Abstract
Alzheimer’s disease (AD) and subcortical ischemic vascular disease (SIVD) are common causes of dementia, often co-occur, and can present quite similarly, making differential diagnosis clinically challenging. This study tested the hypothesis that patients with SIVD retain information better than AD patients. Participants were 35 dementia patients with subcortical lacunes (SIVD group), 27 dementia patients without lacunar infarction (AD group), and 56 normal controls. Results indicated that despite comparable levels of initial acquisition, AD patients showed more rapid forgetting. Further analysis indicated that memory patterns within the SIVD group were heterogeneous, with some participants exhibiting rapid forgetting and some exhibiting good retention. SIVD participants with good retention showed a trend for greater executive impairments relative to SIVD participants with rapid forgetting and AD participants. Results suggest that rapid forgetting in SIVD may imply concomitant AD, whereas the dementia in patients with good retention may be purely vascular in origin. Three SIVD patients with rapid forgetting followed to autopsy all had AD pathology, further supporting the link between memory patterns and AD.
Alzheimer’s disease (AD) and vascular disease are among the most common causes of dementia (Fein et al., 2000). Vascular disease produces a heterogeneous group of behavioral disturbances depending on the type (e.g., ischemic vs. hemorrhagic), location (cortical, subcortical, white matter) and degree of vascular pathology. Patients whose vascular disease is restricted to subcortical structures are most likely to present at dementia clinics. Subcortical ischemic vascular disease (SIVD) is typically the result of occlusions of the deep penetrating arterioles and arteries that feed the basal ganglia, thalamus, white matter, and internal capsule. Unlike large vessel ischemia, which results in cortical strokes with acute onset and focal neurologic dysfunction, SIVD can present similarly to AD, with insidious onset, gradual progression, and without obvious focal neurologic symptoms. These similarities in clinical presentation and age of onset make the differential diagnosis between AD and SIVD challenging.
There have been several attempts to differentiate between AD and SIVD on the basis of neuropsychological findings. Consistent with a subcortical-frontal systems model for SIVD, neuropsychological comparisons have generally reported relative deficits in SIVD compared to AD in executive functions such as verbal fluency, attention, sequencing and problem solving (Lafosse et al., 1997; Libon et al., 2001; Rosenstein, 1998). Relative advantages in SIVD compared to AD have included better memory performance (Hassing & Backman, 1997), fewer intrusion errors (Lukatela, Malloy, Jenkins, & Cohen, 1998), and better recognition memory (Tierney et al., 2001). Libon et al. (1998) demonstrated that patients with AD and SIVD can be dissociated on the basis of differing patterns of memory impairment. In their study, the SIVD group performed as poorly as the AD group on immediate recall of a 9-item CVLT list A. After a delay, however, SIVD participants showed a greater capacity to retain information as evidenced by their significantly higher score on the CVLT recognition discriminability index. This finding of relatively greater preservation of information in SIVD is consistent with the widespread view that information is lost more rapidly in AD than it is in subcortical dementing syndromes (Cummings, 1986; Kramer, Levin, Brandt, & Delis, 1989).
Despite evidence for neuropsychological differences between SIVD and AD, the issue of comorbidity remains an ongoing issue in SIVD research. Both disorders can be present in varying degrees in a high proportion of dementia patients. Vascular disease is common in patients with AD (Pantel et al., 1998) and can present as lacunar infarction and/or white matter disease. Kalaria and Ballard (1999) report that up to 30% of AD cases exhibit cerebrovascular pathology, which includes microvascular degeneration, amyloid angiopathy, and periventricular white matter changes. In addition, up to one third of patients diagnosed with vascular dementia will be found to have Alzheimer’s pathology on autopsy. The presence of AD pathology in some but not all SIVD cases obscures attempts to characterize the dementia due to cerebrovascular disease. Comorbidity may also be one reason why SIVD can be so difficult to diagnose and why there is poor concordance between different diagnostic schema for vascular dementia (Chui, 2001).
