Abstract
Objective
To examine volumetric MRI correlates of longitudinal cognitive decline in normal aging, AD, and subcortical cerebrovascular brain injury (SCVBI).
Background
Previous cross-sectional studies examining the relationship between cognitive impairment and dementia have shown that hippocampal and cortical gray matter atrophy are the most important predictors of cognitive impairment, even in cases with SCVBI. The authors hypothesized that hippocampal and cortical gray matter volume also would best predict rate of cognitive decline in cases with and without SCVBI.
Methods
Subjects were recruited for a multicenter study of contributions to dementia of AD and SCVBI. The sample (n = 120) included cognitively normal, cognitively impaired, and demented cases with and without lacunes identified by MRI. Cases with cortical strokes were excluded. Average length of follow-up was 3.0 years. Measures of hippocampal volume, volume of cortical gray matter, presence of subcortical lacunes, and volume of white matter hyperintensity were derived from MRI. Random effects modeling of longitudinal data was used to assess effects of baseline MRI variables on longitudinal change in a measure of global cognitive ability.
Results
Cortical gray matter atrophy predicted cognitive decline regardless of whether lacunes were present. Hippocampal atrophy predicted decline only in those without lacunes. Neither lacunes nor white matter hyperintensity independently predicted decline.
Conclusions
Results suggest that cortical atrophy is an index of disease severity in both AD and subcortical cerebrovascular brain injury and consequently predicts faster progression. Hippocampal volume may index disease severity and predict progression in AD. The absence of this effect in cases with lacunes suggests that this group is etiologically heterogeneous and is not composed simply of cases of AD with incidental stroke.
AD and cerebrovascular disease (CVD) are generally considered the most common causes of cognitive impairment and dementia in older persons. Recent studies have shown no significant difference in rate of decline in AD and vascular dementia,1-3 but a study from our group4 showed that age at initial evaluation moderated rate of cognitive decline related to AD and CVD. Rate of decline decreased slightly in relation to increasing baseline age in clinical and autopsy samples with AD, whereas rate of decline increased markedly with age in dementia with CVD. This effect was interpreted to result from a relative absence of AD in younger (age < 80) demented cases with CVD, but likely presence of both AD and CVD in the older cases. This highlights the importance of considering AD and CVD as independent pathologic processes that can occur alone or in combination and can have independent, additive, or even synergistic effects on clinical presentation and course. There is cross-sectional evidence that the presence of CVD amplifies effects of AD pathology,5,6 but this type of effect has not been examined in longitudinal studies.
Previous longitudinal studies have typically compared qualitatively different diagnostic groups using only demented cases. There might be distinct advantages to studying individuals with a broader range of cognitive functioning using continuous measures that are closely linked to AD and CVD. This type of study might clarify how underlying brain changes relate to the evolution of cognitive impairment resulting from AD and CVD. Brain imaging would appear to be of particular value, for example, for sensitively measuring hippocampal and cortical atrophy that is well documented in AD and for quantifying strokes and white matter hyperintensity (WMH) associated with CVD. Previous studies have shown that hippocampal atrophy predicts conversion from mild cognitive impairment to dementia,7,8 but there is a need for studies examining broader relationships between qualitatively different structural brain changes and progression of cognitive impairment throughout the continuum of cognitive change.
A previous cross-sectional study from our group9 showed that hippocampal volume (HC) and cortical gray matter volume (CGM) were strong predictors of cognitive ability across multiple domains even in cases with clinically significant subcortical cerebrovascular brain injury (SCVBI). WMH was related to a lesser degree, but subcortical lacunes were minimally related to cognitive function. Deficits identified in cross-sectional analyses and decline demonstrated in longitudinal analyses both should reflect the same underlying biologic processes, and so, these results form the basis for hypotheses to be tested in this study.
