Abstract
Background
Mild cognitive impairment (MCI) is widely viewed as the transition phase between normal aging and Alzheimer disease (AD). Given that MCI can also result from cerebrovascular disease (CVD), the authors used clinical, MRI, and cognitive measures of AD and CVD to test the hypothesis that CVD increases the likelihood of progression from MCI to dementia within 3 years.
Objective
To examine the impact of CVD on progression of MCI to dementia.
Methods
Fifty-two consecutive patients with MCI (71% men) including many with symptomatic CVD were longitudinally evaluated for 3.1 ± 1.3 years. MCI was defined as a Clinical Dementia Rating Scale (CDR) score of 0.5. Dementia was defined as progression to a CDR score of ≥1.0.
Results
Forty-four percent of the MCI patients had MRI infarcts, 50% of which were symptomatic. Thirty-three percent of patients progressed to dementia, and 37.8% of these had MRI infarcts. Clinically probable or possible AD was diagnosed in approximately 82% of converters. Of the clinical and MRI measures, only hippocampal volume was associated with increased risk to progression (hazard ratio [HR] = 0.31 [95% CI 0.1 to 0.92], p = 0.03). When neuropsychological measures were included in the analysis, memory (HR = 0.90 [95% CI 0.84 to 0.96], p = 0.002) and executive function (HR = 0.96 [95% CI 0.92 to 1.0], p = 0.045) were associated with increased risk of dementia progression, whereas APOE genotype, cerebrovascular risk factors, clinical stroke, presence or absence of lacunes, and extent of white matter hyperintensities did not predict progression.
Conclusion
Within a heterogenous group of MCI patients, including many with clinically significant CVD, baseline memory and executive performance significantly predicted likelihood to develop dementia.
Complaints of cognitive impairment are common to the elderly.1 Although causes for these complaints may be multiple,2 incipient Alzheimer disease (AD) is often suspected. This is supported by pathologic evidence of AD in individuals with memory impairment who are not demented,3 raising the possibility that the AD process may begin years before clinical symptoms are evident.4,5 Furthermore, clinical studies of elderly individuals with memory impairment reveal a rapid rate of progression to AD, reaching as high as 15%/year.6 These data therefore suggest that among older individuals, significant memory impairment, short of dementia and often denoted as mild cognitive impairment (MCI), may be a transition phase between the normal aging process and AD.6,7 This is particularly true for highly select populations with patterns and trajectories of memory loss typical of early AD.5
Not all individuals with MCI have memory impairment as the predominant symptom,8 however, and not all individuals with MCI progress to AD.7,9 These data raise concerns that MCI is both a clinically and an etiologically heterogeneous grouping.7,10 Recent evidence suggests that cerebrovascular disease (CVD) may contribute to this heterogeneity.11,12 CVD is common to the elderly and is known to cause cognitive impairment.13-16 Although these studies often show strong relationships between CVD and nonmemory cognitive impairment,16 the likelihood of MCI, as conventionally defined,6 may also be increased by clinical and MRI evidence of CVD11 and in association with pathologic evidence of severe cerebral atherosclerosis.17 Furthermore, considerable evidence has shown that CVD can cause dementia in the absence of AD pathology and also may worsen or accelerate cognitive decline in subjects with AD.18-20 Given this evidence, it seems reasonable to postulate that the presence of CVD would contribute to incident dementia among individuals with MCI. To test this hypothesis, we examined the impact of demographic, clinical, neuropsychological, and MRI measures of both AD and CVD processes on the incidence and type of dementia within a broadly defined group of individuals with MCI.
Methods
Subjects
The subject cohort consisted of the first 52 consecutive MCI patients participating in a prospective longitudinal research project examining the role of CVD and AD on cognition (AG 12435, Helena Chui, principal investigator). The design for this project has been previously described,18,21 but, in brief, all subjects were recruited through one of three memory disorder clinics with expertise in AD and dementia at the University of California at Davis, the University of California at San Francisco, or the University of Southern California. Subjects were systematically selected to have a spectrum of cognitive impairments ranging from normal to demented in the presence of CVD—defined as the presence of one or more lacunar infarcts with or without clinical symptoms—or the absence of CVD. The project recruits and stratifies subjects with a broad spectrum of cognitive abilities for both groups (CVD vs non-CVD), including individuals with normal cognition, MCI, and dementia.
The presence or absence of cognitive impairment was determined on a yearly basis through careful history, physical and neurologic examination, functional assessment, and cognitive performance on a battery of standardized neuropsychological tests including tests of memory, language, visual perception, attention, and concentration. All subjects in this study were re-evaluated at least once. For this analysis, December 2001 served as the end-date for dementia surveillance.
To allow for the possibility that CVD may lead to differing types of cognitive impairment and outcome,8,22 MCI was determined to be broadly inclusive, as defined by any cognitive impairment sufficient to cause a Clinical Dementia Rating Scale (CDR) score23,24 of 0.5, including subjects with clinical stroke involving subcortical structures. The CDR was determined according to previously published protocols that included a semistructured interview of both the patient and the informant by individuals certified to administer the CDR. The CDR score was determined independently of neuropsychological information according to published protocol.23,24
For this analysis, progression to dementia was based solely on a change in CDR score from 0.5 to 1.0 or higher.
Vascular risk factors
As cerebrovascular risk factors are associated with MCI,11,12 we wished to assess the potential impact on progression to dementia. The presence or absence of six cerebrovascular risk factors (stroke, diabetes, hyperlipidemia, TIA, hypertension, and coronary artery disease) was systematically assessed from patient and informant histories as well as review of pertinent medical records as part of each clinical evaluation. To calculate an overall measure of clinical cerebrovascular risk, we created a composite score that was the sum of the factors present ranging from 0 to 6. The composite vascular risk factor score at the baseline visit was used to predict progression from MCI to dementia.
