Abstract
Increased-branching mutants of garden pea (Pisum sativum; ramosus [rms]) and Arabidopsis (Arabidopsis thaliana; more axillary branches) were used to investigate control of cytokinin export from roots in relation to shoot branching. In particular, we tested the hypothesis that regulation of xylem sap cytokinin is dependent on a long-distance feedback signal moving from shoot to root. With the exception of rms2, branching mutants from both species had greatly reduced amounts of the major cytokinins zeatin riboside, zeatin, and isopentenyl adenosine in xylem sap compared with wild-type plants. Reciprocally grafted mutant and wild-type Arabidopsis plants gave similar results to those observed previously in pea, with xylem sap cytokinin down-regulated in all graft combinations possessing branched shoots, regardless of root genotype. This long-distance feedback mechanism thus appears to be conserved between pea and Arabidopsis. Experiments with grafted pea plants bearing two shoots of the same or different genotype revealed that regulation of root cytokinin export is probably mediated by an inhibitory signal. Moreover, the signaling mechanism appears independent of the number of growing axillary shoots because a suppressed axillary meristem mutation that prevents axillary meristem development at most nodes did not abolish long-distance regulation of root cytokinin export in rms4 plants. Based on double mutant and grafting experiments, we conclude that RMS2 is essential for long-distance feedback regulation of cytokinin export from roots. Finally, the startling disconnection between cytokinin content of xylem sap and shoot tissues of various rms mutants indicates that shoots possess powerful homeostatic mechanisms for regulation of cytokinin levels.
Shoot branching is one of the most important determinants of plant architecture and is highly responsive to environmental and endogenous cues. Long-distance signaling is essential for the regulation of axillary shoot branching as it enables coordinated development of distant meristems (for review, see Beveridge, 2006; Dun et al., 2006). Cytokinin, a mobile plant hormone, can influence shoot branching but its precise role is unclear. In several species, direct application of cytokinin to axillary buds promotes outgrowth (e.g. Sachs and Thimann, 1964) and endogenous cytokinin levels have been found to rise in and around axillary buds during growth initiation (Li et al., 1995; Turnbull et al., 1997; Emery et al., 1998). In addition, transgenic plants expressing the bacterial isopentenyl transferase (ipt) gene, which catalyzes cytokinin biosynthesis, exhibit elevated cytokinin levels often accompanied by an increased-branching phenotype (Faiss et al., 1997; Eklöf et al., 2000; Böhner and Gatz, 2001).
Roots have traditionally been considered a primary site of cytokinin biosynthesis, supplying the shoot with cytokinin via the xylem sap. However, evidence for cytokinin synthesis in shoots (e.g. Chen et al., 1985) is now unequivocal (Nordström et al., 2004). Nevertheless, studies by Bangerth (1994) with decapitated bean (Phaseolus vulgaris) plants led to the hypothesis that cytokinin in the xylem sap from roots (xylem sap cytokinin [X-CK]) plays an important role in regulating axillary shoot branching. Following decapitation, bud outgrowth in bean was accompanied by a rise in X-CK. Increases in bud outgrowth and X-CK could both be suppressed by replacement of the apex with exogenous indole-3-acetic acid (IAA). The expression of IPT cytokinin biosynthesis genes in the stem of pea (Pisum sativum) has also been shown to increase after decapitation (Tanaka et al., 2006), and this may be responsible for the rapid increases in stem and axillary bud cytokinin content (Li et al., 1995; Turnbull et al., 1997; Tanaka et al. 2006). Moreover, there is now convincing evidence at the molecular level that auxin can down-regulate cytokinin biosynthesis in shoots (Nordström et al., 2004) but up-regulates some IPT genes in roots (Miyawaki et al., 2004).
However, Faiss et al. (1997) demonstrated that grafting wild-type scions to cytokinin-overproducing ipt rootstocks failed to promote bud outgrowth in the wild-type shoot. In addition, transgenic tobacco (Nicotiana tabacum) plants globally expressing the bacterial ipt gene exhibited increased bud outgrowth at various nodes (Böhner and Gatz, 2001), but local repression of ipt expression in individual buds inhibited their outgrowth. These results indicate that axillary buds may rely on locally synthesized cytokinins for stimulation of branching rather than on cytokinin produced at a distance. Likewise, decapitated stems, whether from plants with roots attached or rootless nodal segments, show the same dynamics of rapid cytokinin increases (Tanaka et al., 2006). Taken together, there is now strong evidence that cytokinins produced in shoot tissues may be a more important influence on axillary branching than X-CK. Questions therefore remain about the role of X-CK in branching control (for review, see Schmülling, 2002) and about the possible wider functions of X-CK and its contribution to shoot cytokinin pools.
The ramosus (rms) increased-branching mutants of pea (rms1–rms5) offer a powerful system in which to simultaneously investigate the role of cytokinin in branching control and the contribution of shoot and root to cytokinin homeostasis (for review, see Beveridge, 2006). Uniquely among reported mutants, X-CK in most rms genotypes is dramatically reduced, up to 40-fold, compared with wild-type plants (Beveridge et al., 1996, 1997b; Morris et al., 2001). Grafting studies indicate that the phenotype of rms mutant shoots depends on long-distance signals, one of whose synthesis requires RMS1 and RMS5 (Morris et al., 2001; Johnson et al., 2006). Physiological and molecular studies indicate that this signal, provisionally termed “shoot multiplication signal” (SMS), is produced in shoot and root, moves acropetally in shoots, and acts as an inhibitor of branching (Foo et al., 2001, 2005; Morris et al., 2001; Beveridge, 2006). SMS is required for auxin to inhibit branching after decapitation (Beveridge et al., 2000), an interaction that may in part be mediated by auxin regulation of RMS1 transcript levels (Foo et al., 2005). In Arabidopsis (Arabidopsis thaliana), this signal is also graft transmissible and is controlled by MORE AXILLARY BRANCHES4 (MAX4) and MAX3, the Arabidopsis orthologs of RMS1 and RMS5, respectively (Turnbull et al., 2002; Sorefan et al., 2003; Booker et al., 2004; Johnson et al., 2006). RMS1/MAX4 and RMS5/MAX3 proteins have homology to carotenoid cleavage enzymes (Sorefan et al., 2003; Booker et al., 2004), and are thus likely to generate as yet unidentified carotenoid derivatives (Schwartz et al., 2004).
The reduced X-CK in several rms mutants appears to be mediated by a second mobile signal that, in contrast to the upwardly mobile SMS, moves in the direction of shoot to root. A strong correlation between increased shoot branching and reduced X-CK is observed in reciprocal grafts between rms1, rms3, or rms4 and wild type, regardless of the genotype of the rootstock that supplies the X-CK. For example, branching is not suppressed in rms3 or rms4 scions grafted to wild-type rootstocks and X-CK is dramatically reduced in these plants, while cytokinin export from rms3 and rms4 rootstocks is normalized by grafting to unbranched wild-type scions (Beveridge et al., 1997a; Beveridge, 2000). This indicates that rms rootstocks are not intrinsically incapable of supplying normal levels of X-CK and that wild-type rootstocks, when connected to branching rms shoots, can exhibit rms-like behavior in terms of X-CK. Conversely, branching is suppressed in rms1 scions grafted to wild-type rootstocks (Beveridge et al., 1997b) and X-CK is not reduced in these grafts (Beveridge, 2000). A small reduction in X-CK is also observed when wild-type plants are induced to branch by cytokinin applied directly to axillary buds (Beveridge, 2000). These findings indicate that regulation of X-CK is dependent on the shoot branching phenotype and requires a long-distance signal that is modulated during the process of axillary bud outgrowth. RMS2 may play a role in the generation of this feedback signal because rms2 is the only rms mutant that does not show down-regulation of X-CK. RMS2 is required for the full suppression of X-CK in rms1 plants as X-CK levels remain elevated in rms1 rms2 double mutants (Beveridge et al., 1997b). This long-distance feedback signal may also regulate RMS1 expression, as RMS1 transcript levels are strongly elevated in rms3, rms4, and rms5 but not rms2 mutant plants (Foo et al., 2005).