MRI remains the most effective tool for determining the presence of subcortical ischemic vascular disease. The challenge for clinicians is to determine when concomitant AD is present. This determination has significant clinical ramifications, since drug treatments for AD are increasingly available. The present study has two primary goals. The first goal is to test the hypothesis that SIVD participants retain information better than AD patients by directly comparing initial and delayed free recall in the two groups using a multivariate design. The second goal is to investigate the patterns of memory performance in SIVD. Heterogeneity of memory patterns would support the view that SIVD patients comprise a mixed group, some with comorbid AD, and some without, and suggest a possible behavioral marker that will assist with differential diagnosis. A secondary aim was to assess for subgroup differences in four MRI variables with known relevance for cognition, cortical atrophy, white matter signal hyperintensities (WMSH), lacunes, and hippocampal atrophy (Fein et al., 2000; Mungas et al., 2001).
METHODS
Participants
We studied 62 patients with dementia and 56 neurologically normal controls. All participants were recruited in California as part of a Program Project Grant on ischemic cerebrovascular disease, and underwent magnetic resonance imaging (MRI), neurological exam, neuropsychological testing, routine labs, and completion of the Clinical Dementia Rating Scale (CDR; Hughes, Berg, Danziger, Coben, & Martin, 1982). Exclusionary criteria included presence of substance abuse, major psychiatric disorder, medications known to interfere with CNS functioning, history of head trauma resulting in sustained loss of consciousness, and history of neurological disorder (e.g., cortical stroke, multiple sclerosis, epilepsy).
All dementia participants had a clinical diagnosis of dementia based on the presence of significant decline in occupational and social functioning and/or a CDR score of 1 or greater. All MRI scans were reviewed by a single neuroradiologist to establish the presence and location of a lacune. The goal of this study was to better understand the role of cerebrovascular disease in dementia. Because lacunar infarction is a marker of cerebrovascular disease, the primary distinction between SIVD and AD used in the present study is that participants defined has having SIVD were demented and had radiologically confirmed subcortical lacunes, whereas participants defined as having AD were demented without lacunes. The 27 demented participants without lacunes in this study all met NINCDS-ADRDA criteria for probable AD. Thirty-five (35) demented participants had evidence for subcortical lacunar infarction and comprised the SIVD group. The 56 neurologically normal control participants all had CDR scores of 0, were clinically rated as being cognitive normal, and had no evidence of lacunar infarcts on MRI. The presence of cortical infarction was an exclusionary criterion for all groups. Demographic data for the three groups are summarized in Table 1.
Table 1.
Demographic Characteristics of AD, SIVD, and Control Groups.
| AD, n = 27
|
SIVD, n = 35
|
Control, n = 56
|
||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Age | 75.2 | 8.0 | 76.6 | 8.7 | 73.2 | 8.2 |
| Education | 14.4 | 3.2 | 12.8 | 3.5 | 14.1 | 2.4 |
| MMSE | 21.1 | 3.8 | 21.9 | 4.0 | 29.1 | 1.2 |
| % female | 40.7 | 40.0 | 58.9 | |||
Note. AD = Alzheimer’s disease.
SIVD = subcortical ischemic vascular disease.
MMSE = Mini-Mental State Exam.
Neuropsychological Procedures
Participants were administered the Memory Assessment Scale List Learning Test (MAS; Williams, 1991) as part of a larger neuropsychological test battery. The MAS List Learning Test consists of a 12-item list that is administered over six learning trials. Each learning trial consisted of an oral presentation of the list at a rate of approximately one word per 2 s followed by the participant’s exhaustive free recall. The delayed free recall trial occurred after a 20-min interval filled with primarily nonverbal tasks. The dependent measure on all trials was the number of words correctly recalled.
The Mini-Mental State Exam (MMSE) served as an index of global cognitive functioning. The test battery also yielded measures of naming (Boston Naming Test), visuoperception (Benton Visual Form Discrimination), category fluency (Animal Naming), working memory (the Mental Control score) and backward digit span raw score from the Wechsler Memory Scale –Revised, and executive functioning (Initiation-Perseveration subscale from the Mattis DRS and Controlled Word Association Test).