Neuropsychological outcome measures
The ability for baseline measures of cognitive ability to predict progression to dementia was measured for memory and executive function based on a priori assumptions regarding the relative specificity of these two cognitive domains for AD- and CVD-related pathology. Item response theory25 methods were used to create psychometrically matched measures of memory and executive function scores. Development and validation of these scales are described in detail elsewhere.26 In brief, item response theory analyses were performed to evaluate basic psychometric properties of items or units of measurement of potential donor scales from the clinical neuropsychological test battery. Items were then selected for the scales used in this study so that the final scales would be matched in reliability across a broad ability range. The memory scale was constructed using trial total scores from the word-list learning task of the Memory Assessment Scales27 as donor scales and included the sum of short delay free recall, short delay cued recall, trial 3 total recall, and trial 1. The executive function scale was constructed from four tests that included the backwards digit span and backwards visual memory span from the Wechsler Memory Scale–Revised,28 FAS letter fluency,29 and the initiation–perseveration subtest of the Mattis Dementia Rating Scale.30 These measures were converted to standard scores based upon the mean and SD of a group of normal controls without lacunes (n = 200) from the parent study. These memory and executive scales had a mean of 100 and SD of 15 in the sample of controls, so that a score of 85 falls 1 SD below the mean of the normal reference sample. As these measures are psychometrically matched and normally distributed, they provide a distinct advantage in terms of precise characterization of cognitive functioning. For example, a score of 80 on either scale falls at the 9th percentile of the normal reference sample, so consequently, applying this cutoff to both scales yields a clear method for defining different combinations of memory and executive impairment using measures that are equally sensitive to deficits in these domains.
MRI measures
Previously published methods were used to segment MRI brain images into gray matter, white matter, CSF, and white matter hyperintensity (WMH) volumes.18 Further manual processing was used to quantify cortical gray matter (cGM) and hippocampal (HC) volumes. In addition, the volumes of MRI-defined infarctions were determined within various brain regions including thalamus, putamen, caudate, globus pallidus, and white matter. Small areas of MRI infarction (>2 mm) were defined as lacunes if there was increased signal intensity relative to CSF on proton density images in subcortical gray and white matter. Careful attention was applied to differentiate lacunes from dilated perivascular spaces, particularly below the anterior commissure, within the putamen and at bends in the course of penetrating arterioles. Isointense lesions on pseudo-proton density MRI (as opposed to “true” proton density, which is obtained when extrapolated to echo time = 0) at the level of the anterior commissure or inferior putamen were termed perivascular spaces; outside that region, they were defined as cavitated lacunes if they were >3 mm at maximum width. Lesions that met either of these criteria were considered lacunes for purposes of data analysis.
HC volumes were derived using a semiautomated, computerized method (Surgical Navigator Systems, Boulder, CO).31,32
Intrarater reliabilities for these measurements based on intraclass correlation coefficients (n = 10) were 0.93 for percentage of white matter, 0.99 for percentage of WMH, 0.95 for cGM, 0.99 for sulcal CSF, and 0.99 for ventricular CSF.
To account for the effect of head size on regional brain measures, all volumes were normalized to total intracranial volume.
Statistical analyses
The two-sample t-test and the χ2 test were used to compare group differences in baseline characteristics between subjects who converted to dementia and those who did not. The Wilcoxon rank sum test and Fisher exact test were used when the assumptions of these tests failed. The primary outcome of interest was time of conversion to dementia. Cox proportional hazards models were used to study whether the domains of brain imaging or neuropsychological tests were better at measuring the causal chain from MCI to dementia, after adjusting for age, education, and gender. Models were first developed using only the brain imaging variables to establish a link between them and conversion to dementia. Final models included both the imaging variables and the neuropsychological tests to see if the two domains contributed independent information on conversion to dementia or if one domain captured information that was further along in the causal pathway from MCI to dementia. Secondary analyses included other clinical variables to the models to see if the clinical domain added anything further to the models. Assumptions of linearity and proportional hazards were checked through both graphic and analytic techniques. All assumptions were reasonably met.
Kaplan–Meier curves were used to illustrate the differences in progression patterns between predefined groups of subjects based on evidence of AD or CVD. For the MRI variables, individuals were categorized as having “high” WMH volumes if the volume was greater than the 75th percentile of the WMH volume distribution for the normal control population based on results from previously reported research.33 Conversely, “low” HC volumes were defined as less than the 25th percentile of HC volume distribution for the normal control population of the larger study based on the same prior research.33 “Low” performance on neuropsychological testing was defined as performance below a standard score of 80 (the 9th percentile) for either memory or executive function. Log-rank tests were used to test for differences in the progression patterns across groups.
All analyses were carried out using SAS and S-plus (Cary, NC), with a p value of <0.05 considered significant.
Results
Subjects
Clinical, demographic, neuropsycho-logical, and MRI volume description of the full cohort and comparison of MCI converters to nonconverts are summarized in table 1. MCI patients were 72.8 ± 7.8 years of age on average (range 56 to 90 years). The mean educational attainment was 14.8 ± 2.7 years (range 8 to 22 years), and the group was predominately men (71%). The average duration of follow-up was 3.1 ± 1.3 years (range 1 to 6 years), and the prevalence of APOE4 genotype was 59%. The MCI group included 16 individuals who were taking cholinesterase inhibitors during the time of observation. Of these 16, 15 were taking Aricept and one was taking Cognex. Individuals taking cholinesterase inhibitors had poorer baseline memory and smaller hippocampi and were more likely to convert to dementia (56%) than those who did not take cholinesterase inhibitors (25%). Consistent with the aims of recruiting a cohort stratified to include CVD, 12 of 52 individuals had clinical stroke and nearly half had MRI evidence of lacunar infarctions. Moreover, only 36% of the subjects were free of any vascular risk factors and 22% had three or more vascular risk factors. The average composite vascular risk score was 1.4 ± 1.5.
Table 1.