As yet, there is scant evidence to suggest that the long-distance feedback signal that reduces X-CK in rms mutants is IAA. Low X-CK might be predicted in plants with high auxin levels or enhanced auxin response. However, shoot IAA level is highest in the rms2 mutant that lacks feedback regulation and is not consistently elevated in other rms mutants (Beveridge et al., 1996; Morris et al., 2001). There is also no evidence for an enhanced auxin response, which in any case would be unlikely to cause an increased-branching phenotype. Instead, response to applied auxin in rms mutants is greatly diminished in terms of inhibiting branching. In Arabidopsis, Bennett et al. (2006) have proposed that the increased-branching phenotype may be caused by the enhanced auxin transport capacity seen in max mutants, although it is not clear how this could affect X-CK (for review, see Dun et al., 2006).
In this study, the rms and max branching mutants were used to investigate the control of X-CK (mostly root derived) in relation to axillary shoot branching. Intact and grafted Arabidopsis max mutants provided an opportunity to test whether feedback regulation of X-CK is conserved across diverse species. Because of the uncertainty about relationships between X-CK and shoot tissue cytokinin, shoot cytokinin content was measured in various rms mutants that display widely differing X-CK levels. The role of the shoot apex and growing axillary shoots in establishing altered X-CK was examined through decapitation experiments and by using mutants that suppress axillary meristem development. Y-grafting studies were undertaken to determine whether the feedback signal is a positive or negative regulator of cytokinin export from the roots. Finally, the role of RMS2 in this feedback process was critically examined through double mutant grafting studies.
RESULTS
Conservation of Feedback in Arabidopsis
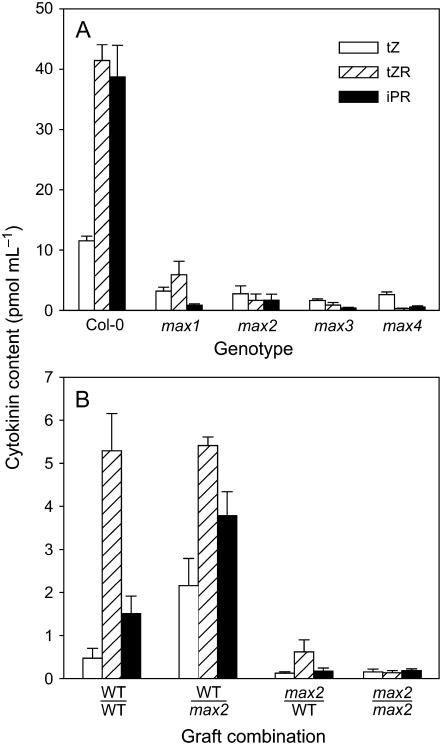
To test whether down-regulation of X-CK is conserved in species other than pea, xylem sap was collected from mature, short-day-grown Arabidopsis plants of Columbia-0 (Col-0; wild type) and branching mutants max1, max2, max3, and max4. Increased-branching phenotypes in max mutants, based on the number of rosette (secondary inflorescence) branches, were similar to those reported by Booker et al. (2005) (data not shown). Predominant X-CKs detected in wild-type xylem sap were trans-zeatin riboside (tZR), isopentenyl adenosine (iPR), and trans-zeatin (tZ; Fig. 1A), together with smaller amounts of cis-ZR (cZR) and isopentenyl adenine (iP; data not shown). The same range of compounds was present in all mutants, but the levels of most were significantly (P < 0.001) reduced. In the case of tZR and iPR, the mutant xylem sap contained between 6-fold less and 50-fold less cytokinin, with levels being almost undetectable in some samples.
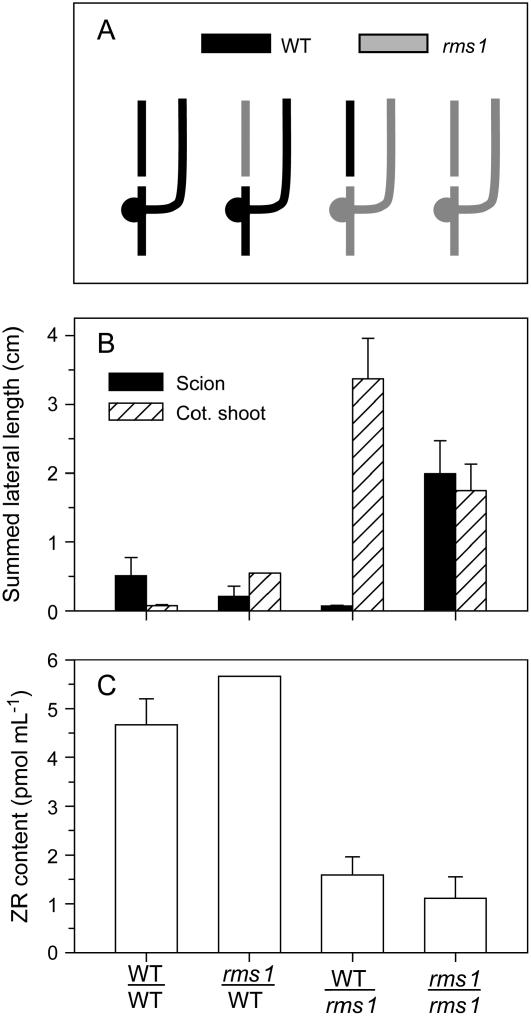
Figure 1.
X-CK content (tZ, tZR, iPR) of Arabidopsis max branching mutants. A, Comparison of wild-type (Col-0) with max1 to max4. Plants were grown under 8-h short days for 2.5 months. B, Reciprocal grafts of max2 with wild-type (WT) plants. Plants were grown under 8-h short days for 2 months. Similar trends were found in independent repeat experiments. Data are means ± se for analyses from individual plants; n = 6.
Based on grafting evidence that pea shoots generate a feedback signal that down-regulates X-CK, we examined X-CK from grafted Arabidopsis plants using reciprocal combinations of max2 and wild type. In these graft combinations, there is no graft-transmissible regulation of branching: max2 scions continue to branch when grafted to wild-type rootstocks, and wild-type scions do not show increased branching when grafted to max2 rootstocks (Booker et al., 2005; data not shown). X-CK was 4- to 8-fold lower in plants with max2 scions than in those with wild-type scions regardless of the rootstock genotype (Fig. 1B; significant at least to P < 0.02). The X-CK content in wild-type ungrafted controls grown alongside these grafted plants were not significantly different from wild-type self-grafts (data not shown).
