Abstract
From breathing to walking, rhythmic movements encompass physiological processes important across the entire animal kingdom. It is thought by many that the generation of rhythmic behavior is operated by a central pattern generator (CPG) and does not require peripheral sensory input. Sensory feedback is, however, required to modify or coordinate the motor activity in response to the circumstances of actual movement. In contrast to this notion, we report here that sensory input is necessary for the generation of Drosophila larval locomotion, a form of rhythmic behavior. Blockage of all peripheral sensory inputs resulted in cessation of larval crawling. By conditionally silencing various subsets of larval peripheral sensory neurons, we identified the multiple dendritic (MD) neurons as the neurons essential for the generation of rhythmic peristaltic locomotion. By recording the locomotive motor activities, we further demonstrate that removal of MD neuron input disrupted rhythmic motor firing pattern in a way that prolonged the stereotyped segmental motor firing duration and prevented the propagation of posterior to anterior segmental motor firing. These findings reveal that MD sensory neuron input is a necessary component in the neural circuitry that generates larval locomotion.
Keywords: rhythmic behavior, sensory feedback, locomotion generation, motor pattern, central pattern generator
Rhythmic behaviors comprise a cyclic, repetitive set of movements, such as locomotion, respiration, and mastication (1, 2). The prevailing model for the neural basis underlying rhythmic behavior is that all rhythmic movements are generated by specialized circuits within the CNS called central pattern generators (CPGs), which can operate without sensory inputs (3–5). Nevertheless, a functional motor program also requires sensory inputs reporting peripheral feedback due to the actual movements to produce the correct behavior (6, 7). This model draws support from reports of sensory feedback affecting the timing and magnitude of motor activity generated by CPGs and from studies based on surgical removal of afferent input (1, 4, 6, 7). Whereas there are electrophysiological demonstrations of the influence of sensory feedback on the output of CPGs, how interactions between sensory neurons in the peripheral and neurons in the CNS contribute to rhythmic behavior in an intact organism is not known. It is therefore important to ask whether sensory inputs are essential for rhythmic behavior and how sensory inputs contribute to rhythmic behavior.
Drosophila is ideal for investigations of neural circuits underlying rhythmic behavior due to its amenability to genetic manipulation. Larval crawling, used for travel across various types of terrain, is accomplished by repeated peristaltic wave-like strides (8) and involves molecules mediating neuronal signaling (9–12). Whereas one study suggests that embryonic assembly of CPG for larval locomotion does not require sensory inputs, the same study reports that interference with the sensory function during embryogenesis severely disrupts the actual patterns of locomotion (13). To explore how sensory inputs contribute to larval locomotion, we conducted a Gal4/upstream activating sequence (UAS)-based screen to identify components of the neural circuitry for larval locomotion, revealing that sensory neuron function is necessary for larval locomotion.
Results and Discussion
Drosophila Larval Sensory Neurons Are Necessary for Locomotion Behavior.
Drosophila larval crawling entails repeated, rhythmic, peristaltic contraction. During each peristaltic stride, muscle contractions are propagated from one end of the body to the other end, passing through all 11 segments one by one [supporting information (SI) Fig. 6 and Movie 1]. To identify sensory neurons important for larval locomotion, we crossed ≈1,000 Gal4 driver lines to UAS-shibirets (UAS-shits) (“ts” indicates temperature sensitive) to transiently reduce the synaptic function of various subsets of Gal4 expressing neurons at the third larval instar and looked for gross locomotion defects in peristalsis rhythmicity and speed. Because shits, a temperature-sensitive mutant form of dynamin, blocks synaptic vesicle recycling and disrupts synaptic transmission only at restrictive temperatures (>29°C) (14, 15), we could raise these larvae at 18°C and ensure normal development of the larval crawling circuitry. We then subjected third instar foraging larvae to restrictive temperature (37°C) for 15 min to silence neurons expressing shits and observed the locomotion behavior.
For the majority of the analyzed Gal4 lines, incubation at restrictive temperature resulted in enhanced locomotion speeds as observed in wild-type larvae (Fig. 1a); faster crawling and increased activity might be a natural avoidance response to high temperatures (16, 17). We isolated 130 Gal4 lines that give rise to different types of defects in larval locomotion (see Methods). Among those 130 lines, UAS-shits expression driven by 10 Gal4 lines resulted in drastically disrupted peristaltic rhythm and reduced locomotion speed. Using these 10 Gal4 lines to drive the expression of UAS–mCD8–GFP to identify the neurons that were silenced in the larvae with defective rhythmic locomotion (18), we found that all 10 Gal4 lines showed expression in some or all peripheral sensory neurons. In particular, the 5-40-Gal4 line drives gene expression in all larval peripheral sensory neurons, but not in CNS neurons (Fig. 2). This Gal4 line (SN-Gal4) allowed us to study the role of peripheral sensory neurons in larval locomotion.