We examined how quantitative MRI measures of SCVBI and CGM and HC relate to subsequent cognitive decline in a diverse sample with a broad range of baseline cognitive functioning and with broad variability of SCVBI. It was hypothesized that, as in the cross-sectional study, hippocampal and cortical atrophy would be more important determinants of cognitive decline than measures of WMH and subcortical lacunes. Because this was a longitudinal study, special emphasis was placed on creating a cognitive measure that broadly assesses domains of cognitive function, does not have floor or ceiling effects, and provides linear measurement in comparison with underlying change in cognitive ability.
Methods
Subjects
Participants in this study were recruited from three academic dementia centers and were evaluated as part of a multicenter collaborative study of contributions of SCVBI and AD to cognitive impairment and dementia. All participants received a comprehensive clinical evaluation that included a detailed medical history, a neurologic examination, appropriate laboratory tests, and neuropsychological testing with a standardized test battery. In addition, participants received a MRI scan of the brain. Participants were diagnosed at a multidisciplinary case conference using National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders Association diagnostic criteria10 for AD and California ADDTC criteria11 for ischemic vascular dementia (IVD). A diagnosis of mixed dementia required that the patient met criteria for both AD and IVD, and in the judgment of the clinical team, both etiologies were believed to contribute in a relatively equal manner to the dementia symptoms. The Institutional Review Boards at all participating institutions approved this study and subjects or their legal representatives gave written informed consent for participating.
Recruitment was targeted to fill six groups defined by three levels of cognitive impairment crossed with presence versus absence of subcortical lacunes. The levels of cognitive impairment were as follows: 1) normal, defined by a Clinical Dementia Rating (CDR)12,13 total score of 0.0; 2) impaired (CDR = 0.5); and 3) demented (CDR ≥ 1.0). A single neuroradiologist reviewed all MRI scans to determine presence of lacunes. Demographic characteristics and global cognitive function (Mini-Mental State Examination) are presented by recruitment group in the table. The clinical diagnosis of demented subjects without lacunes was predominantly probable AD (85.7%). Clinical diagnoses of demented subjects with lacunes included vascular dementia (57.1%), mixed AD/vascular disease (28.6%), and AD (14.3%).
Table.
Number of subjects, demographic characteristics, and MMSE score by recruitment category (CDR category by presence vs absence of lacunes)
| Recruitment group | No. | Education, y, mean (SD) | Age, y, mean (SD) | MMSE, mean (SD) | % Female |
|---|---|---|---|---|---|
| CDR = 0.0 | 47 | 15.4 (3.0) | 71.6 (7.6) | 29.2 (1.0) | 51.1 |
| No lacunes | |||||
| CDR = 0.5 | 16 | 14.6 (2.6) | 74.6 (6.7) | 27.1 (3.1) | 31.3 |
| No lacunes | |||||
| CDR ≥ 1.0 | 7 | 15.4 (1.5) | 78.4 (7.8) | 21.7 (3.3) | 71.4 |
| No lacunes | |||||
| CDR = 0.0 | 21 | 14.2 (2.8) | 75.1 (4.9) | 28.4 (2.0) | 47.6 |
| Lacunes | |||||
| CDR = 0.5 | 22 | 14.0 (2.8) | 70.1 (8.8) | 28.1 (1.7) | 22.7 |
| Lacunes | |||||
| CDR ≥ 1.0 | 7 | 11.3 (3.5) | 75.3 (5.1) | 20.7 (4.1) | 28.6 |
| Lacunes | |||||
| Total | 120 | 14.6 (3.0) | 73.0 (7.4) | 27.6 (3.2) | 42.5 |
MMSE = Mini-Mental State Examination; CDR = Clinical Dementia Rating.
There were a total of 356 assessments for the 120 cases included in this study. Average time from the initial to last assessment was 2.95 years (SD = 1.20, range = 0.67–6.01). Forty-two cases (35.0%) had two assessments, 44 (36.7%) had three, and 34 (28.3%) had four or more.
MRI methods
MRI variables were computerized measures of volume of WMH, CGM, HC, and presence and volume of subcortical stroke (lacunes) within specific structures: thalamus, putamen, caudate, globus pallidus, and white matter. All volumes were normalized to total intracranial volume (ICV).