Cytokinin Homeostasis in the Shoot
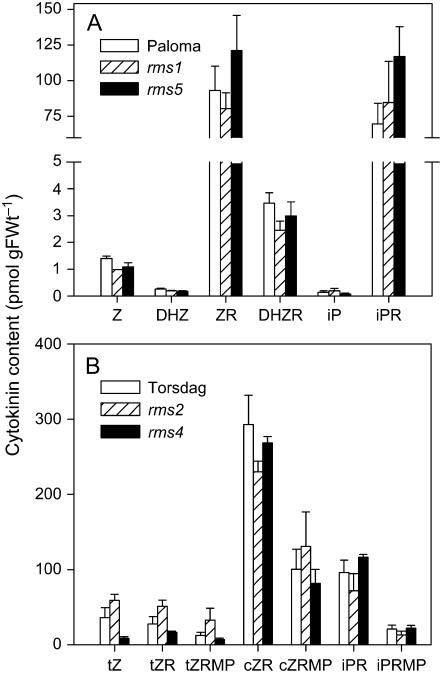
Because of the known substantial differences in X-CK among rms mutants (Beveridge et al., 1997a, 1997b; Beveridge, 2000; Morris et al., 2001), we examined whether there was similar variation in rms shoot cytokinins. Shoot tips of wild-type and rms mutant plants contained broadly similar levels of cytokinins (Fig. 2), as did more mature nodal tissues (data not shown). The predominant cytokinins were ZR and iPR, with these values also including hydrolyzed phosphates in Figure 2A. Smaller quantities of Z and dihydrozeatin riboside (DHZR) were also detected. Where samples were analyzed in more detail, significant amounts of cZR were detected, together with isopentenyl AMP, tZR 5′-monophosphate (tZRMP), and cis-ZRMP (cZRMP; Fig. 2B). Levels of these phosphorylated cytokinins followed similar trends to their ribosyl counterparts. iP was also detected in some samples but not in others, as were very low levels (<0.2 pmol g−1) of zeatin-9-glucoside (Z9G) and dihydrozeatin-9-glucoside (DHZ9G; data not shown). In some instances, a small variation was observed with genotype. For example, shoot tips of rms4-1 contained significantly lower levels of tZ-type compounds (P < 0.05), but not cZR and iPR types. In rms2-1, the only rms mutant with high rather than low X-CK (Beveridge et al., 1997b; Beveridge, 2000), shoot cytokinin levels were not significantly elevated (Fig. 2B). We found similar trends to those reported herein in an experiment with young wild-type and rms4 seedlings in which X-CK was sap collected at shoot transpiration rates (Dodd et al., 2004) and compared with shoot CK content (C. Ngo, I.C. Dodd, C.G.N. Turnbull, and C.A. Beveridge, unpublished data). Overall, there was very little evidence that increased levels of shoot cytokinins were associated with a branching phenotype or that low X-CK leads to low shoot cytokinin.
Figure 2.
Cytokinin content of shoot tissues of rms and wild-type plants. Cytokinin levels in shoot tips of 9-d-old cv Paloma (wild type), rms1-4, and rms5-2 plants (A) and cv Torsdag (wild type), rms2-1, and rms4-1 plants (B) are shown. Data are means ± se; n = 2 to 3 pools of 18 to 20 plants.
Effect of Decapitation and Axillary Shoots on X-CK in rms Mutants
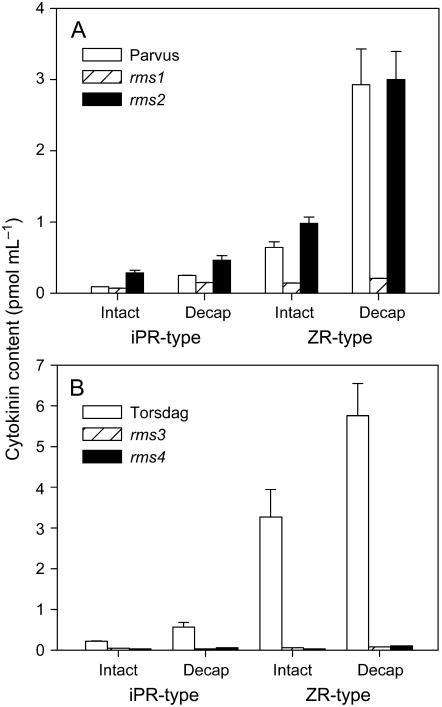
In wild-type plants, shoot tip removal leads to elevated X-CK, but this can be restored by exogenous IAA (Bangerth, 1994). This suggests that auxin from the shoot tip may act to suppress X-CK. Because rms mutations cause reduced X-CK via a long-distance signal, auxin is a potential candidate. If enhanced IAA supply from shoot to root were the primary cause of reduced X-CK in these mutants, then shoot decapitation would be predicted to affect X-CK. To test this, X-CK was monitored in rms plants after decapitation above the highest expanded leaf. As in previous experiments, intact rms1, rms3, and rms4 plants had a significantly reduced ZR-type cytokinin concentration (Fig. 3). Decapitation generally caused at least a doubling in X-CK in wild-type and rms2 plants over 24 h. These differences were significant (P < 0.05) except for the iPR-type X-CK in rms2 plants (Fig. 3A) and ZR-type X-CK in Torsdag plants (Fig. 3B). Small increases in X-CK occurred in rms1, rms3, and rms4 plants, but the levels always remained far below those observed in intact or decapitated wild-type plants (Fig. 3). Similar results were obtained in three independent experiments measuring X-CK at 12 or 24 h after decapitation.
Figure 3.
Effect of decapitation on X-CK concentration of cv Parvus (wild type), rms1-1, and rms2-2 plants (A) and cv Torsdag (wild type), rms3-2, and rms4-1 plants (B). Plants were intact or decapitated above the highest expanded leaf (A, nodes 8–10; B, nodes 16–18) of the main stem. Data are means ± se for analyses from two or three pools of 10 to 20 plants harvested 24 h after decapitation.
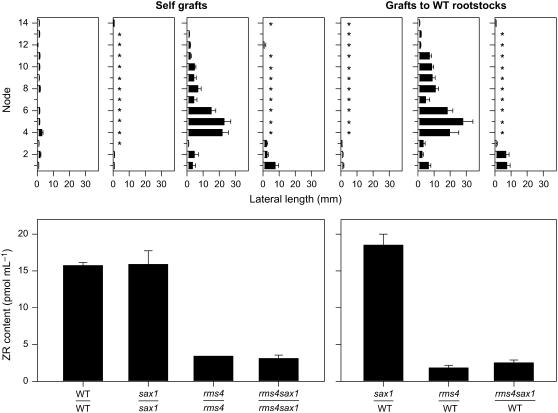
Grafting studies have highlighted that, regardless of shoot or root genotype, there is a correlation between increased shoot branching phenotypes and suppressed X-CK. This raises the possibility that shoots with growing lateral branches are a source of the proposed feedback signal. To test this, we used a suppressed axillary meristem (sax) mutant to suppress axillary branching in an rms background. Due to the absence of many axillary meristems, branching in the rms4-3 sax1-1 double mutant is dramatically reduced compared with rms4-3 plants (Rameau et al., 2002a). Grafting enabled us to determine if the ability of rms4 scions to down-regulate X-CK in wild-type rootstocks is blocked by the sax1 mutation. Xylem sap ZR content of sax1 self-grafts and sax1/wild-type (notation: scion/rootstock) plants, which both have reduced numbers of axillary buds, was similar to wild-type self-grafts (Fig. 4). As observed previously (Beveridge et al., 1996, 1997a), rms4 self-grafts and rms4/wild-type plants were both highly branched and displayed xylem sap ZR concentrations approximately 5- to 10- fold lower than wild-type self-grafts. The rms4 sax1 double mutant has significantly fewer axillary buds and growing branches than rms4 plants (Fig. 4; Rameau et al., 2002a). Despite this reduced branching, rms4 sax1 scions grafted to wild-type rootstocks were as effective as rms4 scions in suppressing cytokinin export from wild-type rootstocks: ZR export from rms4 sax1/wild-type roots was approximately 7-fold lower when compared with that from wild-type self-grafted plants (Fig. 4; P < 0.001). Similar results were obtained from comparisons of rms5 plants with and without basal branches (data not shown).