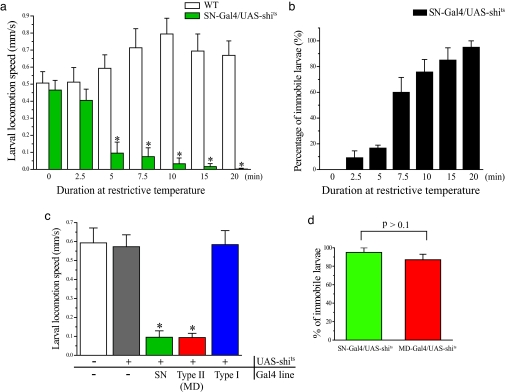
Fig. 1.
MD sensory neurons' function is essential for larval crawling. (a) Locomotion speed of SN-Gal4/UAS-shits larvae decreased with longer exposure to restrictive temperature (37°C) until they ceased crawling. n = 20 per column. (b) Percentage of SN-Gal4/UAS-shits larvae immobilized after different incubation durations at restrictive temperature, reaching ≈100% in 20 min. n = 20 per column. No wild-type larvae were found to be immobile after the same incubation treatment. (c) Larval locomotion speed after inactivation of different types of sensory neurons. All experiments were done after a 10-min exposure to restrictive temperature (37°C). n = 15 per column. ∗, P < 0.001 versus wild-type. (d) Percentage of larvae immobilized after 20 min at restrictive temperature. n = 15 per column. All error bars indicate SE.
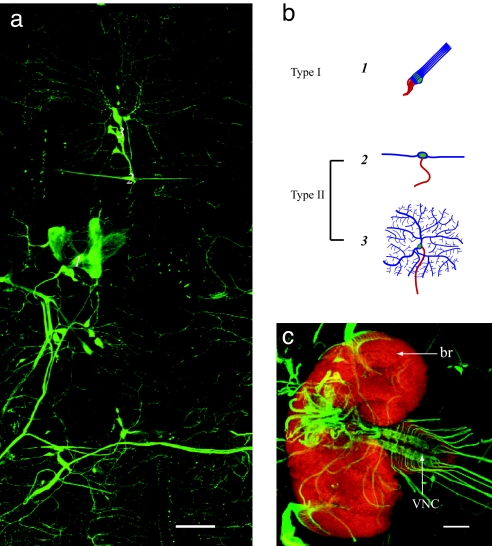
Fig. 2.
SN-Gal4 drives mCD8::GFP expression specifically in peripheral nervous system sensory neuron. (a) SN-Gal4 drives mCD8::GFP expression in all sensory neurons within one hemisegment of the larval body wall. (b) Schematized drawing of different types of sensory neurons. (c) Axonal projection of all sensory neurons (green) in larval ventral nerve cord (VNC) and brain (br) (red). Neuronal staining by anti-elav antibody was at a 1:50 dilution. (Scales bar: 100 μm.)
First, we subjected SN-Gal4/UAS-shits larvae to restrictive temperature (37°C) for different durations to examine more closely the impact of reducing sensory inputs as a consequence of impaired synaptic vesicle recycling in sensory neurons. Whereas wild-type larvae showed either no change or an acceleration of crawling with increases in incubation duration, SN-Gal4/UAS-shits larvae exhibited a progressive decrease in linear locomotion speed and became immobilized after 20 min at 37°C (Fig. 1a and SI Movies 2 and 3). The percentage of immobilized SN-Gal4/UAS-shits larvae also grew with prolonged exposure to 37°C, reaching 100% after 20 min (Fig. 1b), similar to our observations of late embryo and first instar larval locomotion (data not shown). The effect of sensory deprivation at restrictive temperature is reversible; shifting larvae from 37°C to 18°C restored crawling behavior (data not shown). Whereas sensory neuron function was essential for the rhythmic crawling, larvae immobilized by exposure to 37°C still exhibited movements of the mouth hook (SI Movie 3), indicating that some other type of movement remains. To corroborate these results, we used Gal80ts/UAS-inwardly rectifying K+ channel (Kir) as an alternative means to silence all sensory neurons conditionally at third larval instar by acutely overexpressing Kir so that the neurons would be much less excitable (19). We found that larvae carrying both SN-Gal4 and Gal80ts/UAS-Kir became immobilized after exposure to restrictive temperature for 30 min (data not shown).
