the right orbitofrontal cortex and the right anterior insula. In addition, apathy was independently associated with tissue loss in the right vmSFG, disinhibition with tissue loss in the right subgenual cingulate gyrus in the VMPC, and aberrant motor behaviour with tissue loss in the right dorsal ACC and left premotor cortex. These data strongly support the involvement of the right hemisphere in mediating social and emotional behaviour and highlight the importance of distinct regions on the medial wall of the right frontal lobe in regulating different behaviours. Furthermore, the findings underscore the utility of studying patients with dementia for understanding the neuroanatomical basis of social and emotional functions.
Keywords: frontotemporal dementia, neuropsychiatric inventory, voxel-based morphometry, right hemisphere, cingulate
Abbreviations: ACC = anterior cingulate cortex, FTD = frontotemporal dementia, MMSE = Mini-Mental State Examination, NPI = Neuropsychiatric Inventory, OFC = orbitofrontal cortex, ROI = region of interest, SGC = subgenual cingulate gyrus, SPM = statistical parametric mapping, TIV = total intracranial volume, vACC = ventral portion of the right anterior cingulate cortex, VBM = voxel-based morphometry, VMPC = ventromedial prefrontal cortex, vmSFG = ventromedial superior frontal gyrus
Introduction
Disorders of social and emotional functioning are central features of a large number of acquired and developmental disorders ranging from traumatic brain injury and neurodegenerative disease to schizophrenia and autism. New hope for improved treatment of these disorders has arisen over the last few years along with a resurgence of interest in the brain mechanisms underlying social and emotional functions (Dolan, 2002; Blakemore et al., 2004; Ochsner, 2004). Some of the renewed enthusiasm for this area of research has been driven by developments in neuroimaging that allow the study of social and emotional functions in normal subjects, particularly with PET and fMRI (Ochsner, 2004). Additionally, brain injury models have helped to delineate the anatomy of these functions (Harlow, 1868; Eslinger et al., 1984; Adolphs et al., 1996; Hornak et al., 2003).
Neurodegenerative dementing disorders offer a valuable opportunity to study the neural underpinnings of social and emotional behaviour, as they are common (Brookmeyer et al., 1998) and affect structures relevant to emotion and social behaviour including the amygdala, anterior temporal neocortex, ventromedial frontal regions, insula and anterior cingulate cortex (ACC) (Boccardi et al., 2002; Hamann et al., 2002; Rosen et al., 2002a). In some types of dementia, disturbed emotional and social behaviour occur early in the course of the disease (Cummings, 1997) and can even define the central diagnostic features of the illness (Neary et al., 1998). An increasing number of studies assessing brain tissue volumes or metabolic function in dementia are demonstrating that regional tissue loss and hypometabolism correlate with specific cognitive and behavioural impairments in ways that are similar to what has been seen with other, more focal types of pathology (Eustache et al., 2000; Gee et al., 2003; Nadeau, 2003).
Previous studies in patients with neurodegenerative disease suggest that dysfunction or atrophy in the right hemisphere is associated with behavioural disturbances including apathy (Ott et al., 1996; Benoit et al., 2002), delusions (Staff et al., 1999; Edelstyn et al., 2001), antisocial behaviour (Mychack et al., 2001), and behavioural dysfunction in general (Miller et al., 1993; Edwards-Lee et al., 1997; Thompson et al., 2003b; Liu et al., 2004; Williams et al., 2005). Specific brain regions have also been implicated in behaviour, in particular the insula and the medial and ventral frontal regions (Craig et al., 1996; Migneco et al., 2001; Tekin et al., 2001; Benoit et al., 2002; Rosen et al., 2002a; Williams et al., 2005). Some of these studies were limited in that they relied upon region of interest (ROI) type analyses, where relationships between behaviour and a priori regions were examined, leaving open the possibility that other unmeasured regions, or smaller portions of the ROIs, had equally strong or stronger relationships with the behaviour. Also, most of the prior studies used small cohorts of patients and examined only a single behaviour. Since dementias are associated with multiple behavioural abnormalities (Cummings, 1997), it has been hard to conclude that the relationship between the behaviour and the region identified were unique. However, a few studies have demonstrated unique relationships between behaviour and volumes or metabolism in selected brain regions measured with ROI analyses (Craig et al., 1996; Sarazin et al., 2003).
Studies of patients with discrete lesions have also examined the neuroanatomical substrates of social and emotional dysfunction, usually focusing on behaviours associated with damage in specific regions. For example, disinhibition has been noted in association with ventromedial prefrontal and orbitofrontal cortex (OFC) injury (Eslinger and Damasio, 1985; Hornak et al., 1996), particularly in the right hemisphere (Tranel et al., 2002). In these studies, patients had a constellation of behavioural abnormalities including dampened and poorly modulated emotions, poor decision-making, disinhibition and lack of insight (Saver and Damasio, 1991; Tranel et al., 2002), suggesting that all these symptoms could have had a common neuroanatomical basis. Lesion studies have also described apathy in the setting of ACC damage as well as other lesion locations (Carota et al., 2002; Kumral et al., 2002). To date, no studies in either dementia or focal lesion cohorts have systematically examined the neuroanatomical correlates of behavioural abnormalities using approaches capable of finding associations in any part of the brain while including multiple, potentially related behaviours in order to identify neuroanatomical relationships unique to each.
The goal of the current study was to identify, in a large cohort of patients with a variety of dementing conditions, the brain regions where grey matter tissue loss correlated with the severity of twelve different behavioural disturbances characteristic of neurological disease and to determine whether unique relationships between individual behaviours and particular brain regions could be discerned. The main hypothesis was that behavioural disturbances would be associated with tissue loss in focal regions in the right hemisphere including the insula and ventral and medial frontal regions. Prior research allowed more specific hypotheses for some behaviours including that disinhibition would be correlated with atrophy in the right ventromedial prefrontal cortex (VMPC; Tranel et al., 2002) and that apathy would be correlated with tissue loss in the right VMPC (Craig et al., 1996; Sarazin et al., 2003) or ACC (Kumral et al., 2002). The analysis was carried out using voxel-based morphometry (VBM), a technique for assessing regional changes in brain tissue content over the entire brain on a voxel-by-voxel basis without the need for a priori ROI (Ashburner and Friston, 2000).
Methods
Subjects
One hundred and forty-eight patients (mean age 64.8 years, standard deviation = 9.4) with dementia were recruited through the University of California San Francisco Memory and Aging Center. Criteria for inclusion were a diagnosis of dementia, the availability of valid Neuropsychiatric Inventory (NPI) data (see below) and a high-quality research MRI scan within 6 months of the NPI assessment. Dementia diagnoses included frontotemporal dementia (FTD) (n = 39), semantic dementia (n = 23), progressive non-fluent aphasia (n = 13), corticobasal degeneration (n = 12), progressive supranuclear palsy (n = 9) and Alzheimer’s disease (n = 52). Patients were diagnosed using published diagnostic criteria (McKhann et al., 1984; Litvan et al., 1996; Neary et al., 1998; Boeve et al., 2003) after a comprehensive evaluation including neurological history and examination, a specialty nursing evaluation that included a behavioural assessment, and neuropsychological testing of memory, executive function, language and mood using a previously described standard protocol (Rosen et al., 2002a; Kramer et al., 2003). The mean Mini-Mental State Examination (MMSE) was 21 (standard deviation = 7.7), and the mean Clinical Dementia Rating (CDR) (Morris, 1997) score was 0.9 (standard deviation = 0.6).
Identification of behavioural abnormalities
The NPI was used for behavioural assessment (Cummings, 1997). The NPI is a validated, caregiver-based behavioural rating system developed for the assessment of dementia that evaluates the presence or absence, severity (rated 1–3, 3 being most severe) and frequency (rated 1–4, 4 being most frequent) of 12 major behavioural disorders, including delusions, hallucinations, aggression/agitation, depression, anxiety, elation/euphoria, apathy, disinhibition, irritability/lability, aberrant motor behaviour, sleep disturbances and eating disorders. An index of severity is created for each behavioural variable by multiplying the frequency and severity scores, creating a frequency by severity product (F × Sprod—see Levy et al., 1996). Data from the NPI were correlated with regional brain tissue loss (see below).
The NPI was always collected by a geriatric specialist nurse trained in its administration who made the judgement about the consistency and reliability of the data. NPI scores from suspect raters were not used. Although informants questioned for the NPI were not screened for cognitive impairment, they were, in general, the same individuals who provided the history used to make the clinical diagnosis of dementia. When caregivers knowledgeable about the patient provide NPI ratings, between-rater reliability across the NPI domains varies between 93.6 and 100%, while test–retest reliability correlations are 0.79 for frequency and 0.86 for severity (Cummings, 1997).
Acquisition and analysis of MRI data
MRI scanning
Structural MR imaging was accomplished using a 1.5-T Magnetom VISION system (Siemens Inc., Iselin, NJ), a standard quadrature head coil and previously described sequences (Rosen et al., 2002a) to obtain (i) scout views of the brain for positioning subsequent MRI slices, (ii) proton density and T2-weighted MRIs and (iii) T1-weighted (MP-RAGE) images of entire brain. MP-RAGE images were used for analysis.