Baseline comparisons of converters vs nonconverters
| Variable | Total, n = 52 | Converters, n = 17 | Nonconverters, n = 35 | p value |
|---|---|---|---|---|
| Age, y | 72.8 (7.8) | 75.1 (7.9) | 71.7 (7.6) | 0.15 |
| Female, % | 29 | 41 | 23 | 0.20 |
| Education | 14.8 (2.7) | 14.5 (2.6) | 14.9 (2.8) | 0.60 |
| APOE4, % | 59 | 73 | 53 | 0.20 |
| Composite vascular risk | 1.40 (1.50) | 0.94 (1.03) | 1.67 (1.60) | 0.16 |
| Clinical stroke, % | 23 | 12 | 29 | 0.30 |
| cGM* | 37 (3) | 33 (2) | 38 (3) | 0.005 |
| HC* | .30 (0.06) | .26 (0.05) | .31 (0.05) | 0.0014 |
| WMH* | 1.0 (1.0) | 0.9 (1.1) | 1.1 (1.5) | 0.60 |
| MRI lacunes, % | 44 | 35 | 49 | 0.40 |
| Lacunar volume* | 0.04 (0.08) | 0.03 (0.06) | 0.05 (0.09) | 0.30 |
| Memory | 77.4 (16.5) | 62.2 (9.5) | 84.8 (14.0) | <0.0001 |
| Executive function | 82.7 (17.1) | 78.7 (15.5) | 84.6 (17.7) | 0.20 |
Values are means (SD) or percentages.
Expressed as percentage intracranial volume.
cGM = cortical gray matter; HC = hippocampal volume; WMH = white matter hyperintensity volume.
At the end of the observation period (December 2001), 39 of 52 subjects were still actively followed. Of the remaining 13, 10 had died, 2 refused further follow-up, and 1 was lost to follow-up. The single individual lost to follow-up was lost after developing a dementia and had no vascular risk factors. Of the two who refused follow-up, one had already developed dementia. Neither had any vascular risk factors. Therefore, the data from only 1 of the 52 subjects were uninformative. Of the 10 who died, 6 died during 2001 after receiving their yearly evaluation at the correct time. One-half of the individuals dying were demented. Of the five nondemented individuals, four had one vascular risk factor and the other had no vascular risk factors. Comparison of vascular risk between those 39 who were still active at the end of the observation period and those whom died, refused, or were lost to follow-up found no differences in the frequency of vascular risk factors (χ2 = 0.2, p = 0.65).
A total of 17 patients progressed to dementia. Clinical diagnoses at progression to dementia included AD (10/17), mixed AD/vascular dementia (4/17), vascular dementia (2/17), and other (1/17). MCI patients converting to dementia were slightly older, were more likely to be female, and had a higher prevalence of APOE4 genotype. Surprisingly, converters were less likely to have vascular risk factors or clinical stroke. None of these differences was significant, however. Educational achievement was similar for both groups.
MRI variables
Results of MRI analysis for the full cohort including comparison of MCI converters to nonconverters are also summarized in table 1. Mean cGM volumes were consistent with previously reported values for cognitively impaired but nondemented individuals, whereas WMH volumes were nearly twice normal control values and similar in volume to previously reported individuals with subcortical ischemic vascular dementia.18 Within the MCI group, 16 (30%) individuals were identified as having “high” WMH volumes. Mean HC volumes (0.30 ± 0.06) were intermediate between normal control (0.34 ± 0.046) and dementia (0.24 ± 0.051) values obtained from recent analysis of 65 normal subjects and 33 dementia patients from within the larger study cohort. Importantly, within the MCI cohort, 36 (69%) were categorized as having “low” HC volumes.
MRI evidence of CVD was common among the MCI cohort. Approximately 44% of the subjects had lacunes on MRI (23/52): 5 (9.6%) with single lacunes, 5 (9.6%) with two lacunes, and 13 (25%) with three or more lacunes. Seventeen subjects (32.7%) had lacunes in strategic locations such as thalamus or caudate. Ten of the 23 cases (43.5%) with lacunes at baseline had a clinical history of stroke attributable to the lacunar infarction seen on MRI. MCI patients without lacunes (11/29 or 37.9%) were more likely to progress to dementia than patients with lacunes (6/23 or 26.1%), although this difference did not reach significance (χ2 = 0.82, p = 0.37). WMH volumes were also slightly greater in the nonconverters, but this difference was not significant (0.9 ± 1.1 vs 1.1 ± 1.5). HC and cGM volumes, however, were smaller at baseline for converters (0.31 ± 0.05 vs 0.26 ± 0.05; p = 0.0014 for HC and 38 ± 3.0 vs 35 ± 2.0; p = 0.005 for cGM).
Neuropsychological variables
Despite inclusion of subjects with a more broad definition of MCI and a substantial prevalence of CVD by clinical and MRI measures, average memory performance for the entire MCI cohort was 1.5 SD below normal26 with a mean scaled score on the memory outcome variable of 77.4 ± 16.5 (see table 1). Moreover, 63% (33/52) of individuals were categorized as having “low” memory performance and 50% (26/52) were categorized as having “low” executive performance.
Average baseline memory performance of MCI subjects converting to dementia was >2 SD below normal26 (62.2 ± 9.5) and significantly lower than nonconverters (84.8 ± 14; p < 0.0001), whereas executive function performance was not significantly different between converters and nonconverters. In fact, the memory performance of all individuals converting to dementia during the observation period was at least ≥1.33 SD (scaled score of 80) below normal.26 Consistent with previously published prospective longitudinal studies of MCI, individuals with isolated memory impairment6 converted to dementia at a rate of approximately 12%/year (7/17 or 41% over the average 3.1 years of observation), whereas the group with both poor memory and executive performance34 converted to dementia at a higher rate of nearly 20%/year (10/16 or 63% over the average 3.1 years of observation). No individual with isolated executive impairment (10/52) or other cognitive impairment (non-memory, nonexecutive; 9/52) progressed to dementia over the average 3.1 years of observation.
Associations between clinical, MRI variables, and neuro-psychological performance
Composite vascular risk factor scores were inversely associated with executive performance (r = −0.33, p = 0.026), whereas there was a trend toward a similar inverse association with WMH volume and executive performance (r = −0.24, p = 0.086). Neither measure was associated with memory performance (r < 0.10, p > 0.5). Lacunar numbers or volumes were unassociated with either memory or executive performance. Conversely, memory performance was positively associated with HC volume (r = 0.36, p = 0.009).
Multivariate analyses
Cox proportional hazards models were used to assess which clinical or MRI features were most strongly associated with progression to dementia for this cohort (tables 2 and 3). Initial univariate analyses (hazard ratio [HR] [95% CI], p value) revealed significant effects for cGM (0.12 [0.02 to 0.67], 0.016), HC (HR 0.26 [0.10 to 0.65], 0.004), and memory score (0.92 [0.89 to 0.95], <0.0001). No significant univariate effects were found for APOE genotype, clinical stroke, composite vascular risk factor score, lacune number or volume, WMH volume, or executive performance.