Lacunes were small (>2 mm) areas of the brain with increased signal relative to CSF on proton density MRI, in subcortical gray and white matter. Lacunes were differentiated from perivascular spaces, which can be particularly prominent below the anterior commissure and putamen and at bends in the course of penetrating arterioles. Isoin-tense lesions on pseudo proton density MRI (as opposed to “true” proton density, which is obtained when extrapolated to echo time = 0) at the level of the anterior commissure or inferior putamen were termed perivascular spaces; outside that region they were defined as cavitated lacunes if they were ≥3 mm at maximum width. Lesions that met either of these criteria were considered lacunes for purposes of data analysis.
Image acquisition and data management and transmission have been described.14 A computerized segmentation algorithm was used to classify brain MRI pixels into CGM, subcortical gray matter, white matter, WMH, ventricular CSF, and sulcal CSF. In addition, total ICV was computed by summing over all pixels within the intracranial vault. Segmentation methods have been previously reported.14 Intraclass correlation coefficients (n = 10) were 0.93 for percent of white matter; 0.99 for percent of WMH; 0.95 for CGM; 0.99 for sulcal CSF; and 0.99 for ventricular CSF.
The outline of the hippocampus was manually drawn on 1.4-mm MRI slices and volumes were generated by a computer algorithm using previously reported methods.14,15 Accuracy of the manual ratings of HC was subsequently evaluated by comparing manual volumes with volumes independently derived using an automated, computerized method (Surgical Navigator Systems, Boulder, CO) for determining HC.16,17 An analysis of variance approach (n = 92) showed that the automated ratings accounted for 78.8% of the variance in the manual ratings (standardized beta = 0.89), indicating a high level of correspondence between the manual and automated methods.
Neuropsychological tests
All participants received a standardized battery of neuropsychological tests in common clinical use. Several specific tests were used to derive a measure of global cognitive ability (Global Cognition) that had desirable psychometric properties for evaluation of longitudinal change in this study. Donor tests were 1) total recall on Trials 1 and 2 on the Word List Learning Test of the Memory Assessment Scales,18 2) Digit Span forward and backward from the Wechsler Memory Scale–Revised,19 3) letter fluency (the letter “A” from the “FAS” test20), and 4) animal category fluency.21,22 These tests were selected because they broadly assess cognitive domains relevant to AD and SCVBI, have a broad range of measurement without appreciable floor or ceiling effects, can be administered quickly, and can be used even for patients with relatively severe dementia.
Scale construction of the Global Cognition measure was guided by methods associated with item response theory (IRT),23-25 a modern and widely used approach to large-scale psychometric test development. Scale construction methods were based on a larger sample of 400 from this project and are described in detail elsewhere (manuscript submitted). Briefly, IRT analyses yield two important scale level functions or curves that describe the basic psycho-metric properties of the scale. The test information curve (TIC) represents scale reliability at each point on the ability continuum, whereas the test characteristic curve (TCC) describes the expected test score at each ability point. Ability essentially refers to capacity to successfully perform the task or tasks incorporated in the scale, and can be roughly estimated by scale total score. The Global Cognition measure had a TIC showing very high reliability (r ≥ 0.90) from about −2.25 SD below the mean of the overall development sample to 2.25 SD above the mean. This range encompasses all participants in this study, and so, the TIC for the Global Cognition measure indicates high fidelity of measurement across the entire ability continuum relevant to this sample.
The Global Cognition measure was transformed so that scores were referenced to the distribution of the cognitively normal without lacunes recruitment group, and so that the scale of measurement corresponded to a traditional measurement scale with a mean of 100 and SD of 15. Thus a score of 85 represents one SD below the mean of the normal without lacunes group. Traditional internal consistency reliability of this measure was high (coefficient alpha = 0.77). It was highly correlated with other measures of global cognition as well as measures from specific domains including memory, language, attention, and executive function (manuscript submitted). This measure had very high reliability and linear measurement across a broad ability range that encompasses this sample. It also was normally distributed, which made it appropriate for a variety of statistical analyses.
