Abstract
Temperature and daylength act as environmental signals that determine the length of the growing season in boreal evergreen conifers. Climate change might affect the seasonal development of these trees, as they will experience naturally decreasing daylength during autumn, while at the same time warmer air temperature will maintain photosynthesis and respiration. We characterized the down-regulation of photosynthetic gas exchange and the mechanisms involved in the dissipation of energy in Jack pine (Pinus banksiana) in controlled environments during a simulated summer-autumn transition under natural conditions and conditions with altered air temperature and photoperiod. Using a factorial design, we dissected the effects of daylength and temperature. Control plants were grown at either warm summer conditions with 16-h photoperiod and 22°C or conditions representing a cool autumn with 8 h/7°C. To assess the impact of photoperiod and temperature on photosynthesis and energy dissipation, plants were also grown under either cold summer (16-h photoperiod/7°C) or warm autumn conditions (8-h photoperiod/22°C). Photosynthetic gas exchange was affected by both daylength and temperature. Assimilation and respiration rates under warm autumn conditions were only about one-half of the summer values but were similar to values obtained for cold summer and natural autumn treatments. In contrast, photosynthetic efficiency was largely determined by temperature but not by daylength. Plants of different treatments followed different strategies for dissipating excess energy. Whereas in the warm summer treatment safe dissipation of excess energy was facilitated via zeaxanthin, in all other treatments dissipation of excess energy was facilitated predominantly via increased aggregation of the light-harvesting complex of photosystem II. These differences were accompanied by a lower deepoxidation state and larger amounts of β-carotene in the warm autumn treatment as well as by changes in the abundance of thylakoid membrane proteins compared to the summer condition. We conclude that photoperiod control of dormancy in Jack pine appears to negate any potential for an increased carbon gain associated with higher temperatures during the autumn season.
Temperature and daylength are important drivers of physiological changes in boreal evergreen conifers because they determine the length of the growing season. In late summer and early autumn the decrease of the daylength is a signal that initiates cold hardening, a transition of physiological processes that allow hardy plants to survive severe winter conditions. The cold hardening process includes the cessation of growth and long-term changes in the metabolism of the tree (Weiser, 1970; Bigras et al., 2001). Short days are supposed to induce the cold hardening most effectively, whereas decreasing temperatures affect this process only to some extent (Christersson, 1978).
As evergreen conifers keep their needles during winter, the cold hardening process involves a reorganization of the photosynthetic machinery (Ensminger et al., 2004), as the needles retain a substantial amount of chlorophyll (Chl) and proteins and therefore continue to absorb light. Absorption of light under winter conditions can cause photooxidative damage of the photosynthetic apparatus, created by an imbalance between the photochemical generation of electrons and their diminished utilization due to decreasing sink capacity, i.e. the down-regulation of metabolism and growth in winter (Oquist and Huner, 2003). To maintain the balance between light capture and energy utilization under conditions with altered sink capacity, energy flow and photosynthesis have to be adjusted by a process defined as photostasis (Oquist and Huner, 2003). Plants have developed a wide range of mechanisms to achieve this balance and to avoid photooxidative damage. This includes adjustments of the Chl and protein concentration, changes in antenna size and organization, stoichiometry and aggregation of components of the photosynthetic apparatus, and a range of alternative energy dissipation pathways (Demmig-Adams et al., 1996; Asada, 1999; Oquist and Huner, 2003; Munekaga et al., 2004; Horton et al., 2005; Kanervo et al., 2005; Ensminger et al., 2006). Recently, the carotenoid zeaxanthin in combination with the PSII subunit PsbS has gained interest as both play an important role in the safe thermal dissipation of excess energy via nonphotochemical quenching (NPQ; Li et al., 2000, 2002; Savitch et al., 2002; Niyogi et al., 2005). NPQ actually consists of three different components that are commonly defined by referring to their relaxation properties in darkness following a period of illumination (Muller et al., 2001). A dynamic quenching mode is rapidly inducible and driven by the conversion of violaxanthin into zeaxanthin and protonation of the PsbS protein in response to acidification of the thylakoid lumen. State transition quenching relaxes within tens of minutes and is assumed not to be of great importance for photoprotection in terrestrial plants under high light (Niyogi, 1999; Kanervo et al., 2005). The sustained quenching mode (qI) in turn is induced in connection with a decrease in PsbS levels and a loss of functional PSII (Ensminger et al., 2004; Ebbert et al., 2005). In evergreen conifers, this qI is also associated with the termination of growth and induction of frost hardiness (Oquist and Huner, 2003). In this qI state, a reorganization of the photosynthetic apparatus may protect conifer needles by dissipating excess energy as heat independent of a pH gradient (Gilmore and Ball, 2000; Horton and Ruban, 2005; Ensminger et al., 2006). Overwintering evergreen conifers seem to be able to shift between the dynamic and the sustained antenna quenching (qO) modes for dissipating excess energy (Oquist and Huner, 2003). Aside from NPQ of excess energy in the antenna, a zeaxanthin independent way of quenching has been described in the reaction center (RC; Ivanov et al., 2001, 2006; Lee et al., 2001; Sane et al., 2003; Finazzi et al., 2004). In addition to antenna and RC quenching, excess energy can be funneled into a range of alternative electron sinks, most notably photorespiration (Osmond and Grace, 1995; Streb et al., 1998), the water-water cycle (Asada, 1999), and cyclic electron transport (Ivanov et al., 2001; Kanervo et al., 2005). For recent reviews, see Scheibe et al. (2005) and Ensminger et al. (2006).
Over the past decades substantial warming has occurred in northern latitudes, especially in the winter (Serreze et al., 2000). It has been suggested that this is likely to increase the length of the growing season and thus the productivity of northern hemisphere forests (White et al., 2000; Saxe et al., 2001). Increased temperatures in autumn allow trees to maintain a higher quantum yield (Methy et al., 1997). However, little data are available regarding the metabolic responses of evergreens of the boreal forests to extended growing seasons as a result of warmer air temperatures in late autumn. In this study, we focus on the interaction of altered autumn growth conditions and photosynthesis in Jack pine (Pinus banksiana Lamb.), an important evergreen conifer forming dominant stands on dry sandy soils in the western boreal zone of Canada, e.g. Northern Saskatchewan. Climate change will alter the phasing of temperature and daylength, the signals that trigger low temperature acclimation and development during autumn in conifer trees. The question is how the seasonal development is affected once trees experience the naturally decreasing daylength regulating growth cessation and limiting sink capacity while at the same time increased air temperature allows to maintain photosynthetic and respiratory capacity. Here, we used a factorial experiment with 16-h versus 8-h photoperiod (representing long day during July/August and short day during October/November, respectively, at e.g. 54°N, 105°W in northern Saskatchewan) and 22°C versus 7°C (summer and autumn, respectively) to separate the effects of daylength and temperature on photosynthesis and respiration to distinguish which processes are regulated by temperature or daylength only or by a combination of these abiotic factors. In particular, we focused on the effects of these treatments on the acclimation of photosynthetic capacity and energy dissipation and the composition of the photosynthetic apparatus.