Neuroimaging
All participants underwent quantitative brain imaging. Studies were performed on a 1.5 Tesla VISION™ MRI system (Siemens Inc., Iselin, NJ) equipped with a standard quadrature head coil. To minimize motion of the participant’s head, a vacuum-molded head holder (Vac-Pac, Olympic Medical, Seattle, WA) was employed to restrict head movements. The MRI protocol consisted of sagittal T1 weighted localizer scans. This was followed by oblique axial double spin echo (DSE) scans (TR/TE1/TE2 = 5000/20/80 ms), inplane resolution 1.0 × 1.0 mm2, 3 mm thick, contiguous slices, angulated parallel to the optic nerve as seen in the sagittal plane and covering the entire brain from the vertex to the base of the cerebellum. DSE yielded proton density and T2-weighted MR images that were used for semi-automated image tissue segmentation, as described previously (Fein et al., 2000).
Lacunes were operationally defined as small (>3 mm) areas of the brain with increased signal relative to CSF on proton density MRI in subcortical gray and white matter (Carpizzano et al., 2000). Lacunes were differentiated from perivascular spaces (PVS), which can be particularly prominent below the anterior commissure and putamen and at bends in the course of penetrating arterioles. Both are bright on T2 weighted images, but only lacunes are hyperintense relative to CSF on proton density images. A rare cystic lacune may be isointense relative to CSF. Isointense lesions on proton density MRI at the level of the anterior commissure or inferior putamen were termed perivascular spaces; outside that region they were defined as cavitated lacunes if they were 3 mm at maximum width. Lacunes are variably hypointense on T1 weighted MRI. Thus, T1-weighted signal characteristics were not used in our operational definition of lacunes. Images underwent semi-automated image tissue segmentation for quantification of lacunar volume. White matter lesions (WML) were initially segmented as gray matter, then hand edited as abnormal white matter. They appear as hyperintense areas on proton density and T2-weighted MRI in the periventricular white matter and centrum semiovale. These lesions may represent variable degrees of demyelination, edema, or infarction.
A computer algorithm was used to classify brain MRI pixels first into principal tissue types of gray matter, white matter, and cerebrospinal fluid. Subsequently, an operator-guided computer algorithm was applied to further subdivide categories of tissue into the following types: cortical gray matter, subcortical gray matter, white matter, white matter lesions, ventricular cerebrospinal fluid, and sulcal cerebrospinal fluid. In addition, total intracranial volume was computed by summing over all pixels within the intracranial vault. Segmentation methods have been previously reported (Fein et al., 2000).
Automated hippocampal volumetry was carried out using a commercially available high dimensional brain mapping tool (Medtronic Surgical Navigation Technologies, Louisville, CO), which combined a coarse and then a fine transformation to match cerebral MR images with a template brain (Csernansky, Wang, Joshi, et al., 2000). Global landmarks were placed at external boundaries of the target brain by manual adjustment of the angle and dimension of a three-dimensional box in orthogonal MR images. The next step was manual selection of 22 controls points as local landmarks for hippocampal segmentation: one at the hippocampal head, one at the tail, and four per image (i.e. at the superior, inferior, medial and lateral boundaries) on five equally spaced images perpendicular to the long axis of the ipsilateral hippocampus. This step was repeated for the contralateral hippocampus. Marking both hippocampi required less than 10 min for each participant. Using both the global and local landmarks, a coarse transformation was computed using landmark matching. Automated hippocampal morphometry was then performed by a fluid image matching transformation (Haller, Christensen, Joshi, et al., 1996). Hippocampal and cortical grey matter volumes were adjusted for total intracranial volume.
RESULTS
Demographic data are summarized in Table 1. There were no statistically significant group differences in age, education, or male:female ratio. In addition, the two demented groups had very similar total MMSE scores (means of around 21), indicating comparable levels of dementia severity.
Initial Acquisition and Retention of Information Over Delays
The MAS consists of multiple learning trials and a 20-min delayed recall trial. The initial step in data analysis examined group differences in immediate recall using repeated measures ANOVA, with a main effect indicating group differences in overall recall, and a Group × Trial interaction indicating differences in the rate of acquisition across the learning trials. The second step in the data analysis using repeated measures ANOVA to examine how well information was retained over the delay.