Figure 4.
Branching phenotype (top) and xylem sap ZR content (bottom) of 47-d-old cv Térèse (WT), sax1, rms4-3, and rms4-3 sax1 scions grafted to self or wild-type rootstocks. Top: * indicates the node was devoid of a lateral bud; n = 9 to 12. Bottom: n = 2 or 3 pools of 10 plants. Data are means ± se.
X-CK Levels in Y-Grafted Plants
By monitoring X-CK in Y-grafted plants that allow two shoots of different genotypes to grow on the same rootstock, we were able to consider whether feedback regulation of X-CK is due to inhibitory action from branching rms shoots or a stimulus from wild-type shoots. We chose rms1 and wild type because the wild-type/rms1 (notation: scion/rootstock plus cotyledonary shoot) combination results in plants with one branched rms1 cotyledonary shoot and one nonbranched wild-type scion connected to the same rms1 rootstock (Foo et al., 2001; Fig. 5A). In standard wild-type/rms1 grafts with a single nonbranched wild-type scion, the X-CK is similar to that of wild-type self-grafts (Beveridge, 2000). In contrast, the xylem sap ZR level in wild-type/rms1 Y-grafted plants, which have both a branched rms1 cotyledonary shoot and an unbranched wild-type shoot, was similar to rms1 self-grafts and approximately 2-fold lower than in wild-type self-grafts (Fig. 5B; P < 0.05). In rms1/wild-type Y-grafted plants, branching was suppressed in both rms1 and wild-type shoots (Foo et al., 2001; Fig. 5B), and the xylem sap ZR concentration was similar to wild-type self-graft levels (Fig. 5C) and comparable to standard single-shoot rms1/wild-type grafts (Beveridge, 2000). It therefore appears likely that branching rms shoots generate an inhibitory influence on X-CK that is effective even in the presence of a nonbranching wild-type shoot. Similar results were obtained with Y-grafts between rms5-3 and cv Torsdag (wild-type) plants (data not shown), consistent with RMS1 and RMS5 having a similar gene function (Morris et al., 2001).
Figure 5.
Influence of Y-grafting on shoot branching and sap ZR content of 40-d-old Y-grafted cv Weitor (WT) and rms1-2 plants. A to C, Graft configuration (A), branching phenotype (B), and xylem sap ZR content (C). For B, n = 6 to 12 (except rms1/wild type, where n = 2). For C, n = 3 pools of three to five plants (except rms1/wild type, where n = 1 pool of two plants). Graft combinations are shown as scion over rootstock, with the cotyledonary shoot having the same genotype as the rootstock. Data are means ± se.
The Role of RMS2 in Modulating Cytokinin Export from Roots
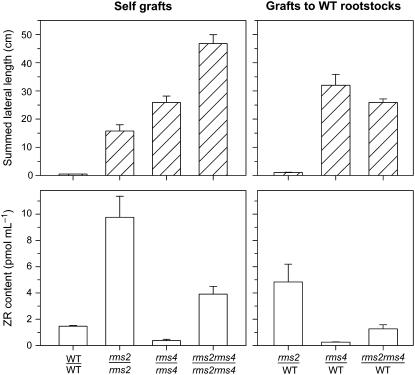
Previous studies demonstrated that, unlike other rms mutants, rms2 plants display elevated X-CK and that RMS2 is required for the suppression of X-CK in rms1 plants (Beveridge et al., 1997b). Here, we investigated rms2 rms4 and rms2 rms5 double mutant plants to determine whether RMS2 also affects the suppression of X-CK by rms4 and rms5 mutations. In contrast to the low X-CK observed in rms4 and rms5 plants, X-CK was elevated approximately 2- to 3- fold in rms2 rms4 and rms2 rms5 double mutants compared with wild-type controls (Fig. 6; data not shown). However, X-CK in double mutants was not as high as in rms2 plants. These double mutants showed an additive branching phenotype compared with the single mutants, based on summed lateral length (Fig. 6) and the number of primary and secondary branches (data not shown; Murfet and Symons, 2000b).
Figure 6.
Branching phenotype (top) and xylem sap ZR content (bottom) of 25-d-old reciprocally grafted cv Torsdag (WT), rms2-1, rms4-1, and rms2-1 rms4-1 plants. Top: n = 15 to 25. Bottom: n = 3 pools of four to eight plants. Data are means ± se.
To investigate the role of RMS2 in long-distance regulation of X-CK, rms2 rms4 double mutants, along with rms2 and rms4 single mutants, were grafted with wild-type plants. Consistent with previous reports, rms2/wild-type plants displayed massively reduced branching compared with rms2 self-grafts, while X-CK was intermediate between rms2 and wild-type self-grafted plants (Fig. 6; Beveridge, 2000). Unlike rms2, branching was not suppressed in rms4 shoots grafted to wild-type rootstocks and X-CK was reduced in these plants compared with wild-type self-grafts (Fig. 6; Beveridge et al., 1997a). Branching in rms2 rms4 double mutant scions grafted to wild-type rootstocks was slightly reduced compared with rms2 rms4 self-grafts and similar to that observed in rms4/wild-type plants. X-CK in rms2 rms4/wild-type grafts was similar to wild-type self-grafts, and intermediate between that of rms2/wild-type and rms4/wild-type plants. Therefore, the effect of the long-distance signal generated by rms4 scions is diminished without RMS2 function. These differences in branching and X-CK content were significant at least at the level of P < 0.01.
DISCUSSION
Feedback Regulation of X-CK Operates in Pea and Arabidopsis
We previously demonstrated that reduced X-CK in rms1, rms3, rms4, and rms5 plants is due to a shoot-derived long-distance feedback signal (Beveridge et al., 1997a; Beveridge, 2000; Morris et al., 2001; Fig. 7, no. 6). Here, we show that max branching mutants of Arabidopsis display the same phenomenon. All four max mutants examined showed substantially reduced X-CK (Fig. 1A), indicating this is due to disruption of a common regulatory mechanism related to control of shoot branching. MAX2 is the ortholog of RMS4 (Johnson et al., 2006) and, like rms3 and rms4 mutants of pea, branching is not suppressed in max2 shoots grafted to wild-type rootstocks (data not shown; Booker et al., 2005). Again, like rms3 and rms4 grafts, X-CK was significantly reduced in wild-type rootstocks grafted with max2 shoots and was restored in max2 rootstocks grafted with wild-type shoots (Fig. 1B). This indicates the presence of a long-distance signal in Arabidopsis that moves from shoot to root and causes down-regulation of X-CK. We conclude that feedback regulation of X-CK may be a generic process in plants and that the mechanism is intrinsically linked to regulation of shoot branching.
