Abstract
Objective
To examine how baseline and change of volumetric MRI relate to cognitive decline in older individuals.
Background
Memory is associated with hippocampal integrity, whereas executive function has been linked to impaired frontal lobe function. Previous studies have shown that hippocampal and cortical atrophy are more strongly related to cognition than are measures of subcortical cerebrovascular disease (CVD). The authors hypothesized that memory (MEM) decline would be related to change in hippocampal volume (HC), whereas decline in executive function (EXEC) would be related to change of cortical gray matter volume (CGM) and measures of subcortical CVD.
Methods
Subjects from a multicenter study (n = 103) included cognitively normal, mildly impaired, and demented cases with and without subcortical lacunes. All had longitudinal cognitive evaluation (mean = 4.8 years) and two or more MRI scans at least one year apart (mean = 3.4 years). MRI measures included HC, CGM, total lacune volume (LAC), and white matter hyperintensity volume (WMH). Random effects modeling of longitudinal data assessed effects of MRI baseline and MRI change on baseline and change of psychometrically matched measures of MEM and EXEC.
Results
Change in MEM was related to HC baseline and HC change. Change in EXEC was related to baseline CGM and to change in CGM, HC, and LAC. Results were unchanged when demented cases were excluded. WMH was not associated with change in MEM or EXEC independent of HC, CGM, and LAC.
Conclusion
Hippocampal volume was the primary determinant of memory decline, whereas executive function (EXEC) decline was related to multiple brain components. Results support a hypothesis that MEM decline is strongly influenced by Alzheimer disease (AD), whereas EXEC decline may be complexly determined by cerebrovascular disease and AD.
Alzheimer disease (AD) and cerebrovascular disease (CVD) are common causes of cognitive impairment and dementia in older persons.1-4 Brain imaging has important applications for examining contributions of AD and CVD to cognitive decline, e.g., for sensitively measuring hippocampal and cortical atrophy that is well documented in AD and for quantifying strokes and white matter hyperintensity (WMH) associated with CVD. Previous studies have shown that hippocampal atrophy predicts conversion from mild cognitive impairment to dementia,5,6 presumably because it indexes an AD process, but there is a need for studies examining broader relationships between different structural brain changes and progression of cognitive impairment throughout the continuum of cognitive change.
Previous studies from our group showed that baseline cognitive ability and cognitive decline is primarily associated with cortical and hippocampal changes, whereas lacunes and WMH have more limited relationships independent of cortical and hippocampal effects.7-9 Our work to date has examined the relationship of baseline MRI findings with baseline cognitive performance and subsequent longitudinal cognitive decline. Examining relationships of longitudinal MRI change with cognitive decline might add important information to clarify the effects of these MRI variables and, potentially, of AD and CVD processes that underlie the MRI changes, i.e., MRI changes associated with AD and CVD might mediate cognitive decline associated with these diseases.
In this study, we sought to examine how baseline MRI and longitudinal change in MRI relate to longitudinal change in cognition. Two specific cognitive domains, memory (MEM) and executive function (EXEC), were selected as primary outcomes. MEM is strongly related to hippocampal structure and function and is a sensitive indicator of AD.5,10-12 EXEC is primarily determined by frontal lobe structure and function and is particularly sensitive to disruption of frontal-subcortical loops that are affected by subcortical CVD.13 Thus, these two cognitive domains have relevance for examining differential effects of AD and CVD processes. Psychometrically matched cognitive outcome measures were used to facilitate unambiguous interpretation of differential effects. Our primary hypotheses were that memory decline would be related to change in the hippocampal volume (HC), whereas decline of EXEC would be related to change of cortical gray matter volume (CGM) and measures of subcortical CVD. Previous studies from this project have shown that cognitive effects of lacunes and WMH are limited after controlling for CGM, and so we expected lacunes and WMH effects to be attenuated when examined jointly with CGM.
Methods
Subjects
Participants were recruited from three academic dementia centers as part of a multicenter collaborative study of contributions of subcortical CVD and AD to cognitive impairment and dementia. All participants received a comprehensive clinical evaluation that included a medical history, a neurologic examination, appropriate laboratory tests, and neuropsychological testing with a standardized test battery. In addition, participants received a standardized MRI scan of the brain at the baseline evaluation as well as at least one subsequent MRI scan. The institutional review boards at all participating institutions approved this study, and subjects or their legal representatives gave written informed consent.
Recruitment was targeted to fill six groups defined by three levels of cognitive impairment crossed with presence vs absence of subcortical lacunes. These groups were used for recruitment stratification to ensure broad variability of cognitive function and CVD and for descriptive data analyses to characterize the sample; primary data analyses used continuous measures of cognition and structural brain volumes. The levels of cognitive impairment were 1) normal, defined by a Clinical Dementia Rating (CDR)14,15 total score of 0.0, 2) impaired (CDR score = 0.5), and 3) demented (CDR score ≥1.0). A single neuroradiologist reviewed all MRI scans to determine presence of lacunes. Summary data on demographic characteristics and global cognitive function (Mini-Mental State Examination) are presented in table 1. The current sample included participants (n = 103) with two or more MRI scans and two or more neuropsychological evaluations. There were 58 subjects at baseline (15 with lacunes); 14 progressed to impaired and one to demented by the last follow-up. There were 34 impaired at baseline (19 with lacunes); four were normal at the last evaluation, 18 remained impaired, and 12 became demented. There were 11 demented patients (six with lacunes) at baseline. Six demented patients had baseline clinical diagnoses of AD, three vascular dementia, and two mixed AD/vascular dementia.