Voxel-based morphometry
VBM is a technique for voxel-wise analysis of local changes in brain tissue content which has been used to study many brain disorders including dementia (Abell et al., 1999; Krams et al., 1999; Ashburner and Friston, 2000; Mummery et al., 2000; Good et al., 2001b; Burton et al., 2002; Rosen et al., 2002a; Boxer et al., 2003; Critchley et al., 2003; Gorno-Tempini et al., 2004; Williams et al., 2005). VBM involves several preprocessing steps before the images are analysed. The VBM preprocessing procedures employed for this study included two recently described procedures developed to optimize spatial normalization and segmentation: (i) creation of a study specific template for normalization made up of the average of all subjects included in the study (Good et al., 2001a) and (ii) optimization of spatial normalization using grey matter images to determine the final normalization parameters (Good et al., 2001b). Preprocessing was implemented as follows. All subject images were first spatially normalized using the Montreal Neurological Institute (MNI) brain provided with statistical parametric mapping (SPM) (linear followed by non-linear 8 × 8 × 7 parameter transform). These images were segmented to create normalized grey, white and cerebrospinal fluid compartments, which were averaged across subjects to create a new averaged whole brain, and grey, white and cerebrospinal fluid images to be used as normalization templates (Good et al., 2001a) and as custom prior probability templates for tissue classification (Testa et al., 2004). The original images were then segmented in native space and the resultant grey matter images were normalized to the study specific grey matter template (same normalization parameters). The parameters obtained from the latter normalization were then applied to the original T1-weighted image, which was segmented again after normalization. Grey matter voxel values were then multiplied by the Jacobian determinants derived from the spatial normalization step in order to preserve the initial volumes (Good et al., 2001b). Finally, the grey matter images were smoothed with a 12 mm full width at half-maximum isotropic Gaussian kernel to reduce error related to intersubject variability in local gyral anatomy and to render the imaging data more normally distributed. Smoothing imaging data with a 12 mm Gaussian filter in the preprocessing stages imposes limits on the analysis such that unique relationships for areas that are <12 mm apart cannot be discerned. The 12 mm kernel used here is one of the more commonly used kernels for VBM and this filter kernel minimizes the risk of false positive findings (Salmond et al., 2002).
All image preprocessing steps and statistical analysis were implemented in the SPM2 software package (www.fil.ion.ucl.ac.uk/spm).
For statistical analysis, the image and the NPI F × Sprod for each participant were entered into a design matrix and the relationships between changes in grey matter content and NPI F × Sprod were analysed using the general linear model. The significance of each effect of interest was determined using the theory of Gaussian fields. For all analyses, we accepted a threshold of P < 0.05, (SPM family-wise error, corrected for multiple comparisons) for statistical significance. Total intracranial volume (TIV) was always used as a covariate.
Statistical analysis was carried out in two steps. First, NPI scores for each of the 12 behaviours were entered into separate ‘covariates only’ design matrices. The relationship between voxel values and the behaviour of interest was examined with a –1 t-contrast, assuming that increasing severity of the behaviour would be associated with decreased tissue content. This allowed identification of those variables that showed significant effects for the purposes of data reduction. For this step, diagnosis was not included in the model, because previous research has shown that some behavioural abnormalities are characteristic of FTD and semantic dementia, while other behaviours are more similar across FTD and semantic dementia and other degenerative diseases (Levy et al., 1996; Bozeat et al., 2000; Bathgate et al., 2001; Liu et al., 2004).
Behaviours that showed regions of significant correlation in this first step were entered into a second ‘conditions and covariates’ analysis including diagnostic group (FTD or semantic dementia versus other), age, sex and MMSE as covariates. This design allowed analysis of the average effects for the behavioural disorders identified in the first step and independent contrasts of the individual behaviours. Behavioural effects for each diagnostic group were modelled separately so that covariate-by-condition interactions could be assessed. Figure 1 depicts the final design matrix for the four behaviours ultimately included in the final analysis (see Results section for elaboration). Specific brain–behaviour relationships were tested for these four behaviours using t-contrasts including the average effects across the four behaviours in each group [(–1 0 –1 0 –1 0 –1 0) and (0 –1 0 –1 0 –1 0 –1) with additional zeros for condition and nuisance covariates] and across groups [(–1 –1 –1 –1 –1 –1 –1 –1)], and unique effects for each behaviour in each group [e.g. (–1, 0, 0, 0, 0, 0, 0, 0) and (0, –1, 0, 0, 0, 0, 0, 0)] and across groups [e.g. (–1, –1, 0, 0, 0, 0, 0, 0)].
Fig. 1.
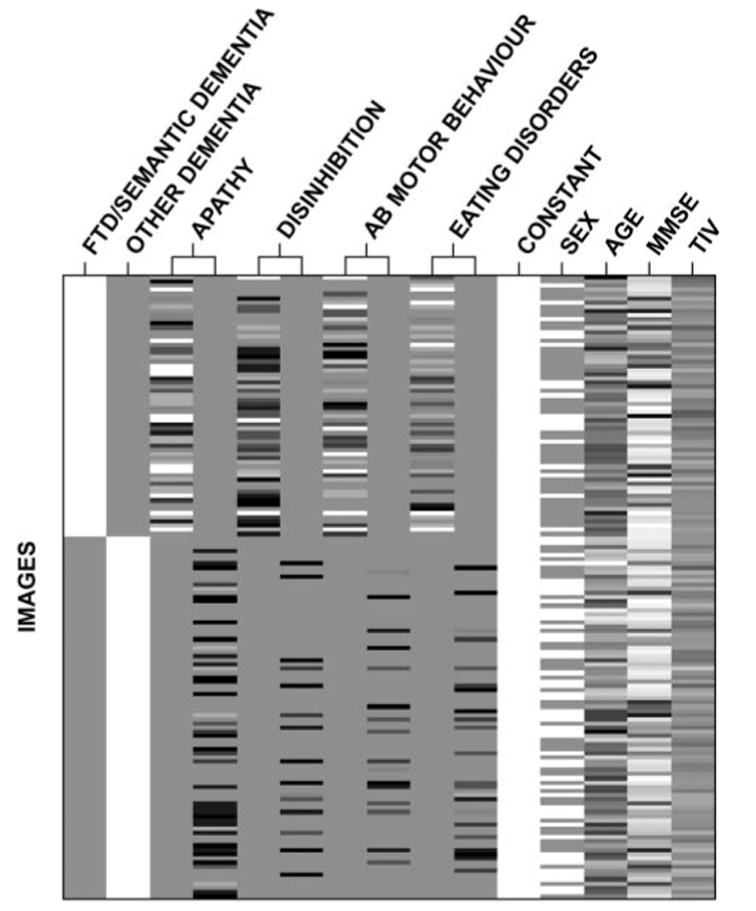
Study design matrix including two conditions and four behavioural covariates with condition by covariate interactions modelled.
Data were analysed in this latter step at the whole brain level and after the application of a ROI for small volume correction based on a priori hypotheses generated in previous studies (Rosen et al., 2002a). The ROI was created from the AAL brain atlas (Tzourio-Mazoyer et al., 2002) and applied to the SPM dataset using WFU-Pickatlas (Maldjian et al., 2003); it included the orbital parts of the superior, middle and inferior frontal gyri, gyrus rectus and olfactory cortex, the medial superior frontal gyrus, the supplementary motor area, the anterior and median cingulate and paracingulate regions, and the insula. Anatomic localization of clusters with significant effects was accomplished by overlaying the t-maps on the study specific template and using the AAL and Brodmann’s atlases that accompany the MRIcro software package (Rorden and Brett, 2000).
Relationships between NPI scores and the other independent variables were examined with Pearson correlation coefficients and linear regression using the SPSS software package (version 10.1 for Windows, SPSS Inc., Chicago, IL). Also, in order to better understand the unique effects contributed by all variables in the regression model and to test for violations of the assumptions of linear regression and multicollinearity, the linear regression performed in SPM was duplicated in SPSS for all peak voxels using raw values from the smoothed grey matter images and the same independent variables entered into the SPM design matrix. The linear form of the relationship of the dependent variable with each independent variable and homoscedasticity of the residuals was examined with scatterplots of the residuals plotted against the independent variables and predicted values with superimposed zero-lines and Lowess curves. Normality of the residuals was inspected with normal q–q plots. Multicollinearity was evaluated with the value inflation factor and comparison of the beta coefficients to the corresponding zero-order correlation coefficients (Cohen et al., 2003).
The study was approved by the UCSF committee on human research. All subjects, or their surrogates provided informed consent before participating.
Results
Incidence of behavioural abnormalities and behavioural correlations
Table 1 shows the frequency of each behaviour in the patient group. The most frequent was apathy (62%) and the least frequent was hallucinations (3%). None of the behavioural variables was significantly correlated with MMSE. Age and sex showed a significant correlation with several behaviours (Table 2).
Table 1.