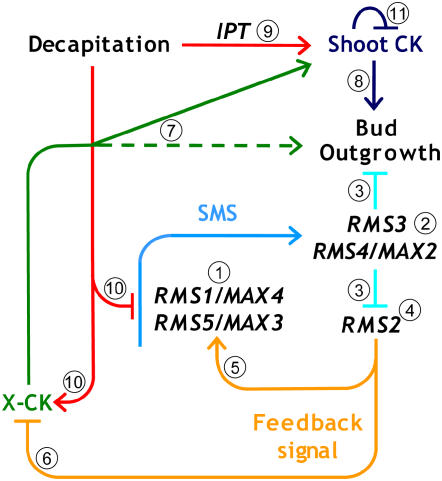
Figure 7.
Model of cytokinin regulation by the shoot branching network. Similar models are presented by Foo et al. (2005) and Johnson et al. (2006), and reviewed by Beveridge (2006) and Dun et al. (2006). New data presented here are incorporated in this model together with the findings of Tanaka et al. (2006). 1, Synthesis of a mobile branching inhibitor (SMS) in the rootstock and shoot is dependent on RMS1/MAX4 and RMS5/MAX3. 2, SMS perception/transduction is dependent on RMS4/MAX2; phenotypic grafting evidence shows that this F-box protein acts predominantly in the shoot and is required for SMS action. 3, Outputs of the RMS4/MAX2 signal transduction pathway include separate inhibition of bud outgrowth and repression of RMS2; suppression of bud formation or branching does not prevent activation of the feedback signal (Fig. 4). 4, RMS2 affects long-distance feedback and exhibits graft-transmissible action (Fig. 6). 5, The long-distance feedback signal activates SMS synthesis in shoot and roots; RMS1 and RMS5 expression is minimal in rms2 mutants. 6, The feedback signal also represses xylem cytokinin from the roots; all rms mutants except rms2 have low xylem cytokinin. 7, It is possible that X-CK directly stimulates bud outgrowth (Fig. 6). 8, Shoot cytokinin stimulates bud outgrowth; direct addition of cytokinin to buds generally causes increased growth. 9, Decapitation activates IPT and enhances local shoot cytokinin content. 10, Decapitation also reduces SMS by suppressing RMS1 and RMS5 gene expression and increases X-CK independently of RMS2 action (Fig. 3). 11, Total shoot cytokinin levels in intact plants are maintained by an unknown homeostatic system (Figs. 1 and 2). Solid arrows are interpretations based on direct evidence; broken arrows are tentative or poorly understood relationships. [See online article for color version of this figure.]
It has been suggested that this feedback signal also regulates the SMS branching inhibitor pathway controlled by RMS1 and RMS5 in pea (Beveridge et al., 1997b; Beveridge, 2006; Fig. 7, no. 5). For example, grafts between rms4 and wild type indicate that RMS1 expression is regulated by a shoot-derived signal (Foo et al., 2005). The SMS pathway in Arabidopsis is regulated by MAX3 and MAX4, orthologous to RMS5 and RMS1, respectively (Sorefan et al., 2003; Booker et al., 2004; Johnson et al., 2006). Conservation of feedback regulation of X-CK between pea and Arabidopsis suggests that the feedback system in Arabidopsis may also regulate the SMS pathway in this species. Consistent with this hypothesis, MAX4 promoter-β-glucuronidase (GUS) fusion studies indicated that expression of MAX4 is somewhat elevated in max2 hypocotyls (Bainbridge et al., 2005) although not to the extent expected from the results in pea. Because low X-CK content is associated with high RMS1 expression (Foo et al., 2005), it is possible that cytokinin directly or indirectly suppresses RMS1 expression. Indeed, in Arabidopsis, exogenous cytokinin largely prevents up-regulation of MAX4 by auxin (Bainbridge et al., 2005).
Cytokinin Levels in the Shoot Tissue of rms Mutants Are Near Wild Type
An examination of cytokinin levels in rms mutants indicates that increased or decreased X-CK does not result in corresponding changes in the cytokinin content of shoot tissues. Despite rms1, rms4, and rms5 mutant plants displaying massive reduction in the concentration of several major cytokinins in xylem sap (Beveridge et al., 1997b; Morris et al., 2001; Figs. 3–6), the shoot cytokinin levels in these mutants remained similar to wild type (Fig. 2). The near-normal shoot cytokinin levels suggest that either X-CK contributes very little to shoot cytokinin pools or that shoots possess homeostatic mechanisms to maintain their cytokinin status (Fig. 7, no. 11). In rms2, the only rms mutant with high rather than low X-CK, shoot cytokinin levels were similar to that of other rms mutants and wild-type plants. The disconnection between X-CK and shoot tissue cytokinin levels indicates that processes in the shoot can have a dramatic influence on whole-plant cytokinin homeostasis. This may explain why rms mutant shoots do not display phenotypes expected from cytokinin deficiency (Werner et al., 2003) and provides further evidence that tissues outside the roots play an important role in regulating cytokinin status. Because flux of X-CK into most rms shoots is greatly diminished, normal levels of shoot cytokinins are probably maintained by altered local cytokinin biosynthesis and/or metabolism (Chen et al., 1985; Nordström et al., 2004; Gaudinová et al., 2005). However, future studies should also evaluate cytokinin export to the root via the phloem and cytokinin transfer between xylem and phloem. Such processes occur with other xylem- and phloem-mobile hormones, such as abscisic acid (Wilkinson and Davies, 2002).
Role of Axillary Buds, Lateral Branches, and Decapitation in X-CK Feedback
One attractive hypothesis to explain the role of axillary buds and auxin in X-CK feedback is that auxin acts as the shoot-to-root feedback signal that regulates X-CK, because auxin is known to negatively regulate cytokinin levels in shoots (e.g. Nordström et al., 2004) and decapitation, which depletes shoot IAA levels, enhances X-CK (Bangerth, 1994). However, in contrast to the significant changes in comparable wild-type plants, decapitation of rms1, rms3, or rms4 plants caused only minor effects on X-CK, and thus patently failed to restore X-CK to wild-type levels (Fig. 3). Therefore, basipetal transport of auxin, which would most likely emanate from the shoot tip, appears not to be the cause of X-CK suppression in rms mutants. These data also indicate that normalized delivery of root-derived X-CK to the shoot is not necessary for the rapid initiation of bud outgrowth stimulated by decapitation in rms mutants (Beveridge et al., 2000; Morris et al., 2005). Instead, stem-derived cytokinins may play a role following decapitation-induced activation of IPT genes (Tanaka et al., 2006; Fig. 7, no. 9).
Y-grafting studies with rms1 and rms5 plants revealed that the mobile feedback signal is most likely produced by branching shoots and acts by suppressing X-CK (Fig. 7, no. 6). X-CK content is not reduced in rms1 or rms5 rootstocks with wild-type scions unless a branching mutant shoot is allowed to grow from the rootstock (Fig. 5; data not shown; Beveridge, 2000). This indicates that branching rms shoots probably produce elevated levels of an inhibitor of X-CK, rather than wild-type shoots producing a stimulus of X-CK. A less likely alternative is that wild-type shoots produce a stimulus that enhances X-CK, and, in Y-grafted wild-type/rms1 or wild-type/rms5 plants, dilution of this signal means that it fails to reach a threshold for X-CK promotion.