Analysis of the six learning trials on the MAS indicated a robust main effect for group, F(2, 115) = 145.8, p <.001 and a significant Group × Trial interaction, F(10, 575) = 13.9, p <.001. Post hoc analyses of the main and interaction effects were carried out using two-group MANOVAs. Controls had superior recall and faster learning relative to the AD group (interaction effect F(5, 405) = 17.9, p <.001) and the SIVD group (interaction effect F(5, 445) = 18.0, p < .001), but there were no significant differences between the AD and the SIVD group in number of words recalled or rate of acquisition (interaction effect F(5, 300) = 6.5, p >.15). In addition, the SIVD and AD groups did not differ in recall on the final (6th) learning trial.
Decline in recall over the 20-min delay was assessed using repeated measures ANOVA, with Trial (learning Trial 6 vs. delayed free recall) as the within-subject factor and Group as the between-subjects factor. Planned contrasts were SIVD versus AD, and SIVD versus Controls. When the two dementia groups were compared with one another, there was a significant Group × Trial interaction, F(1, 60) = 7.7, p <.01, reflecting the fact that the AD patients had a steeper decline in recall over the delay than did the SIVD patients (see Fig. 1). Analyses comparing the SIVD group with controls also yielded a Group × Trial interaction, F(1, 89) = 31.4, p < .001, indicating that SIVD participants had a steeper decline in recall over the delay than did the controls.
Fig. 1.
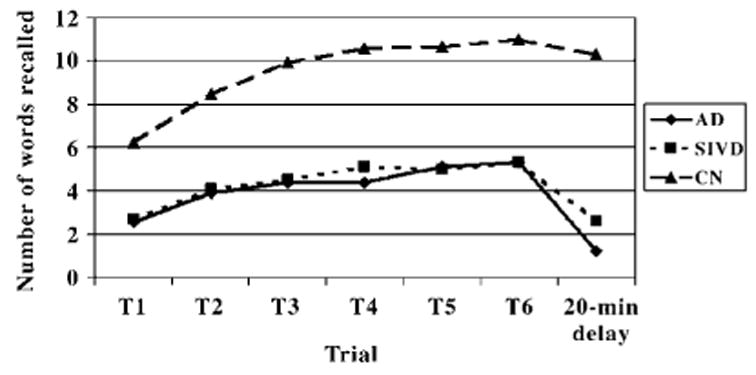
MAS performance of the AD, SIVD, and Normal Control (NC) groups over the six learning trials and the delayed recall trial.
Heterogeneity of Group Memory Performance
Participants were divided into three groups based on how well they retained information over the delay period. Classification rules were developed on the basis of face validity. Participants who recalled three or fewer items on the 6th MAS learning trial were classified as indeterminate, since their low levels of acquisition made analysis of forgetting rates problematic. Participants with sufficient acquisition (i.e., those who recalled four or more items on Trial 6) who lost 40% or more items over the delay were classified as having rapid forgetting, and participants with sufficient acquisition who lost less than 40% of the items over the delay were classified as having good retention.
Table 2 reports the number of participants within the AD, SIVD, and Control groups that were classified into rapid forgetting, good retention, and indeterminate subgroups. All of the controls exhibited good retention of the list. Within the AD group, over 81% exhibited rapid forgetting of the list, whereas only 7.4% showed good retention and 11% had poor initial acquisition. Within the SIVD group, however, the three memory patterns were about evenly represented. About 34% of the SIVD cases exhibited rapid forgetting of the list, 37% showed good retention and almost 29% had poor initial acquisition. Differences between the two dementia groups were assessed with a chi square analysis, which indicated that the three types of memory patterns were differentially represented (df = 2, chi square = 88.8, p <.001). Mean percentage loss was 81.8% for the AD group, 82.9% for the SIVD: Rapid Forgetting group, 11.8% for the SIVD: Good Retention group, and 6.8% for the controls.
Table 2.
Classification of AD, SIVD, and Control Subjects into Rapid Forgetting, Good Retention, and Indeterminate Subgroups.
| Rapid forgetting | Good retention | Indeterminate | |
|---|---|---|---|
| AD | 22 (81.5%) | 2 (7.4%) | 3 (11.1%) |
| SIVD | 12 (34.3%) | 13 (37.1%) | 10 (28.6%) |
| Controls | 0 | 56 (100%) | 0 |
Note. AD = Alzheimer’s disease.
SIVD = subcortical ischemic vascular disease.