Table 1.
Demographic characteristics and global cognitive status of subject sample
| Baseline cognitive status |
||||
|---|---|---|---|---|
| CDR = 0.0, n = 58 | CDR = 0.5, n = 34 | CDR ≥1.0, n = 11 | All, n = 103 | |
| Sex, no. (%) | ||||
| Male | 27 (46.6) | 27 (79.4) | 6 (54.5) | 60 (58.3) |
| Female | 31 (53.4) | 7 (20.6) | 5 (45.5) | 43 (41.7) |
| Education, y | ||||
| Mean (SD) | 15.7 (2.9) | 14.2 (3.1) | 13.8 (1.9) | 15.0 (3.0) |
| Range | 10–24 | 8–22 | 11–16 | 8–24 |
| Age, y | ||||
| Mean (SD) | 74.1 (6.7) | 72.6 (7.1) | 76.5 (9.7) | 73.8 (7.2) |
| Range | 58–87 | 56–85 | 56–86 | 56–87 |
| MMSE | ||||
| Mean (SD) | 29.0 (1.4) | 27.0 (2.6) | 23.6 (3.4) | 27.8 (2.7) |
| Range | 21–30 | 20–30 | 18–29 | 18–30 |
MMSE = Mini-Mental State Examination.43
MRI methods
MRI variables were volumetric, computerized measures of WMH, CGM, HC, and total lacune volume (LAC) within specific structures: thalamus, putamen, caudate, globus pallidus, and white matter. These measures were obtained for both the baseline scan and follow-up scans.
LAC was small (>2 mm) areas of subcortical gray and white matter with increased signal relative to CSF on proton density MRI. LAC was differentiated from perivascular spaces, which can be particularly prominent below the anterior commissure and putamen and at bends in the course of penetrating arterioles. Isoin-tense lesions on pseudo proton density MRI at the level of the anterior commissure or inferior putamen were termed perivascular spaces; outside that region, they were defined as cavitated lacunes if they were ≥3 mm at maximum width. Lesions that met either of these criteria were considered lacunes for purposes of data analysis.
Image acquisition and data processing previously have been described.7 A computerized segmentation algorithm was used to classify brain MRI pixels into CGM (subcortical gray matter), white matter (WMH), ventricular CSF, and sulcal CSF.16 In addition, total intracranial volume (ICV) was computed by summing over all pixels within the intracranial vault. Intraclass correlation coefficients across short-term repeated scans (n = 10) were 0.92 for the percentage of white matter, 0.80 for the percentage of WMH, 0.95 for CGM, 0.96 for sulcal CSF, and 0.99 for ventricular CSF.
Automated hippocampal volumetry was carried out using a commercially available high-dimension brain mapping tool (Medtronic Surgical Navigation Technologies, Louisville, CO).17 After manual identification of both global and local landmarks, a coarse transformation was computed using landmark matching. Automated hippocampal morphometry was then performed by a fluid image-matching transformation.18,19 This software was validated by comparison with a manual method to trace the hippocampus20 using same-day back-to-back MRI studies on 60 subjects (normal elderly, mild cognitively impaired subjects, and patients with AD). Correlation between automated and manual measurements of HCs was 0.92. Automated measurements achieved an intraclass correlation coefficient across scans of 0.94 compared to 0.99 for manual measurements.
Neuropsychological measures
All participants received a standardized battery of neuropsychological tests. Several tests were used to derive psychometrically matched measures of MEM and EXEC that were the primary outcomes in this study. Details of scale derivation and validation have been previously reported.21 MEM was a composite measure derived from delayed and cued recall, and mostly supraspan performance on selected immediate recall trials of the word list learning task of the Memory Assessment Scales.22 EXEC was a composite scale that used letter fluency (F, A, and S),23 digit span backward,24 visual span backward,24 and the Initiation-Perseveration subscale of the Mattis Dementia Rating Scale25 as donor scales.
Scale construction of the MEM and EXEC measures was guided by methods associated with item response theory,26-28 and was based on a larger sample of 400 from this project.21 Both measures have high reliability (r ≥ 0.90) from about −2.0 SD below the mean of the overall development sample to 2.0 SD above the mean. These measures do not have appreciable floor or ceiling effects for participants in this sample and have linear measurement properties across a broad ability range.21 They also are near-normally distributed, which presents advantages for statistical analyses.
The MEM and EXEC measures were transformed so that scores were referenced to the distribution of the cognitively normal without lacunes recruitment group in the development sample from this project and so that the scale of measurement corresponded to a traditional scale with a mean of 100 and SD of 15. Thus, a score of 85 represents 1 SD below the mean of the normal individuals without lacunes.