Feedback control of X-CK is not due to a constitutive effect of rms mutations in the shoot because X-CK is restored in plants where branching is suppressed, such as in rms1 or rms5 shoots grafted to wild-type rootstocks (Fig. 5; Beveridge, 2000; data not shown). Likewise, low X-CK content from rms rootstocks is not constitutive because such rootstocks can deliver normal X-CK levels when grafted to nonbranched wild-type shoots. As suggested previously by Beveridge (2000), down-regulation of X-CK is therefore a phenomenon associated with the shoot branching phenotype and not shoot genotype. However, we asked the question of whether presence of growing branches was essential for activation of the feedback signal. By monitoring X-CK in rms sax double mutants that display a reduced number of axillary buds and shoots, it appears that feedback regulation of X-CK is probably induced by processes that occur independently from axillary shoots or buds themselves (Fig. 7, no. 3). Presence of the sax1 mutation resulted in a 7-fold reduction in total lateral branch length compared with rms4 scions and suppression of axillary meristem formation at a majority of nodes, yet these rms4 sax1 double mutant scions were as effective as rms4 scions at suppressing X-CK in wild-type rootstocks (Fig. 4). Similarly, at least for rms5, X-CK was still reduced in individuals that had failed to initiate axillary bud outgrowth from basal nodes (data not shown). We conclude that the low X-CK observed in rms plants is unlikely to be caused by a signal emanating only from axillary buds or growing branches. Therefore, feedback regulation of X-CK in rms mutants appears to be controlled by processes upstream of axillary bud formation.
RMS2 Is Central to Down-Regulation of X-CK
Decapitated rms2 plants showed the same increase in X-CK as in wild-type plants, indicating that control of X-CK in this situation is at least partly RMS2 independent (Fig. 7, no. 10). However, double mutant and grafting studies show that RMS2 is required for the full suppression of X-CK in rms1, rms4, and rms5 plants. While rms1, rms4, and rms5 single mutants exhibit greatly reduced X-CK compared with wild type, this is not the case in rms1 rms2, rms2 rms4, and rms2 rms5 double mutants (Fig. 6; data not shown; Beveridge et al., 1997b). Moreover, rms2 rms4 scions have a reduced ability to suppress X-CK in wild-type rootstocks compared with rms4 scions. It therefore appears that RMS2 is required in other intact rms mutants for down-regulation of X-CK to levels below wild type. However, if RMS2 acts downstream of RMS4 in the same feedback pathway (Fig. 7, no. 4), then double null mutants would not have an additive branching phenotype. According to our model (Fig. 7), the rms4 mutation causes derepression of RMS2, but this would have no effect in rms2 rms4 plants because the lack of RMS2 function would result in derepression of X-CK content. One explanation consistent with these genes acting as suggested (Beveridge et al., 1997b; Fig. 7) is that available rms2 mutations may retain some partial function. Future studies should investigate whether RMS2 acts on a different pathway.
What Is the Role of X-CK in Shoot Branching?
Given that feedback regulation of X-CK, but not cytokinin content in shoot tissue, is affected in comparable branching mutants from divergent species, one can presume that this process is important. What then is its role? One hypothesis, supported by these findings together with the effect of rms2 on X-CK and the additive phenotype of rms2 double mutants, is that the reduced X-CK may reduce the outgrowth of secondary or accessory buds, such as those that are produced in double mutants with rms2 (Beveridge et al., 1997b; Murfet and Symons, 2000a, 2000b; data not shown). In the case of the rms2 mutant, although the trends are correlative, the magnitude of differences in X-CK content do not provide convincing evidence that elevated X-CK content alone causes the branching phenotype in various graft combinations involving rms2 (Fig. 6). This observation (Fig. 7, no. 7) is consistent with the finding of Faiss et al. (1997) that cytokinin overproduction in roots is inadequate to stimulate shoot branching. However, it reveals the possibility that X-CK may play a significant role under certain developmental or physiological conditions. Future studies should pay attention to stages of axillary bud outgrowth that may be receptive to cytokinin and other long-distance signals and to the role of signal cross-talk (for review, see Beveridge, 2006; Dun et al., 2006; Beveridge et al., 2007).
CONCLUSION
Conservation of regulation of X-CK via a shoot-derived long-distance feedback signal in Arabidopsis and pea (revealed via the max and rms mutants, respectively) suggests that the process is likely to have an important function. Evidence from decapitation studies, although clearly involving many effects other than auxin depletion, indicates that the feedback signal is probably auxin independent and may therefore be novel. The signal appears to be activated by factors that promote bud outgrowth, but is not produced by axillary buds or branches themselves. We suggest that feedback control of X-CK may be one essential component of the homeostatic control of shoot branching (Fig. 7). Future studies need to address the issues of relative rates of biosynthesis, degradation, import, and export of cytokinins in shoots and roots, and the impact of rms and max mutations on each of these processes.
MATERIALS AND METHODS
Plant Material, Growth Conditions, and Statistical Analysis
All pea (Pisum sativum) cultivars used in this study have a quantitative, long-day growth habit and all mutants described are recessive (Arumingtyas et al., 1992). The tall rms branching lines and double mutants are described by Beveridge et al. (1994, 1996, 1997b), Murfet and Symons (2000a, 2000b), and Foo et al. (2005), whereas the dwarf rms4 and sax lines are described by Rameau et al. (1997, 2002a, 2002b). Plants were grown under long-day conditions as described by Beveridge et al. (1997a) and Morris et al. (2001) unless otherwise stated. Nodes were numbered acropetally from the first scale leaf (node 1).
Arabidopsis (Arabidopsis thaliana) seeds (Col-0, max1-1, max2-1, max3-9, and max4-1) were sown directly onto the surface of compost (Levingtons F2S:vermiculite, 4:1 mixture) and placed in darkness at 4°C for 3 d. Plants were then grown at 23°C with an 8-h photoperiod provided by cool-white fluorescent lights at approximately 120 μmol m−2 s−1 supplemented with low intensity tungsten lamps.
Tests of significance between treatments in the various experiments were performed by Student's t tests or by one-way ANOVA followed by Tukey's honestly significant difference test. Unless stated otherwise, treatments were considered significantly different where P < 0.05.
Grafting Experiments
Epicotyl-epicotyl pea grafts were performed between 6- to 7- d-old seedlings as described by Beveridge et al. (1994). Y-grafts are constructed identically but a single cotyledonary shoot is allowed to grow from the rootstock, enabling generation of a plant with shoots of two different genotypes connected to the same rootstock (Beveridge and Murfet, 1996). In grafts performed with the sax1 lines, the largest lateral bud or branch at each of nodes 1 to 3 was removed 10 d before scoring and xylem sap harvest.
For Arabidopsis, hypocotyl-hypocotyl grafts using the collar method described by Turnbull et al. (2002) were constructed between max2-1 and Wassilewskija-2 plants carrying a GUS reporter gene for the purpose of ensuring accurate identification of scion and rootstock genotypes. Xylem sap was then collected as described below.
Harvest of Xylem Sap and Cytokinin Quantification
For pea X-CK analysis, xylem sap was harvested using the suction method described by Beveridge et al. (1997a) and involved collection of xylem sap following removal of the shoot at the epicotyl. It may therefore be considered as essentially root-derived xylem sap. In the case of Y-grafted plants, the cotyledonary shoot was removed prior to sap suction. Cytokinin extraction from harvested xylem sap and liquid chromatography-tandem mass spectrometry (LC-MS-MS) analyses were usually performed as described by Morris et al. (2001), except that 1 ng μL−1 of [2H5]ZR (Apex Organics) was added as an internal standard.
For Figure 3, X-CK was quantified as described by Beveridge et al. (1997a) using ELISA with anti-ZR and anti-iPR antibodies. This method was validated previously for these sample types using MS (Beveridge et al., 1997a). As the anti-ZR antibody also detects Z, the 5′-monophosphate of ZR, and dihydrozeatin, the cytokinins quantified in this experiment are described as ZR-type cytokinins. Similarly, the anti-iPR antibody detects iP and iPR.