Percentage of patients with each behavioural abnormality on the NPI
| Feature | Percent |
|---|---|
| Delusions | 19 |
| Hallucinations | 3 |
| Agitation | 44 |
| Depression | 39 |
| Anxiety | 43 |
| Elation | 20 |
| Apathy | 62 |
| Disinhibition | 41 |
| Irritability | 41 |
| Aberrant motor behaviour | 40 |
| Sleep disorders | 26 |
| Eating disorders | 43 |
Table 2.
Correlations between variables entered into the VBM matrix
| FTD/semantic dementia | Apathy | Disinhibition | Aberrant motor behaviour | Eating disorders | Male | Age | MMSE | TIV | |
|---|---|---|---|---|---|---|---|---|---|
| FTD/semantic dementia | – | 0.58* | 0.55* | 0.53* | 0.54* | 0.26* | –0.21* | –0.03 | –0.16 |
| Apathy | 0.56* | – | 0.39* | 0.49* | 0.49* | 0.18 | –0.14 | –0.02 | 0.03 |
| Disinhibition | 0.55* | 0.4* | – | 0.63* | 0.56* | 0.06 | –0.26* | –0.01 | –0.14 |
| Aberrant motor behaviour | 0.54* | 0.49* | 0.63* | – | 0.5* | 0.1 | –0.26* | 0.06 | –0.03 |
| Eating disorders | 0.54* | 0.49* | 0.56* | 0.5* | – | –0.02 | –0.17* | –0.06 | –0.01 |
| Male | 0.26* | 0.18 | 0.06 | 0.1 | –0.02 | – | 0.04 | 0.05 | –0.07 |
| Age | –0.21* | –0.14 | –0.26* | –0.26* | –0.17* | 0.04 | – | –0.02 | 0.07 |
| MMSE | –0.03 | –0.02 | –0.01 | 0.06 | –0.06 | 0.05 | –0.02 | – | 0.06 |
| TIV | –0.16 | 0.03 | –0.14 | –0.03 | –0.01 | –0.07 | 0.07 | 0.06 | – |
Value is significant at P < 0.05 (two-tailed).
First step neuroimaging analysis
Of the 12 behaviours examined in the first step of the analysis, 4 showed regions where local decreases in grey matter tissue content were significantly correlated with increased F × Sprod scores. These were apathy, disinhibition, eating disorders, and aberrant motor behaviours. For the most part, the regions identified were in the right hemisphere, and there appeared to be substantial overlap in the regions associated with each condition. The regions identified included the lateral OFC in all four behaviours, the rostromedial prefrontal cortex including the ventral anterior cingulate cortex (vACC) and the adjacent ventromedial superior frontal gyrus (vmSFG) in apathy and eating disorders, the ACC more dorsally (dACC) in apathy and aberrant motor behaviour, the caudate head/ ventral striatum in apathy and eating disorders, the subgenual cingulate gyrus (SGC) in the most medial posterior part of the OFC (or VMPC) and the right temporal pole in disinhibition, and the insula in eating disorders and aberrant motor behaviour. These four behavioural variables were carried forward to the second step analysis.
Second step neuroimaging analysis (combined analysis using apathy, disinhibition, aberrant motor behaviour and eating disorders)
The only four behaviours showing significant correlations with regional tissue loss were those known to be common in FTD and semantic dementia (Levy et al., 1996; Bozeat et al., 2000; Snowden et al., 2001; Liu et al., 2004). These earlier findings suggested that regions correlated with behaviour in the first analysis could also be regions correlated with diagnosis of FTD or semantic dementia and thus were not specific to behaviour per se. Figure 2 illustrates the distribution of scores for the four behaviours in this study using box plots and shows that the median scores for these behaviours and the maximum scores for these behaviours were indeed higher in FTD and semantic dementia than in the group of patients with non-FTD/semantic dementia diagnoses. Since the goal of the analysis was to identify regions specifically related to behaviour, the effect of diagnostic group (FTD or semantic dementia versus other) was included in the analysis as a condition along with the F ×Sprod scores for apathy, disinhibition, eating disorders and aberrant motor behaviour (with condition by covariate interactions modelled, see Methods) and MMSE, age, sex and TIV as nuisance covariates.
Fig. 2.
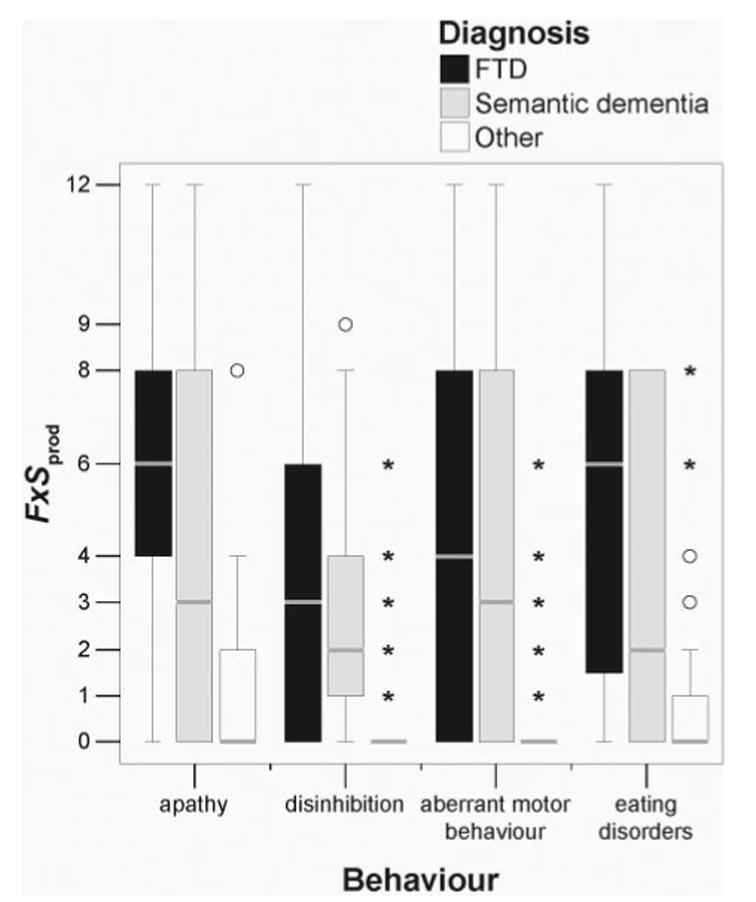
Boxplots of F × Sprod scores for four behaviours in FTD, semantic dementia and other neurodegenerative diseases. Horizontal grey lines mark the median score, the ends of each box mark the first and third quartiles, and the outer lines represent the range of scores or 1.5 box-lengths from the median. Circles mark scores >1.5 box-lengths from the median, and asterisks mark scores >3 box-lengths from the median.
Average effects across all four behaviours
Analysis of the four behaviours as a group revealed a group by behaviour interaction. In the FTD/semantic dementia group, increasing F × Sprod scores across these four behaviours was correlated with decreasing tissue content in the right frontal lobe, with significant clusters in the vACC, extending anteriorly into the vmSFG, the more posterior VMPC including the SGC, the MFG on the lateral surface, the caudate head, the OFC and the anterior insula (Table 3, Fig. 3A). Limiting the analysis to the ventral/medial frontal and insula ROI bilaterally revealed an additional cluster in the right OFC. No voxels correlated with these behaviours as a group in the non-FTD/semantic dementia diagnostic group.
Table 3.
Regions where F × Sprod scores for the four behaviours—apathy, disinhibition, eating disorders and aberrant motor behaviour—in the FTD/semantic dementia group were inversely correlated with decreased grey matter tissue content
| Anatomical region* | BA† | X, Y, Z‡ | Cluster size§ | Z-score |
|---|---|---|---|---|
| Whole brain level, P < 0.05, corrected | ||||
| R vACC/vmSFG | 32/10 | 8, 51, 8 | 15 738 | 5.33 |
| R VMPC | 11 | 1, 33, −17 | – | 5.04 |
| R vACC/vmSFG | 10/11 | 10, 50, −7 | – | 4.81 |
| R MFG | 45 | 51, 48, 27 | 98 | 5.05 |
| R caudate head | – | 10, 16, −2 | 1707 | 4.98 |
| R OFC | 11 | 20, 9, −20 | – | 4.81 |
| R anterior insula | 47 | 33, 24, −4 | 11 | 4.58 |
| Additional after SVC within ventral/medial frontal and insula ROI, P < 0.05, corrected | ||||
| R OFC | 11 | 28, 35, −16 | 22 | 4.06 |
Based on the AAL brain, R = right/L = left. OFC = orbitofrontal cortex; ACC = anterior cingulate cortex; MFG = middle frontal gyrus; VMPC = ventromedial prefrontal cortex (posterior, medial orbital frontal region).
BA = Brodmann area. Based on the Brodmann map image provided with MRIcro.
Coordinate for peak voxel in the cluster.
Cluster size on P < 0.05 corrected statistical map. Regions that were within the same cluster are listed consecutively. Peak voxels >12 mm apart within the same cluster were labelled separately.
Fig. 3.