Data analysis
Descriptive analyses were performed to characterize baseline status and rate of change of cognitive and MRI variables. For MRI variables, means were calculated for each cognitive impairment recruitment category (normal, impaired, demented) for the first scan and the last scan. For CGM and HC, average annualized percentage of change from the baseline value was calculated as the percentage of change from the first to last scan divided by the intervening time in years. Average annualized absolute change was calculated for LAC and WMH. Simple analyses of variance were used to compare cognitive impairment group means on baseline MRI values and average rate of change for each MRI variable type. Baseline cognitive scores by cognitive recruitment category were similarly compared with analyses of variance. Random effects regression analyses were used to evaluate cognitive group differences in rate of change of MEM and EXEC.
Primary outcomes of interest were the longitudinal cognitive measures MEM and EXEC. MRI measures (CGM, HC, WMH, and LAC) at baseline and longitudinal assessments were the independent variables. Multivariate growth models29 were used in the primary analyses. These models have many of the same properties as univariate random effects models30 but enable explicit modeling of correlated outcomes. An important advantage of the multivariate model is that it facilitates separation of general effects on cognition from specific effects on MEM and EXEC. Random effects for baseline level and rate of change for each cognitive outcome were included in the models and were fit with an unstructured covariance matrix for random effects using SAS Proc Mixed.31
In the first step of model building, demographic variables were entered into univariate random effects models along with time and time by demographic variable interactions. Education, gender, and age had significant effects on baseline cognitive scores and gender was related to EXEC change. Subsequent models controlled for baseline effects of gender, education, and age; for baseline ICV; and for the effect of gender on cognitive change. The second step in the analyses employed multivariate growth models with joint modeling of the two cognitive outcomes with respect to each type of MRI variable, separately. Baseline and longitudinal (time varying) MRI values were added as independent variables along with demographic covariates. The time-varying MRI variables were coded as the change from the baseline scan. There were fewer MRI assessments than cognitive assessments. When an MRI assessment was not available to match a cognitive assessment within 6 months, the values from the closest MRI assessment were used. Terms were included to test for effects of baseline MRI on baseline cognition and cognitive change and of longitudinal MRI (change from baseline) on cognitive change. The third step used a multivariate growth model including CGM and HC measures jointly as predictors because these were hypothesized to be the major independent variables related to cognitive change. Subsequent analyses added LAC and WMH variables to identify a final multivariate model. This final model was then fit using MRI variables that were standardized based on cognitively normal cases at baseline. Regression coefficients could then be interpreted as the magnitude of difference in MEM or EXEC associated with a 1-SD difference in the MRI variable, and strength of effects across MRI variables could be directly compared. Finally, a secondary analysis using the final model was done excluding the 11 demented cases. All assumptions of the random effects models were checked graphically and analytically and were reasonably met by the data.
Results
There were 421 neuropsychological assessments for the 103 cases included in this study. Average time from the initial to last assessment was 4.8 years (SD = 2.0, range = 1.1 to 9.0). Fifteen cases (14.6%) had two assessments, 23 (22.3%) had three, 27 (26.2%) had four, 21 (20.4%) had five, and 17 (16.5%) had six or more. Eighty (77.7%) had two MRI scans and 23 (22.3%) had three. Average time from the baseline scan to the last scan was 3.4 years (SD = 1.4, range = 1.1 to 6.9).
Table 2 presents baseline means and average annual rate of change of cognitive measures by cognitive impairment category (normal, impaired, demented). Baseline MEM performance substantially differed across all cognitive groups. For EXEC, baseline differences were smaller; impaired and demented groups did not differ but both differed from normal. MEM change was significant only for the demented group (p = 0.04), but group differences were not significant (p = 0.09). EXEC change was significant for the demented group, which significantly differed from both the normal and impaired groups. Table 3 shows baseline means and average annual rate of change for MRI variables. Baseline HC differed among all three groups. Baseline CGM was greater in subjects, baseline LAC was lower in subjects, and baseline WMH was higher in the demented group. Rate of change of CGM and HC differed across groups (all p values = 0.01); subjects differed from the other two groups for both. Correlations among baseline MRI variables included CGM with baseline WMH (r = −0.46, p < 0.0001), CGM with HC (r = 0.48, p = 0.0001), WMH with LAC (r = 0.31, p = 0.001), and WMH with HC (r = −0.29, p < 0.003). CGM change was correlated with HC change (r = 0.36, p = 0.0002) and LAC change (r = −0.21, p < 0.04). Baseline HC was correlated with HC change (r = 0.36, p = 0.0002).
Table 2.
Mean baseline cognitive scores (SD in parentheses) and annual rate of change (standard errors in parentheses) of cognitive variables by baseline cognitive status
| Baseline cognitive status |
||||
|---|---|---|---|---|
| Cognitive variable | Assessment | CDR = 0.0, n = 58 | CDR = 0.5, n = 34 | CDR ≥ 1.0, n = 11 |
| Memory | Baseline | 107.1 (15.5) | 80.4 (17.1) | 65.5 (12.6) |
| Average change | 0.14 (0.36) | −0.62 (0.58) | −2.33 (1.14) | |
| Executive | Baseline | 98.4 (12.1) | 85.7 (19.3) | 80.0 (20.6) |
| Average change | −0.36 (0.28) | −0.44 (0.44) | −4.62 (0.86) | |
| Time cognitive, y | First to last | 5.6 (1.3–9.0) | 3.8 (1.1–7.2) | 3.4 (1.5–6.1) |
The average time between the first and last cognitive evaluation (range in parentheses) is presented by cognitive status. Annual change is in standard score points (mean = 100, SD = 15).