Data analysis
A mixed model, random effects regression analysis was used to model longitudinal change in global cognitive ability and evaluate effects of quantitative MRI variables on rate of cognitive decline.26,27 Random effects modeling of longitudinal data has also been referred to as a two-stage model for repeated measurement.26 It can be conceptualized as a method in which regression coefficients to account for within-subject change of scores across time are simultaneously estimated for all individuals in the sample (stage 1), and in the same analysis, between-subject predictors of these within-subject change indices are evaluated (stage 2). Random effects models have significant advantages for analysis of longitudinal data26,28 This approach utilizes all available data and accounts for within-person correlation across time, which results in increased statistical power for estimating effects. Effects related to baseline scores and change in independent variables can be evaluated, and predictive effects of multiple independent variables on baseline scores and change across time can be evaluated. Subjects can have missed follow-up visits and varying time intervals between assessments, making this a flexible approach to analysis.
This analysis, performed with SAS Proc Mixed, incorporated random effects terms to account for subject (baseline score) and subject by time (rate of change) differences in performance on the Global Cognitive measure. Terms were included to account for effects on baseline cognitive scores of age, education, sex, and quantitative MRI variables. Age, but not other demographic variables, was related to longitudinal change in preliminary analyses, so a term to account for age effects on longitudinal change was included in regression models. Imaging variables were first entered individually into a model incorporating effects for age, education, and sex as predictors of baseline score and age as a predictor of change. Terms were added individually to this baseline model to evaluate the effects of lacunes, WMH, CGM, and HC on baseline score and time-related change. These analyses for WMH, CGM, and HC included a categorical variable coding for presence versus absence of lacunes, and an effect for the interaction of the MRI variable with presence of lacunes. The analysis for lacunes included only presence versus absence of lacunes. A final model simultaneously entered all MRI variables and their interactions with lacunes.
Random effects analyses are sensitive to assumptions that the random effects are linear and normally distributed. These assumptions were examined using graphical and statistical diagnostics. Residuals and random effects were examined to assure that they were normally distributed, and plots of residuals against predicted values and effects were examined to verify that nonlinear trends in the data were not present. Additional diagnostics included evaluation of variance components related to random effects and within-subject error variance to address adequacy of statistical estimation procedures associated with the random effects modeling.
Results
Presence of lacunes was related to baseline Global Cognition (p = 0.02) and to change across time (p = 0.03) when entered individually into the regression model along with demographic variables. WMH was related to the baseline score (p = 0.007) but not to change (p = 0.36). The interaction of lacunes and WMH was not related (p > 0.13) to baseline score or change. CGM was related to baseline score (p = 0.0003) and this effect did not differ according to presence versus absence of lacunes (p = 0.57). Baseline CGM was also related to change (p = 0.002), and this effect did not differ according to lacunes (p = 0.41). HC had a significant effect on baseline Global Cognition (p < 0.0001), and the lacune by HC interaction was not significant (p = 0.88). The HC (p = 0.01) and lacune by HC interaction (p = 0.02) effects on cognitive change were both significant. These results show that lacunes, WMH, CGM, and HC were all related to baseline Global Cognition. Lower baseline CGM predicted more rapid decline for cases with and without lacunes. Lower baseline HC also predicted decline, but the relationship between HC and cognitive decline differed across lacune groups.
When MRI variables were entered jointly, HC (p < 0.0001) and WMH (p = 0.02) were independent predictors of baseline score. Cognitive change was related to CGM (p = 0.02) and the lacune by HC interaction (p = 0.01). Figure 1 shows the average rate of change in Global Cognition as a function of baseline CGM for the lacune and no-lacune groups. These results represent CGM effects independent of all other MRI variables in the simultaneous model. CGM was similarly related to change in both groups, and indeed, showed a slightly stronger relationship in the group with lacunes. In contrast, HC was strongly related to change in the no lacune group (p = 0.004) but was not related in the lacune group (p = 0.32).
Figure 1.