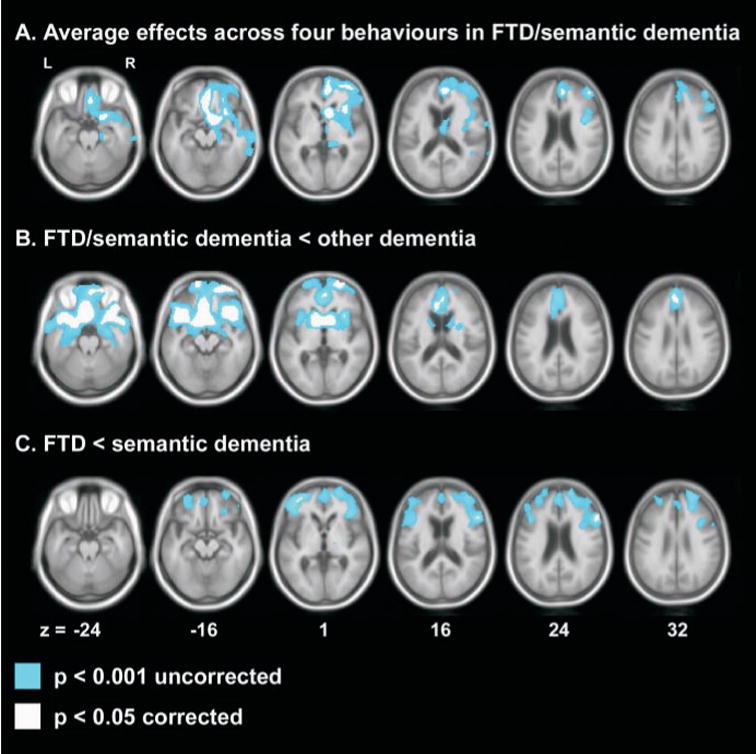
Regions of grey matter tissue loss associated with diagnosis and behaviour. (A) Average effects across four behaviours: apathy, disinhibition, aberrant motor behaviours and eating disorders in the FTD/semantic dementia group. (B) Tissue loss in FTD or semantic dementia versus any other dementia diagnosis. (C) Tissue loss in FTD versus semantic dementia. Slice thickness is 1 mm.
Regional tissue loss associated with an FTD or semantic dementia diagnosis
In order to understand how the regions correlated with behaviour compared with the regions associated with diagnosis, separate analyses were conducted using diagnostic groups as conditions and MMSE, age, sex and TIV as covariates. These analyses demonstrated that a diagnosis of FTD or semantic dementia in this group was associated, on the average, with significant tissue loss in the inferior and medial portions of the frontal lobes bilaterally (Fig. 3B). Thus, the regions associated with behaviour in FTD/semantic dementia appeared to be a subset of those associated with a diagnosis of FTD/ semantic dementia. When FTD was specifically compared with semantic dementia, FTD showed bilateral frontal atrophy, which was significant at a few locations in the left and right lateral frontal cortex (Fig. 3C). Semantic dementia showed bitemporal atrophy compared with FTD (data not shown).
Unique effects for individual behaviours
Apathy, aberrant motor behaviour and disinhibition showed significant correlations with specific brain regions (Fig. 4, Table 4).
Fig. 4.
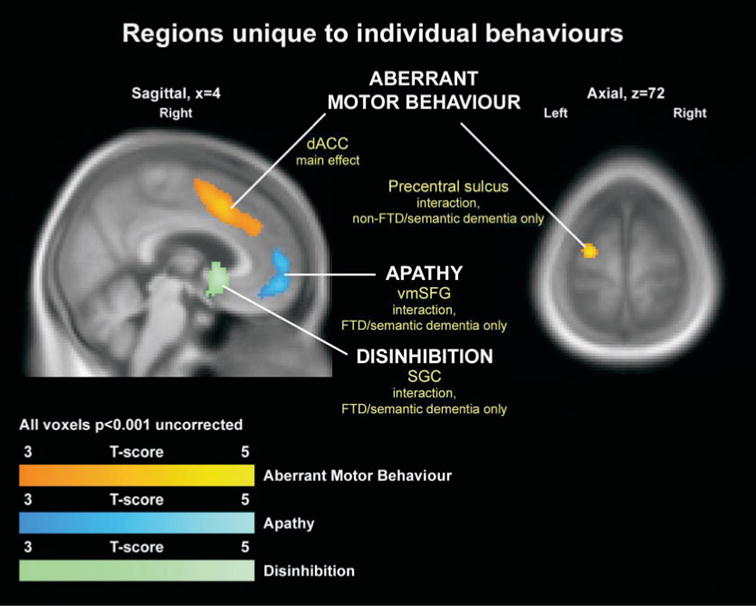
Regions where individual behaviours showed unique associations with focal regions of tissue loss.
Table 4.
Regions where F × Sprod scores for specific behaviours were uniquely inversely correlated with tissue loss
| Anatomical regiona (association) | BAb | X, Y, Zc | Cluster sized | Z-Score | βe | Partial correlation | |
|---|---|---|---|---|---|---|---|
| R vmSFG (FTD/semantic dementia, apathy) | 10 | 9, 57, 3 | 96 | 4.13 | FTD/semantic dementia
Non-FTD/semantic dementia |
–0.554
–0.069 |
–0.344*
–0.065 |
| R Dacc (main effect, AMB) | 32 | 4, 15, 45 | 45 | 4.10 | FTD/semantic dementia
Non-FTD/semantic dementia |
–0.488
–0.251 |
–0.315*
–0.236* |
| L premotor cortex (non-FTD/semantic dementia, AMB) | 6 | –25, –11, 72 | 90 | 4.83 | FTD/semantic dementia
Non-FTD/semantic dementia |
–0.147
–0.433 |
–0.100
–0.390* |
| R SGC (FTD/semantic dementia, disinhibition) | 25 | 2, 11, –3 | 19 | 4.05 | FTD/semantic dementia
Non-FTD/semantic dementia |
–0.529
–0.004 |
–0.335*
–0.003 |
Association = group(s) contributing to the effect at the peak voxel. AMB = aberrant motor behaviour.
Same as in Table 3.
β = standardized regression coefficient.
Value is significant at P < 0.05 (one-tailed).
For apathy, a significant effect was detected in FTD/ semantic dementia, but not non-FTD/semantic dementia, with behaviour being correlated with tissue loss in the vmSFG, extending back to the cingulate sulcus (P < 0.05 corrected, using a ventral/medial frontal and insula ROI for small volume correction—see Methods).
Aberrant motor behaviour showed both a main effect and an interaction. Increasing aberrant motor behaviour was correlated with tissue loss in the right dACC, across the whole dementia group (P < 0.05 corrected using the ventral/medial frontal and insula ROI for small volume correction). This region appeared to extend up into the supplementary motor area. In the non-FTD/semantic dementia group, increasing aberrant motor behaviour was correlated with tissue loss in the left premotor cortex (P < 0.05 corrected at the whole brain level).
Disinhibition showed an interaction, with increasing dis-inhibition correlating with tissue loss in the SGC in FTD/ semantic dementia, but not non-FTD/semantic dementia (P < 0.05 using the ventral/medial frontal and insula ROI for small volume correction).
Figure 5 depicts the relationship between adjusted voxel values and behaviour for apathy, disinhibition and aberrant motor behaviour at the peak voxels where these behaviours showed unique effects. Behavioural scores are well distributed for the FTD/semantic dementia group throughout the range of NPI F × Sprod scores. For disinhibition and aberrant motor behaviour, non-FTD/semantic dementia patients tended to cluster in the very low score ranges. For apathy, a substantial number of non-FTD/semantic dementia patients had evidence of apathy, but only a few had the very high scores seen in FTD/semantic dementia.
Fig. 5.
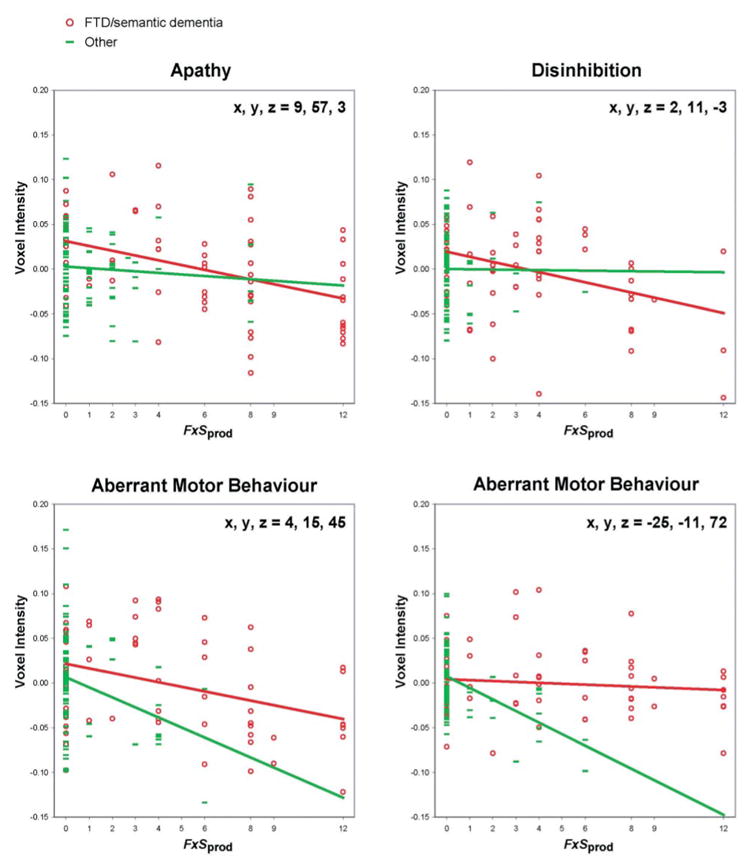
Relationships between adjusted voxel intensity and NPI F × Sprod score at the four peaks where individual behaviours showed unique relationships with tissue loss.