CDR = Clinical Dementia Rating score.
Table 3.
Mean values (SD in parentheses) for first and last assessments for MRI variables and annual percentage of change (CGM and HC) and annual absolute change (WMH and LAC) by baseline cognitive status
| Baseline cognitive status |
||||
|---|---|---|---|---|
| Cognitive/MRI variable | Assessment | CDR = 0.0, n = 58 | CDR = 0.5, n = 34 | CDR ≥ 1.0, n = 11 |
| CGM | First | 0.393 (0.022) | 0.372 (0.031) | 0.363 (0.034) |
| Last | 0.383 (0.029) | 0.353 (0.039) | 0.336 (0.046) | |
| Annual % change | −0.6 (1.8) | −2.1 (3.6) | −2.8 (5.3) | |
| HC | First | 0.0035 (0.0004) | 0.0030 (0.0005) | 0.0026 (0.0006) |
| Last | 0.0033 (0.0004) | 0.0029 (0.0006) | 0.0023 (0.0005) | |
| Annual % change | −1.1 (1.4) | −2.4 (3.7) | −2.9 (2.0) | |
| LAC | First | 0.00014 (0.00042) | 0.00068 (0.00101) | 0.00067 (0.00129) |
| Last | 0.00014 (0.00042) | 0.00065 (0.00100) | 0.00064 (0.00119) | |
| Annual change (mm3) | −0.002 (0.12) | −0.009 (0.17) | 0.015 (0.14) | |
| WMH | First | 0.0061 (0.0071) | 0.0099 (0.0106) | 0.0198 (0.0208) |
| Last | 0.0077 (0.0092) | 0.0120 (0.0126) | 0.0221 (0.0189) | |
| Annual change (mm3) | 0.59 (1.3) | 1.35 (5.8) | 0.69 (4.2) | |
| Time MRI, y | First to last | 4.0 (1.1–6.9) | 2.6 (1.2–4.8) | 2.9 (1.1–5.1) |
The average time between the first and last MRI scans (range in parentheses) is presented by cognitive status. MRI variables are normalized to total intracranial volume.
CGM = cortical gray matter volume; HC = hippocampal volume; LAC = total lacune volume; WMH = white matter hyperintensity volume; CDR = Clinical Dementia Rating score.
Initial multivariate growth models investigated the associations between each type of MRI measure and baseline level and change for both cognitive measures. Baseline CGM was associated with baseline MEM (p = 0.03) and baseline EXEC (p = 0.005). Baseline CGM predicted change in MEM (p = 0.02) and EXEC (p = 0.007). CGM change from baseline was associated with EXEC change (p = 0.004). Baseline HC was strongly related to both baseline MEM and baseline EXEC (p values = 0.0001), baseline HC predicted MEM change, (p = 0.0005), and HC change from baseline was associated with change in MEM (p = 0.01) and EXEC (p = 0.002). Baseline LAC was related to baseline EXEC (p = 0.0006) and predicted EXEC change (p = 0.04), and LAC change was associated with EXEC change (p = 0.01). Baseline WMH was related to baseline EXEC (p = 0.0008) and to change in EXEC (p = 0.02).
The next analytic step used a multivariate growth model to investigate the joint effects of the CGM and HC variables on MEM and EXEC. Baseline HC was associated with baseline MEM and EXEC (p values < 0.0005), but CGM was not related to baseline cognition independent of HC. HC baseline predicted MEM change (p = 0.007) and CGM baseline predicted EXEC change (p = 0.02). HC change was related to MEM change (p = 0.005), and HC change and CGM change were independently related to EXEC change (p = 0.006 [HC] and p = 0.02 [CGM]).
LAC variables were added in the next step and were independently related to EXEC but not MEM scores. WMH variables were added in a subsequent joint model but were not related to MEM or EXEC independent of CGM, HC, and LAC. The model incorporating effects of CGM, HC, and LAC was chosen as the final model and was applied using standardized MRI values so that effect sizes could be directly compared. The correlation between baseline MEM and EXEC random effects in this analysis was 0.25, and the correlation between MEM and EXEC change random effects was 0.32. Table 4 presents the results from this analysis.
Table 4.
Results of joint random effects modeling of MEM and EXEC baseline and change in association with MRI baseline and change from baseline measures
| Dependent variable | Independent variable | Regression coefficient | Standard error | p |
|---|---|---|---|---|
| Memory baseline | CGM baseline | 2.10 | 2.45 | 0.39 |
| HC baseline | 7.48 | 1.46 | 0.0001 | |
| LAC baseline | −0.35 | 1.03 | 0.73 | |
| Memory change | CGM baseline | 0.18 | 0.32 | 0.56 |
| HC baseline | 0.69 | 0.25 | 0.008 | |
| LAC baseline | 0.11 | 0.18 | 0.54 | |
| CGM change | −1.62 | 1.10 | 0.14 | |
| HC change | 5.72 | 1.82 | 0.002 | |
| LAC change | −2.08 | 1.44 | 0.14 | |
| Executive baseline | CGM baseline | 2.20 | 2.00 | 0.27 |
| HC baseline | 4.38 | 1.19 | 0.0003 | |
| LAC baseline | −2.66 | 0.83 | 0.002 | |
| Executive change | CGM baseline | 0.55 | 0.25 | 0.03 |
| HC baseline | 0.03 | 0.20 | 0.90 | |
| LAC baseline | −0.24 | 0.14 | 0.09 | |
| CGM change | 1.71 | 0.83 | 0.04 | |
| HC change | 4.01 | 1.37 | 0.004 | |
| LAC change | −2.97 | 1.08 | 0.006 |
Regression coefficients, standard errors, and p values are from simultaneous modeling of memory (MEM) and executive function (EXEC) using baseline and change in cortical gray matter volume (CGM), hippocampal volume (HC), and lacune volume (LAC) as jointly entered independent variables. Standardized MRI variables were used for this analysis. Regression coefficients indicate differences in memory and executive function measures associated with a 1.0 SD difference in the respective MRI variable.
