Abstract
Seed dormancy is a common phase of the plant life cycle, and several parts of the seed can contribute to dormancy. Whole seeds, seeds lacking the testa, embryos, and isolated aleurone layers of Arabidopsis (Arabidopsis thaliana) were used in experiments designed to identify components of the Arabidopsis seed that contribute to seed dormancy and to learn more about how dormancy and germination are regulated in this species. The aleurone layer was found to be the primary determinant of seed dormancy. Embryos from dormant seeds, however, had a lesser growth potential than those from nondormant seeds. Arabidopsis aleurone cells were examined by light and electron microscopy, and cell ultrastructure was similar to that of cereal aleurone cells. Arabidopsis aleurone cells responded to nitric oxide (NO), gibberellin (GA), and abscisic acid, with NO being upstream of GA in a signaling pathway that leads to vacuolation of protein storage vacuoles and abscisic acid inhibiting vacuolation. Molecular changes that occurred in embryos and aleurone layers prior to germination were measured, and these data show that both the aleurone layer and the embryo expressed the NO-associated gene AtNOS1, but only the embryo expressed genes for the GA biosynthetic enzyme GA3 oxidase.
The seeds of most angiosperms are dormant at maturity, and dormancy must be lost before germination can occur (Bewley, 1997). This pause in the plant life cycle allows germination to occur under conditions favorable for growth of the seedling and in a season that provides sufficient time for completion of the next generation. Dormancy is a property of an intact seed, but several parts within the seed can contribute to seed dormancy (Bewley, 1997; Koornneef et al., 2000; Finch-Savage and Leubner-Metzger, 2006). In many cases, seed coverings, such as remnants of the fruit, the testa, and the endosperm, are significant barriers to embryo outgrowth (Groot and Karssen, 1987; Sanchez et al., 1990; Dahal et al., 1997; Debeaujon et al., 2000). This seed coat-imposed dormancy is widespread and more common than true embryo dormancy, where the embryo fails to initiate growth even when removed from the constraints imposed by the seed coverings (Bewley and Black, 1994; Ogawa et al., 2003). Seed coats are thought to restrain the growth of the embryo, and weakening of the seed coats, perhaps combined with an increase in the growth potential of the embryo axis, can result in radicle protrusion (Nabors and Lang, 1971). The contribution of seed coat weakening relative to increased embryo growth potential for dormancy loss, however, remains controversial (see discussion in Gong et al., 2005). Arabidopsis (Arabidopsis thaliana) is like many seeds in that both the embryo and seed coats have been implicated in the control of dormancy and germination (Bewley and Black, 1994; Debeaujon and Koornneef, 2000; Finch-Savage and Leubner-Metzger, 2006; Müller et al., 2006).
Dormancy is genetically determined, and seeds with some genotypes are dormant after months or years of dry storage, whereas seeds with other genotypes lose dormancy within weeks (Koornneef et al., 2000). This process of dormancy loss can be hastened or slowed by environmental conditions. For example, a period of cold and damp, referred to as stratification, often removes dormancy, but seeds may be more dormant when imbibed at temperatures of 28°C to 37°C (Bewley and Black, 1994).
The molecular and biochemical parameters that underlie seed dormancy remain unknown despite a century of research in this area. Genetic evidence indicates strongly that abscisic acid (ABA) is central to the establishment and maintenance of seed dormancy (Hilhorst and Karssen, 1992; Jullien et al., 2000) and that gibberellin (GA) is important for germination (Debeaujon and Koornneef, 2000; Ogawa et al., 2003; Kucera et al., 2005). Recent data have shown that nitric oxide (NO) is a likely component of a signaling pathway that promotes a loss of dormancy (Bethke et al., 2004b, 2006b). Data from Arabidopsis suggest that NO might decrease the sensitivity of seeds to ABA (Bethke et al., 2006a), but a relationship between NO and GA signaling has not been established.
Arabidopsis has been the subject of research on dormancy and germination for several decades. Arabidopsis ecotypes vary widely in their depth of dormancy (Clerkx et al., 2004). Dormancy in dry seeds is lost after a few weeks at 25°C for seeds of the Columbia (Col) and Landsberg erecta (Ler) ecotypes, but several months are required for seeds of the C24 ecotype to become nondormant. Seeds of highly dormant ecotypes such as Cape Verde Islands (Cvi) and Kashmir-2 (Kas2) require almost 1 year to fully after ripen (Clerkx et al., 2004). The optimal germination temperature for Arabidopsis is approximately 15°C, and seeds imbibed at higher temperatures may have increased dormancy.