Table 4 provides the standardized regression coefficients and partial correlation coefficients for apathy, aberrant motor behaviour and disinhibition at voxels where they showed unique effects. Tests for multicollinearity and violations of the assumptions of linear regression revealed no discernible bias in the estimation of the regression coefficients or their standard errors.
Discussion
The primary goals of this study were to examine the neuroanatomical correlates of behavioural abnormalities in dementia and to identify unique relationships between individual behaviours and particular brain regions that were independent of more general brain–behaviour relationships. Four behaviours: apathy, disinhibition, eating disorders, and aberrant motor behaviour were significantly correlated with tissue loss in several regions in the right frontal lobe. In addition, three of these behaviours: apathy, disinhibition and aberrant motor behaviour were associated with tissue loss in specific brain regions, independent of the effects of the other behaviours. These findings support the results of previous studies indicating that specific behavioural disorders are associated with right frontal dysfunction. In addition, the data indicate that neuronal dysfunction in different regions within the medial frontal cortex has specific implications for social and emotional behaviour that could be elucidated with more directed studies. Furthermore, they provide evidence that specific neuroanatomical–behavioural relationships can be delineated in patients with dementia.
Behavioural dysfunction and the right hemisphere
Behavioural dysfunction was correlated with tissue loss in several cortical regions in the right hemisphere including the vACC and adjacent vmSFG, the VMPC including the SGC, the OFC, the lateral MFG and the anterior insula. The association between behaviour and the right hemisphere supports the results from previous studies by other groups in patients with dementia (Miller et al., 1993; Ott et al., 1996; Edwards-Lee et al., 1997; Staff et al., 1999; Edelstyn et al., 2001; Thompson et al., 2003b) and focal neurological lesions (Tranel et al., 2002).
The specific processes that underlie the specialization of the right hemisphere for regulation of social behaviour are poorly understood. In the case of language, neurophysiological approaches such as fMRI have linked the left hemisphere with basic functions including word processing, lexical semantics and grammatical processing. These studies have helped to further define the role for the left hemisphere in language indicated by lesion studies (Demonet et al., 2005). In contrast, specific links between the right hemisphere and putative functions underlying social and emotional behaviour have not been demonstrated as consistently. For instance, while lesion studies have mostly focused on the role of the right hemisphere in emotional processing (Bowers et al., 1991; Adolphs et al., 1996; Borod et al., 1998; Rosen et al., 2002b), data from physiological studies have suggested that both hemispheres make contributions to emotion (Davidson et al., 1990; Canli, 1999; Wager et al., 2003). Another putatively right hemispheric function suggested by lesion studies is self-awareness, which may be an important component in regulating some social and emotional functions (Craik et al., 1999; Fossati et al., 2003). While the connections between regulation of behaviour and more elemental functions in the right hemisphere are not completely established, the current results add to the growing body of literature indicating that functions mediated by the right hemisphere make unique contributions to the regulation of social and emotional behaviour.
Correlations between specific behaviours and specific regions
Three regions in different parts of the medial frontal cortex had unique associations with specific behaviours. Apathy correlated with tissue loss in the ventral portion of the vmSFG adjacent to the vACC, disinhibition with tissue loss in the SGC, posterior to the region associated with apathy, and aberrant motor behaviour with tissue loss in the dACC and premotor cortex. These findings are largely in agreement with available data from prior studies and suggest unique contributions of each of these regions in regulating behaviour.
Prior studies in patients with neurodegenerative disease have found specific regional associations with behaviour, particularly for apathy. Medial frontal metabolism (Craig et al., 1996; Migneco et al., 2001; Benoit et al., 2002) and plaque burden in the ACC at autopsy (Tekin et al., 2001) have both been correlated with apathy in patients with AD. One study examined the metabolic correlates of emotional and social dysfunction with PET and ROI analysis in a mixed population of patients with frontal lobe pathology including strokes, traumatic brain injury and FTD in about two-thirds of the sample. They found apathy to be correlated with hypometabolism in the right medial BA 10, essentially in the same location as the current analysis (Sarazin et al., 2003). Another group recently demonstrated that the areas of hypometabolism in the medial frontal region associated with apathy in FTD appeared to be anterior to the medial frontal regions associated with disinhibition (Franceschi et al., 2005).
Focal lesion studies are also in close agreement with the current findings. Disinhibition has been associated with lesions to the VMPC (Eslinger and Damasio, 1985; Hornak et al., 1996) and one study based on six patients suggested that the phenomenon was predominantly associated with right-sided VMPC injury (Tranel et al., 2002). Apathy is often considered as part of a larger spectrum of disorders characterized by decreased spontaneous goal directed behaviour that also includes abulia and akinetic mutism (Vijayaraghavan et al., 2002). These disorders are most commonly described in the setting of medial frontal lesions affecting the ACC or adjacent SFG and may be more common with right hemispheric lesions (Kumral et al., 2002). However, they have been described in the setting of a variety of lesion locations including the basal ganglia and thalamus (Kumral et al., 1999; Carota et al., 2002; Tekin and Cummings, 2002). The current study suggests that although apathy may occur from damage to multiple regions, the vmSFG may have the most specific relationship to apathy and be a critical component in a larger system for motivation and goal directed behaviour that includes subcortical and possibly other cortical structures.
The current results also suggest that atrophy in different regions in the medial frontal cortex results in differing behavioural profiles. The distinction between apathy and disinhibition is particularly interesting because the associated regions are both closely linked to emotional processing and sometimes behave similarly in functional imaging paradigms (Simpson et al., 2001). Prior studies have indicated that the vmSFG region is the most frequently activated region in functional imaging studies involving emotion (Phan et al., 2002) and activity in this region has also been associated with fluctuations in basal peripheral autonomic activity (Critchley et al., 2000; Critchley, 2003), self-reflective thinking (Gusnard et al., 2001), a ‘default’ state of the brain when specific tasks are not being performed (Raichle et al., 2001) and tracking the expected reward value of particular stimuli (Knutson et al., 2003). In contrast the SGC has shown increased activity with sadness and active depression (Mayberg et al., 1999), and has been implicated in retention of extinction in fear conditioning (Phelps et al., 2004). The SGC region also appears to be at the centre of the VMPC lesions that lead to impaired decision-making based on future consequences, which has been hypothetically linked to the absence of a ‘somatic marker’ (Bechara et al., 1994). Similar to the vmSFG region, the SGC has been implicated in autonomic function (Devinsky et al., 1995) and tracking of reward value (Rolls, 2000, 2004). While the existing literature on the SGC and vmSFG suggests some overlap in their functional roles, as well as potential distinctions, the fact that these regions are associated with behaviours that appear in many ways so disparate, namely disinhibition and apathy, suggests that further work distinguishing their physiological properties will have important implications for the study of human behavioural regulation.
The analysis of aberrant motor behaviours generated an unexpected but intriguing finding. These behaviours were associated with tissue loss in the dACC. This item on the NPI captures repetitive motor acts such as tapping, pacing, restlessness and frequent rummaging through drawers. These behaviours can be ritualistic, particularly in FTD (Miller et al., 1995; Snowden et al., 2001) and bear a strong resemblance to behaviours seen in obsessive-compulsive disorder (OCD). Functional imaging has linked OCD symptoms to several brain regions including the medial OFC, vACC and dACC (Mataix-Cols et al., 2004). Dorsal ACC is associated with major functional roles in both the cognitive and motor domains that may be relevant to these behavioural abnormalities. In the cognitive domain, dACC has been linked to mediating between responses when conflicting choices are available (van Veen and Carter, 2002). Conceivably, inability to mediate these conflicts may result in repetitive, non-directed behaviour. On the other hand, in the motor domain dACC and the adjacent supplementary motor area participate in planning of complex movements (Picard and Strick, 1996). Notably, the regions of tissue loss associated with aberrant motor behaviour included the dACC and the adjacent supplementary motor area in FTD/semantic dementia as well as premotor cortex in non-FTD/semantic dementia, suggesting that further research might directly investigate the role of motor planning in the development of aberrant motor behaviour.
Unlike the other behaviours included in this analysis, eating behaviours did not uniquely associate with any specific region. From a statistical point of view, this indicates that this behaviour was well correlated with the variance in atrophy common to the other three behaviours. Eating behaviours in FTD are complex and varied, and include carbohydrate craving, overeating with weight gain, obsessions for particular foods and occasionally oral exploration of non-food objects, which may not always coexist in an individual patient (Miller et al., 1995; Ikeda et al., 2002). This variability may have impacted the likelihood of finding unique associations with eating disorders in this analysis.