In a previous study, Suster and Bate (13) reported that they used a tetanus toxin (TNT) transgene to inactivate all peripheral sensory neurons throughout development without abolishing the ability of the CPG to generate peristaltic waves in the embryo, albeit the waves were abnormal. How might this study be reconciled with our results? One possibility is that the Gal4 drivers used in these two studies have different strengths. Another possibility might be that homeostatic processes could make CPGs sensitive to acute withdrawal of a sensory input in our study but resistant to chronic withdrawal in the study of Suster and Bate (13, 20). We therefore did a side-by-side comparison of the driver (PO163-Gal4) used by Suster and Bate and our driver (SN-Gal4). Compared with GFP expression driven by SN-Gal4, GFP expression driven by PO-163-Gal4 was weaker and further diminished during development. Moreover, TNT or Kir expression by PO-163-Gal4 allowed larvae to crawl, albeit abnormally, and to survive to the third instar, similar to the results reported by Suster and Bate. In contrast, TNT or Kir expression driven by SN-Gal4 rendered the embryos immobilized and unable to hatch (n = 28). It thus seems likely that the PO-163-Gal4 driver is not sufficiently strong to produce enough TNT light chain in the sensory neurons to completely inactivate all of the sensory neurons, hence producing the weaker effect (13).
Larval Locomotion Requires Multiple Dendritic (MD) Sensory Neurons.
The Drosophila larval peripheral nervous system is composed of segmentally repeated sensory neurons, which are further divided into type I and type II neurons (21). Type I neurons have ciliated monopolar dendrites, whereas wild type II neurons (also known as MD neurons) have MD projections (Fig. 2b). To determine which sensory neurons are necessary for rhythmic crawling behavior, we selectively expressed shits in either or both subtypes of peripheral sensory neurons by using Gal4 lines, including dcirl-Gal4/UAS-shits (Type I-Gal4/UAS-shits) for shits expression in type I sensory neurons (chordotonal and external sensory organs) and 21-7-Gal4/UAS-shits (MD-Gal4/UAS-shits) for shits expression only in type II or MD neurons. Whereas depriving inputs from type I sensory neurons had no obvious effect on crawling, reducing pan-MD or pan-sensory input drastically decreased the crawling speed (0.094 ± 0.022 mm/s, and 0.095 ± 0.033 mm/s, respectively), about six times slower than control values (Fig. 1c and SI Movie 4). After 20 min at restrictive temperature, MD-Gal4/UAS-shits larvae were immobile (Fig. 1d), implicating MD neurons as the type of sensory neurons that relay sensory inputs essential for generating larval locomotion. No locomotion defects were observed when all those Gal4 lines larvae were exposed to restrictive temperature (data not shown).
Sensory Inputs from MD Neurons Is Required for Normal Peristaltic Contraction Duration.
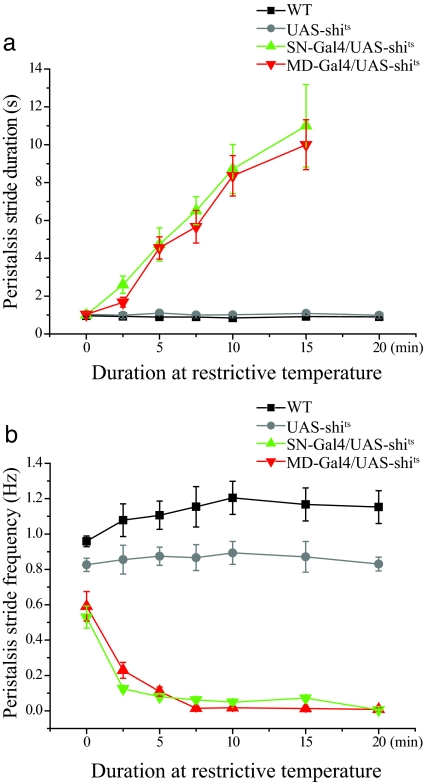
How might impaired MD neuron function have reduced the speed of larval crawling? Larvae move forward by making rhythmic peristaltic contractions. A reduction of speed could arise from either a delay in finishing each peristaltic wave, resulting in prolonged stride duration and reduced frequency, or a reduction in stride length. To better understand how MD neurons may regulate crawling, we used high-magnification video recordings to analyze the phenotype of larvae with partially inactivated sensory neurons (those that had been exposed to the restrictive temperature for durations <20 min). We focused on studying the peristaltic movement during each contraction stride and assessed the peristalsis wave duration and stride length (Fig. 3a and data not shown). Over time, at 37°C, the duration of each peristalsis wave of motion remained ≈1 sec for wild type larvae. In contrast, the peristalsis wave duration increased progressively as MD-Gal4/UAS-shits and SN-Gal4/UAS-shits larvae remained at 37°C, and the muscle contractions became more arrhythmic and sustained for longer periods of time (SI Movies 2 and 4). There was a corresponding decrease in peristalsis wave frequency (Fig. 3b) but no significant reduction in stride length (data not shown). Thus, reduction of MD sensory neuron function results in a dramatic delay for each peristalsis wave and eventually a complete arrest of peristalsis activity, implying that the circuitry that generates larval locomotion depends on MD neuron inputs.