The seed coats of Arabidopsis consist of the dead testa and a layer of living aleurone cells. The development and structure of the testa has been described in detail (Windsor et al., 2000; Debeaujon et al., 2003). The testa arises from cells of the inner and outer integuments of the ovule, and the outer cell layer of the outer integument produces mucilage and thickened cell walls, which give the outer surface of the seed its characteristic pattern (Windsor et al., 2000). Proanthocyanadin pigments are deposited in the testa and contribute to Arabidopsis seed dormancy (Debeaujon et al., 2000, 2001, 2003). The living, single-cell-layered aleurone is the sole endosperm tissue, and it accumulates storage lipid and protein during seed maturation (Declercq et al., 1990; Penfield et al., 2004). A role for the Arabidopsis aleurone layer in supplying sugars to the growing seedling has been described (Penfield et al., 2004), and mutants with impaired lipid metabolism in the aleurone layer and embryo have been identified that show defects in germination and early seedling growth (Eastmond et al., 2000; Footitt et al., 2002). The mobilization of stored lipid in the Arabidopsis aleurone layer requires GA and is not blocked by ABA (Penfield et al., 2004).
Here we report on experiments designed to examine the contribution of the Arabidopsis embryo, aleurone layer, and testa to seed dormancy and to determine where in the seed NO is perceived. The data indicate that under conditions of high water potential, the aleurone layer is the most important determinant of seed dormancy. The data also show clearly that the aleurone layer is responsive to NO, as well as to GA and ABA, in ways that are consistent with the physiology of dormancy and germination in this species.
RESULTS
Arabidopsis Seeds Remain Dormant When the Testa Is Removed
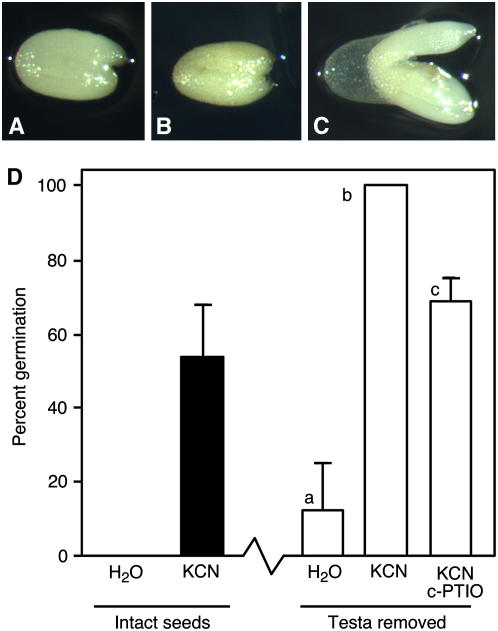
Mature Arabidopsis seeds contain an embryo enveloped in an aleurone layer that is in turn surrounded by the testa. Previous work has shown convincingly that pigments in the testa contribute significantly to seed dormancy (Debeaujon et al., 2000). Removal of the testa from imbibed, dormant C24 Arabidopsis seeds, however, was not sufficient to remove seed dormancy as long as an intact aleurone layer was present. These testa-less seeds, such as those shown in Figure 1, A to C, could remain viable and dormant for over 1 month without germinating or greening when incubated on agarose in the light. Dormancy was lost when the aleurone layer was damaged or removed (see below). To demonstrate that testa-less seeds retained the capacity to germinate, they were treated with vapors from a KCN solution. In a previous report, it was shown that KCN vapors effectively reduced Arabidopsis seed dormancy (Bethke et al., 2006b). Testa-less seeds treated with KCN vapors germinated 100% after 5 d, whereas testa-less seeds exposed to water vapor failed to germinate readily, and only 10% germinated within 5 d (Fig. 1D). Previous experiments with the NO scavenger 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (c-PTIO) suggested that NO participates in the loss of Arabidopsis seed dormancy resulting from KCN treatment (Bethke et al., 2006b). The germination of testa-less seeds treated with KCN vapor was reduced by 100 μm c-PTIO, suggesting that NO plays a role in that process as well (Fig. 1D).
Figure 1.
Arabidopsis seeds remain dormant when the testa is removed but germinate when exposed to cyanide vapors. Seeds lacking the testa are shown in A to C 3 d (A) and 28 d (B and C) after testa removal. The seed in C was exposed to KCN vapors for 3 d beginning 25 d after testa removal. D, Germination percentages after 5 d for intact seeds exposed to water vapor or KCN vapor for 2 d, and for seeds with the testa removed exposed to water vapor, KCN vapor, or KCN vapors in the presence of c-PTIO. Each experiment was repeated at least three times with five to 14 seeds per treatment. Bars with different lowercase letters are significantly different with P < 0.05.