For Arabidopsis, the suction method for pea described above was adapted for 2- to 3-month-old plants. The whole leafy rosette was removed by cutting at the top of the hypocotyl. In this case the X-CK is entirely root-derived xylem sap. After washing with distilled water, a short length of silicone tubing (2-mm i.d.) was placed over the hypocotyl stump and secured by tying tightly with gift ribbon. The 2-mm tubing was slid inside a piece of larger diameter tubing connected to a hypodermic syringe. Xylem sap was collected under vacuum into the syringe for 90 to 100 min, and then frozen in liquid N2 and stored at −70°C until extraction. Cytokinins were analyzed directly by LC-MS-MS after filtration and addition of the following [2H]-labeled cytokinin internal standards: [2H5]Z, [2H5]ZR, [2H3]dihydrozeatin ([2H3]DHZ), [2H3]DHZR, [2H6]iP, [2H6]isopentenyl adenosine-9-glucoside, [2H6]iPR, and [2H3]DHZ9G (OlChemIm).
Harvest of Shoot Tissue and Cytokinin Quantification from Shoots
Plants were grown in a glasshouse with natural photoperiod extended to 16 h and day/night temperature of 23°C/15°C. Growth media consisted of a 4:1 peat compost:perlite mix. Shoot tips, consisting of all tissue above and including node 3, was harvested from 8- or 9-d-old plants, and then frozen in liquid N2 and stored at −80°C.
Frozen pea tissue (1–4 g fresh weight) was ground in liquid N2 and extracted in methanol:water (1:1) containing 2 ng g−1 cytokinin internal standards. Extracts were centrifuged at 10,000g for 5 min and supernatants decanted. Pellets were re-extracted twice in methanol:water (1:1) and centrifuged as above. Combined supernatants were evaporated to an aqueous phase under vacuum. Samples were then resuspended in water.
Following extraction, samples were reacted with 60 units of alkaline phosphatase (Sigma-Aldrich), passed through a C18 Sep-Pak cartridge, and purified through cytokinin immunoaffinity columns as described by Morris et al. (2001).
LC-MS-MS analyses were performed largely as described by Prinsen et al. (1995). Z, ZR, DHZ, and DHZR and their corresponding glucosides were chromatographically separated from iP and iPR using the conditions described by Morris et al. (2001), or for some samples a longer solvent program completely resolved all measured compounds: gradient of acetonitrile in 10 mm ammonium acetate (pH 3.4), initially 5% for 4 min, rising to 14% at 20 min and 32% at 25 min, using a flow rate of 200 μL min−1 through a Phenomenex 3-μm C18 Luna 100- × 2-mm column on an Agilent 1100 Binary LC system, coupled to an Applied Biosystems Q-Trap hybrid mass spectrometer fitted with a TurboIonspray (electrospray) source operating in positive ion multiple reaction monitoring mode. Dwell time was 30 ms for each MS-MS ion pair. In some samples, the [2H5]Z, [2H5]Z9G, and [2H5]ZR standards were used to estimate DHZ, DHZ9G, and DHZR, respectively, and [2H5]ZR and [2H5]ZRMP were used to estimate cZR and cZRMP, respectively. Quantitation was essentially as described by Prinsen et al. (1995) including correction for isotopic purity and application of a linear calibration curve.
Acknowledgments
We thank Tatsuo Kakimoto (Osaka University) for providing the GUS Arabidopsis lines that we used to highlight grafting success and Elizabeth Dun for helpful discussions. Thanks to Chuong Ngo and Ian Dodd for our additional endogenous cytokinin measurements mentioned herein.
This work was supported by the Australian Research Council, the Queensland Smart State Initiative Fund, and the Biotechnology and Biological Sciences Research Council (BBSRC; grant no. 32/P18282). E.F., K.P., and S.M. were funded by Australian Postgraduate Awards, and N.Y. was supported by a BBSRC Committee Studentship.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Christine A. Beveridge (c.beveridge@uq.edu.au).
Some figures in this article are displayed in color online but in black and white in the print edition.
Open Access articles can be viewed online without a subscription.
References
- Arumingtyas EL, Floyd RS, Gregory MJ, Murfet IC (1992) Branching in Pisum: inheritance and allelism test with 17 ramosus mutants. Pisum Genet 24 17–31 [Google Scholar]
- Bainbridge K, Sorefan K, Ward S, Leyser O (2005) Hormonally controlled expression of the Arabidopsis MAX4 shoot branching regulatory gene. Plant J 44 569–580 [DOI] [PubMed] [Google Scholar]
- Bangerth F (1994) Response of cytokinin concentration in the xylem exudate of bean (Phaseolus vulgaris L.) plants to decapitation and auxin treatment, and relationship to apical dominance. Planta 194 439–442 [Google Scholar]
- Bennett T, Sieberer T, Willett B, Booker J, Luschnig C, Leyser O (2006) The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport. Curr Biol 16 553–563 [DOI] [PubMed] [Google Scholar]
- Beveridge CA (2000) Long-distance signalling and a mutational analysis of branching in pea. Plant Growth Regul 32 193–203 [Google Scholar]
- Beveridge CA (2006) Axillary bud outgrowth: sending a message. Curr Opin Plant Biol 9 35–40 [DOI] [PubMed] [Google Scholar]
- Beveridge CA, Mathesius U, Rose RJ, Gresshoff PM (2007) Common regulatory themes in meristem development and whole-plant homeostasis. Curr Opin Plant Biol 10 44–51 [DOI] [PubMed] [Google Scholar]
- Beveridge CA, Murfet IC (1996) The gigas mutant in pea is deficient in the floral stimulus. Physiol Plant 96 637–645 [Google Scholar]
- Beveridge CA, Murfet IC, Kerhoas L, Sotta B, Miginiac E, Rameau C (1997. a) The shoot controls zeatin riboside export from pea roots: evidence from the branching mutant rms4. Plant J 11 339–345 [Google Scholar]
- Beveridge CA, Ross JJ, Murfet IC (1994) Branching mutant rms-2 in Pisum sativum. Grafting studies and endogenous indole-3-acetic acid levels. Plant Physiol 104 953–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge CA, Ross JJ, Murfet IC (1996) Branching in pea. Action of genes Rms3 and Rms4. Plant Physiol 110 859–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge CA, Symons GM, Murfet IC, Ross JJ, Rameau C (1997. b) The rms1 mutant of pea has elevated indole-3-acetic acid levels and reduced root-sap zeatin riboside content but increased branching controlled by graft-transmissible signals. Plant Physiol 115 1251–1258 [Google Scholar]
- Beveridge CA, Symons GM, Turnbull CGN (2000) Auxin inhibition of decapitation-induced branching is dependent on graft-transmissible signals regulated by genes Rms1 and Rms2. Plant Physiol 123 689–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhner S, Gatz C (2001) Characterisation of novel target promoters for the dexamethasone-inducible/tetracycline-repressible regulator TGV using luciferase and isopentenyl transferase as sensitive reporter genes. Mol Gen Genet 264 860–870 [DOI] [PubMed] [Google Scholar]
- Booker J, Auldridge M, Wills S, McCarty D, Klee H, Leyser O (2004) MAX3/CCD7 is a carotenoid cleavage dioxygenase required for the synthesis of a novel plant signalling molecule. Curr Biol 14 1232–1238 [DOI] [PubMed] [Google Scholar]