Table 2.
Multivariate analysis of demographic and MRI variables
| Predictor | Estimate | SE | p value | HR | 95% CI |
|---|---|---|---|---|---|
| Age | 0.003 | 0.04 | 0.94 | 1.00 | 0.92–1.10 |
| Education | −0.02 | 0.12 | 0.87 | 0.98 | 0.78–1.24 |
| Gender | 0.67 | 0.61 | 0.27 | 1.96 | 0.59–6.52 |
| cGM | −2.30 | 1.48 | 0.12 | 0.10 | 0.01–1.81 |
| HC | −1.18 | 0.56 | 0.03 | 0.31 | 0.10–0.92 |
| Lacunes | 0.10 | 0.58 | 0.86 | 1.11 | 0.36–3.42 |
| WMH | −0.31 | 0.38 | 0.41 | 0.73 | 0.35–1.54 |
HR = hazard ratio; cGM = cortical gray matter; HC = hippocampal volume; WMH = white matter hyperintensity volume.
Table 3.
Multivariate analysis of demographic, MRI, and neuropsychological variables
| Predictor | Estimate | SE | p value | HR |
|---|---|---|---|---|
| Age | 0.004 | 0.06 | 0.96 | 1.00 |
| Education | 0.25 | 0.14 | 0.07 | 1.28 |
| Gender | 1.29 | 0.73 | 0.08 | 3.62 |
| Memory | −0.11 | 0.03 | 0.002 | 0.90 |
| Executive | −0.04 | 0.02 | 0.046 | 0.96 |
| cGM | −3.33 | 2.90 | 0.25 | 0.04 |
| HC | 0.19 | 0.69 | 0.78 | 1.21 |
| Lacunes | 1.05 | 0.86 | 0.22 | 2.88 |
| WMH | −0.18 | 0.27 | 0.50 | 0.83 |
HR = hazard ratio; cGM = cortical gray matter; HC = hippocampal volume; WMH = white matter hyperintensity volume.
Initial multivariate analysis (see table 2) examined the independent association between MRI variables and progression to dementia. HC was the only variable significantly associated with progression to dementia for this model. Inclusion of APOE genotype did not alter this relationship (data not shown).
The second analysis (see table 3) examined the additional impact of memory and executive performance in association with progression to dementia in conjunction with MRI measures. In the full model, reduced memory and executive performance were significantly associated with progression to dementia in the cohort, whereas the effect of HC was no longer significant. The addition of APOE geno-type did not alter this relationship (data not shown).
Kaplan–Meier curves
Finally, Kaplan–Meier curves were used to assess categorical effects that may be more clinically relevant. Two separate analyses were performed. In the first analysis (figure 1), cognitive scores were categorized as “high” or “low” based on scaled score values of 80 (which corresponds to approximately the 9th percentile or 1.33 SD below the mean). No individual with “high” memory performance converted to dementia over the observation period. Within the “low” memory group, individuals with “high” executive function had a significantly better survival than did individuals with the combination of both “low” memory and “low” executive function (log-rank test comparing the four progression curves: p value = 0.005). In the second analysis (figure 2), WMH was categorized as “high” or “low” based on the 75th percentile of normal individuals within the study (16 had “high” WMH), whereas HC was categorized as “high” or “low” based on the 25th percentile of normal individuals within the study (69% had “low” HC volumes). No individual with “low” WMH and “high” HC converted to dementia over the observation period. Individuals with “high” WMH and “high” HC showed a modest rate of progression to dementia within the first year of the observation period, but no further progressions throughout the remainder. The presence of “low” HC was associated with substantially increased rates of progression to dementia independent of WMH category, although the differences in progression patterns did not quite reach significance (log-rank test comparing the four progression curves: p value = 0.07).
Figure 1.
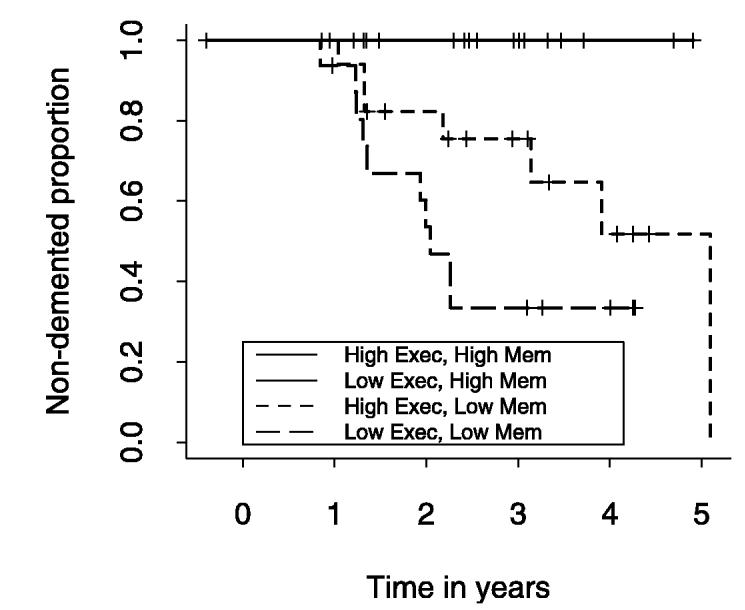
Graphic display of Kaplan-Meier survival curves for four classes of cognition based on memory (Mem) and executive (Exec) performance. See text for details.
Figure 2.