RESULTS
Gas Exchange
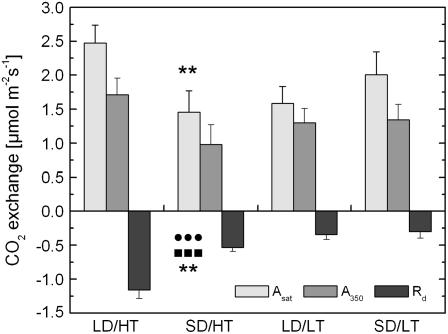
Needle level gas exchange was performed on samples of seedlings of all four treatments (Fig. 1). At 1,000 μmol photons m−2 s−1, we observed the highest rate of light-saturated net assimilation (Asat) in the summer conditions with 16-h photoperiod and 22°C (LD/HT) treatment. In the natural autumn control with 8-h photoperiod/7°C (SD/LT), Asat was decreased by 19%. Within the other two treatments, Asat was considerably lower, with the warm autumn conditions with 8-h photoperiod/22°C treatment (SD/HT) showing 41% lower values than LD/HT and 28% lower values than SD/LT. This pattern was also observed when net assimilation was measured at growth light conditions (Fig. 1). While assimilation did not show any clear effect of either temperature or photoperiod and only showed a significant difference in the interaction of both factors, we observed a clear response of respiration to either factor plus the interaction of both factors. Within the warm temperature treatments, the respiration rate in LD/HT was more than twice the rate observed in SD/HT (Fig. 1), suggesting an effect of the shorter photoperiod. Low temperature imposed an additional effect on dark respiration, being responsible for a further decrease in cold summer conditions with 16-h photoperiod/7°C (LD/LT) and SD/LT needles. These results are also valid when data are expressed on a fresh weight basis because the ratio of fresh weight to leaf area (grams per meter) only changes minimally between treatments over the duration of the experiment (LD/HT, 369 ± 40; SD/HT, 342 ± 19; LD/LT, 341 ± 21; SD/LT, 366 ± 37).
Figure 1.
The effect of daylength and temperature on needle level CO2 assimilation and respiration per needle area. Bars indicate light-saturated net CO2 assimilation, measured at 1,000 μmol photons m−2 s−1 PPFD (Asat), CO2 assimilation at growth light conditions of 350 μmol photons m−2 s−1 PPFD (A350). Black bars indicate dark respiration measured after 20 min of dark acclimation. All measurements were performed at growth temperature (22°C in LD/HT and SD/HT, 7°C in LD/LT and SD/LT plants). Each bar represents the average of n = 7 to 8 ± se biological replicates. •, ▪, and *, Significant differences due to daylength, temperature, and their interactive effect, respectively. Two symbols, P ≤ 0.01; three symbols, P ≤ 0.001.
Chl Fluorescence
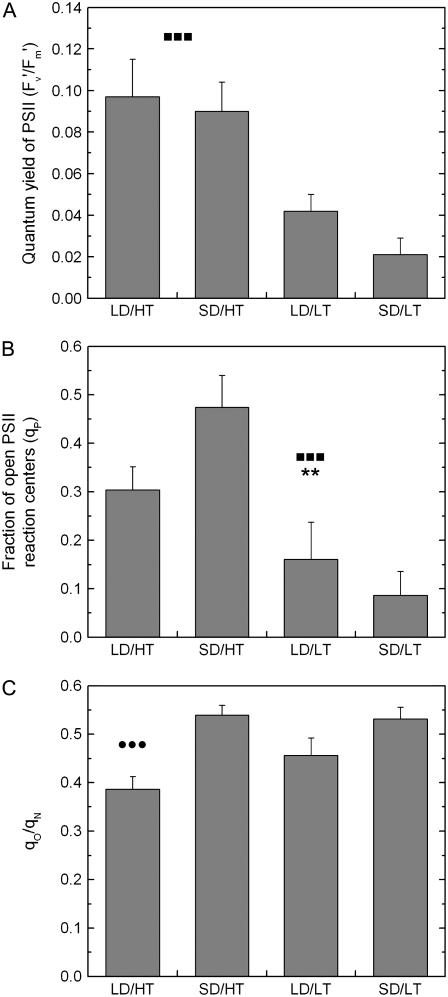
Chl a fluorescence was measured simultaneously with gas exchange. Maximum photochemical efficiency of PSII in the dark-adapted state (Fv/Fm) was highest in the LD/HT control (Table I). The sole effect of a decreased length of the photoperiod resulted in a 10% decrease of Fv/Fm in SD/HT plants. Low temperature had a much stronger effect on decreasing Fv/Fm in the LD/LT compared to the LD/HT treatment. The effect was additive when combining both factors in the SD/LT treatment (51% decrease) with no interactive effect between daylength and temperature. In both high temperature treatments the quantum yield of PSII did not differ significantly from each other, but low temperature decreased the yield to less than one-half in LD/LT and to about one-quarter in SD/LT compared to the LD/HT treatment (Fig. 2A). qP as a measure of the fraction of open PSII RCs revealed large differences that were temperature dependent but did also depend on the interactive effect of daylength and temperature; e.g. values of the SD/HT treatment were 56% higher than in the summer control LD/HT treatment (Fig. 2B). Using nonphotochemical (qN) and antenna quenching (qO), we calculated the ratio of qO/qN to indicate the relative component of the qO from all nonphotochemical processes (Table I). qO/qN values were lowest during the typical LD/HT treatment, whereas under SD/HT conditions or under SD/LT conditions, the respective values were 40% and 38% higher than in LD/HT (Fig. 2C), showing solely a photoperiod dependence.
Table I.