Fig. 3.
MD neuron function is required for the completion of locomotive peristalsis. (a) Peristalsis duration increased with the duration of exposing MD-Gal4/UAS-shits larvae to 37°C. Peristalsis duration is not shown for the 20-min time point, because the larvae are immobile by then. n = 20 per symbol. (b) Peristalsis frequency decreased as the MDGal4/UAS-shits larvae were exposed to 37°C. n = 20 per symbol.
MD Neuron Function Is Required for the Formation of Rhythmic Locomotive Motor Pattern.
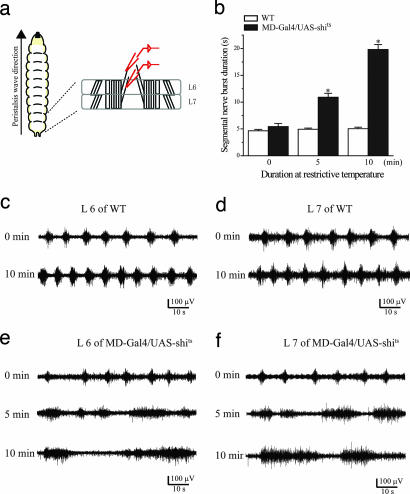
The larval peristaltic crawling behavior is accompanied by rhythmic firing of motoneuron axons in the segmental nerves, which exhibit intersegmental coordination of neuronal activity as revealed by an en passant recording from larval segmental nerves in semiintact larvae displaying rhythmic peristaltic contraction (22) (Fig. 4a). In wild-type larvae with smooth and coordinated peristaltic muscle contraction, the concurrent rhythmic motor firing exhibited stereotyped burst duration and interburst interval (Fig. 4 c and d). With increasing exposure to 37°C, burst frequency increased, whereas burst duration remained unchanged (Fig. 4 c and d). When MD-Gal4/UAS-shits larvae were brought to 37°C, however, there was progressively more arrhythmic and irregular motor neuron firing: The burst duration became prolonged and irregular with longer exposure to the restrictive temperature (Fig. 4 e and f), accounting for the dramatic delay in peristalsis stride duration observed in intact animals (Fig. 3a). These results clearly indicate that neural circuits within the larval CNS cannot maintain a basic fictive firing pattern without sensory feedback involving the MD neuron inputs.
Fig. 4.
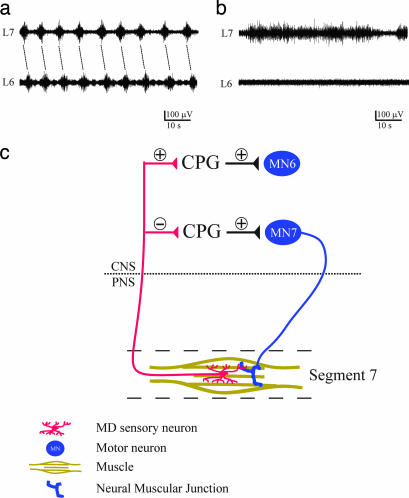
MD sensory neuron input is crucial for the stereotyped firing duration of segmental nerves. (a) Diagram of segmental nerve and body wall muscles for an en passant nerve recording from two consecutive segments (L6 and L7). (b) Segmental nerve burst duration increased with prolonged inactivation of sensory neuron (n = 10). (c and d) Wild-type larvae retained rhythmic motor firing at 37°C in both segment L6 (c) and L7 (d). (e and f) Prolonged burst duration in MDGal4/UAS-shits at 37°C in both segment L6 (e) and L7 (f). All error bars indicate SE. ∗, P < 0.001 versus wild type.