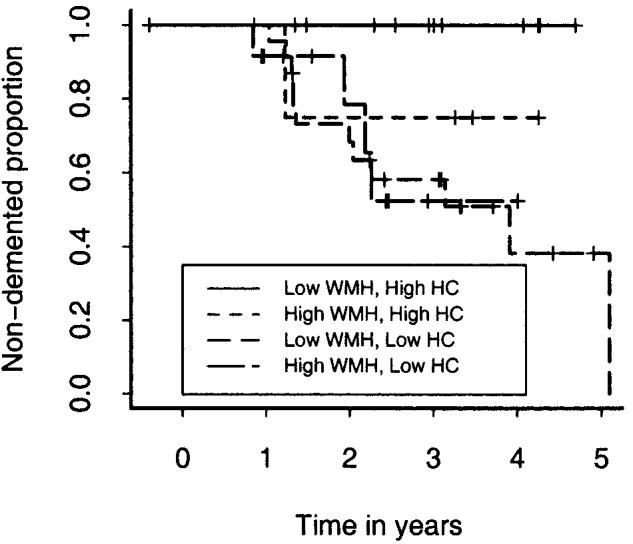
Graphic display of Kaplan-Meier survival curves for four classes of MRI measure based on hippocampal (HC) and white matter hyperintensity (WMH) volumes. See text for details.
Discussion
In this study, we assessed the impact of clinical, neuropsychological, and MRI measures on incident dementia within a heterogeneous group of MCI patients that included 23 individuals with CVD-related brain injury. Many of these individuals also had multiple vascular risk factors consistent with previous epidemiologic studies that report increased risk for MCI in association with CVD.12 Given that vascular risk factors are known to increase the likelihood of both MCI and expressed dementia, we hypothesized that clinical and MRI estimates of CVD would significantly contribute to incident dementia within this more broadly defined group of MCI individuals. Despite recruiting a more diverse cohort of MCI patients, including those with substantial CVD, however, average memory performance for the group was 77.4 ± 16.5 or approximately 1.5 SD below normal. In addition, the relation between memory performance and yearly rate of progression to dementia was nearly identical to previously reported studies.6,34 Finally, multivariate analyses found that neuropsychological testing of memory and executive function best predicted those MCI patients who would convert to dementia over the 3.1-year period of observation. From these results, we conclude that even moderate amounts of CVD (as evidenced by vascular risk factors, subcortical stroke, MRI lacunes, or extensive WMH) do not substantially alter the clinical course of MCI when memory impairment is significantly less than normal. Moreover, neuropsychological measures better predicted conversion to dementia than did a variety of MRI measures. Role of CVD. Whereas considerable epidemiologic evidence suggests that cerebrovascular risk factors are associated with various types of dementia, including AD,35-40 only limited prospective information exists regarding the impact of CVD on incident dementia.20,41 In one of these studies,41 qualitative estimates of white matter lucencies (leukoariosis)42 seen on x-ray CT were associated with increased risk of progression to dementia within a group of 27 MCI patients. The extent of leukoariosis was estimated according to a previously published assessment scale.43 With use of this scale, approximately one-half of the subjects had leukoariosis on CT. Individuals converting to dementia had leukoariosis scores nearly five times those who did not, and 7 of 8 subjects converting to dementia had leukoariosis on CT as compared with 5 of 19 who did not convert.41 Linear measures of medial temporal lobe were also significantly associated with progression. The authors conclude that both cerebrovascular and AD processes likely contribute to dementia progression among cognitively impaired individuals. Although it is difficult to compare qualitative estimates of leukoariosis defined by CT to WMH volumes determined from MRI, it is generally believed that white matter abnormalities are more severe when detected by CT. It is possible, therefore, that the group examined in this study41 included individuals with more severe CVD than did our group, suggesting that a threshold of cerebrovascular brain injury may be necessary to impact on cognition.14,44 Although our own data lend some support to this hypothesis, as individuals categorized as having “high” WMH volumes showed an intermediate rate of progression to dementia, the prevalence of clinical stroke, number of MRI lacunes, and number of vascular risk factors were actually lower in those who converted to dementia. Moreover, large WMH volumes were relatively uncommon in our group (only 16 MCI patients, of which there were only 4 converters), reducing the power to detect an effect. Finally, these authors41 made no mention of prevalent cerebrovascular risk factors, clinical stroke, or lacunar infarctions, further limiting comparison to the group reported here.
A second study20 had both a larger cohort (1,015 participants) and a more consistent 3-year duration of follow-up as compared with our follow-up duration that ranged from 10 months to 6 years. The prevalence of MCI, however, was not determined within their dementia-free cohort. A total of 30 individuals converted to dementia (approximately 1%/year). Prevalent silent lacunar infarction and incident silent lacunar infarctions were both associated with an increased risk to develop dementia or rapid cognitive decline, although this was particularly true for individuals with incident lacunar infarction.20 Interestingly, memory performance did not decline significantly for individuals with incident lacunar infarctions. Finally, the prevalence of APOE4 genotype was much lower in this group of nondemented elderly20 as compared with the individuals reported here.
Contrasting the results of these studies examining the impact of MRI measures of CVD on incident dementia20,41 with our own data raises interesting questions about the role of CVD on progression from MCI to dementia. Although it is evident that CVD can cause cognitive decline13,14,16,20 and is more prevalent among individuals with MCI,11,12 for this small group of individuals, CVD did not appear to influence progression to dementia when memory impairment was severe. One explanation for our inability to detect such a relationship may have been our use of the CDR23,24 as the outcome measure. This scale emphasizes memory-related cognitive impairments common to AD. CVD does not appear to impact significantly on memory performance as shown by our data and data previously reported.20 Future studies of individuals with more severe CVD using outcome measures less dependent on memory performance may help to reconcile the differences between our own study and those previously published. Selection bias may be another explanation. All subjects were recruited to this study through memory impairment clinics, and average memory performance was significantly reduced compared with normal. Moreover, the prevalence of APOE4 genotype was substantially enriched in this group of subjects, likely reflecting the selection bias of referrals to memory disorder clinics and suggesting a high probability of coincident AD. Therefore, despite our attempts to broaden the sample through inclusion of individuals with substantial vascular disease, use of memory impairment clinics for recruitment may still have resulted in our sample being enriched with individuals having considerable coincident AD. Future studies with either a community-based sample with more typical distributions of APOE4 or a sample enriched for nonmemory impairment (e.g., impaired in executive performance alone) may better serve to examine the impact and time course of CVD on cognitive decline and progression to dementia. A final explanation may be that CVD exerts a more pernicious effect on cognition. For example, CVD may take years to move an individual from normal cognition through MCI and on to dementia. The brief observation period used in this study, therefore, may favor identification of prognostic factors for neurodegenerative diseases such as AD where progression is likely to occur quickly. Future analysis of this cohort after a longer observation period may better elucidate the impact of CVD on dementia incidence.