The effect of daylength and temperature on Chl fluorescence parameters
Fv/Fm, qO, and qN, measured under saturating light conditions (1,000 μmol photons m−2 s−1); n = 8 ± se. Two-way ANOVA analysis indicates statistically significant differences due to daylength, temperature, or an interactive effect of both factors. •, ▪, and *, Significant differences due to daylength, temperature, and their interactive effect, respectively. One symbol, P ≤ 0.05; two symbols, P ≤ 0.01; three symbols, P ≤ 0.001; n.s., not significant.
| Treatment
|
Significance
|
Interactive Effect | |||||
|---|---|---|---|---|---|---|---|
| LD/HT | SD/HT | LD/LT | SD/LT | Daylength | Temp | ||
| Fv/Fm | 0.80 ± 0.01 | 0.72 ± 0.02 | 0.51 ± 0.05 | 0.39 ± 0.02 | • • • | ▪ ▪ ▪ | n.s. |
| qO | 0.36 ± 0.03 | 0.52 ± 0.02 | 0.35 ± 0.05 | 0.38 ± 0.03 | • • | ▪ | * |
| qN | 0.93 ± 0.01 | 0.96 ± 0.01 | 0.74 ± 0.06 | 0.71 ± 0.05 | n.s. | ▪ ▪ ▪ | n.s. |
Figure 2.
A, The effect of daylength and temperature on quantum yield of PSII under steady-state condition at 1,000 μmol photons m−2 s−1 PPFD. B, Estimated qP. C, qO/qN as an estimate of the amount of qO. All measurements were performed at growth temperature (22°C for LD/HT and SD/HT and 7°C for LD/LT and SD/LT). Each bar represents the average of n = 8 ± se biological replicates. •, ▪, and *, Significant differences due to daylength, temperature, and their interactive effect, respectively. Two symbols, P ≤ 0.01; three symbols, P ≤ 0.001.
Effects of Photoperiod and Temperature on the Aggregation State of Chl-Protein Complexes
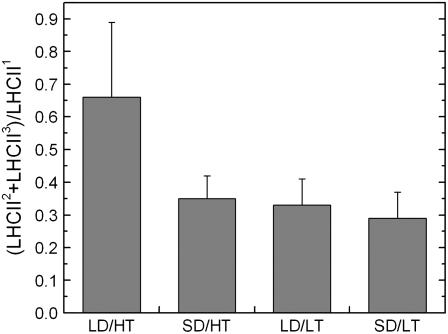
Nondenaturing SDS-PAGE was used to estimate the amount of Chl-binding protein complexes in the thylakoid membrane. The ratio of monomeric light-harvesting complex of PSII (LHCII3) and dimeric LHCII (LHCII2) to oligomeric LHCII (LHCII1) was determined from gel scans to assess the degree of aggregation of LHCII, with a low ratio indicating a high aggregation state of the LHCIIs (Fig. 3). The bulk of LHCII in all four treatments was found in form of oligomeric LHCII1; only one-fifth to one-third, depending on the treatment, was found as LHCII2 and LHCII3. In LD/HT controls, the ratio of LHCII2+3/LHCII1 was 0.66 ± 0.23 and thus about twice the ratio determined in SD/HT (0.35 ± 0.07), LD/LT (0.33 ± 0.08), and SD/LT (0.29 ± 0.08), which indicates a much higher aggregation state in the latter treatments.
Figure 3.
The effect of daylength and temperature on the degree of aggregation of LHCII. Bars indicate the ratio of LHCII3 and LHCII2 to LHCII1 as determined from nondenaturing SDS-PAGE. Each bar represents the average of n = 4 ± se biological replicates.
Photosynthetic Pigments
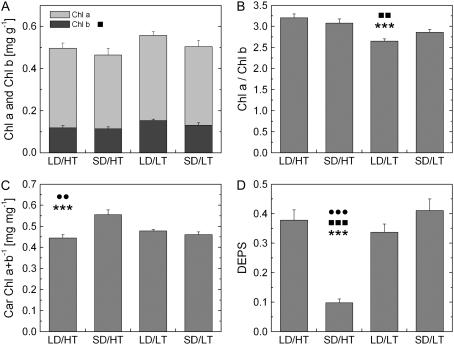
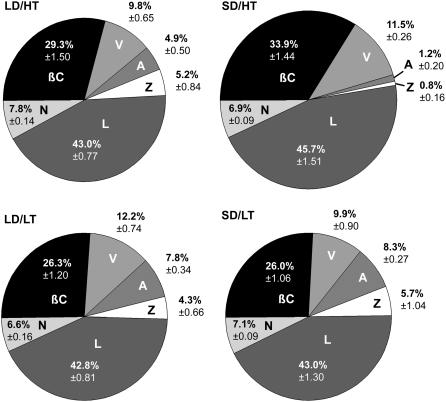
Chl a and Chl b levels differed by less than 10% in the two warm temperature treatments, and Chl b increased significantly in the two cold treatments. Photoperiod had no effect on Chl levels (Fig. 4A). However, because the low temperature increases in Chl b exceeded the slight increases in Chl a, we observed a temperature-dependent decrease in Chl a/Chl b (Fig. 4B), which also showed an interactive effect. Total carotenoids per total Chl was constant among all treatments, except for SD/HT needles, which revealed a significant increase of about 15% compared to the other three treatments (Fig. 4C). In general, the share of neoxanthin and lutein made up approximately one-half of the carotenoid pool and did not vary much between treatments (Fig. 5). In contrast, the fractions of the remaining carotenoids were more variable, indicated, for example, by an increase in β-carotene and an almost complete absence of zeaxanthin in the SD/HT treatment. In turn, this treatment showed the highest amount of violaxanthin but the least amount of antheraxanthin. As a result, the deepoxidation state was considerably lower in SD/HT, reaching only about one-quarter of the values obtained in the other treatments (Fig. 4D). This effect has been observed in two independent experiments carried out in two separate years.
Figure 4.
The effect of daylength and temperature on the composition of photosynthetic pigments in needles of Jack pine. A, Total Chl per fresh weight; Chl b was affected by temperature. B, Chl a to Chl b ratio. C, Total carotenoids per total Chl. D, Deepoxidation status of the xanthophyll cycle pigments, calculated as (0.5A + Z)/(V + A + Z). Each bar represents the average of n = 8 ± se biological replicates. •, ▪, and *, Significant differences due to daylength, temperature, and their interactive effect, respectively. One symbol, P ≤ 0.05; two symbols, P ≤ 0.01; three symbols, P ≤ 0.001.
Figure 5.
The effect of daylength and temperature on the carotenoid composition in needles of Jack pine. Each carotenoid component is normalized to the total amount of carotenoids. The relative size of each pie reflects the amount of carotenoids based on a per total Chl basis. βC, β-carotene; V, violaxanthin; A, antheraxanthin; Z, zeaxanthin; L, lutein; N, neoxanthin. Values represent the average of n = 8 ± se biological replicates.