Neuroanatomical correlates of apathy and disinhibition were only found in our FTD/semantic dementia group, while many of the prior studies that found regional correlates of apathy included patients with AD (Craig et al., 1996; Migneco et al., 2001; Tekin et al., 2001; Benoit et al., 2002). The fact that no relationship was found between tissue loss and some behaviours in the non-FTD/semantic dementia group is probably due to a lack of power. In the case of disinhibition this is understandable because disinhibition was infrequent in the non-FTD/semantic dementia group. Although apathy was the most common behavioural disorder in the non-FTD/semantic dementia group, few of the non-FTD/semantic dementia patients had scores >4, whereas the F × Sprod scores in the FTD/semantic dementia group were well distributed throughout the range of potential scores. Based on the previous work described above, we believe that, at least in the case of apathy, the relationship with vmSFG may be true regardless of dementia subtype.
Methodological issues
VBM has advantages over ROI techniques in allowing analysis of brain–behaviour relationships across the entire brain, but there are some caveats. Some of the regions in the right hemisphere that correlate with the average of all four behaviours may be appearing due to co-atrophy. The VMFC, OFC, insula and ACC (particularly the ventral portion) have all been linked to social and emotional processing through lesion studies (Eslinger and Damasio, 1985; Saver and Damasio, 1991; Calder et al., 2000; Craig, 2003; Hornak et al., 2003; Vogt et al., 2003) and functional imaging studies (Mayberg, 1994; Phillips et al., 1998; Phan et al., 2002; Knutson et al., 2003; Kringelbach and Rolls, 2003; Phelps et al., 2004). Although the caudate nucleus has a putative role in emotional processing (and hence in social function) as indicated by lesion studies (Kumral et al., 1999) and functional imaging (Lane et al., 1997; Phillips et al., 1998), the precise role of the caudate nucleus in emotional and social processing is still a matter of debate (Milders et al., 2003). The strong interconnections between the caudate and the OFC and ACC (Alexander et al., 1986; Tekin and Cummings, 2002) may have resulted in this region atrophying along with the others, and caudate atrophy may or may not relate to specific behavioural abnormalities. Similarly the dorsolateral frontal cortex also has putative roles in emotion (for instance in regulation—see Ochsner et al., 2002), but this region is traditionally viewed as part of a system for cognitive processing (Alexander et al., 1986; Tekin and Cummings, 2002). Dorsolateral frontal cortex might have appeared in this analysis because of some relationship to the more medial frontal structures, or because of generalized atrophy in the right hemisphere in patients with behavioural dysfunction. More complex implementations of VBM or ROI approaches that directly account for atrophy in different regions of the brain, as well as the inclusion of more explanatory variables, are potentially useful approaches to this problem.
The regional atrophy correlated with each of the individual behaviours is less susceptible to this confound of co-atrophy because a relatively small set of voxels was identified relating to each behaviour. However, our findings do not imply that these are the only regions relating to these behaviours. Rather, the specific medial frontal regions highlighted may be part of a larger network of regions that must be damaged to cause the behaviour of interest. An excess of damage to one part of the system superimposed on damage to the system as a whole may result in more severe behaviour of a certain type.
The underlying assumptions of VBM have also been the subject of criticism, mainly because VBM cannot comprehensively differentiate changes in tissue content from local misregistration of images (Bookstein, 2001). VBM as currently implemented also assumes simple linear relationships that are qualitatively similar throughout the brain, and thus it may be insensitive to other types of relationships (Davatzikos, 2004). Although the theory behind these criticisms is correct, it has been pointed out that current approaches to VBM attempt to minimize the effects of misregistration through techniques such as tissue-specific normalization templates, and that these caveats appear to have limited applicability to real imaging data (Ashburner and Friston, 2001). Other voxel-based analyses, such as deformation-based morphometry (Studholme et al., 2004; Thompson et al., 2003a) and more elaborate models of possible brain–behaviour relationships (Davatzikos, 2004) can address these issues more directly. Direct comparisons have shown that VBM produces data that is comparable, though not precisely the same as ROI analyses (Good et al., 2002; Testa et al., 2004).
Implications for the neuroscience of behaviour
The current results, along with other recent studies (Galton et al., 2001; Boxer et al., 2003; Gee et al., 2003; Grundman et al., 2003; Kassubek et al., 2004; Williams et al., 2005), indicate that quantitative analysis of tissue loss can reveal important associations between cognitive or behavioural deficits and circumscribed brain regions, even in patients with diffuse disease. Neurodegenerative disorders are ubiquitous, and show injury in regions seldom selectively affected by other disease processes. In addition, the ability to study a changing process in individuals over time behaviourally and with imaging may allow better control for differences across individuals, reducing noise in analyses. Voxel based analyses of patients with dementia can reveal important information about localized neurological functions, particularly those involved in social and emotional behaviour. In the future, voxel-based studies in dementia (including VBM and other approaches) should be combined with ROI studies and traditional focal lesion studies to achieve the most accurate description of these relationships.
Acknowledgments
This work was supported by National Institute on Aging (NIA) grants 1K08AG020760-01, AG10129, P50-AG05142, and AG16570, the State of California Alzheimer’s Disease Research Center of California (ARCC) grant 01-154-20, NIH grant number M01 RR00079 (UCSF General Clinical Research Center) and the Hillblom Network. We also thank Joel Kramer and Kristine Yaffe for their careful readings of the manuscript.
References
- Abell F, Krams M, Ashburner J, Passingham R, Friston K, Frackowiak R, et al. The neuroanatomy of autism: a voxel-based whole brain analysis of structural scans. Neuroreport. 1999;10:1647–51. doi: 10.1097/00001756-199906030-00005. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Damasio H, Tranel D, Damasio AR. Cortical systems for the recognition of emotion in facial expressions. J Neurosci. 1996;16:7678–87. doi: 10.1523/JNEUROSCI.16-23-07678.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–81. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuro-image. 2000;11:805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Why voxel-based morphometry should be used. Neuroimage. 2001;14:1238–43. doi: 10.1006/nimg.2001.0961. [DOI] [PubMed] [Google Scholar]
- Bathgate D, Snowden JS, Varma A, Blackshaw A, Neary D. Behaviour in frontotemporal dementia, Alzheimer’s disease and vascular dementia. Acta Neurol Scand. 2001;103:367–78. doi: 10.1034/j.1600-0404.2001.2000236.x. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Benoit M, Koulibaly PM, Migneco O, Darcourt J, Pringuey DJ, Robert PH. Brain perfusion in Alzheimer’s disease with and without apathy: a SPECT study with statistical parametric mapping analysis. Psychiatry Res. 2002;114:103–11. doi: 10.1016/s0925-4927(02)00003-3. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Winston J, Frith U. Social cognitive neuroscience: where are we heading? Trends Cogn Sci. 2004;8:216–22. doi: 10.1016/j.tics.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Boccardi M, Pennanen C, Laakso MP, Testa C, Geroldi C, Soininen H, et al. Amygdaloid atrophy in frontotemporal dementia and Alzheimer’s disease. Neurosci Lett. 2002;335:139–43. doi: 10.1016/s0304-3940(02)01169-2. [DOI] [PubMed] [Google Scholar]
- Boeve BF, Lang AE, Litvan I. Corticobasal degeneration and its relationship to progressive supranuclear palsy and frontotemporal dementia. Ann Neurol. 2003;54(Suppl 5):S15–S19. doi: 10.1002/ana.10570. [DOI] [PubMed] [Google Scholar]
- Bookstein FL. Voxel-based morphometry” should not be used with imperfectly registered images. Neuroimage. 2001;14:1454–62. doi: 10.1006/nimg.2001.0770. [DOI] [PubMed] [Google Scholar]
- Borod JC, Cicero BA, Obler LK, Welkowitz J, Erhan HM, Santschi C, et al. Right hemisphere emotional perception: evidence across multiple channels. Neuropsychology. 1998;12:446–58. doi: 10.1037//0894-4105.12.3.446. [DOI] [PubMed] [Google Scholar]
- Bowers D, Blonder LX, Feinberg T, Heilman KM. Differential impact of right and left hemisphere lesions on facial emotion and object imagery. Brain. 1991;114:2593–609. doi: 10.1093/brain/114.6.2593. [DOI] [PubMed] [Google Scholar]