MEM was associated with HC, but CGM and LAC had no effects independent of HC. A baseline difference in HC of 1.0 SD magnitude in subjects was associated with a 7.5-point (0.5 SD) difference in the baseline MEM score and lower volume of this magnitude predicted a subsequent decline of 0.7 points per year. (Demented cases had mean baseline HC about 2.25 SD below the mean of the subjects.) HC change from baseline of a 1.0-SD magnitude was associated with corresponding MEM decline of 5.7 points. EXEC was complexly associated with all three MRI variables. Baseline EXEC was independently associated with HC (a 1.0-SD difference corresponded to a 4.4-point difference in EXEC) and LAC (a 1.0-SD increase corresponded to a 2.7-point decrease in EXEC). EXEC change was independently associated with CGM change (1.71 points per 1.0 SD), HC change (4.0 points), and LAC change (3.0) points. CGM baseline also predicted EXEC change (0.6 points per 1.0-SD difference).
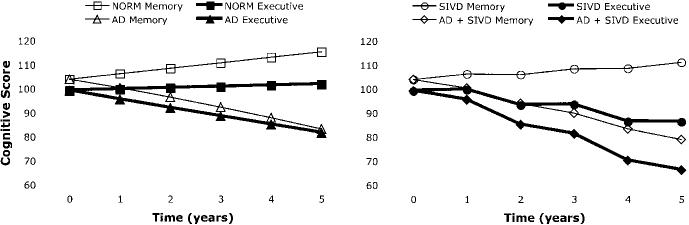
These results are demonstrated graphically in the figure. The left side presents model-predicted trajectories for MEM and EXEC for two hypothetical cases corresponding to normal MRI (NORM) and prototypical AD progressing from normal baseline MRI (AD). The right side shows model-predicted trajectories for two additional cases, one with subcortical ischemic vascular disease defined by incident lacunes in years 2 and 4 and otherwise normal MRI (SIVD) and the other with prototypical AD and incident lacunes in years 2 and 4 (SIVD + AD). For the NORM case, HC and CGM values that were respective means for cognitively normal individuals in this sample were entered into the derived regression model at baseline and at all subsequent time points, and LAC values of zero were entered at all time points. The AD case had the same starting values of HC and CGM as well as LAC values of zero, but HC declined at a rate of 5% per year, consistent with a previous report,32 and CGM at each year was the value predicted by a linear regression of CGM on HC. The SIVD case is the same as the NORM case, except that nonzero LAC were entered in years 2 and 4. The average LAC of cases with one lacune was used to determine the LAC to be added at the time points after the incident lacunes. These results show average baseline performance in all groups. EXEC is stable in NORM, but MEM improves, which most likely reflects a practice effect since the same test was repeated at all assessments. The AD case shows a steady decline, of equal magnitude, in MEM and EXEC. The SIVD case shows a clear stepwise decline in EXEC associated with the incident lacunes, but little effect on MEM. The SIVD + AD case shows decline in both domains, but unlike the AD case, EXEC declines more rapidly with stepwise decrements associated with the incident lacunes. These figures demonstrate graphically the independent and additive effects of structural brain changes of AD and SIVD.
Figure.
Longitudinal trajectories of memory and executive function representing model-predicted average scores at yearly time points for four hypothetical cases. The NORM case was defined by normal MRI at all time points. The Alzheimer disease (AD) case had a normal baseline MRI, but cortical gray matter volume and hippocampal volume decline at a rate commensurate with typical AD. The subcortical ischemic vascular disease (SIVD) case was defined by incident lacunes in years 2 and 4. The SIVD + AD case was defined by a combination of typical AD and incident lacunes in years 2 and 4. Details about how these cases were defined are presented in the text.
When demented cases were excluded from an additional secondary analysis using the model incorporating CGM, HC, and LAC, results were very similar. The salient differences were that CGM change was no longer related to EXEC change, but LAC change was a stronger associate of EXEC change with a 1.0-SD increase associated with a 4.0-point decline in EXEC.
Discussion
This study examined the association of MRI measures with longitudinal change in MEM and EXEC. Longitudinal decline in HC was associated with declining MEM, as would be expected, but in addition, individuals with smaller baseline HC declined more rapidly independent of the rate of subsequent HC atrophy. Longitudinal change in EXEC was determined by multiple brain components. Those with greater initial cortical atrophy and greater rates of cortical and hippocampal atrophy declined more rapidly. Increasing LAC across scans was associated with a decline in EXEC. WMH was not related to change in MEM or EXEC independent of the other brain components. When demented cases were excluded, LAC change had a stronger effect on EXEC decline.