Changes in Proteins of Photosynthesis and Carbohydrate Metabolism
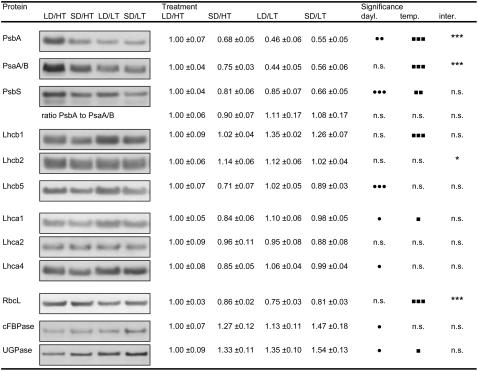
The acclimation in response to daylength and temperature (Fig. 6) shows the highest levels of RC proteins of PSII and PSI (PsbA and PsaA/B, respectively) under LD/HT conditions compared to decreased levels in cold temperature treatments with lowest values in the LD/LT treatment. However, although the overall amount of RC proteins was variable among the treatments, the ratio of PsaA/B over PsbA remained constant (Fig. 6). PsbS levels decreased by 19% in response to short photoperiod and by 15% in response to low temperature as compared to the summer control. The additive effect of decreased photoperiod and low temperature resulted in PsbS levels in the SD/LT treatment to be 34% lower than in LD/HT, indicating that its regulation is independent with no interactive effect of temperature and daylength (Fig. 6).
Figure 6.
The effect of daylength and temperature on the expression levels of key proteins of photosynthesis in needles of Jack pine. The average optical density of the LD/HT treatment was arbitrarily scaled to 1. Typical bands from the original western blots are shown next to the values, with each lane loaded on an equal protein basis. Each value represents the average of n = 8 ± se biological replicates. Two-way ANOVA analysis indicates statistically significant differences due to daylength, temperature, or an interactive effect of both factors. •, ▪, and *, Significant differences due to daylength, temperature, and their interactive effect, respectively. One symbol, P ≤ 0.05; two symbols, P ≤ 0.01; three symbols, P ≤ 0.001; n.s., not significant.
There was also a clear response of the LHC proteins Lhca1, Lhca4, and Lhcb5 to daylength (Fig. 6). Protein levels in LD/HT and LD/LT clearly exceeded the levels in SD/HT and SD/LT, while there was no effect of temperature. By contrast, Lhcb1 was significantly affected by temperature, as indicated by the accumulation of this protein, with LD/LT being 35% and SD/LT being 26% higher than LD/HT and SD/HT levels (Fig. 6). Lhca2 and Lhcb2 exhibited minimal differences between LD/HT and the three other treatments (Fig. 6). The accumulation of the large subunit of Rubisco (RbcL) followed the same pattern observed for the RC proteins of PSII and PSI. Cytosolic Fru-1,6-bisphosphatase (cFBPase) and UDP-Glc pyrophosphorylase (UGPase), both cytosolic enzymes that are required to convert triose phosphate exported from the chloroplast into Suc, were clearly affected by daylength and UGPase also by temperature. Short-day conditions alone induced the accumulation of cFBPase by 27% and exposure to low temperature by 13%. A similar pattern was observed in the expression of UGPase (Fig. 6).
DISCUSSION
Short Photoperiod and Low Temperature Promote Down-Regulation of Photosynthetic Capacity
It is remarkable that net CO2 assimilation in the SD/HT treatment is considerably lower than in the LD/HT and comparable to that of the two low temperature treatments (Fig. 1). Photosynthesis therefore appears to be not only temperature dependent but is also strongly influenced by the length of the photoperiod. In conifers, shortened daylength acts as a signal for the induction of terminal budset and the cessation of growth (Repo et al., 2001). In combination with low temperature, this is an early autumn prerequisite to induce freezing resistance and therefore an important mechanism to prepare for the harsh winter conditions in northern forest environments (Bigras et al., 2001). This actual effect of daylength on photosynthesis probably reflects feedback regulation through an overall decrease in metabolic activity and growth. Such down-regulation of photosynthesis due to a reduced sink capacity is associated with the cessation of growth (Savitch et al., 2000). A decreased rate of CO2 uptake is therefore likely due to the cessation of growth in pine after cold acclimation. Carbohydrate partitioning undergoes a tremendous shift as growth ceases and metabolic activity decreases under short photoperiod and low temperature. Increased expression of cFBPase, one of the key regulatory enzymes controlling the conversion of triose phosphate exported from the chloroplast into Suc (Strand et al., 2000), is associated with short photoperiod (Fig. 6) and indicates increased capacity for Suc synthesis. There is a dual role for Suc during autumn; it can be exported to the roots to be used for starch synthesis for winter storage and it may serve as a cryoprotectant to increase frost resistance in needle tissue. This clearly shows that the regulation of an important pathway for the cold hardening process strictly requires proper phasing of low temperature and daylength. However, levels of UGPase, acting downstream of cFBPase in the Suc synthesis pathway (Kleczkowski et al., 2004), did not show this exclusive increase due to short daylength but increased also in response to temperature (Fig. 6).
Down-regulation of metabolic processes in response to low temperature and short photoperiod was also reflected in decreased rates of dark respiration in SD/HT, LD/LT, and SD/LT compared to LD/HT. The response of plants to a shorter daylength was a more than 50% decrease in the rate of respiration. However, as a result of warmer temperatures, the respiration rate in the SD/HT treatment remained higher than in the SD/LT treatment.