Exploratory analyses addressed the possibility of neuropsychological and neuroanatomical differences between the SIVD memory subgroups and participants with AD. SIVD participants with poor initial learning had significantly lower MMSE scores (Tukey’s Honestly Significant Difference; ps <.05) than the AD participants with rapid forgetting or the SIVD participants with either rapid forgetting or good retention, whereas the latter three groups performed comparably. Consequently, all subsequent analyses only compared AD participants, SIVD participants with good retention, and SIVD participants with rapid forgetting. Data are presented in Table 3.
Table 3.
Means (and Standard Deviations) on Neuropsychological and MRI Variables for the AD, SIVD Rapid Forgetting, and SIVD Good Retention Subgroups.
| AD
|
SIVD: Rapid forgetting
|
SIVD: Good retention
|
||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Lacune volume (cm3) | 0.0 | 0.0 | 1.0 | 1.0 | 1.6 | 1.5 |
| WMSH (cm3) | 14.2 | 16.7 | 35.3 | 32.6 | 34.9 | 32.8 |
| Hippocampal volumes | 3.1 | 0.9 | 3.5 | 1.0 | 3.8 | 0.6 |
| Cortical gray | 457.2 | 33.5 | 450.5 | 55.1 | 449.7 | 42.8 |
| FAS | 27.2 | 9.7 | 30.2 | 20.6 | 18.1 | 13.9 |
| Initiation-Perseveration | 27.8 | 6.6 | 27.9 | 6.2 | 23.1 | 6.7 |
| Digits Backward | 4.9 | 1.8 | 5.1 | 2.3 | 4.3 | 1.7 |
| Mental Control | 4.8 | 1.5 | 4.2 | 1.9 | 3.9 | 1.6 |
| Boston Naming | 38.6 | 16.2 | 38.7 | 12.2 | 38.5 | 12.4 |
| Benton Form Discrimination | 15.5 | 3.5 | 15.6 | 3.1 | 13.5 | 4.9 |
Note. MRI = magnetic resonance imaging.
AD = Alzheimer’s disease.
SIVD = subcortical ischemic vascular disease.
WMSH = white matter signal hyperintensities.
One-way ANOVAs were carried out to determine if there were MRI differences between the AD and two SIVD groups. SIVD subjects with good retention had significantly greater lacune volume than the SIVD subjects with rapid forgetting ( p <.05). The AD group had smaller hippocampal volumes and less WMSH than the SIVD subgroups; the SIVD subgroups did not differ from one another. There were no differences in white matter signal hyperintensity or cortical gray matter.
There were no group differences on the Boston Naming, Animal Naming, or Benton Visual Form Discrimination. One-way ANOVAs yielded a significant group effect on DRS Initiation-Perseveration, F(2, 45) = 3.47, p <.05. Post hoc analyses indicated that the SIVD Good Retention group performed significantly less well than the AD and SIVD Rapid Forgetting groups. Group differences on FAS and working memory tasks like backward digit span and WMS–R Mental Control did not reach significance, although the direction of group differences was in the same direction, with the SIVD Good Retention group obtaining lower mean scores than the other two groups.
Autopsy Findings
The neuropsychological data raise the possibility that subcortical lacunar infarction patients with rapid forgetting may be most likely to have concomitant AD. To date, three participants within the SIVD-Rapid Forgetting subgroup have gone to autopsy. In each case, neuropathology has indicated that in addition to cerebrovascular disease, these participants also had AD, with Braak and Braak staging of 4 or greater (Braak & Braak, 1995) and neuropathological diagnoses of mixed dementia. None of the participants within the SIVD Good Retention subgroup have died. However, in the larger database, five cases with mild cognitive impairment and evidence for lacunar infarcts on MRI have died. None of these cases showed neuropathological indications of AD on autopsy.
DISCUSSION
When subcortical lacunes are found in a patient with progressive dementia, it is often difficult to infer what role cerebrovascular disease might play in the dementia, and whether the patient has AD in addition to cerebrovascular disease. Clinical criteria that reliably distinguish between pathologically verified AD, SIVD, and mixed dementia are not available.