Unique aspects of this study were that both baseline MRI measures and longitudinal change in multiple volumetric measures were used as independent variables, and the cognitive outcome measures were carefully matched according to psychometric characteristics. The use of psychometrically matched measures and of multivariate modeling of change facilitates delineation of domain-specific effects from more nonspecific cognitive changes. Another important aspect of this study was that primary analyses used continuous measures of cognition and brain volumes and were based on a sample with broad variability of cognitive function and, presumably, of AD and CVD pathology. AD and CVD are inherently continuous processes, as are the consequent cognitive and structural brain changes. The design and analytic approach used in this study facilitate sensitive characterization of how brain changes relate to cognitive decline and specifically permit examination of how individual cognitive trajectories are influenced by brain structure.
HC had particularly robust effects on baseline cognition and cognitive change for both MEM and EXEC, although effects on MEM were approximately 50% stronger than effects on EXEC. These results are noteworthy because average rates of HC decline were modest and variability of MEM change was limited in this predominantly nondemented sample. The average rate of HC change in the demented cases was 2.9% per year. A recent study on the rate of hippocampal atrophy in AD found an average annual rate of change of 4.9%,32 an estimate 60% higher than that for the demented cases in this sample. This difference likely reflects heterogeneity of etiologies for dementia in this sample and specifically the inclusion of non-AD dementias. Even though overall rates of HC decline were modest, differences in HC decline had important clinical sequelae. In the 34 cases in this study who were impaired at baseline, HC did not decline in four who reverted to normal; the annual rate of HC decline was 1.7% in 18 who did not change, but the annual change was 5.1% in 12 who converted to dementia, a clearly significant differences (not shown). The relationship of HC to conversion in this sample has been previously reported,33 but these results demonstrate the practical impact of individual differences in the HC atrophy rate.
These results have implications for two important clinical issues: prognosis and diagnosis. This study included predominantly nondemented individuals, and results were very similar when the demented cases at baseline were excluded. There is considerable interest in cognitive decline and progression to dementia in mildly impaired individuals. This study has direct relevance for using structural MRI to predict cognitive decline. It differs from previous research in some important respects. Nondemented cases were heterogeneous and included cognitively normal and nonamnestic as well as amnestic cases with mild cognitive impairment. By design, there was broad variability with respect to subcortical CVD.
Similar to previous studies focusing on amnestic mild cognitive impairment, results showed that baseline HC predicted cognitive decline in this sample, but only for MEM. The ability of baseline CGM to predict EXEC decline is a new finding but is consistent with a previous study from our group that showed that baseline CGM predicted decline on a measure of global cognition.8 Overall, results show the prognostic value of baseline MRI results but, in addition, indicate that CGM and HC make independent and perhaps domain-specific contributions to cognitive decline.
Results also have implications regarding underlying pathologic processes and ultimately diagnosis. Effects of AD and CVD are of considerable interest and are most relevant given the design and subject sample for this study. It is important to stress, however, that the sample was predominantly nondemented, and, consequently, neither of these pathologies was sufficiently severe to result in dementia in most cases. The joint but differential effects on cognition of structural brain changes associated with AD and CVD are demonstrated graphically in the figure, which shows the AD effects on both MEM and EXEC and a clear impact of CVD on EXEC but not MEM.
The hippocampus has been well established as the major anatomic substrate of MEM. A relationship between the rate of hippocampal atrophy and the rate of MEM decline can be explained as a direct effect of hippocampal tissue loss. The ability of baseline HC to predict subsequent MEM decline requires a more indirect explanation. Lower HC at baseline might result from a progressive disease process that leads to progressive MEM decline. This disease process presumably would affect brain systems beyond the hippocampus because the baseline HC effect could not be entirely explained by declining HC. AD is a likely candidate because atrophy5,6,34,35 and neurofibrillary degeneration36,37 of the hippocampus are early indicators of AD, a progressive disorder that also affects cortical regions. Hippocampal sclerosis could contribute to hippocampal atrophy,38 but it is less clear that this is a sufficiently prevalent and progressive disease process to presage broader cognitive decline.
Presumably, the relationship of baseline CGM with EXEC reflects a progressive process (or processes) affecting frontal cortex. EXEC, although anatomically distributed according to task and modality, broadly has a common substrate in prefrontal cortex. Dysfunction of prefrontal cortex may arise from multiple pathways, including direct neuronal loss, loss of input from neocortical association areas, and damage to frontal-subcortical circuits.13,39-41 Neither the anatomic pathway nor the etiology of this dysfunction is established by the present data. AD eventually spreads to frontal regions and also causes generalized cortical atrophy, but there is accumulating evidence that CVD may be an equally important determinant of cortical atrophy.7-9 Therefore, the effects of baseline CGM and CGM change on EXEC decline could be explained by AD, CVD, or a combination of these two pathologic processes. The association between increasing LAC and EXEC decline, especially in the nondemented cases, is consistent with a recent study42 and points to a direct effect of subcortical CVD on EXEC decline. It is noteworthy that LAC effects were stronger in this study than in previous studies from this project,8,9 which may reflect greater sensitivity associated with longitudinal measurement of LAC in this study.