Acclimation of Photosynthetic Energy Conversion and Composition of the Photosynthetic Apparatus to Growth Conditions
Decreased sink capacity requires acclimation of the energy partitioning process to balance the flow of energy between energy captured by the light reactions and the energy utilized metabolically. This may be achieved by changing properties of the thylakoid membrane-bound LHC proteins, thereby altering the efficiency of capture, conversion, and dissipation of light energy. Several of the LHC proteins showed a response to photoperiod. Lhca1, Lhca4, and Lhcb5 reached the lowest levels in the SD/HT treatment (Fig. 6). This coincided with a low deepoxidation state of the xanthophyll cycle pigments and a lack of zeaxanthin (Figs. 4 and 5), pigments that are preferentially bound in bulk by these proteins (Morosinotto et al., 2002). The down-regulation of photosynthesis in autumn was reflected in partial losses of PSII and PSI RCs as well as the RbcL content, which clearly reflects adjustment of photosynthesis to the reduced need for photosynthates under cold temperatures (see also Zarter et al., 2006). However, in the SD/HT treatment, the decrease of PsbA was balanced by an increase in the fraction of open RCs of PSII, resulting in a similar yield as in LD/HT (Figs. 2, A and B, and 6). While in LD/HT the photosynthetic apparatus remains fully functional and associated with a considerable sink for photosynthates, in low temperature treatments a reduced sink capacity was clearly accompanied by a decrease in the photochemical efficiency. A decrease in photochemical efficiency was not apparent in SD/HT plants, reflecting an energetic imbalance in these plants. Apparently this imbalance was not reflected by qP (Fig. 2B), suggesting alternative sinks for electrons that could include photorespiration, water-water cycle, and cyclic electron transport. Increased photorespiration has previously been shown to be an effective photoprotective strategy in high mountain plants under low temperature (Streb et al., 1998). Ivanov et al. (2001) suggested that cyclic electron transport around PSI is necessary to dissipate excess energy and to retain functionality of the photosynthetic apparatus in winter-stressed pine needles. Our observation of a significant increase in β-carotene in SD/HT (Fig. 5) points toward an increased formation of singlet-excited oxygen (1O2*), which can efficiently be quenched by β-carotene (Cantrell et al., 2003; Krieger-Liszkay, 2005; Telfer, 2005). 1O2* is generated from singlet-excited Chl via the Chl triplet state, if the energy of excited Chl cannot be used for photochemistry or dissipated as heat (Adams et al., 2004). There are at least two possible sites of an increased production of 1O2* (Krieger-Liszkay, 2005). It could either originate from the antenna, where singlet Chl cannot be quenched due to low concentrations of zeaxanthin and hence passes its energy down to produce 1O2*, or in the RC of PSII via charge recombination. Here, β-carotene is the principal quencher of excess energy because triplet Chl cannot be quenched directly. In this case, charge recombination would contribute to filling the gap between electrons generated, as measured by the yield and electrons used by CO2 fixation in the SD/HT treatment.
Dissipation of Excess Energy Depends on the Aggregation State of LHCII
Not only did the yield of photochemistry differ between treatments but we also observed different strategies to dissipate energy that is in excess to be used for photochemistry. It has been suggested that high qO in relation to qN is an indicator for high qO (Bukhov et al., 2001; Sane et al., 2003). We found qO/qN values in the LD/HT treatment considerably lower than in the other treatments (Fig. 2C). Previous work also showed that a higher amount of aggregated LHCs is associated with improved photoprotection in overwintering evergreens and other plants (Horton et al., 1991; Ruban et al., 1993; Ottander et al., 1995; Gilmore and Ball, 2000; Krol et al., 2002). Reorganization of pigment-protein complexes, which is indicated by an increased aggregation state of LHCII, was observed in all but the LD/HT control treatment (Fig. 3) and typically coincides with higher qO in these treatments. Short photoperiod, as well as low temperature, seem to provide a separate signal that invokes elements of the cold acclimation pathway, resulting in a structural reorganization of the photosynthetic apparatus. Through a constitutive and nonregulated quenching component facilitated by aggregated antenna complexes, SD/HT plants that have been grown under the same temperature regime as the LD/HT plants do not require to accumulate large quantities of zeaxanthin and hence show a much lower deepoxidation of the xanthophyll cycle pigments. Based on these observations, we conclude that zeaxanthin is not the main component required to facilitate the quenching of excess energy in the SD/HT treatment.
Our observations support the LHCII conformation model for NPQ proposed by Horton et al. (2005). In this model, the amount of quenching is regulated within minutes by the aggregation state of LHCII in combination with binding of either violaxanthin or zeaxanthin, with the highest quenching efficiency resulting from an aggregated configuration binding zeaxanthin. A similar mechanism might be responsible for the acclimation to seasonal changes in photoperiod and temperature (Fig. 7), with the zeaxanthin-binding aggregated state representing the qI component of NPQ (Fig. 7C).
Figure 7.
Model of extended energy quenching including LHCII aggregation. The model depicts the amount of photochemical and NPQ controlled by the deepoxidation state of the xanthophyll cycle and the aggregation state of LHCII, based on the model of Horton et al. (2005). Energy absorbed (yellow arrows) is quenched either nonphotochemically (black arrows) in the antenna complex (green rectangles) or photochemically through the photosystems (white parts, open RCs; hatched parts, closed RCs) and used for CO2 fixation (gray arrows). Depending on the aggregation state of LHCII (represented by the proximity of the green rectangles) and the xanthophyll configuration, energy is preferentially quenched one way or the other (the thickness of the arrows marks the efficiency of the respective processes). State A refers to the situation found in our LD/HT treatment, with a low aggregation state of LHCII in combination with a high deepoxidation state. State B is dominant in the SD/HT treatment, where high aggregation of LHCII coincides with a very low zeaxanthin concentration. This results in nonphotochemical and photochemical quenching that compares to the situation observed in state A, except that not all of the energy provided through the photochemical quenching process is used for CO2 fixation. In this situation, alternative electron sinks, including photorespiration, water-water cycle, and charge recombination, play a prominent photoprotective role to support the photochemical quenching process. State C refers to both the LD/LT and SD/LT treatments, with high aggregation and high deepoxidation states; a large portion of the incident light is quenched in the antenna, photochemical quenching is relatively small. See text for further details.
Does Increased Autumn Air Temperature Increase Photosynthetic Gain?
Our results indicate an experimentally extended growing season does not necessarily result in increased CO2 uptake and carbon gain in an evergreen conifer. On the contrary, short-day photoperiod and warm temperatures might even have the opposite effect due to increased rates of respiration and decreased maximum capacity for carbon uptake. Based on these experiments using seedlings in climate controlled chambers, we cannot predict how short photoperiod and warm autumn temperature might affect entire boreal forests in the future. In addition, in our treatments we used a relatively large temperature difference in the SD/LT (7°C/5°C) versus the SD/HT (22°C/18°C, warm autumn) treatment compared to the predicted increase of mean annual land air surface temperature that is in the range of 4°C to 6°C by the end of 2100 (IPCC, 2001). Nonetheless, our results suggest that increased autumn air temperature has the potential to interrupt the regulation of the seasonal development in conifer trees. One can now speculate that there is a temperature within the range of predicted climate change at which field-grown trees cannot behave optimally and thus cannot exploit the growing season optimally. The temperature increase apparently alters the phasing of the two critical environmental stimuli, thereby not only decreasing the sink capacity of the trees but even turning it into a potential source for the respiratory release of CO2. Thus, photosynthetic down-regulation due to photoperiodic control of growth cessation during the autumn appears to offset any potential carbon gain resulting from a prolonged growing season in the autumn (Saxe et al., 2001). In response to experimentally increased autumn temperature, Jack pine seedlings exhibit an energy-quenching mechanism that does not follow the well-described PsbS- and zeaxanthin-dependent dissipation pathways. Thus, further investigations are essential to determine whether our findings from controlled environment experiments are consistent with climatic changes under natural environmental conditions on an ecosystem scale.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Rooted Jack pine (Pinus banksiana Lamb.) seedlings were obtained from a local nursery (Somerville Seedlings) and planted in a mixture of ProMix (Premier Horticulture) and low nutrient mineral sand (1:2, v/v). The plants were kept outside underneath a light shelter for 1 year. In the second year, plants were transferred to controlled experimental summer conditions at the end of July, 2005 (Conviron growth chambers). Following 10 d at experimental summer conditions (see below) to allow for acclimation to chamber conditions, plants were exposed for 4 weeks to either 22°C/18°C (day/night) with a photoperiod of 16 h (LD/HT), 22°C/18°C with an 8-h photoperiod (SD/HT), 7°C/5°C with a 16-h photoperiod (LD/LT), or 7°C/5°C with an 8-h photoperiod (SD/LT). All treatments were provided with a photosynthetic photon flux density (PPFD) of 350 μmol photons m−2 s−1.