Neuropsychological vs MRI measures to predict progression of MCI to dementia
A number of studies have examined clinical and imaging predictors associated with increased risk to convert from MCI to dementia,6,34,45-49 but we are unaware of any previous study to directly compare neuropsychological performance to MRI measures within the same group of individuals.
Substantial evidence from quantitative MRI studies suggests that HC atrophy is present before dementia onset46,47,50 and progresses with progression to clinically apparent disease.51 A large prospective study of MCI patients47 found a fourfold increase in the percentage of individuals converting to dementia within 5 years when HC size was 2.5 SD below ageand gender-defined norms. Similar findings were noted in a second study, although memory scores were also significant predictors.46 Another study using qualitative estimates of HC size found similar results.52
Similarly, reports of neuropsychological testing among individuals with MCI suggest that the severity of memory impairment strongly predicts progression to dementia,6,53 although associated deficits in language or impairments in other cognitive domains may contribute to more rapid progression.34 It appears, therefore, that the more closely cognitive impairments are to AD, the greater the likelihood an individual will progress to dementia, particularly if the cognitive complaints are accompanied by care-giver reports of impaired daily function.54,55
As HC volume is associated with AD pathology and surviving neurons52,56 as well as memory impairment,57 it would be expected that neuropsychological testing and MRI measures should show comparable and highly correlated associations with dementia progression. Although our data do show significant associations between HC measures and memory performance, it is not at all surprising to find that impairments in both memory and executive performance—indicative of individuals closer to the clinical definition of dementia—were better predictors of dementia progression. There are a number of plausible explanations for this finding. For example, our measures of HC may have been less accurate than those of other reported studies.46,47,50,58 This seems unlikely given that approximately 50% of our “low” HC group converted to dementia in 3 years, a figure remarkably similar to other published results.47,58 Alternatively, the neuropsychological measures may capture processes not accurately measured by the more discrete MRI measures. For example, memory impairments are known to occur from vascular disease through disruption of frontal cortical systems59 that would not be accounted for by HC measurement. Moreover, subtle cognitive impairments related to WMH may be captured by executive function measures21,60 that could impact additively on likelihood to convert to dementia. Whatever the explanation, it appears the neuropsychological assessment of memory and executive function is a better predictor than MRI measures of progression to dementia within a group of MCI patients with prominent memory impairment.
We therefore conclude that among a small group of broadly defined MCI patients, including individuals with substantial amounts of CVD, the presence of impairments in memory and executive performance best predicted future likelihood of progression to dementia. These results likely reflect the strong effect of the AD process when MCI individuals have substantial memory impairment but may also reflect the clinical utility of neuropsychological measures to more fully describe individuals at risk for progression when AD is the suspected cause for MCI. Moreover, these findings support the notion that the concept of MCI is applicable to the general population where comorbid diseases such as CVD are common. Future studies of a larger cohort of MCI patients with nonmemory impairment and a normal prevalence of the APOE4 genotype may help to clarify the impact of CVD on incident dementia among MCI patients. Until further information is available, however, the presence of moderate WMH, subcortical stroke, or MRI evidence of lacunar infarction does not appear to impact substantially on the course of MCI patients presenting with substantial memory impairment. In addition, this small study does not offer conclusive evidence for quantitative MRI assessment of HC or cGM to identify individuals at high risk for progression to dementia among a group of MCI subjects identified as having substantial memory impairment and a high prevalence of APOE4 genotype.
Footnotes
Supported by grants P01 AG12435, P30 AG10129, R01 AG021028, and R01 AG10220.
References
- 1.Cutler SJ, Grams AE. Correlates of self-reported everyday memory problems. J Gerontol Soc Sci. 1988;43:S82–S90. doi: 10.1093/geronj/43.3.s82. [DOI] [PubMed] [Google Scholar]
- 2.O'Connor DW, Pollitt PA, Roth M, Brook PB, Reiss BB. Memory complaints and impairment in normal, depressed and demented elderly persons identified in a community survey. Arch Gen Psychiatry. 1990;47:224–227. doi: 10.1001/archpsyc.1990.01810150024005. [DOI] [PubMed] [Google Scholar]
- 3.Morris JC, Price AL. Pathologic correlates of nondemented aging, mild cognitive impairment, and early-stage Alzheimer's disease. J Mol Neurosci. 2001;17:101–118. doi: 10.1385/jmn:17:2:101. [DOI] [PubMed] [Google Scholar]
- 4.Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer's disease. Ann Neurol. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 5.Morris JC, Storandt M, Miller JP, et al. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 6.Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 7.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 8.Graham JE, Rockwood K, Beattie BL, et al. Prevalence and severity of cognitive impairment with and without dementia in an elderly population. Lancet. 1997;349:1793–1796. doi: 10.1016/S0140-6736(97)01007-6. [DOI] [PubMed] [Google Scholar]