Larval locomotion is a multisegmented movement that requires intersegmental coordination of muscle contraction, just as the walking of human beings requires coordination among limb muscles and body muscles (23). In wild-type larvae, the posterior-to-anterior rhythmic peristaltic contraction is associated with a transition of motor bursting from one to the next segment (22). This transition was still well maintained after 15 min at 37°C (Fig. 5a). However, in MD-Gal4/UAS-shits larvae after 15 min at the restrictive temperature, this phase transition is lost: The burst duration in the most posterior (L7) segment was prolonged, and, during this time, the immediately anterior (L6) segment remained silent (Fig. 5b). Accordingly, we observed that the L7 muscle kept on contracting, and the contraction could not be propagated from L7 to L6. The failure of the propagation of segmental nerve firing was observed during anterior-to-posterior propagation as well (data not shown). Consistent with the finding that the bursting pattern from isolated ventral nerve ganglia was arrhythmic and uncoordinated among segments (22), unlike the rhythmic firing detected by an en passant recording of segmented nerves with preserved sensory inputs (Fig. 5a), this result explains how a lack of sensory inputs finally abolished rhythmic peristaltic contraction.
Fig. 5.
Blocking MD sensory neuron input prevents propagation of segmental nerve firing from L7 to L6. (a) Coordinated phase shift of segmental nerve firing in wild-type larvae after 15 min at 37°C. (b) Firing of L7, but not L6, segmental nerves in MDGal4/UAS-shits larvae after 15 min at 37°C. (c) Hypothetical model for the role of MD neuron in generating the intersegmentally coordinated motor neuron (MN) firing for forward peristaltic locomotion. In the larval CNS, sensory inputs from L7 segment MD neurons is hypothesized to restrict the firing duration of motor neurons in the same segment (MN7) by inhibiting the CPG circuit activity and to activate motor neurons in the preceding segment (MN6) by exciting the CPG circuit activity in a process that likely involves the CPG but critically depends on MD sensory neuron activity.
By using genetic manipulations in an intact animal, this study demonstrates that the generation of larval locomotion requires sensory inputs. Using 5-40-Gal4, a pan sensory neuron driver, to block all larval sensory inputs, we show that sensory inputs are essential for larval locomotion behavior. Similar results obtained with 21-7-Gal4, which is active in all MD neurons, implicate MD neurons as the type of sensory neurons that are required for peristaltic locomotion. Moreover, by recording the motor firing pattern underlying locomotive peristalsis, we found that reduction of MD neuron function drastically prolonged motor burst duration and eliminated the propagation of motor burst from one segmental nerve to the next. These electrophysiology data suggest that MD neuron activities are required for terminating the motor firing in one segment and initiating another motor burst in the next segment to produce the larval locomotion, probably through interaction between MD neurons and CPG circuits (see the model in Fig. 5c).
By using both approaches for acute and chronic withdrawal of sensory inputs, we have shown that a block of sensory inputs leads to the arrest of Drosophila larval crawling. To reconcile our findings with a previous study in which locomotion persisted despite the overexpression of TNT light chain by another Drosophila pan-sensory Gal4, PO163-Gal4, notwithstanding a reduction in peristalsis contraction frequency in embryo and crawling speed in first instar larvae (13), we compared PO163-Gal4 with the two Gal4 drivers used in this study, SN-Gal4 and MD-Gal4, and found that the expression of UAS-TNT or UAS-Kir driven by PO163-Gal4 but not SN-Gal4 or MD-Gal4 yielded mobile third instar larvae. It thus appears that PO163-Gal4 does not produce enough TNT light chain in the sensory neurons to completely block sensory inputs. Another method used by Suster and Bate to remove sensory input involves a senseless mutant. However, they pointed out that a significant number of MD-type sensory neurons persisted in the senseless mutant (13). Therefore, this experiment suffers from the same caveat, i.e., the failure to completely remove sensory inputs. The importance of the sensory input in the generation of larval locomotion may not be fully appreciated in studies involving incomplete removal of sensory inputs.
Historically, there have been two main hypotheses about the neural mechanisms underlying rhythmic behaviors: peripheral control and central control (1). The first hypothesis emphasizes that rhythmic patterns are achieved through the use of sensory inputs from the moving parts of the body and that loss of the normal sensory inputs disrupts the behavior. The alternative central control theory holds that CPGs within the CNS are intrinsically capable of providing the proper timing of muscle activation without sensory inputs. It seems likely that both central and sensory contributions are important for the production of normal rhythmic behavior (4, 23), but it is generally assumed that CPG outputs generate the basic motor oscillation, the core rhythm, whereas sensory feedback exerts a modulating or coordinating role (13). This conclusion mainly relies on two arguments: (i) There is no unequivocal experimental data to support the hypothesis that peripheral sensory feedback is necessary for the generation of properly timed rhythmic motor outputs. (ii) Isolated, deafferented nervous systems are still able to generate properly timed rhythmic output in the absence of peripheral feedbacks. However, both of those arguments have their limitations when considering whole-animal behavior in an intact organism.