- Boxer AL, Kramer JH, Du A-T, Schuff N, Weiner MW, Miller BL, et al. Focal right inferotemporal atrophy in AD with disproportionate visual constructive impairment. Neurology. 2003;61:1485–91. doi: 10.1212/01.wnl.0000090568.34810.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozeat S, Gregory CA, Ralph MA, Hodges JR. Which neuropsychiatric and behavioural features distinguish frontal and temporal variants of fronto-temporal dementia from Alzheimer’s disease? J Neurol Neurosurg Psychiatry. 2000;69:178–86. doi: 10.1136/jnnp.69.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88:1337–42. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton EJ, Karas G, Paling SM, Barber R, Williams ED, Ballard CG, et al. Patterns of cerebral atrophy in dementia with Lewy bodies using voxel-based morphometry. Neuroimage. 2002;17:618–30. [PubMed] [Google Scholar]
- Calder AJ, Keane J, Manes F, Antoun N, Young AW. Impaired recognition and experience of disgust following brain injury. Nat Neurosci. 2000;3:1077–8. doi: 10.1038/80586. [DOI] [PubMed] [Google Scholar]
- Canli T. Hemispheric asymmetry in the experience of emotion: a perspective from functional imaging. Neuroscientist. 1999;5:201–7. [Google Scholar]
- Carota A, Staub F, Bogousslavsky J. Emotions, behaviours and mood changes in stroke. Curr Opin Neurol. 2002;15:57–69. doi: 10.1097/00019052-200202000-00010. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correla-correlation analysis for the behavioral sciences. Mahwah, NJ: Lawrence Erlbaum Associates; 2003. [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500–5. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craig AH, Cummings JL, Fairbanks L, Itti L, Miller BL, Li J, et al. Cerebral blood flow correlates of apathy in Alzheimer disease. Arch Neurol. 1996;53:1116–20. doi: 10.1001/archneur.1996.00550110056012. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Moroz TM, Moscovitch M, Stuss DT, Winocur G, Tulving E, et al. In search of the self: a positron emission tomography study. Psychol Sci. 1999;10:24–36. [Google Scholar]
- Critchley H. Emotion and its disorders. Br Med Bull. 2003;65:35–47. doi: 10.1093/bmb/65.1.35. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Elliott R, Mathias CJ, Dolan RJ. Neural activity relating to generation and representation of galvanic skin conductance responses: a functional magnetic resonance imaging study. J Neurosci. 2000;20:3033–40. doi: 10.1523/JNEUROSCI.20-08-03033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Good CD, Ashburner J, Frackowiak RS, Mathias CJ, Dolan RJ. Changes in cerebral morphology consequent to peripheral autonomic denervation. Neuroimage. 2003;18:908–16. doi: 10.1016/s1053-8119(03)00011-9. [DOI] [PubMed] [Google Scholar]
- Cummings JL. The neuropsychiatric inventory: assessing psychopathology in dementia patients. Neurology. 1997;48:S10–S16. doi: 10.1212/wnl.48.5_suppl_6.10s. [DOI] [PubMed] [Google Scholar]
- Davatzikos C. Why voxel-based morphometric analysis should be used with great caution when characterizing group differences. Neuroimage. 2004;23:17–20. doi: 10.1016/j.neuroimage.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Ekman P, Saron CD, Senulis JA, Friesen WV. Approach-withdrawal and cerebral asymmetry: emotional expression and brain physiology. I J Pers Soc Psychol. 1990;58:330–41. [PubMed] [Google Scholar]
- Demonet JF, Thierry G, Cardebat D. Renewal of the neurophysiology of language: functional neuroimaging. Physiol Rev. 2005;85:49–95. doi: 10.1152/physrev.00049.2003. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Dolan RJ. Emotion, cognition, and behavior. Science. 2002;298:1191–4. doi: 10.1126/science.1076358. [DOI] [PubMed] [Google Scholar]
- Edelstyn NM, Oyebode F, Barrett K. The delusions of Capgras and intermetamorphosis in a patient with right-hemisphere white-matter pathology. Psychopathology. 2001;34:299–304. doi: 10.1159/000049328. [DOI] [PubMed] [Google Scholar]
- Edwards-Lee T, Miller BL, Benson DF, Cummings JL, Russell GL, Boone K, et al. The temporal variant of frontotemporal dementia. Brain. 1997;120:1027–40. doi: 10.1093/brain/120.6.1027. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, Damasio AR. Severe disturbance of higher cognition after bilateral frontal lobe ablation: patient EVR. Neurology. 1985;35:1731–41. doi: 10.1212/wnl.35.12.1731. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, Damasio H, Graff-Radford N, Damasio AR. Examining the relationship between computed tomography and neuropsychological measures in normal and demented elderly. J Neurol Neurosurg Psychiatry. 1984;47:1319–25. doi: 10.1136/jnnp.47.12.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eustache F, Desgranges B, Aupee AM, Guillery B, Baron JC. Functional neuroanatomy of amnesia: positron emission tomography studies. Microsc Res Tech. 2000;51:94–100. doi: 10.1002/1097-0029(20001001)51:1<94::AID-JEMT10>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Fossati P, Hevenor SJ, Graham SJ, Grady C, Keightley ML, Craik F, et al. In search of the emotional self: an FMRI study using positive and negative emotional words. Am J Psychiatry. 2003;160:1938–45. doi: 10.1176/appi.ajp.160.11.1938. [DOI] [PubMed] [Google Scholar]
- Franceschi M, Anchisi D, Pelati O, Zuffi M, Matarrese M, Moresco RM, et al. Glucose metabolism and serotonin receptors in the frontotemporal lobe degeneration. Ann Neurol. 2005;57:216–25. doi: 10.1002/ana.20365. [DOI] [PubMed] [Google Scholar]
- Galton CJ, Patterson K, Graham K, Lambon-Ralph MA, Williams G, Antoun N, et al. Differing patterns of temporal atrophy in Alzheimer’s disease and semantic dementia. Neurology. 2001;57:216–25. doi: 10.1212/wnl.57.2.216. [DOI] [PubMed] [Google Scholar]
- Gee J, Ding L, Xie Z, Lin M, DeVita C, Grossman M. Alzheimer’s disease and frontotemporal dementia exhibit distinct atrophy-behavior correlates: a computer-assisted imaging study. Acad Radiol. 2003;10:1392–401. doi: 10.1016/s1076-6332(03)00543-9. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude I, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage. 2001a;14:685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001b;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Good CD, Scahill RI, Fox NC, Ashburner J, Friston KJ, Chan D, et al. Automatic differentiation of anatomical patterns in the human brain: validation with studies of degenerative dementias. Neuroimage. 2002;17:29–46. doi: 10.1006/nimg.2002.1202. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55:335–46. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundman M, Jack CR, Jr, Petersen RC, Kim HT, Taylor C, Datvian M, et al. Hippocampal volume is associated with memory but not nonmemory cognitive performance in patients with mild cognitive impairment. J Mol Neurosci. 2003;20:241–8. doi: 10.1385/jmn:20:3:241. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci USA. 2001;98:4259–64. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann S, Monarch ES, Goldstein FC. Impaired fear conditioning in Alzheimer’s disease. Neuropsychologia. 2002;40:1187–95. doi: 10.1016/s0028-3932(01)00223-8. [DOI] [PubMed] [Google Scholar]
- Harlow JM. Recovery from the passage of an iron bar through the head. Massachussets Med Soc Publ. 1868;2:327–46. [Google Scholar]
- Hornak J, Rolls ET, Wade D. Face and voice expression identification in patients with emotional and behavioural changes following ventral frontal lobe damage. Neuropsychologia. 1996;34:247–61. doi: 10.1016/0028-3932(95)00106-9. [DOI] [PubMed] [Google Scholar]
- Hornak J, Bramham J, Rolls ET, Morris RG, O’Doherty J, Bullock PR, et al. Changes in emotion after circumscribed surgical lesions of the orbito-frontal and cingulate cortices. Brain. 2003;126:1691–712. doi: 10.1093/brain/awg168. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Brown J, Holland AJ, Fukuhara R, Hodges JR. Changes in appetite, food preference, and eating habits in frontotemporal dementia and Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2002;73:371–6. doi: 10.1136/jnnp.73.4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassubek J, Juengling FD, Ecker D, Landwehrmeyer GB. Thalamic atrophy in Huntington’s disease co-varies with cognitive performance: a morphometric MRI analysis. Cereb Cortex. 2005;16:846–53. doi: 10.1093/cercor/bhh185. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuroimage. 2003;18:263–72. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Jurik J, Sha SJ, Rankin KP, Rosen HJ, Johnson JK, et al. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn Behav Neurol. 2003;16:211–8. doi: 10.1097/00146965-200312000-00002. [DOI] [PubMed] [Google Scholar]
- Krams M, Quinton R, Ashburner J, Friston KJ, Frackowiak RS, Bouloux PM, et al. Kallmann’s syndrome: mirror movements associated with bilateral corticospinal tract hypertrophy. Neurology. 1999;52:816–22. doi: 10.1212/wnl.52.4.816. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. Neural correlates of rapid reversal learning in a simple model of human social interaction. Neuroimage. 2003;20:1371–83. doi: 10.1016/S1053-8119(03)00393-8. [DOI] [PubMed] [Google Scholar]
- Kumral E, Evyapan D, Balkir K. Acute caudate vascular lesions. Stroke. 1999;30:100–8. doi: 10.1161/01.str.30.1.100. [DOI] [PubMed] [Google Scholar]