- 9.Ritchie K, Artero S, Touchon J. Classification criteria for mild cognitive impairment. A population-based validation study. Neurology. 2001;56:37–42. doi: 10.1212/wnl.56.1.37. [DOI] [PubMed] [Google Scholar]
- 10.DeCarli C. Defining mild cognitive impairment: prevalence, prognosis, etiology and treatment. Lancet Neurol. 2003;2:15–21. doi: 10.1016/s1474-4422(03)00262-x. [DOI] [PubMed] [Google Scholar]
- 11.DeCarli C, Miller BL, Swan GE, et al. Cerebrovascular and brain morphologic correlates of mild cognitive impairment in the National Heart, Lung, and Blood Institute Twin Study. Arch Neurol. 2001;58:643–647. doi: 10.1001/archneur.58.4.643. [DOI] [PubMed] [Google Scholar]
- 12.Lopez OL, Jagust WJ, Dulberg C, et al. Risk factors for mild cognitive impairment in the cardiovascular health study cognition study: part 2. Arch Neurol. 2003;60:1394–1399. doi: 10.1001/archneur.60.10.1394. [DOI] [PubMed] [Google Scholar]
- 13.Launer LJ, Masaki K, Petrovich H, Foley D, Havlik RJ. The association between mid-life blood pressure levels and late-life cognitive function. The Honolulu–Asia Aging Study. JAMA. 1995;274:1846–1851. [PubMed] [Google Scholar]
- 14.DeCarli C, Murphy DG, Tranh M, et al. The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology. 1995;45:2077–2084. doi: 10.1212/wnl.45.11.2077. [DOI] [PubMed] [Google Scholar]
- 15.Swan GE, DeCarli C, Miller BL, et al. Association of midlife blood pressure to late-life cognitive decline and brain morphology. Neurology. 1998;51:986–993. doi: 10.1212/wnl.51.4.986. [DOI] [PubMed] [Google Scholar]
- 16.Swan GE, DeCarli C, Miller BL, et al. Biobehavioral characteristics of nondemented older adults with subclinical brain atrophy. Neurology. 2000;54:2108–2114. doi: 10.1212/wnl.54.11.2108. [DOI] [PubMed] [Google Scholar]
- 17.Riley KP, Snowdon DA, Markesbery WR. Alzheimer's neurofibrillary pathology and the spectrum of cognitive function: findings from the Nun Study. Ann Neurol. 2002;51:567–577. doi: 10.1002/ana.10161. [DOI] [PubMed] [Google Scholar]
- 18.Fein G, Di Sclafani V, Tanabe J, et al. Hippocampal and cortical atrophy predict dementia in subcortical ischemic vascular disease. Neurology. 2000;55:1626–1635. doi: 10.1212/wnl.55.11.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mungas D, Reed B, Ellis WG, Jagust WJ. The effects of age on rate of progression of Alzheimer disease and dementia with associated cerebrovascular disease. Arch Neurol. 2001;58:1243–1247. doi: 10.1001/archneur.58.8.1243. [DOI] [PubMed] [Google Scholar]
- 20.Vermeer SE, Prins ND, den Heijer T, et al. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 21.Mungas D, Jagust WJ, Reed BR, et al. MRI predictors of cognition in subcortical ischemic vascular disease and Alzheimer's disease. Neurology. 2001;57:2229–2235. doi: 10.1212/wnl.57.12.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rockwood K, Wentzel C, Hachinski V, et al. Prevalence and outcomes of vascular cognitive impairment. Vascular Cognitive Impairment Investigators of the Canadian Study of Health and Aging. Neurology. 2000;54:447–451. doi: 10.1212/wnl.54.2.447. [DOI] [PubMed] [Google Scholar]
- 23.Berg L, Hughes CP, Coben LA, et al. Mild senile dementia of Alzheimer type: research diagnostic criteria, recruitment, and description of a study population. J Neurol Neurosurg Psychiatry. 1982;45:962–968. doi: 10.1136/jnnp.45.11.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris JC, McKeel DW, Fulling K, Torack RM, Berg L. Validation of clinical diagnostic criteria for Alzheimer's disease. Ann Neurol. 1988;24:17–22. doi: 10.1002/ana.410240105. [DOI] [PubMed] [Google Scholar]
- 25.Hambleton RK, Swaminathan H. Item response theory. Principles and applications. Kluwer-Nijhoff; Boston: 1985. [Google Scholar]
- 26.Mungas D, Reed BR, Kramer JH. Psychometrically matched measures of global cognition, memory, and executive function for assessment of cognitive decline in older persons. Neuropsychology. 2003;17:380–392. doi: 10.1037/0894-4105.17.3.380. [DOI] [PubMed] [Google Scholar]
- 27.Williams J. Memory Assessment Scales. Psychological Assessment Resources; Odessa: 1991. [Google Scholar]
- 28.Wechsler D. Wechsler Adult Intelligence Scale–Revised. Psychological Corp.; San Antonio: 1981. [Google Scholar]
- 29.Benton AL, Hamsher K. Multilingual Aphasia Examination. University of Iowa; Iowa City: 1976. [Google Scholar]
- 30.Mattis S. Dementia Rating Scale. Psychological Assessment Resources; Odessa, FL: 1988. [Google Scholar]
- 31.Haller JW, Christensen GE, Joshi SC, et al. Hippocampal MR imaging morphometry by means of general pattern matching. Radiology. 1996;199:787–791. doi: 10.1148/radiology.199.3.8638006. [DOI] [PubMed] [Google Scholar]
- 32.Haller JW, Banerjee A, Christensen GE, et al. Three-dimensional hippocampal MR morphometry with high-dimensional transformation of a neuroanatomic atlas. Radiology. 1997;202:504–510. doi: 10.1148/radiology.202.2.9015081. [DOI] [PubMed] [Google Scholar]
- 33.Wu CC, Mungas D, Petkov CI, et al. Brain structure and cognition in a community sample of elderly Latinos. Neurology. 2002;59:383–391. doi: 10.1212/wnl.59.3.383. [DOI] [PubMed] [Google Scholar]
- 34.Bozoki A, Giordani B, Heidebrink JL, Berent S, Foster NL. Mild cognitive impairments predict dementia in nondemented elderly patients with memory loss. Arch Neurol. 2001;58:411–416. doi: 10.1001/archneur.58.3.411. [DOI] [PubMed] [Google Scholar]
- 35.Slooter AJ, van Duijn CM, Bots ML, et al. Apolipoprotein E genotype, atherosclerosis, and cognitive decline: the Rotterdam Study. J Neural Transm (Suppl) 1998;53:17–29. doi: 10.1007/978-3-7091-6467-9_3. [DOI] [PubMed] [Google Scholar]