One major finding of the present study is that distinct differences are present in the memory performances of AD and SIVD participants well matched for age and degree of cognitive impairment. Although these two dementia groups performed comparably in their immediate recall of verbal material, patients with AD exhibit a steeper rate of decline in their recall over a 20-min delay than do SIVD patients. The SIVD group also had a steeper rate of decline for verbal material than did controls. Our findings support Libon et al.’s (1998) conclusion that there are different rates of forgetting between AD and SIVD, and provide a firmer foundation for that conclusion by demonstrating the differences with repeated measures analyses and using comparable immediate and delayed memory indices.
A second important finding is that patterns of memory performance are more heterogeneous in dementia patients with lacunes than in dementia patients without lacunes. Over 80% of our AD participants exhibited rapid forgetting. In contrast, within the SIVD group, participants were more evenly distributed into those with rapid forgetting, those with good retention, and those with poor initial acquisition. Exploratory analyses comparing these different subgroup with AD participants suggest that SIVD participants with good retention perform less well on executive tasks than AD participants and SIVD participants with rapid forgetting. Analysis of the MRI data indicated that AD subjects had smaller hippocampal volumes than either SIVD subgroup. In addition, the SIVD Good Retention group had significantly greater lacunar volume that the SIVD Rapid Forgetting group.
Results suggest that pattern of cognitive performance may be useful in differential diagnosis. Rapid forgetting is generally associated with AD (Hart, Kwentus, Harkins, & Taylor, 1988; Huppert & Kopelman, 1989; Reed, Paller, & Mungas, 1998), so AD pathology can be suspected in SIVD patients with exhibit poor retention of information over delays. In contrast, adequate retention of information is more typically reported in subcortical dementia patients who present with greater executive impairment. Thus, the combination of relatively spared memory and greater impairment on executive tasks might be more suggestive of pure SIVD (Tierney et al., 2001). The few cases in our sample who have gone on to autopsy provide support for this. Three of three SIVD cases who exhibit rapid forgetting were found to have pathological evidence for AD, whereas none of five lacunar infarct cases with mild cognitive impairment and good retention of information drawn from the larger program project database demonstrated AD pathology.
Several factors pose persistent challenges for clinicians attempting to parse the relative contributions of vascular and AD pathology in dementia patients. Vascular disease can produce cerebral changes that are much more widespread than the subcortical lacunes that defined our cohort. Lafosse et al. (1997) reported more cortical atrophy in their SIVD group than in their AD group, and declines in neuropsychological functioning were highly correlated with cortical atrophy, irrespective of whether the diagnosis was AD or SIVD. Hippocampal atrophy has also been reported in SIVD (Crystal et al., 1993; Laakso et al., 1996; Pantel et al., 1998). Fein et al. (2000) and Mungas et al. (2001) have reported that the dementia in SIVD correlated best with hippocampal and cortical atrophy rather than with the number or total volume of lacunes. This pattern of correlations is similar to that found in AD. Other similarities between AD and SIVD have also been reported. Patients with vascular dementia have been found to have cholinergic deficits in basal forebrain (Kalaria & Ballard, 1999) and postmortem studies of patients with pure vascular pathology have reported hippocampal volume loss (Pantoni, Garcia, & Brown, 1996).
A separate body of literature implicates diffuse white matter disease in the memory deficits seen in SIVD patients. For example, depressed patients with moderate-to-severe deep white matter hyper-intensities demonstrated worse performance on general and delayed recall memory indices than depressed patients without such lesions and normal elderly participants (Kramer-Ginsberg et al., 1999). Even in normal participants, white-matter foci not adjacent to the lateral ventricles were related to performance on immediate visual memory (Baum, Schulte, Girke, Reischies, & Felix, 1996). However, Fein et al. (2000) reported that while measures of WMSH were correlated with severity of cognitive impairment in SIVD, hippocampal atrophy and cortical gray matter explained a much larger percentage of the variance in cognitive ability than did WMSH.
Differentiating between SIVD, AD, and mixed dementia remains an important goal with implications for prevention and treatment. Radiological evidence for subcortical ischemic vascular disease is insufficient for inferring the absence of AD. Although SIVD and AD overlap in many ways and present in a continuous rather than dichotomous fashion, the results of this study suggest that patterns of neuropsychological test performance might offer clues about the mechanisms underlying a patient’s dementia and, when used in tandem with other clinical information, may improve differential diagnosis. Inclusion of a broader range of cognitive measures might also prove useful, since several investigators have proposed that compared to AD, SIVD is characterized by better preservation of recognition memory and fewer intrusion errors (Chui, 2001; Lafosse et al., 1997; Libon et al., 1998; Tierney et al., 2001). How well the pattern of cognitive deficits predicts underlying pathology, however, remains to be established.