- Kumral E, Bayulkem G, Evyapan D, Yunten N. Spectrum of anterior cerebral artery territory infarction: clinical and MRI findings. Eur J Neurol. 2002;9:615–24. doi: 10.1046/j.1468-1331.2002.00452.x. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Bradley MM, Lang PJ, Ahern GL, Davidson RJ, et al. Neuroanatomical correlates of pleasant and unpleasant emotion. Neuropsychologia. 1997;35:1437–44. doi: 10.1016/s0028-3932(97)00070-5. [DOI] [PubMed] [Google Scholar]
- Levy ML, Miller BL, Cummings JL, Fairbanks LA, Craig A. Alzheimer disease and frontotemporal dementias. Behavioral distinctions Arch Neurol. 1996;53:687–90. doi: 10.1001/archneur.1996.00550070129021. [DOI] [PubMed] [Google Scholar]
- Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47:1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- Liu W, Miller BL, Kramer JH, Rankin K, Wyss-Coray C, Gearhart R, et al. Behavioral disorders in the frontal and temporal variants of frontotemporal dementia. Neurology. 2004;62:742–8. doi: 10.1212/01.wnl.0000113729.77161.c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D, Wooderson S, Lawrence N, Brammer MJ, Speckens A, Phillips ML. Distinct neural correlates of washing, checking, and hoarding symptom dimensions in obsessive-compulsive disorder. Arch Gen Psychiatry. 2004;61:564–76. doi: 10.1001/archpsyc.61.6.564. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Frontal lobe dysfunction in secondary depression. J Neuropsychiatry Clin Neurosci. 1994;6:428–42. doi: 10.1176/jnp.6.4.428. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–82. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Migneco O, Benoit M, Koulibaly PM, Dygai I, Bertogliati C, Desvignes P, et al. Perfusion brain SPECT and statistical parametric mapping analysis indicate that apathy is a cingulate syndrome: a study in Alzheimer’s disease and nondemented patients. Neuroimage. 2001;13:896–902. doi: 10.1006/nimg.2000.0741. [DOI] [PubMed] [Google Scholar]
- Milders M, Crawford JR, Lamb A, Simpson SA. Differential deficits in expression recognition in gene-carriers and patients with Huntington’s disease. Neuropsychologia. 2003;41:1484–92. doi: 10.1016/s0028-3932(03)00079-4. [DOI] [PubMed] [Google Scholar]
- Miller BL, Chang L, Mena I, Boone K, Lesser IM. Progressive right fronto-temporal degeneration: clinical, neuropsychological and SPECT characteristics. Dementia. 1993;4:204–13. doi: 10.1159/000107324. [DOI] [PubMed] [Google Scholar]
- Miller BL, Darby AL, Swartz JR, Yener GG, Mena I. Dietary changes, compulsions and sexual behavior in frontotemporal degeneration. Dementia. 1995;6:195–9. doi: 10.1159/000106946. [DOI] [PubMed] [Google Scholar]
- Morris JC. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr. 1997;9:173–6. doi: 10.1017/s1041610297004870. discussion 177–8. [DOI] [PubMed] [Google Scholar]
- Mummery C, Patterson K, Price C, Ashburner J, Frackowiak R, Hodges J. A voxel-based morphometry study of semantic dementia: relationship between temporal lobe atrophy and semantic memory. Ann Neurol. 2000;47:36–45. [PubMed] [Google Scholar]
- Mychack P, Kramer JH, Boone KB, Miller BL. The influence of right frontotemporal dysfunction on social behavior in frontotemporal dementia. Neurology. 2001;56:S11–S15. doi: 10.1212/wnl.56.suppl_4.s11. [DOI] [PubMed] [Google Scholar]
- Nadeau SE. Alzheimer’s disease as a window to neural mechanisms of cognition. Neurology. 2003;61:1470–1. doi: 10.1212/wnl.61.11.1470. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–54. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Ochsner KN. Current directions in social cognitive neuroscience. Curr Opin Neurobiol. 2004;14:254–8. doi: 10.1016/j.conb.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–29. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ott BR, Noto RB, Fogel BS. Apathy and loss of insight in Alzheimer’s disease: a SPECT imaging study. J Neuropsychiatry Clin Neurosci. 1996;8:41–6. doi: 10.1176/jnp.8.1.41. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–48. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Scott SK, Calder AJ, Andrew C, Giampietro V, et al. Neural responses to facial and vocal expressions of fear and disgust. Proc Biol Sci. 1998;265:1809–17. doi: 10.1098/rspb.1998.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard N, Strick PL. Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex. 1996;6:342–53. doi: 10.1093/cercor/6.3.342. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. The orbitofrontal cortex and reward. Cereb Cortex. 2000;10:284–94. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The functions of the orbitofrontal cortex. Brain Cogn. 2004;55:11–29. doi: 10.1016/S0278-2626(03)00277-X. [DOI] [PubMed] [Google Scholar]
- Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol. 2000;12:191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Gorno-Tempini ML, Goldman WP, Perry RJ, Schuff N, Weiner M, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002a;58:198–208. doi: 10.1212/wnl.58.2.198. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Perry RJ, Murphy J, Kramer JH, Mychack P, Schuff N, et al. Emotion comprehension in the temporal variant of frontotemporal dementia. Brain. 2002b;125:2286–95. doi: 10.1093/brain/awf225. [DOI] [PubMed] [Google Scholar]
- Salmond CH, Ashburner J, Vargha-Khadem F, Connelly A, Gadian DG, Friston KJ. Distributional assumptions in voxel-based morphometry. Neuroimage. 2002;17:1027–30. [PubMed] [Google Scholar]
- Sarazin M, Michon A, Pillon B, Samson Y, Canuto A, Gold G, et al. Metabolic correlates of behavioral and affective disturbances in frontal lobe pathologies. J Neurol. 2003;250:827–33. doi: 10.1007/s00415-003-1087-z. [DOI] [PubMed] [Google Scholar]
- Saver JL, Damasio AR. Preserved access and processing of social knowledge in a patient with acquired sociopathy due to ventromedial frontal damage. Neuropsychologia. 1991;29:1241–9. doi: 10.1016/0028-3932(91)90037-9. [DOI] [PubMed] [Google Scholar]
- Simpson JR, Jr, Drevets WC, Snyder AZ, Gusnard DA, Raichle ME. Emotion-induced changes in human medial prefrontal cortex: II. During anticipatory anxiety. Proc Natl Acad Sci USA. 2001;98:688–93. doi: 10.1073/pnas.98.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden JS, Bathgate D, Varma A, Blackshaw A, Gibbons ZC, Neary D. Distinct behavioural profiles in frontotemporal dementia and semantic dementia. J Neurol Neurosurg Psychiatry. 2001;70:323–32. doi: 10.1136/jnnp.70.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staff RT, Shanks MF, Macintosh L, Pestell SJ, Gemmell HG, Venneri A. Delusions in Alzheimer’s disease: spet evidence of right hemispheric dysfunction. Cortex. 1999;35:549–60. doi: 10.1016/s0010-9452(08)70818-9. [DOI] [PubMed] [Google Scholar]
- Studholme C, Cardenas V, Blumenfeld R, Schuff N, Rosen HJ, Miller B, et al. Deformation tensor morphometry of semantic dementia with quantitative validation. Neuroimage. 2004;21:1387–98. doi: 10.1016/j.neuroimage.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: an update. J Psychosom Res. 2002;53:647–54. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- Tekin S, Mega MS, Masterman DM, Chow T, Garakian J, Vinters HV, et al. Orbitofrontal and anterior cingulate cortex neurofibrillary tangle burden is associated with agitation in Alzheimer disease. Ann Neurol. 2001;49:355–61. [PubMed] [Google Scholar]
- Testa C, Laakso MP, Sabattoli F, Rossi R, Beltramello A, Soininen H, et al. A comparison between the accuracy of voxel-based morphometry and hippocampal volumetry in Alzheimer’s disease. J Magn Reson Imaging. 2004;19:274–82. doi: 10.1002/jmri.20001. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, de Zubicaray G, Janke AL, Rose SE, Semple J, et al. Dynamics of gray matter loss in Alzheimer’s disease. J Neurosci. 2003a;23:994–1005. doi: 10.1523/JNEUROSCI.23-03-00994.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SA, Patterson K, Hodges JR. Left/right asymmetry of atrophy in semantic dementia: behavioral-cognitive implications. Neurology. 2003b;61:1196–203. doi: 10.1212/01.wnl.0000091868.28557.b8. [DOI] [PubMed] [Google Scholar]
- Tranel D, Bechara A, Denburg NL. Asymmetric functional roles of right and left ventromedial prefrontal cortices in social conduct, decision-making, and emotional processing. Cortex. 2002;38:589–612. doi: 10.1016/s0010-9452(08)70024-8. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol Behav. 2002;77:477–82. doi: 10.1016/s0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- Vijayaraghavan L, Krishnamoorthy ES, Brown RG, Trimble MR. Abulia: a delphi survey of British neurologists and psychiatrists. Mov Disord. 2002;17:1052–7. doi: 10.1002/mds.10194. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Berger GR, Derbyshire SW. Structural and functional dichotomy of human midcingulate cortex. Eur J Neurosci. 2003;18:3134–44. doi: 10.1111/j.1460-9568.2003.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. Neuroimage. 2003;19:513–31. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Williams GB, Nestor PJ, Hodges JR. Neural correlates of semantic and behavioural deficits in frontotemporal dementia. Neuroimage. 2005;24:1042–51. doi: 10.1016/j.neuroimage.2004.10.023. [DOI] [PubMed] [Google Scholar]