Because the role of sensory inputs in rhythmic behavior has often been assessed based on surgical operations to remove sensory inputs, it raises such concerns about whether all sensory inputs are blocked or whether motor outputs remain intact after surgical operation (1), which might be the reason why different laboratories achieved different results with the same protocols (24–27). It is also difficult to study animal behavior following the surgical operation. By using a genetics approach in an intact animal, we can overcome this uncertainty and show that sensory inputs are indispensable in generating rhythmic behavior.
The CPG was originally defined as a neural circuit that can produce a repetitive, rhythmic neural output (1, 28, 29) (this is the definition we used in this study), but it has been widely used to refer to a neural circuit that is capable of generating a rhythmic behavior. In reality, the majority of rhythmic behaviors are multisegmented movements, such as walking, typing, and in a simpler organism, larval crawling, all of which require a well coordinated spatiotemporal sequence of muscle activations in different body segments. For example, walking is based on the coordinated movement sequences of all muscles on the legs, and it has been proposed to be comprised of multiple CPGs, each of which controls a single muscle of the leg (6). Therefore, for multisegmented behavior, coordinating the temporal sequence of individual CPGs should be necessary for the generation of rhythmic movements.
Our findings identify an essential role for MD neurons of the Drosophila larval peripheral nervous system in rhythmic locomotion. Whereas the physiological function of MD neurons is poorly understood, it is clear that MD neurons spread their dendrites along the epidermis and tile the internal epithelial surface of the entire larval body wall (30). A previous study has demonstrated that the MD neuron counterparts in Manduca larvae respond to the depression and stretch of the body wall (31). In adult tsetse flies, the ventral cluster of MD neurons is sensitive to body wall distention during blood feeding (32). Similar to Drosophila larvae, the leech also contains segmentally repeated MD neurons innervating its body wall. Interestingly, these neurons hyperpolarize in response to body wall stretching and depolarize upon the release of body wall stretching (33). The implication that MD neurons in these animals serve a proprioceptive function to sense the stretch of the animal body wall is consistent with our hypothesis that MD neuron in Drosophila larvae could sense the contraction and the release of the contraction of each segment, and this information is subsequently sent back to the CNS and used to construct a chain of reflex action that underlies peristaltic locomotion.
In vertebrates, human patients who lose somesthetic afferent input as a result of acquired large-fiber sensory neuropathy are consequently unable to control their movement and posture (34, 35). However, it is still unclear how loss of sensory inputs deprive movement ability. Here, by using a genetic approach to silence sensory neurons in intact animals, we created a similar dysfunctional movement phenotype in Drosophila larvae. This and similar combinations of behavior, electrophysiology, and genetic approaches should make it feasible to better decipher the role of sensory feedback in the generation of rhythmic behavior.
Materials and Methods
Fly Stocks.
Flies were kept on standard media at 25°C. Gal4 drivers used for screening were provided by U. Heberlein (University of California, San Francisco). The UAS-shits flies were acquired from T. Kitamoto (University of Iowa, Iowa City, IA) (14). 21-7-Gal4 was provided by H. Lee and Y. Xiang (both at the University of California, San Francisco). At third instar, 21-7-Gal4 drives gene expression exclusively in all MD sensory neurons. dcirl-Gal4 was generated by cloning 1–1,018 base pairs upstream of the dcirl translational start into pPTGAL vector. At the third instar, dcirl-Gal4 drives gene expression in all type I sensory neurons and in some interneurons in the ventral nerve cord.
Larval Locomotion Analysis.
Third-instar foraging stage larvae were plated on grape agar to allow larvae to crawl out of food medium. Clean larvae were then selected and placed on 2% agar plates. These larvae were placed in a 37°C incubator for a given time period (VWR Scientific, West Chester, PA). Their locomotion behavior was subsequently videotaped under high-magnification by using a digital camcorder (3CCD Handycam; Sony, Tokyo, Japan) attached to a microscope (MZ FLIII; Leica, Deerfield, IL). During recording, the restrictive temperature was maintained for heat-shocked larvae by placing the agar plates on a 37°C heat block (Sybron, Portsmouth, NH). Larvae were videotaped for up to 60 sec at one frame per 0.033 sec.