Acknowledgments
This work was supported by a grant from the National Institute on Aging, PO1 AG12435 (HCC).
References
- Baum KA, Schulte C, Girke W, Reischies FM, Felix R. Incidental white-matter foci on MRI in ‘‘healthy’’ subjects: Evidence of subtle cognitive dysfunction. Neuroradiology. 1996;38:755–760. doi: 10.1007/s002340050342. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Capizzano AA, Schuff N, Amend DL, Tanabe JL, Norman D, Maudsley AA, Jagust W, Chui HC, Fein G, Segal MR, Weiner MW. Subcortical ischemic vascular dementia: Assessment with quantitative MR imaging and 1H MR spectroscopy. American Journal of Neuroradiology. 2000;21:621–630. [PMC free article] [PubMed] [Google Scholar]
- Chui H. Dementia due to subcortical ischemic vascular disease. Clinical Cornerstone. 2001;3:40–51. doi: 10.1016/s1098-3597(01)90047-x. [DOI] [PubMed] [Google Scholar]
- Crystal HA, Dickson DW, Sliwinski MJ, Lipton RB, Grober E, Marks-Nelson H, Antis P. Pathological markers associated with normal aging and dementia in the elderly. Annals of Neurology. 1993;34:566–573. doi: 10.1002/ana.410340410. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Wang L, Joshi S, Miller JP, Gado M, Kido D, McKeel D, Morris JC, Miller MI. Early DAT is distinguished from aging by high-dimensional mapping of the hippocampus. Neurology. 2000;55:1636–1643. doi: 10.1212/wnl.55.11.1636. [DOI] [PubMed] [Google Scholar]
- Cummings JL. Subcortical dementia: Neuropsychology, neuropsychiatry and pathophysiology. British Journal of Psychiatry. 1986;149:682–697. doi: 10.1192/bjp.149.6.682. [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Tanabe J, Cardenas V, Weiner MW, Jagust WJ, Reed BR, Norman D, Schuff N, Kusdra L, Greenfield T, Chui H. Hippocampal and cortical atrophy predict dementia in subcortical ischemic vascular disease. Neurology. 2000;55:1626–1635. doi: 10.1212/wnl.55.11.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller JW, Christensen GE, Joshi SC, Newcomer JW, Miller MI, Csernansky JG, Vannier MW. Hippocampal MR imaging morphometry by means of general pattern matching. Radiology. 1996;199:787–791. doi: 10.1148/radiology.199.3.8638006. [DOI] [PubMed] [Google Scholar]
- Hart RP, Kwentus JA, Harkins SW, Taylor JR. Rate of forgetting in mild Alzheimer’s-type dementia. Brain and Cognition. 1988;7:31–38. doi: 10.1016/0278-2626(88)90019-x. [DOI] [PubMed] [Google Scholar]
- Hassing L, Backman L. Episodic memory functioning in population-based samples of very old adults with Alzheimer’s disease and vascular dementia. Dement and Geriatric Cognitive Disorders. 1997;8:376–383. doi: 10.1159/000106658. [DOI] [PubMed] [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. British Journal of Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- Huppert FA, Kopelman MD. Rates of forgetting in normal ageing: A comparison with dementia. Neuropsychologia. 1989;27:849–860. doi: 10.1016/0028-3932(89)90008-0. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, Ballard C. Overlap between pathology of Alzheimer disease and vascular dementia. Alzheimer Disease and Associated Disorders. 1999;13(Suppl 3):S115–S123. doi: 10.1097/00002093-199912003-00017. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Levin BE, Brandt J, Delis DC. Differentiation of Alzheimer’s, Huntington’s, and Parkinson’s disease patients on the basis of verbal learning performance. Neuropsychology. 1989;3:111–120. [Google Scholar]