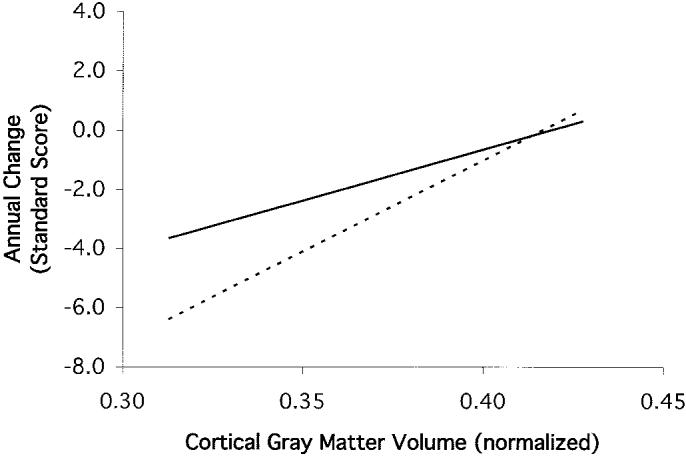
Predicted average annual rate of change of Global Cognition by baseline cortical gray matter volume (CGM) for participants with and without lacunes. Estimates are derived from random effects modeling of longitudinal change and indicate CGM effects that are independent of demographic variables and other MRI variables. Slopes of regression lines for the lacune and nolacune groups do not significantly differ. Solid line = least squares regression line for cases without lacunes; dashed line = least squares regression line for cases with lacunes.
Secondary analyses were performed to help clarify the lacune by HC interaction effect on rate of change. First, a simple random effects model was used to estimate individual trajectories of cognitive change across time. Individual estimates of rate of change were generated for each subject using a model in which time was entered as the lone fixed effect and subjects and subjects by time were included as random effects. These estimates, which correspond to the slopes of the within-subject regression lines, were then plotted against HC for the lacune and no lacune groups, and linear regression lines were plotted to represent the bivariate relationship between individual rate of change and HC (figure 2). Results in figure 2 show that HC is more strongly related to decline in the no lacunes group.
Figure 2.

Scatterplot of estimated annual change in Global Cognition for each participant by baseline hippocampal volume (HC). Annual change estimates are derived from random effects modeling of longitudinal change as a function of time and represent estimated slopes of within-subject regression lines. Results show a significant interaction of HC and lacunes. HC is significantly related to Global Cognition in the no-lacunes group but not in the lacunes group. ● = Participants without lacunes; □ = participants with lacunes; solid line = least squares regression line for cases without lacunes; dashed line = least squares regression line for cases with lacunes.
There are two additional noteworthy features of figure 2. First, there is considerable variability in rate of change in the lacunes group, but this variability is minimally related to HC. Second, 11 of 14 (78.6%) cases with very low HC (≤0.3% of total ICV) were in the no lacunes group. These 11 cases without lacunes had a distribution of cognitive decline that was clearly different than that of the no-lacunes cases with higher HC. In contrast, the distribution of cognitive decline for the three cases with lacunes and low HC was broadly variable across the entire distribution of cognitive change in the overall sample. The clinical etiologic diagnosis for the 11 cases without lacunes was predominantly AD (seven probable, two possible). Of the remaining two, one had cognitive impairment not meeting criteria for dementia and the other had a clinical diagnosis of CVD not meeting criteria for IVD. When the 14 cases with low HC were excluded, the slope of the regression line for the no lacunes group was very similar to that for the lacunes group. Thus, the relationship between HC and rate of change in the no lacunes group appears to be largely determined by the cases with very low HC, most of whom had a clinical diagnosis of AD.
Cases with lacunes had lower mean CGM (p = 0.03; mean [SEM] no lacunes = 0.385 [0.004], lacunes = 0.372 [0.004]). Lacune and no-lacune groups clearly differed on mean WMH (p < 0.0001; no lacunes = 0.0063 [0.0015], lacunes = 0.0160 [0.0017]). Mean HC did not differ across lacune groups (p = 0.89), but range and variance of HC was greater in the no-lacunes group. This was largely due to the predominance of very low HC cases in the nolacunes group and is readily apparent in figure 2.