Photosynthetic Gas Exchange
CO2 exchange rates were measured on detached current year needles using a LiCor 6262 infrared gas analyzer connected to a modified LD2/3 cuvette (Hansatech). The needles were collected right before the measurement in the early afternoon, after seedlings had been exposed to growth light of 350 μmol m−2 s−1 for at least 4 h. The CO2 concentration was maintained at 375 ppm in air with 21% O2. Dark respiration was measured in these needles after 20 min of dark adaptation. Subsequently, plants were exposed to a PPFD of 350 μmol m−2 s−1 for 7 min to obtain measurements of steady-state photosynthesis, followed by a shift to a saturating PPFD of 1,000 μmol m−2 s−1 for another 7 min. Steady-state photosynthesis was usually attained within 3 to 6 min, depending on actinic light intensity and temperature. Gas exchange rates are averages over a measuring period of 30 s. All gas exchange measurements were performed at growth temperature.
Chl Fluorescence
Chl a fluorescence was measured with a PAM 2100 Chl fluorometer (Heinz Walz). The fiber optic of the PAM 2100 was connected to the LD2/3 Hansatech cuvette via a custom-made port to allow simultaneous fluorescence and gas exchange measurements after seedlings had been exposed to growth light of 350 μmol m−2 s−1 for at least 4 h (see above). Initial (minimum) PSII fluorescence in the dark-adapted state (F0) and Fm were determined after 20 min of dark adaptation in the cuvette. F0′, Fm′, and transient fluorescence (Ft) were obtained concomitantly with the gas exchange measurements after steady-state photosynthesis was achieved (Sveshnikov et al., 2006). Optimum quantum efficiency of PSII was calculated as Fv/Fm = (Fm − F0)/Fm and the quantum yield of PSII in the light as Fv′/Fm′ = (Fm′ − Ft)/Fm′ (Genty et al., 1989). The fraction of PSII RCs in an open state was estimated as qP = (Fm′ − Ft)/(Fm′ − F0′) (Schreiber and Bilger, 1987). Nonphotochemical and antenna quenching were calculated as qN = 1 − (Fm′ − F0′)/(Fm − F0) and qO = 1 − F0′/F0, according to Rees et al. (1990).
Isolation of Thylakoid Membranes and Separation of Chl-Protein Complexes
Thylakoids of fresh needles were isolated at 4°C according to Krol et al. (2002) by grinding needles in 50 mm Tricine, pH 7.8, containing 0.4 m sorbitol, 10 mm NaCl, 5 mm MgCl2, and 20% (w/v) polyethylene glycol 4000. The ground samples were filtered through Miracloth, followed by three washing steps in double-distilled water, 1 mm EDTA, and washing buffer, containing 50 mm Tricine, pH 7.8, 10 mm NaCl, and 5 mm MgCl2 by centrifugation at 4°C at 5,000g for 10 min. The pellets were solubilized in 300 mm Tricine, pH 8.8, containing 13% (v/v) glycerol, 0.1% (w/v) SDS, and 0.45% (w/v) dodecylmaltoside to give a SDS + dodecylmaltoside:Chl ratio of 20:1 (w/w). The Chl-protein complexes were separated in the dark at 4°C in nondenaturing 1.3 m Tris-HCl polyacrylamide gels with a Tris/Gly running buffer that contained 0.2% (w/v) Deriphat 160. The gels were scanned at 671 nm and the relative amount of Chl in each complex was determined as ratio of the peak area to the total area.
Photosynthetic Pigments
Needle samples for the pigment extraction were taken around noon after seedlings had been exposed for 4 h to growth light of 350 μmol m−2 s−1. Needles were ground to a fine powder in liquid nitrogen and extracted for 2 h in the dark on ice in 100% acetone buffered with NaHCO3. Extracts were separated by HPLC with a Spherisorb ODS-1 analytical column (S.P.E.), modified from Gilmore and Yamamoto (1991) as described in detail by Krol et al. (2002). Total Chl and total carotenoids were estimated spectrophotometrically according to Lichtenthaler (1987). The deepoxidation state was calculated as (0.5A + Z)/(V + A + Z), where V is violaxanthin, A is antheraxanthin, and Z is zeaxanthin.
Protein Extraction, SDS-PAGE, and Immunoblotting
For protein extraction, needles were ground to a fine powder in liquid nitrogen. Forty milligrams of sample were extracted in 800 μL of ice-cold extraction buffer for 15 min on ice followed by 15 min of extraction at room temperature. The extraction buffer consisted of 60 mm Tris-HCl, pH 6.8, containing 4% (w/v) SDS, 15% (w/v) Suc, 20 mm dithiothreitol, and Complete, EDTA-free, proteinase inhibitor cocktail (Roche Diagnostics). Membrane proteins were solubilized for 5 min at 75°C, cooled on ice for 1 min, and then briefly centrifuged to remove debris from the supernatant. The total concentration of extracted protein was determined after Lowry et al. (1951) using the RC DC protein assay kit from Bio-Rad. Protein (7 μg/lane) was loaded and separated electrophoretically at 200 V for 30 min on 10% (w/v) BisTris gels (Nupage, Invitrogen) using the XCell Midi gel system and a MES/SDS buffer system (Invitrogen). Following separation, proteins were transferred to a nitrocellulose membrane (0.2-μm pore size, Bio-Rad) and probed with antibodies against PsbA, PsbS, RbcL, cFBPase, UGPase, the LHC proteins Lhca1, Lhca2, Lhca4, Lhcb1, Lhcb2, and Lhcb5 (Agrisera) as well as against PSI. Goat anti-rabbit and rabbit anti-chicken IgG conjugated with horseradish peroxidase (Sigma-Aldrich) were used as secondary antibodies to allow for chemiluminescent detection of the proteins (ECL detection kit, Amersham) bound to the membrane exposed to x-ray film (Biomax Light, Eastman Kodak). The optical density of each band on the film was quantified using the Scion software package.