- Booker J, Sieberer T, Wright W, Williamson L, Willett B, Stirnberg P, Turnbull C, Srinivasan M, Goddard P, Leyser O (2005) MAX1 encodes a cytochrome P450 family member that acts downstream of MAX3/4 to produce a carotenoid-derived branch-inhibiting hormone. Dev Cell 8 443–449 [DOI] [PubMed] [Google Scholar]
- Chen CM, Ertl JR, Leisner SM, Chang CC (1985) Localization of cytokinin biosynthetic sites in pea (Pisum sativum) plants and carrot (Daucus carota) roots. Plant Physiol 78 510–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd IC, Ngo C, Turnbull CGN, Beveridge CA (2004) Effects of nitrogen supply on xylem cytokinin delivery, transpiration and leaf expansion of pea genotypes differing in xylem-cytokinin concentration. Funct Plant Biol 31 903–911 [DOI] [PubMed] [Google Scholar]
- Dun E, Ferguson B, Beveridge CA (2006) Apical dominance and shoot branching: divergent opinions or divergent mechanisms. Plant Physiol 142 812–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklöf S, Åstot C, Sitbon F, Moritz T, Olsson O, Sandberg G (2000) Transgenic tobacco plants co-expressing Agrobacterium IAA and IPT genes have wild-type hormone levels but display both auxin- and cytokinin-overproducing phenotypes. Plant J 23 279–284 [DOI] [PubMed] [Google Scholar]
- Emery RJN, Longnecker NE, Atkins CA (1998) Branch development in Lupinus angustifolius L. II. Relationship with endogenous ABA, IAA and cytokinins in axillary and main stem buds. J Exp Bot 49 555–562 [Google Scholar]
- Faiss M, Zalubílová J, Strnad M, Schmülling T (1997) Conditional transgenic expression of the IPT gene indicates a function for cytokinins in paracrine signaling in whole tobacco plants. Plant J 12 401–415 [DOI] [PubMed] [Google Scholar]
- Foo E, Bullier E, Goussot M, Foucher F, Rameau C, Beveridge CA (2005) The branching gene RAMOSUS1 mediates interactions among two novel signals and auxin in pea. Plant Cell 17 464–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo E, Turnbull CGN, Beveridge CA (2001) Long distance signalling and the control of branching in the rms1 mutant of pea. Plant Physiol 126 203–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudinová A, Dobrev PI, Šolcová B, Novák O, Strnad M, Friedecký D, Motyka V (2005) The involvement of cytokinin oxidase/dehydrogenase and zeatin reductase in regulation of cytokinin levels in pea (Pisum sativum L.) leaves. J Plant Growth Regul 24 188–200 [Google Scholar]
- Johnson X, Brcich T, Dun E, Goussot M, Haurogné K, Beveridge CA, Rameau C (2006) Branching genes are conserved across species: genes controlling a novel signal in pea are co-regulated by other long-distance signals. Plant Physiol 142 1014–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CJ, Guevara E, Gerrera J, Bangerth F (1995) Effect of apex excision and replacement by 1-naphthylacetic acid on cytokinin concentration and apical dominance in pea plants. Physiol Plant 94 465–469 [Google Scholar]
- Miyawaki K, Matsumoto-Kitano M, Kakimoto T (2004) Expression of cytokinin biosynthetic isopentenyltransferase genes in Arabidopsis: tissue specificity and regulation by auxin, cytokinin, and nitrate. Plant J 37 128–138 [DOI] [PubMed] [Google Scholar]
- Morris SE, Cox M, Ross JJ, Krisanti S, Beveridge CA (2005) Auxin dynamics after decapitation are not correlated with the initial outgrowth of axillary buds. Plant Physiol 138 1665–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SE, Turnbull CGN, Murfet IC, Beveridge CA (2001) Mutational analysis of branching in pea. Evidence that Rms1 and Rms5 regulate the same novel signal. Plant Physiol 126 1205–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murfet IC, Symons GC (2000. a) Double mutant rms2 rms5 expresses a transgressive, profuse branching phenotype. Pisum Genet 32 33–38 [Google Scholar]
- Murfet IC, Symons GC (2000. b) The pea rms2-1 rms4-1 double mutant phenotype is transgressive. Pisum Genet 32 59–60 [Google Scholar]
- Nordström A, Tarkowski P, Tarkowska D, Norbaek R, Åstot C, Dolezal K, Sandberg G (2004) Auxin regulation of cytokinin biosynthesis in Arabidopsis thaliana: a factor of potential importance for auxin-cytokinin-regulated development. Proc Natl Acad Sci USA 101 8039–8044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinsen E, Redig P, Van Dongen W, Esmans EL, Van Onckelen HA (1995) Quantitative analysis of cytokinins by electrospray tandem mass spectrometry. Rapid Commun Mass Spectrom 9 948–953 [Google Scholar]
- Rameau C, Bellec Y, Grillot P, Parmenter KS, Beveridge CA, Turnbull CGN (2002. a) Mutations at several loci suppress vegetative axillary meristem initiation in pea. Pisum Genet 34 15–19 [Google Scholar]
- Rameau C, Bodelin C, Cadier D, Murfet IC (1997) New ramosus mutants at loci Rms1 Rms3 and Rms4 resulting from mutation breeding program at Versailles. Pisum Genet 29 7–12 [Google Scholar]
- Rameau C, Murfet IC, Laucou V, Floyd RS, Morris SE, Beveridge CA (2002. b) Pea rms6 mutants exhibit increased basal branching. Physiol Plant 115 458–467 [DOI] [PubMed] [Google Scholar]
- Sachs T, Thimann KV (1964) Release of lateral buds from apical dominance. Nature 201 939–940 [Google Scholar]
- Schmülling T (2002) New insights into the functions of cytokinins in plant development. J Plant Growth Regul 21 40–49 [DOI] [PubMed] [Google Scholar]
- Schwartz SH, Qin X, Loewen MC (2004) The biochemical characterization of two carotenoid cleavage enzymes from Arabidopsis indicates that a carotenoid-derived compound inhibits lateral branching. J Biol Chem 279 46940–46945 [DOI] [PubMed] [Google Scholar]
- Sorefan K, Booker J, Haurogné K, Goussot M, Bainbridge K, Foo E, Chatfield S, Ward S, Beveridge C, Rameau C, et al (2003) MAX4 and RMS1 are orthologous dioxygenase-like genes that regulate shoot branching in Arabidopsis and pea. Genes Dev 17 1469–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Takei K, Kojima M, Sakakibara H, Mori H (2006) Auxin controls local cytokinin biosynthesis in the nodal stem in apical dominance. Plant J 45 1028–1036 [DOI] [PubMed] [Google Scholar]
- Turnbull CGN, Booker JP, Leyser HMO (2002) Micrografting techniques for testing long-distance signalling in Arabidopsis. Plant J 32 255–262 [DOI] [PubMed] [Google Scholar]
- Turnbull CGN, Raymond MAA, Dodd IC, Morris SE (1997) Rapid increases in cytokinin concentration in lateral buds of chickpea (Cicer arietinum L.) during release of apical dominance. Planta 202 271–276 [Google Scholar]
- Werner T, Motyka V, Laucou V, Smets R, Van Onckelen H, Schmülling T (2003) Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 15 2532–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S, Davies WJ (2002) ABA-based chemical signalling: the co-ordination of responses to stress in plants. Plant Cell Environ 25 195–210 [DOI] [PubMed] [Google Scholar]