- 36.Launer LJ, Ross GW, Petrovitch H, et al. Midlife blood pressure and dementia: the Honolulu–Asia Aging Study. Neurobiol Aging. 2000;21:49–55. doi: 10.1016/s0197-4580(00)00096-8. [DOI] [PubMed] [Google Scholar]
- 37.Skoog I, Lernfelt B, Landahl S, et al. 15-year longitudinal study of blood pressure and dementia. Lancet. 1996;347:1141–1145. doi: 10.1016/s0140-6736(96)90608-x. [DOI] [PubMed] [Google Scholar]
- 38.Kivipelto M, Helkala EL, Hanninen T, et al. Midlife vascular risk factors and late-life mild cognitive impairment: a population-based study. Neurology. 2001;56:1683–1689. doi: 10.1212/wnl.56.12.1683. [DOI] [PubMed] [Google Scholar]
- 39.Kivipelto M, Helkala EL, Laakso MP, et al. Midlife vascular risk factors and Alzheimer's disease in later life: longitudinal, population based study. Br Med J. 2001;322:1447–1451. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kivipelto M, Laakso MP, Tuomilehto J, Nissinen A, Soininen H. Hypertension and hypercholesterolaemia as risk factors for Alzheimer's disease: potential for pharmacological intervention. CNS Drugs. 2002;16:435–444. doi: 10.2165/00023210-200216070-00001. [DOI] [PubMed] [Google Scholar]
- 41.Wolf H, Ecke GM, Bettin S, Dietrich J, Gertz HJ. Do white matter changes contribute to the subsequent development of dementia in patients with mild cognitive impairment? A longitudinal study. Int J Geriatr Psychiatry. 2000;15:803–812. doi: 10.1002/1099-1166(200009)15:9<803::aid-gps190>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 42.Hachinski VC, Potter P, Merskey H. Leuko-araiosis: an ancient term for a new problem. Can J Neurol Sci. 1986;13(suppl 4):5533–5534. doi: 10.1017/s0317167100037264. [DOI] [PubMed] [Google Scholar]
- 43.Lopez OL, Becker JT, Jungreis CA, et al. Computed tomography— but not magnetic resonance imaging—identified periventricular white-matter lesions predict symptomatic cerebrovascular disease in probable Alzheimer's disease. Arch Neurol. 1995;52:659–664. doi: 10.1001/archneur.1995.00540310029012. [DOI] [PubMed] [Google Scholar]
- 44.Boone KB, Miller BL, Lesser IM, Mehringer CM, Hill E, Berman N. Cognitive deficits with white-matter lesions in healthy elderly Arch Neurol. 1992;49:549–554. doi: 10.1001/archneur.1992.00530290141024. [DOI] [PubMed] [Google Scholar]
- 45.Marquis S, Moore MM, Howieson DB, et al. Independent predictors of cognitive decline in healthy elderly persons. Arch Neurol. 2002;59:601–606. doi: 10.1001/archneur.59.4.601. [DOI] [PubMed] [Google Scholar]
- 46.Visser PJ, Scheltens P, Verhey FR, et al. Medial temporal lobe atrophy and memory dysfunction as predictors for dementia in subjects with mild cognitive impairment. J Neurol. 1999;246:477–485. doi: 10.1007/s004150050387. [DOI] [PubMed] [Google Scholar]
- 47.Jack CR, Jr, Petersen RC, Xu YC, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Petersen RC, Waring SC, Smith GE, Tangalos EG, Thibodeau SN. Predictive value of APOE genotyping in incipient Alzheimer's disease. Ann NY Acad Sci. 1996;802:58–69. doi: 10.1111/j.1749-6632.1996.tb32599.x. [DOI] [PubMed] [Google Scholar]
- 49.Tierney MC. Cognitive tests that best discriminate between presymptomatic AD and those who remain nondemented. Neurology. 2001;57:163–164. doi: 10.1212/wnl.57.1.163. [DOI] [PubMed] [Google Scholar]
- 50.Kaye JA, Swihart T, Howieson D, et al. Volume loss of the hippocampus and temporal lobe in healthy elderly persons destined to develop dementia. Neurology. 1997;48:1297–1304. doi: 10.1212/wnl.48.5.1297. [DOI] [PubMed] [Google Scholar]
- 51.Fox NC, Warrington EK, Stevens JM, Rossor MN. Atrophy of the hippocampal formation in early familial Alzheimer's disease. A longitudinal MRI study of at-risk members of a family with an amyloid precursor protein 717Val-Gly mutation. Ann NY Acad Sci. 1996;777:226–232. doi: 10.1111/j.1749-6632.1996.tb34423.x. [DOI] [PubMed] [Google Scholar]
- 52.de Leon M, George A, Convit A, et al. The radiologic prediction of Alzheimer disease: the atrophic hippocampal formation. AJNR Am J Neuroradiol. 1993;14:897–906. [PMC free article] [PubMed] [Google Scholar]
- 53.Tierney MC, Szalai JP, Snow WG, et al. A prospective study of the clinical utility of ApoE genotype in the prediction of outcome in patients with memory impairment. Neurology. 1996;46:149–154. doi: 10.1212/wnl.46.1.149. [DOI] [PubMed] [Google Scholar]
- 54.Tabert MH, Albert SM, Borukhova-Milov L, et al. Functional deficits in patients with mild cognitive impairment: prediction of AD. Neurology. 2002;58:758–764. doi: 10.1212/wnl.58.5.758. [DOI] [PubMed] [Google Scholar]
- 55.Albert SM, Tabert MH, Dienstag A, Pelton G, Devanand E. The impact of mild cognitive impairment on functional abilities in the elderly. Curr Psychiatry Rep. 2002;4:64–68. doi: 10.1007/s11920-002-0015-8. [DOI] [PubMed] [Google Scholar]
- 56.Jack CR, Dickson DW, Parisi JE, et al. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology. 2002;58:750–757. doi: 10.1212/wnl.58.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Petersen RC, Jack CR, Jr, Xu YC, et al. Memory and MRI-based hippocampal volumes in aging and AD. Neurology. 2000;54:581–587. doi: 10.1212/wnl.54.3.581. [DOI] [PubMed] [Google Scholar]
- 58.Jack CR, Jr, Petersen RC, Xu YC, et al. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer's disease. Neurology. 1997;49:786–794. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reed BR, Eberling JL, Mungas D, Weiner MW, Jagust WJ. Memory failure has different mechanisms in subcortical stroke and Alzheimer's disease. Ann Neurol. 2000;48:275–284. [PMC free article] [PubMed] [Google Scholar]
- 60.Kramer JH, Reed BR, Mungas D, Weiner MW, Chui HC. Executive dysfunction in subcortical ischaemic vascular disease. J Neurol Neurosurg Psychiatry. 2002;72:217–220. doi: 10.1136/jnnp.72.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]