Statistics
The effects of daylength and temperature on photosynthetic properties were statistically analyzed by two-way ANOVA at P < 0.05 using SPSS version 14.0. All significant differences mentioned in the text refer to the two-way ANOVA results.
Acknowledgments
The authors are grateful to Dr. P.E. Jensen (The Royal Veterinary and Agricultural University, Copenhagen) for providing the PSI antibody.
This work was supported by the European Union (PhysConFor, Marie-Curie fellowship, contract no. MOIF–CT–2004–002476 to I.E.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Ingo Ensminger (ensminger@mpimp-golm.mpg.de).
Open Access articles can be viewed online without a subscription.
References
- Adams WW, Zarter CR, Ebbert V, Demmig-Adams B (2004) Photoprotective strategies of overwintering evergreens. Bioscience 54 41–49 [Google Scholar]
- Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50 601–639 [DOI] [PubMed] [Google Scholar]
- Bigras FJ, Ryyppö A, Lindström A, Stattin E (2001) Cold acclimation and deacclimation of shoots and roots of conifer seedlings. In FJ Bigras, SJ Colombo, eds, Conifer Cold Hardiness. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 57–88
- Bukhov NG, Heber U, Wiese C, Shuvalov VA (2001) Energy dissipation in photosynthesis: does the quenching of chlorophyll fluorescence originate from antenna complexes of photosystem II or from the reaction center? Planta 212 749–758 [DOI] [PubMed] [Google Scholar]
- Cantrell A, McGarvey DJ, Truscott TG, Rancan F, Bohm F (2003) Singlet oxygen quenching by dietary carotenoids in a model membrane environment. Arch Biochem Biophys 412 47–54 [DOI] [PubMed] [Google Scholar]
- Christersson L (1978) The influence of photoperiod and temperature on the development of frost hardiness in seedlings of Pinus silvestris and Picea abies. Physiol Plant 44 288–294 [Google Scholar]
- Demmig-Adams B, Gilmore AM, Adams WW (1996) Carotenoids. 3. In vivo functions of carotenoids in higher plants. FASEB J 10 403–412 [DOI] [PubMed] [Google Scholar]
- Ebbert V, Adams WW, Mattoo AK, Sokolenko A, Demmig-Adams B (2005) Up-regulation of a photosystem II core protein phosphatase inhibitor and sustained D1 phosphorylation in zeaxanthin-retaining, photoinhibited needles of overwintering Douglas fir. Plant Cell Environ 28 232–240 [Google Scholar]
- Ensminger I, Busch F, Huner NPA (2006) Photostasis and cold acclimation: sensing low temperature through photosynthesis. Physiol Plant 126 28–44 [Google Scholar]
- Ensminger I, Sveshnikov D, Campbell DA, Funk C, Jansson S, Lloyd J, Shibistova O, Oquist G (2004) Intermittent low temperatures constrain spring recovery of photosynthesis in boreal Scots pine forests. Glob Change Biol 10 995–1008 [Google Scholar]
- Finazzi G, Johnson GN, Dallosto L, Joliot P, Wollman FA, Bassi R (2004) A zeaxanthin-independent nonphotochemical quenching mechanism localized in the photosystem II core complex. Proc Natl Acad Sci USA 101 12375–12380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron-transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990 87–92 [Google Scholar]
- Gilmore AM, Ball MC (2000) Protection and storage of chlorophyll in overwintering evergreens. Proc Natl Acad Sci USA 97 11098–11101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore AM, Yamamoto HY (1991) Resolution of lutein and zeaxanthin using a non-endcapped, lightly carbon-loaded C-18 high-performance liquid-chromatographic column. J Chromatogr 543 137–145 [Google Scholar]
- Horton P, Ruban A (2005) Molecular design of the photosystem II light-harvesting antenna: photosynthesis and photoprotection. J Exp Bot 56 365–373 [DOI] [PubMed] [Google Scholar]
- Horton P, Ruban AV, Rees D, Pascal AA, Noctor G, Young AJ (1991) Control of the light-harvesting function of chloroplast membranes by aggregation of the lhcii chlorophyll protein complex. FEBS Lett 292 1–4 [DOI] [PubMed] [Google Scholar]
- Horton P, Wentworth M, Ruban A (2005) Control of the light harvesting function of chloroplast membranes: the LHCII-aggregation model for non-photochemical quenching. FEBS Lett 579 4201–4206 [DOI] [PubMed] [Google Scholar]
- Houghton JT, Ding Y, Griggs DJ, Noguer M, van der Linden PJ, Xiaosu D, editors (2001) Climate Change 2001. The Scientific Basis: Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, UK
- Ivanov AG, Sane PV, Krol M, Gray GR, Balseris A, Savitch LV, Oquist G, Huner NPA (2006) Acclimation to temperature and irradiance modulates PSII charge recombination. FEBS Lett 580 2797–2802 [DOI] [PubMed] [Google Scholar]
- Ivanov AG, Sane PV, Zeinalov Y, Malmberg G, Gardestrom P, Huner NPA, Oquist G (2001) Photosynthetic electron transport adjustments in overwintering Scots pine (Pinus sylvestris L.). Planta 213 575–585 [DOI] [PubMed] [Google Scholar]
- Kanervo E, Suorsa M, Aro EM (2005) Functional flexibility and acclimation of the thylakoid membrane. Photochem Photobiol Sci 4 1072–1080 [DOI] [PubMed] [Google Scholar]
- Kleczkowski LA, Geisler M, Ciereszko I, Johansson H (2004) UDP-glucose pyrophosphorylase: an old protein with new tricks. Plant Physiol 134 912–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger-Liszkay A (2005) Singlet oxygen production in photosynthesis. J Exp Bot 56 337–346 [DOI] [PubMed] [Google Scholar]
- Krol M, Hurry V, Maxwell DP, Malek L, Ivanov AG, Huner NPA (2002) Low growth temperature inhibition of photosynthesis in cotyledons of jack pine seedlings (Pinus banksiana) is due to impaired chloroplast development. Can J Bot 80 1042–1051 [Google Scholar]
- Lee HY, Hong YN, Chow WS (2001) Photoinactivation of photosystem II complexes and photoprotection by non-functional neighbours in Capsicum annuum L. leaves. Planta 212 332–342 [DOI] [PubMed] [Google Scholar]
- Li XP, Bjorkman O, Shih C, Grossman AR, Rosenquist M, Jansson S, Niyogi KK (2000) A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 403 391–395 [DOI] [PubMed] [Google Scholar]