Locomotion was analyzed by using two methods. First, movies were analyzed by using Digital Imaging Analysis Software (DIAS) (Solltech, Oakdale, IA) as described in ref. 8. For each frame, the position of the larval centroid was determined. Centroid paths were generated by plotting the movement of the larvae over the duration of the film. “Speed” was computed automatically. Second, “peristalsis duration” and “peristalsis frequency” were analyzed manually from QuickTime files. Peristalsis duration was defined as the time required for the propagation of muscle contractions to travel from the posterior to the anterior segments.
SN-Gal4;Gal80ts/UAS-Kir larvae were also raised at 18°C until third instar to prevent the expression of Kir. To turn on the expression of Kir, these larvae were exposed to restrictive temperature (37°C) for 30 min to inactivate the inhibitory function of Gal80 on Gal4.
Gal4 Screen.
We observed a variety of different crawling defects after blocking synaptic transmission within different sets of neurons, including paralysis (53 lines), locomotion orientation change (30 lines), peristalsis length and locomotion speed change (37 lines), and peristalsis rhythmicity and locomotion speed change (10 lines). To understand the mechanisms underlying peristalsis locomotion, we only focused on studying those Gal4 lines that generated both peristalsis rhythmicity and locomotion speed change.
Electrophysiology.
Third-instar foraging larvae were dissected in Ca2+-free haemolymph-like (HL3) solution. The larva was dissected through the dorsal midline, and the CNS, brain lobes, ventral ganglia, and segmental nerves were left intact (22). Most of the dissected larvae presented spontaneous locomotion ability, characterized by muscle contraction waves that were propagated forward or backward. For recordings, the preparation was continuously superfused with oxygenated HL3 solution containing 1.5 mM Ca2+. An extracellular recording from segmental nerves was recorded by using a suction electrode with a diameter of 7–10 μm (36). Voltage signals were amplified by an Axoclamp 200B (Axon Instruments, Foster City, CA) with bandwidth filters set at 0.1 and 10 kHz and digitized directly to a disk with DigiData 1200.
Statistical Analysis.
All data were analyzed by one-way ANOVA by using Origin 7.0 (OriginLab, Northampton, MA).
Supplementary Material
Acknowledgments
We thank U. Heberlein for making the 1,000 Gal4 lines available to us; T. Kitamoto, H. H. Lee, and Y. Xiang for providing fly stocks; L. E. Fox for advice about extracellular recording of segmental nerve, S. B. Yang for technical help; and members of the Y.N.J. Laboratory for discussion. This work is supported by National Institutes of Health Grant R01NS40929 (to Y.N.J.). Y.N.J. and L.Y.J. are investigators of the Howard Hughes Medical Institute.
Abbreviations
- CPG
central pattern generator
- Kir
inwardly rectifying K+ channel
- MD
multiple dendritic
- shits
shibirets
- TNT
tetanus toxin
- ts
temperature sensitive
- UAS
upstream activating sequence.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0700895104/DC1.
References
- 1.Delcomyn F. Science. 1980;210:492–498. doi: 10.1126/science.7423199. [DOI] [PubMed] [Google Scholar]
- 2.Nusbaum MP, Beenhakker MP. Nature. 2002;417:343–350. doi: 10.1038/417343a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearson KG. Curr Opin Neurobiol. 1995;5:786–791. doi: 10.1016/0959-4388(95)80107-3. [DOI] [PubMed] [Google Scholar]
- 4.Marder E, Bucher D. Curr Biol. 2001;11:R986–96. doi: 10.1016/s0960-9822(01)00581-4. [DOI] [PubMed] [Google Scholar]
- 5.Grillner S. Neuron. 2006;52:751–766. doi: 10.1016/j.neuron.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Buschges A. J Neurophysiol. 2005;93:1127–1135. doi: 10.1152/jn.00615.2004. [DOI] [PubMed] [Google Scholar]