The fact that change in HC predicted change in EXEC (independently of CGM) argues against the idea that CVD alone explains EXEC change. CVD can affect the hippocampus, but the likelihood that these effects would either be so common and so uniformly progressive in the overall sample as to produce this association seems small. Progressive hippocampal atrophy is strongly linked to AD and thus change in HC likely serves as an index of AD progression.
Further research is required to clarify the importance of these findings for understanding the contributions of AD and CVD to cognitive change. An important limitation of this study is that AD and CVD pathology was not directly measured; inferences about these pathologies were made based on MRI findings. Further research with neuropathologically characterized samples will be important to verify or refute our hypotheses about how the MRI findings correspond to underlying pathologies. Further research is needed to clarify the relationship between subcortical CVD and CGM. Consistent findings from this project are that CGM has important effects on cognition and appears to be related to CVD, and neuropathologic studies will be important for determining specific types of CVD pathology that are associated with CGM atrophy.
Acknowledgment
David Norman, MD, reviewed all MRI scans to identify lacunes.
Footnotes
Supported in part by grants AG123435, AG10129 from the National Institute on Aging, Bethesda, MD, and by the California Department of Health Services Alzheimer's Disease Program, contracts 94-20354, 94-20355, 98-14970, and 98-14971.
Disclosure: The authors report no conflicts of interest.
References
- 1.Lobo A, Launer LJ, Fratiglioni L, et al. Neurologic Diseases in the Elderly Research Group Prevalence of dementia and major subtypes in Europe: a collaborative study of population based cohorts. Neurology. 2000;54(11 suppl 5):S4–S9. [PubMed] [Google Scholar]
- 2.Nyenhuis DL, Gorelick PB. Vascular dementia: a contemporary review of epidemiology, diagnosis, prevention, and treatment. J Am Geriatr Soc. 1998;46:1437–1448. doi: 10.1111/j.1532-5415.1998.tb06015.x. [DOI] [PubMed] [Google Scholar]
- 3.Lopez OL, Kuller LH, Fitzpatrick A, Ives D, Becker JT, Beauchamp N. Evaluation of dementia in the cardiovascular health cognition study. Neuroepidemiology. 2003;22:1–12. doi: 10.1159/000067110. [DOI] [PubMed] [Google Scholar]
- 4.Knopman DS, Parisi JE, Boeve BF, et al. Vascular dementia in a population based autopsy study. Arch Neurol. 2003;60:569–575. doi: 10.1001/archneur.60.4.569. [DOI] [PubMed] [Google Scholar]
- 5.Visser PJ, Scheltens P, Verhey FR, et al. Medial temporal lobe atrophy and memory dysfunction as predictors for dementia in subjects with mild cognitive impairment. J Neurol. 1999;246:477–485. doi: 10.1007/s004150050387. [DOI] [PubMed] [Google Scholar]
- 6.Jack CR, Jr, Petersen RC, Xu YC, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fein G, Di Sclafani V, Tanabe J. Hippocampal and cortical atrophy predict dementia in subcortical ischemic vascular disease. Neurology. 2000;55:1626–1635. doi: 10.1212/wnl.55.11.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mungas D, Reed BR, Jagust WJ. Volumetric MRI predicts rate of cognitive decline related to AD and cerebrovascular disease. Neurology. 2002;59:867–873. doi: 10.1212/wnl.59.6.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mungas D, Jagust WJ, Reed BR. MRI predictors of cognition in subcortical ischemic vascular disease and Alzheimer's disease. Neurology. 2001;57:2229–2235. doi: 10.1212/wnl.57.12.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albert MS, Moss MB, Tanzi R, Jones K. Preclinical prediction of AD using neuropsychological tests. J Int Neuropsychol Soc. 2001;7:631–639. doi: 10.1017/s1355617701755105. [DOI] [PubMed] [Google Scholar]
- 11.Cahn DA, Sullivan EV, Shear PK. Structural MRI correlates of recognition memory in Alzheimer's disease. J Int Neuropsychol Soc. 1998;4:106–114. doi: 10.1017/s1355617798001064. [DOI] [PubMed] [Google Scholar]
- 12.Reed BR, Eberling JL, Mungas D, Weiner MW, Jagust WJ. Memory failure has different mechanisms in subcortical stroke and Alzheimer's disease. Ann Neurol. 2000;48:275–284. [PMC free article] [PubMed] [Google Scholar]
- 13.Cummings JL. Frontal subcortical circuits and human behavior. Arch Neurol. 1993;50:873–880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- 14.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 15.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 16.Cardenas VA, Ezekiel F, Di Sclafani V, Gomberg B, Fein G. Reliability of tissue volumes and their spatial distribution for segmented magnetic resonance images. Psychiatry Res. 2001;106:193–205. doi: 10.1016/s0925-4927(01)00075-0. [DOI] [PubMed] [Google Scholar]