Discussion
Results of this study showed that baseline CGM and HC were associated with change in global cognitive ability. CGM volume predicted subsequent decline in cases with and without lacunes, whereas HC predicted decline in cases without lacunes but not in those with lacunes. Whereas WMH and presence of lacunes were related to baseline cognitive scores, lacunes but not WMH predicted cognitive decline. However, the lacune effect was not independent of effects of CGM and HC.
Previous literature has established the importance of the hippocampus in the progression of AD. The hippocampus typically has extensive pathologic involvement early in the course of AD.29-32 In cases of mild cognitive impairment, hippocampal atrophy is predictive of subsequent cognitive decline and conversion to dementia, and has been considered an early marker for a progressive AD process.7,8 Thus it is not surprising that HC would predict cognitive decline related to AD, which is the most likely explanation for the relationship between HC and cognitive decline in the cases without lacunes in this study. The relationship between baseline hippocampal atrophy and cognitive decline in this group appeared to be largely due to rapid progression in cases with severe hippocampal atrophy, most of whom had a clinical diagnosis of AD.
CGM was significantly correlated with WMH (r = −0.42) and, to a lesser degree, with total lacune volume (r = −0.21) in this study and in previous reports from this project.9,14 Cortical atrophy in demented cases was equivalent in lacune and nolacune groups, and neuropathologically diagnosed cases with SCVBI but without neocortical neurofibrillary pathology showed cortical atrophy similar to that in cases with a pathologic diagnosis of AD.14 These results converge to indicate that cortical atrophy that cannot be explained by AD occurs in association with SCVBI. In the earlier cross-sectional study, cortical atrophy was shown to be an equally strong predictor of cognitive performance in lacune and no-lacune cases,9 and in this study, equally predicted cognitive decline.
This constellation of results most likely indicates that cortical atrophy is a common cause of cognitive impairment resulting from both AD and CVD, and also predicts subsequent cognitive decline in both conditions. In AD, the typical progression of neurofibrillary pathology begins in the medial temporal lobes and later spreads to neocortex.29,33 Memory is most strongly related to HC, but non-memory cognitive abilities are strongly related to CGM independent of HC.9 Thus one would expect more rapid decline of global cognition in AD cases with neocortical pathology than in early AD without neocortical involvement. In SCVBI, cortical atrophy may well reflect more severe and extensive cerebrovascular disease that, one could argue based on this study, is more likely to be progressive.
An alternate explanation for the relationship between CGM and cognitive decline in cases with lacunes is that AD is present in these cases and accounts for both the cortical atrophy and cognitive decline. Indeed, AD most likely is present and influences results to some degree in this group. Three of seven demented cases with lacunes had a clinical diagnosis of AD. However, the lacunes group clearly differed in many respects from the no-lacunes group, and specifically, had much more prominent CVD and lesser prevalence of severe hippocampal atrophy. This would suggest that CVD is a more important etiologic factor and AD is less salient in these cases. The most compelling argument, though, that the relationship between cortical atrophy and cognitive decline is not simply due to AD comes from the lack of a relationship between HC and cognitive decline in the lacune group. If AD were the explanation for cognitive decline in this group, then one would expect a relationship to HC similar to that observed in the no-lacune group.
The absence of a clear relationship between HC and cognitive decline in the lacunes group merits further discussion. First, it may relate to the heterogeneity of both rate of cognitive decline and disease this group. In particular, patients in this group may have had progression of CVD subsequent to the baseline MRI that might have contributed to variability in rate of cognitive change and obscured effects of baseline hippocampal atrophy. Sample size also might have affected results for this group. The relationship between HC and cognitive decline in the no-lacunes group appeared to be driven by a relatively small group of individuals with very low HC. There were only three cases with very low HC in the lacunes group, and a larger sample with more cases combining lacunes and low HC might show a significant effect of baseline HC in this group.
This study does not directly address the issue of relative rate of cognitive decline associated with AD and SCVBI. This study and previous work from this project support a hypothesis that cortical atrophy is a final common pathway of both disorders and makes a major contribution to broad impairment of cognitive abilities. It seems reasonable to expect that individuals with both pathologies would progress at a more rapid rate than with either pathology alone. However, neuropathologic diagnosis, which was not available for this study, is required to definitively address the relative independent effects of SCVBI and AD on rate of progression, as well as to clearly delineate their additive and possibly synergistic effects.