- Kramer-Ginsberg E, Greenwald BS, Krishnan KR, Christiansen B, Hu J, Ashtari M, Patel M, Pollack S. Neuropsychological functioning and MRI signal hyperintensities in geriatric depression. American Journal of Psychiatry. 1999;156:438–444. doi: 10.1176/ajp.156.3.438. [DOI] [PubMed] [Google Scholar]
- Laakso MP, Partanen K, Riekkinen P, Lehtovirta M, Helkala EL, Hallikainen M, Hanninen T, Vainio P, Soininen H. Hippocampal volumes in Alzheimer’s disease, Parkinson’s disease with and without dementia, and in vascular dementia: An MRI study. Neurology. 1996;46:678–681. doi: 10.1212/wnl.46.3.678. [DOI] [PubMed] [Google Scholar]
- Lafosse JM, Reed BR, Mungas D, Sterling SB, Wahbeh H, Jagust WJ. Fluency and memory differences between ischemic vascular dementia and Alzheimer’s disease. Neuropsychology. 1997;11:514–522. doi: 10.1037//0894-4105.11.4.514. [DOI] [PubMed] [Google Scholar]
- Libon DJ, Bogdanoff B, Cloud BS, Skalina S, Giovannetti T, Gitlin HL, Bonavita J. Declarative and procedural learning, quantitative measures of the hippocampus, and subcortical white alterations in Alzheimer’s disease and ischaemic vascular dementia. Journal of Clinical and Experimental Neuropsychology. 1998;20:30–41. doi: 10.1076/jcen.20.1.30.1490. [DOI] [PubMed] [Google Scholar]
- Libon DJ, Bogdanoff B, Leopold N, Hurka R, Bonavita J, Skalina S, Swenson R, Gitlin HL, Ball SK. Neuropsychological profiles associated with subcortical white matter alterations and Parkinson’s disease: Implications for the diagnosis of dementia. Archives of Clinical Neuropsychology. 2001;16:19–32. [PubMed] [Google Scholar]
- Lukatela K, Malloy P, Jenkins M, Cohen R. The naming deficit in early Alzheimer’s and vascular dementia. Neuropsychology. 1998;12:565–572. doi: 10.1037//0894-4105.12.4.565. [DOI] [PubMed] [Google Scholar]
- Mungas D, Jagust WJ, Reed BR, Kramer JH, Weiner MW, Schuff N, Norman D, Mack WJ, Willis L, Chui HC. MRI predictors of cognition in subcortical ischemic vascular disease and Alzheimer’s disease. Neurology. 2001;57:2229–2235. doi: 10.1212/wnl.57.12.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantel J, Schroder J, Essig M, Jauss M, Schneider G, Eysenbach K, von Kummer R, Baudendistel K, Schad LR, Knopp MV. In vivo quantification of brain volumes in subcortical vascular dementia and Alzheimer’s disease. An MRI-based study. Dement and Geriatric Cognitive Disorders. 1998;9:309–316. doi: 10.1159/000017082. [DOI] [PubMed] [Google Scholar]
- Pantoni L, Garcia JH, Brown GG. Vascular pathology in three cases of progressive cognitive deterioration. Journal of Neurological Sciences. 1996;135:131–139. doi: 10.1016/0022-510x(95)00273-5. [DOI] [PubMed] [Google Scholar]
- Reed BR, Paller KA, Mungas D. Impaired acquisition and rapid forgetting of patterned visual stimuli in Alzheimer’s disease. Journal of Clinical and Experimental Neuropsychology. 1998;20:738–749. doi: 10.1076/jcen.20.5.738.1123. [DOI] [PubMed] [Google Scholar]
- Rosenstein LD. Differential diagnosis of the major progressive dementias and depression in middle and late adulthood: A summary of the literature of the early 1990s. Neuropsychology Review. 1998;8:109–167. doi: 10.1023/a:1025628925796. [DOI] [PubMed] [Google Scholar]
- Tierney MC, Black SE, Szalai JP, Snow G, Fisher RH, Nadon G, Chui HC. Recognition memory and verbal fluency differentiate probable Alzheimer disease from subcortical ischemic vascular dementia. Archives of Neurology. 2001;58:1654–1659. doi: 10.1001/archneur.58.10.1654. [DOI] [PubMed] [Google Scholar]
- Williams JM. Memory Assessment Scales. Odessa: Psychological Assessment Resources; 1991. [Google Scholar]