- 7.Rossignol S, Dubuc R, Gossard JP. Physiol Rev. 2006;86:89–154. doi: 10.1152/physrev.00028.2005. [DOI] [PubMed] [Google Scholar]
- 8.Wang JW, Sylwester AW, Reed D, Wu DA, Soll DR, Wu CF. J Neurogenet. 1997;11:231–254. doi: 10.3109/01677069709115098. [DOI] [PubMed] [Google Scholar]
- 9.Osborne KA, Robichon A, Burgess E, Butland S, Shaw RA, Coulthard A, Pereira HS, Greenspan RJ, Sokolowski MB. Science. 1997;277:834–836. doi: 10.1126/science.277.5327.834. [DOI] [PubMed] [Google Scholar]
- 10.Budnik V, Zhong Y, Wu CF. J Neurosci. 1990;10:3754–3768. doi: 10.1523/JNEUROSCI.10-11-03754.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cattaert D, Birman S. J Neurobiol. 2001;48:58–73. doi: 10.1002/neu.1042. [DOI] [PubMed] [Google Scholar]
- 12.Wang JW, Soll DR, Wu CF. J Neurogenet. 2002;16:45–63. doi: 10.1080/01677060213106. [DOI] [PubMed] [Google Scholar]
- 13.Suster ML, Bate M. Nature. 2002;416:174–178. doi: 10.1038/416174a. [DOI] [PubMed] [Google Scholar]
- 14.Kitamoto T. J Neurobiol. 2001;47:81–92. doi: 10.1002/neu.1018. [DOI] [PubMed] [Google Scholar]
- 15.Manoli DS, Foss M, Villella A, Taylor BJ, Hall JC, Baker BS. Nature. 2005;436:395–400. doi: 10.1038/nature03859. [DOI] [PubMed] [Google Scholar]
- 16.Rosenzweig M, Brennan KM, Tayler TD, Phelps PO, Patapoutian A, Garrity PA. Genes Dev. 2005;19:419–424. doi: 10.1101/gad.1278205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klose MK, Chu D, Xiao C, Seroude L, Robertson RM. J Neurophysiol. 2005;94:3563–3572. doi: 10.1152/jn.00723.2005. [DOI] [PubMed] [Google Scholar]
- 18.Lee T, Luo L. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- 19.Liu G, Seiler H, Wen A, Zars T, Ito K, Wolf R, Heisenberg M, Liu L. Nature. 2006;439:551–556. doi: 10.1038/nature04381. [DOI] [PubMed] [Google Scholar]
- 20.Nargeot R. J Neurosci. 2003;23:4803–4808. doi: 10.1523/JNEUROSCI.23-12-04803.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bodmer R, Jan YN. Roux's Arch Dev Biol. 1987;196:69–77. doi: 10.1007/BF00402027. [DOI] [PubMed] [Google Scholar]
- 22.Fox LE, Soll DR, Wu CF. J Neurosci. 2006;26:1486–1498. doi: 10.1523/JNEUROSCI.4749-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dickinson MH, Farley CT, Full RJ, Koehl MA, Kram R, Lehman S. Science. 2000;288:100–106. doi: 10.1126/science.288.5463.100. [DOI] [PubMed] [Google Scholar]
- 24.Gray J, Lissmann HW, Pumphery RJ. J Exp Biol. 1938;15:408–430. [Google Scholar]
- 25.Gray J, Lissmann HW. J Exp Biol. 1938;15:506–517. [Google Scholar]
- 26.Kristan WB, Jr, Calabrese RL. J Exp Biol. 1976;65:643–668. doi: 10.1242/jeb.65.3.643. [DOI] [PubMed] [Google Scholar]
- 27.Stent GS, Kristan WB, Jr, Friesen WO, Ort CA, Poon M, Calabrese RL. Science. 1978;200:1348–1357. doi: 10.1126/science.663615. [DOI] [PubMed] [Google Scholar]
- 28.Bullock TH. Behaviour. 1961;17:48–59. [Google Scholar]
- 29.Holst EV. Experientia. 1948;4:374–381. doi: 10.1007/BF02145189. [DOI] [PubMed] [Google Scholar]
- 30.Grueber WB, Ye B, Moore AW, Jan LY, Jan YN. Curr Biol. 2003;13:618–626. doi: 10.1016/s0960-9822(03)00207-0. [DOI] [PubMed] [Google Scholar]
- 31.Grueber WB, Graubard K, Truman JW. J Comp Neurol. 2001;440:271–283. doi: 10.1002/cne.1385. [DOI] [PubMed] [Google Scholar]
- 32.Anderson M, Finlayson LH. Physiol Entomol. 1978;3:157–167. [Google Scholar]
- 33.Blackshaw SE, Thompson SW. J Physiol. 1988;396:121–137. doi: 10.1113/jphysiol.1988.sp016954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanes JN, Mauritz KH, Evarts EV, Dalakas MC, Chu A. Proc Natl Acad Sci USA. 1984;81:979–982. doi: 10.1073/pnas.81.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smetacek V, Mechsner F. Nature. 2004;432:21. doi: 10.1038/432021a. [DOI] [PubMed] [Google Scholar]
- 36.Wu CF, Ganetzky B, Jan LY, Jan YN, Benzer S. Proc Natl Acad Sci USA. 1978;75:4047–4051. doi: 10.1073/pnas.75.8.4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.