- Li XP, Muller-Moule P, Gilmore AM, Niyogi KK (2002) PsbS-dependent enhancement of feedback de-excitation protects photosystem II from photoinhibition. Proc Natl Acad Sci USA 99 15222–15227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148 350–382 [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193 265–275 [PubMed] [Google Scholar]
- Methy M, Gillon D, Houssard C (1997) Temperature-induced changes of photosystem II activity in Quercus ilex and Pinus halepensis. Can J For Res 27 31–38 [Google Scholar]
- Morosinotto T, Baronio R, Bassi R (2002) Dynamics of chromophore binding to Lhc proteins in vivo and in vitro during operation of the xanthophyll cycle. J Biol Chem 277 36913–36920 [DOI] [PubMed] [Google Scholar]
- Muller P, Li XP, Niyogi KK (2001) Non-photochemical quenching: a response to excess light energy. Plant Physiol 125 1558–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munekaga Y, Hashimoto M, Miyaka C, Tomizawa KI, Endo T, Tasaka M, Shikanai T (2004) Cyclic electron flow around photosystem I is essential for photosynthesis. Nature 429 579–582 [DOI] [PubMed] [Google Scholar]
- Niyogi KK (1999) Photoprotection revisited: genetic and molecular approaches. Annu Rev Plant Physiol Plant Mol Biol 50 333–359 [DOI] [PubMed] [Google Scholar]
- Niyogi KK, Li XP, Rosenberg V, Jung HS (2005) Is PsbS the site of non-photochemical quenching in photosynthesis? J Exp Bot 56 375–382 [DOI] [PubMed] [Google Scholar]
- Oquist G, Huner NPA (2003) Photosynthesis of overwintering evergreen plants. Annu Rev Plant Biol 54 329–355 [DOI] [PubMed] [Google Scholar]
- Osmond CB, Grace SC (1995) Perspectives on photoinhibition and photorespiration in the field: quintessential inefficiencies of the light and dark reactions of photosynthesis. J Exp Bot 46 1351–1362 [Google Scholar]
- Ottander C, Campbell D, Oquist G (1995) Seasonal changes in photosystem-II organization and pigment composition in Pinus sylvestris. Planta 197 176–183 [Google Scholar]
- Rees D, Noctor GD, Horton P (1990) The effect of high-energy-state excitation quenching on maximum and dark level chlorophyll fluorescence yield. Photosynth Res 25 199–211 [DOI] [PubMed] [Google Scholar]
- Repo T, Nilsson JE, Rikala R, Ryyppö A, Sutinen ML (2001) Cold hardiness of Scots pine (Pinus sylvestris L.). In FJ Bigras, SJ Colombo, eds, Conifer Cold Hardiness. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 463–493
- Ruban AV, Young AJ, Horton P (1993) Induction of nonphotochemical energy-dissipation and absorbency changes in leaves: evidence for changes in the state of the light-harvesting system of photosystem-II in vivo. Plant Physiol 102 741–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sane PV, Ivanov AG, Hurry V, Huner NPA, Oquist G (2003) Changes in the redox potential of primary and secondary electron-accepting quinones in photosystem II confer increased resistance to photoinhibition in low-temperature-acclimated Arabidopsis. Plant Physiol 132 2144–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitch LV, Harney T, Huner NPA (2000) Sucrose metabolism in spring and winter wheat in response to high irradiance, cold stress and cold acclimation. Physiol Plant 108 270–278 [Google Scholar]
- Savitch LV, Leonardos ED, Krol M, Jansson S, Grodzinski B, Huner NPA, Oquist G (2002) Two different strategies for light utilization in photosynthesis in relation to growth and cold acclimation. Plant Cell Environ 25 761–771 [Google Scholar]
- Saxe H, Cannell MGR, Johnsen B, Ryan MG, Vourlitis G (2001) Tree and forest functioning in response to global warming. New Phytol 149 369–399 [DOI] [PubMed] [Google Scholar]
- Scheibe R, Backhausen JE, Emmerlich V, Holtgrefe S (2005) Strategies to maintain redox homeostasis during photosynthesis under changing conditions. J Exp Bot 56 1481–1489 [DOI] [PubMed] [Google Scholar]
- Schreiber U, Bilger W (1987) Rapid assessment of stress effects on plant leaves by chlorophyll fluorescence measurements. In JD Tenhunen, FM Catarino, OL Lange, WC Oechel, eds, Plant Response to Stress. Functional Analysis in Mediterranean Ecosystems, Vol 15. NATO ASI Series. Springer, Berlin, pp 27–53
- Serreze MC, Walsh JE, Chapin FS, Osterkamp T, Dyurgerov M, Romanovsky V, Oechel WC, Morison J, Zhang T, Barry RG (2000) Observational evidence of recent change in the northern high-latitude environment. Clim Change 46 159–207 [Google Scholar]
- Strand A, Zrenner R, Trevanion S, Stitt M, Gustafsson P, Gardeström P (2000) Decreased expression of two key enzymes in the sucrose biosynthesis pathway, cytosolic fructose-1,6-bisphatase and sucrose phosphate synthase, has remarkably different consequences for photosynthetic carbon metabolism in transgenic Arabidopsis thaliana. Plant J 23 759–770 [DOI] [PubMed] [Google Scholar]
- Streb P, Shang W, Feierabend J, Bligny R (1998) Divergent strategies of photoprotection in high-mountain plants. Planta 207 313–324 [Google Scholar]
- Sveshnikov D, Ensminger I, Ivanov AG, Campbell DA, Lloyd J, Funk C, Hüner NPA, Öquist G (2006) Excitation energy partitioning and quenching during cold acclimation in Scots pine. Tree Physiol 26 325–336 [DOI] [PubMed] [Google Scholar]
- Telfer A (2005) Too much light? How beta-carotene protects the photosystem II reaction centre. Photochem Photobiol Sci 4 950–956 [DOI] [PubMed] [Google Scholar]
- Weiser CJ (1970) Cold resistance and injury in woody plants: knowledge of hardy plant adaptations to freezing stress may help us to reduce winter damage. Science 169 1269–1278 [DOI] [PubMed] [Google Scholar]
- White A, Cannell MGR, Friend AD (2000) The high-latitude terrestrial carbon sink: a model analysis. Glob Change Biol 6 227–245 [Google Scholar]
- Zarter CR, Adams WW, Ebbert V, Adamska I, Jansson S, Demmig-Adams B (2006) Winter acclimation of PsbS and related proteins in the evergreen Arctostaphylos uva-ursi as influenced by altitude and light environment. Plant Cell Environ 29 869–878 [DOI] [PubMed] [Google Scholar]