- 17.Csernansky JG, Wang L, Joshi S. Early DAT is distinguished from aging by high dimensional mapping of the hippocampus. Dementia of the Alzheimer type. Neurology. 2000;55:1636–1643. doi: 10.1212/wnl.55.11.1636. [DOI] [PubMed] [Google Scholar]
- 18.Haller JW, Christensen GE, Joshi SC. Hippocampal MR imaging morphometry by means of general pattern matching. Radiology. 1996;199:787–791. doi: 10.1148/radiology.199.3.8638006. [DOI] [PubMed] [Google Scholar]
- 19.Haller JW, Banerjee A, Christensen GE, et al. Three-dimensional hippocampal MR morphometry with high-dimensional transformation of a neuroanatomic atlas. Radiology. 1997;202:504–510. doi: 10.1148/radiology.202.2.9015081. [DOI] [PubMed] [Google Scholar]
- 20.Hsu YY, Schuff N, Du AT. Comparison of automated and manual MRI volumetry of hippocampus in normal aging and dementia. J Magn Reson Imaging. 2002;16:305–310. doi: 10.1002/jmri.10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mungas D, Reed BR, Kramer JH. Psychometrically matched measures of global cognition, memory, and executive function for assessment of cognitive decline in older persons. Neuropsychology. 2003;17:380–392. doi: 10.1037/0894-4105.17.3.380. [DOI] [PubMed] [Google Scholar]
- 22.Williams JM. Memory Assessment Scales. Psychological Assessment Resources; Odessa, FL: 1991. [Google Scholar]
- 23.Benton AL, Hamsher KD. Multilingual Aphasia Examination. University of Iowa; Iowa City: 1976. [Google Scholar]
- 24.Wechsler D. Wechsler Memory Scale-Revised (WMS-R) The Psychological Corporation; San Antonio, TX: 1987. [Google Scholar]
- 25.Mattis S. Dementia Rating Scale. Psychological Assessment Resources; Odessa, FL: 1988. [Google Scholar]
- 26.Baker FB. The basics of item response theory. Heine-man Publishing; Portsmouth, NH: 1985. [Google Scholar]
- 27.Hambleton RK, Swaminathan H. Item response theory. Principles and applications. Kluwer-Nijhoff Publishing; Boston: 1985. [Google Scholar]
- 28.Hambleton RK, Swaminathan H, Rogers HJ. Fundamentals of item response theory. Sage Publications; Newbury Park, CA: 1991. [Google Scholar]
- 29.Reinsel G. Multivariate repeated-measurement or growth curve models with multivariate random-effects covariance structure. J Am Stat Assoc. 1982;77:190–195. [Google Scholar]
- 30.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 31.Harvey DJ, Beckett LA, Mungas DM. Multivariate modeling of two associated cognitive outcomes in a longitudinal study. Alzheimer Dis Assoc Disord. doi: 10.3233/jad-2003-5502. in press. [DOI] [PubMed] [Google Scholar]
- 32.Jack CR, Jr, Shiung MM, Gunter JL, et al. Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology. 2004;62:591–600. doi: 10.1212/01.wnl.0000110315.26026.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeCarli C, Mungas D, Harvey D, et al. Memory impairment, but not cerebrovascular disease, predicts progression of MCI to dementia. Neurology. 2004;63:220–227. doi: 10.1212/01.wnl.0000130531.90205.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anstey KJ, Maller JJ. The role of volumetric MRI in understanding mild cognitive impairment and similar classifications. Aging Ment Health. 2003;7:238–250. doi: 10.1080/1360786031000120732. [DOI] [PubMed] [Google Scholar]
- 35.Detoledo-Morrell L, Sullivan MP, Morrell F, Wilson RS, Bennett DA, Spencer S. Alzheimer's disease: in vivo detection of differential vulnerability of brain regions. Neurobiol Aging. 1997;18:463–468. doi: 10.1016/s0197-4580(97)00114-0. [DOI] [PubMed] [Google Scholar]
- 36.Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW. The topical and neuroanatomical distribution of neurofibrillary tangles and senile plaques in the cerebral cortex of patients with Alzheimer's disease. Cereb Cortex. 1991;1:103–116. doi: 10.1093/cercor/1.1.103. [DOI] [PubMed] [Google Scholar]
- 37.Braak H, Braak E, Bohl J. Staging of Alzheimer-related cortical destruction. Eur Neurol. 1993;33:403–408. doi: 10.1159/000116984. [DOI] [PubMed] [Google Scholar]
- 38.Jack CR, Jr., Dickson DW, Parisi JE, et al. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology. 2002;58:750–757. doi: 10.1212/wnl.58.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lafosse JM, Reed BR, Mungas D, Sterling SB, Wahbeh H, Jagust WJ. Fluency and memory differences between ischemic vascular dementia and Alzheimer's disease. Neuropsychology. 1997;11:514–522. doi: 10.1037//0894-4105.11.4.514. [DOI] [PubMed] [Google Scholar]
- 40.Libon DJ, Bogdanoff B, Leopold N. Neuropsychological profiles associated with subcortical white matter alterations and Parkinson's disease: Implications for the diagnosis of dementia. Arch Clin Neuropsychol. 2001;16:19–32. [PubMed] [Google Scholar]
- 41.Cummings J. Vascular subcortical dementias: clinical aspects. Dementia. 1994;5:177–180. doi: 10.1159/000106718. [DOI] [PubMed] [Google Scholar]
- 42.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MMB. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 43.Folstein M, Folstein S, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]