Cognitive decline associated with dementia is notoriously variable and difficult to predict, and results of this study may have clinical relevance for using MRI to determine prognosis for cognitive decline. Results suggest that cortical atrophy regardless of the cause is predictive of faster subsequent decline. Severe hippocampal atrophy also predicts faster decline, particularly in the absence of SCVBI. Because cortical and hippocampal atrophy had additive effects on cognitive decline, their combined presence would suggest a greater rate of decline than would either alone. An equally important implication is that these results suggest that MRI indicators of SCVBI, WML and lacunes, are not predictive of cognitive decline in the absence of cortical and hippocampal atrophy. Of course, this study utilized resource intensive quantitative image analysis methods that are not routinely available to the clinician and involved a sample that may not be representative of typical clinical practice. Nevertheless, this type of quantitative research is an important first step leading to practical clinical methods, although additional research is required to translate these results into practical rating scales that can be used effectively in clinical settings.
Acknowledgment
David Norman, MD, reviewed all MRI scans to identify lacunes.
Footnotes
Supported in part by grants AG12435, AG10129, and AG10220 from the National Institute on Aging, Bethesda, MD, and by the California Department of Health Services Alzheimer's Disease Program, contracts 94-20354, 94-20355, 98-14970, and 98-14971.
References
- 1.Corey-Bloom J, Galasko D, Hofstetter CR, et al. Clinical features distinguish large cohorts with possible AD, probable AD, and mixed dementia. J Am Geriatr Soc. 1993;41:31–37. doi: 10.1111/j.1532-5415.1993.tb05944.x. [DOI] [PubMed] [Google Scholar]
- 2.Agüero-Torres H, Fratiglioni L, Guo Z, Viitanen M, Winblad B. Prognostic factors in very old demented adults: a seven-year follow-up from a population based survey in Stockholm. J Am Geriatr Soc. 1998;46:444–452. doi: 10.1111/j.1532-5415.1998.tb02464.x. [DOI] [PubMed] [Google Scholar]
- 3.Ballard C, Patel A, Oyebode F, Wilcock G. Cognitive decline in patients with Alzheimer's disease, vascular dementia and senile dementia of Lewy body type. Age Ageing. 1996;25:209–213. doi: 10.1093/ageing/25.3.209. [DOI] [PubMed] [Google Scholar]
- 4.Mungas D, Reed BR, Ellis WG, Jagust WJ. Age affects rate of progression of Alzheimer's disease and dementia with associated cerebrovascular disease. Arch Neurol. 2001;58:1243–1247. doi: 10.1001/archneur.58.8.1243. [DOI] [PubMed] [Google Scholar]
- 5.Snowdon DA, Greiner LH, Mortimer JA, et al. Brain infarction and the clinical expression of Alzheimer disease: the nun study. J Am Med Assoc. 1997;277:813–817. [PubMed] [Google Scholar]
- 6.Nagy ZS, Esiri MM, Jobst KA, et al. The effects of additional pathology on the cognitive deficit of Alzheimer's disease. Neuropathol Exp Neurol. 1997;56:165–170. doi: 10.1097/00005072-199702000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Visser PJ, Scheltens P, Verhey FR, et al. Medial temporal lobe atrophy and memory dysfunction as predictors for dementia in subjects with mild cognitive impairment. J Neurol. 1999;246:477–485. doi: 10.1007/s004150050387. [DOI] [PubMed] [Google Scholar]
- 8.Jack CR, Jr, Petersen RC, Xu YC, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mungas D, Jagust WJ, Reed BR, et al. MRI predictors of cognition in subcortical ischemic vascular disease and Alzheimer's disease. Neurology. 2001;57:2229–2235. doi: 10.1212/wnl.57.12.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 11.Chui HC, Victoroff JI, Margolin D, Jagust W, Shankle R, Katz-man R. Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer's Disease Diagnostic and Treatment Centers. Neurology. 1992;42:473–480. doi: 10.1212/wnl.42.3.473. [DOI] [PubMed] [Google Scholar]
- 12.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 13.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:11. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 14.Fein G, DiScalfani V, Tanabe J, et al. Hippocampal and cortical atrophy predict dementia in subcortical ischemic vascular disease. Neurology. 2000;55:1626–1635. doi: 10.1212/wnl.55.11.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Scalfani V, Bloomer C, Clark H, Norman D, Hannauer D, Fein G. Abstinent chronic cocaine and cocaine/alcohol abusers evidence normal hippocampal volumes on MRI despite cognitive impairments. Addict Biol. 1998;3:261–270. doi: 10.1080/13556219872074. [DOI] [PubMed] [Google Scholar]
- 16.Haller JW, Christensen GE, Joshi SC, et al. Hippocampal MR imaging morphometry by means of general pattern matching. Radiology. 1996;199:787–791. doi: 10.1148/radiology.199.3.8638006. [DOI] [PubMed] [Google Scholar]
- 17.Haller JW, Banerjee A, Christensen GE, et al. Three-dimensional hippocampal MR morphometry with high-dimensional transformation of a neuroanatomic atlas. Radiology. 1997;202:504–510. doi: 10.1148/radiology.202.2.9015081. [DOI] [PubMed] [Google Scholar]
- 18.Williams JM. Memory Assessment Scales. Psychological Assessment Resources; Odessa, FL: 1991. [Google Scholar]
- 19.Wechsler D. Wechsler Memory Scale–Revised (WMS-R) The Psychological Corporation; San Antonio, TX: 1987. [Google Scholar]
- 20.Benton AL, Hamsher Kd. Multilingual Aphasia Examination. University of Iowa; Iowa City, IA: 1976. [Google Scholar]
- 21.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's Disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 22.Welsh KA, Butters N, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part V. A normative study of the neuropsychological battery. Neurology. 1994;44:609–614. doi: 10.1212/wnl.44.4.609. [DOI] [PubMed] [Google Scholar]
- 23.Baker FB. The basics of item response theory. Heineman Publishing; Portsmouth, NH: 1985. [Google Scholar]
- 24.Hambleton RK, Swaminathan H. Item response theory. Principles and applications. Kluwer-Nijhoff Publishing; Boston: 1985. [Google Scholar]
- 25.Hambleton RK, Swaminathan H, Rogers HJ. Fundamentals of item response theory. Sage Publications; Newbury Park, CA: 1991. [Google Scholar]
- 26.Mattis S. Dementia Rating Scale. Psychological Assessment Resources; Odessa, FL: 1988. [Google Scholar]
- 27.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 28.Diggle PJ, Liang K-Y, Zeger SL. Analysis of longitudinal data. Clarendon Press; Oxford: 1994. [Google Scholar]
- 29.Beckett L. Analysis of longitudinal data. In: Gorelick PB, Attes M, editors. Handbook of neuroepidemiology. Vol. 31. Marcel Dekker, Inc.; New York: 1994. p. 62. [Google Scholar]
- 30.Braak H, Braak E, Bohl J. Staging of Alzheimer-related cortical destruction. Eur Neurol. 1993;33:403–408. doi: 10.1159/000116984. [DOI] [PubMed] [Google Scholar]
- 31.Convit A, de Leon MH, Golomb J, et al. Hippocampal atrophy in early Alzheimer's disease: anatomic specificity and validation. Psychiatry Q. 1993;64:371–387. doi: 10.1007/BF01064929. [DOI] [PubMed] [Google Scholar]
- 32.Laasko MP, Soininen H, Partanen K, et al. MRI of the hippocampus in Alzheimer's disease: sensitivity, specificity, and analysis of incorrectly classified subjects. Neurobiol Aging. 1997;19:23–31. doi: 10.1016/s0197-4580(98)00006-2. [DOI] [PubMed] [Google Scholar]
- 33.Jack CR, Petersen RC, Xu Y, et al. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer's disease. Neurology. 1997;49:786–794. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]