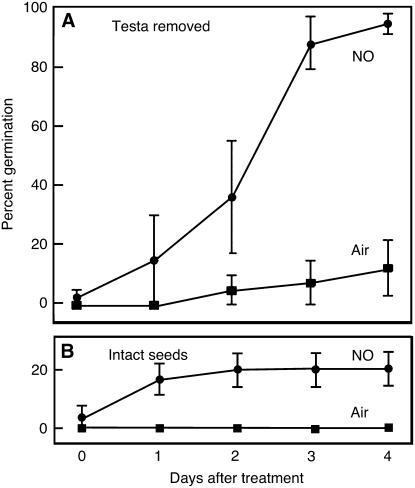
We tested whether testa-less seeds responded to NO by exposing them to gaseous NO, as described previously (Libourel et al., 2006). As shown in Figure 2A, over 90% of testa-less seeds germinated within 4 d of treatment with NO gas compared with less than 20% for similar testa-less seeds treated with air. The effect of NO gas on testa-less seeds was much more dramatic than the effect on intact seeds treated for 48 h with the same gas mixture (Fig. 2B). Whereas germination percentages 4 d after a 24-h treatment with NO were approximately 95% for testa-less seeds, they were only about 20% for intact seeds treated for 48 h (Fig. 2B). It was observed, however, that testa-less C24 seeds were less dormant than intact seeds, because about 15% of testa-less seeds germinated 4 d after being exposed to air for 24 h, whereas 0% of intact seeds exposed to air germinated at this time. These experiments with testa-less seeds demonstrate that the major determinant of seed dormancy resides in the aleurone layer and/or embryo and that either the aleurone layer or embryo was responsive to KCN and NO. The fact that testa-less seeds (Figs. 1D and 2A) are less dormant than intact seeds in the absence of KCN or NO treatment (Figs. 1D and 2B) confirms prior observations showing that the testa contributes to dormancy in Arabidopsis (Debeaujon et al., 2000), perhaps by acting as a barrier against passage of small molecules.
Figure 2.
Germination of Arabidopsis seeds lacking the testa is stimulated by NO gas. Purified NO gas or air were passed over seeds with the testa removed for 1 d (A) or over intact seeds for 2 d (B), and germination was scored every day for 4 d. Data are means ± se for six to 17 seeds.
Embryos Removed from Dormant Arabidopsis Seeds Are Not Dormant
To differentiate between components of seed dormancy that originate in the embryo and those that originate in the aleurone layer, the responses of isolated embryos and isolated seed coats were studied. Seed coats consisted of the living aleurone layer and the dead, adhering testa, and throughout this article we refer to these as Arabidopsis aleurone layers. This terminology is consistent with that used for cereal aleurone layers, which are the aleurone layer with adhering testa and pericarp.
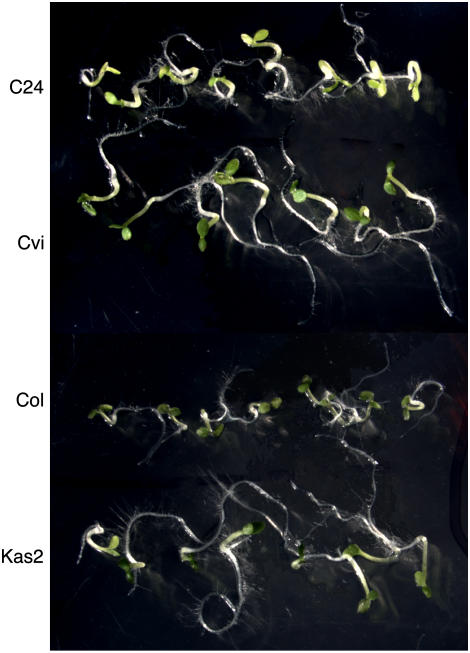
Figure 3 shows photographs of embryos that were removed from several ecotypes of Arabidopsis seeds within hours of imbibition and then incubated on agarose. The Col seeds used for this experiment were fully after ripened and showed no dormancy. The C24, Cvi, and Kas2 ecotype seeds were dormant and had final germination percentages of less than 5%. Regardless of the dormancy status of the seed, however, none of these seeds had true embryo dormancy. All isolated embryos grew and greened within 3 to 4 d of removal from the seed coats. Indeed, under the conditions of our experiment, embryos from highly dormant Cvi and Kas2 ecotypes grew more vigorously than embryos from nondormant Col seeds (Fig. 3).
Figure 3.
Embryos from dormant C24, Cvi, and Kas2 ecotype Arabidopsis seeds and from nondormant Col ecotype seeds grow when removed from the seed. All embryos were photographed 5 d after isolation. [See online article for color version of this figure.]
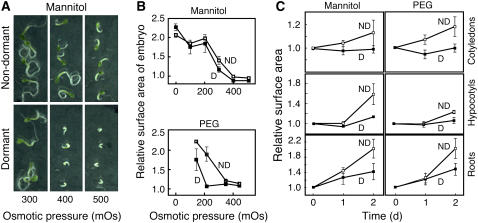
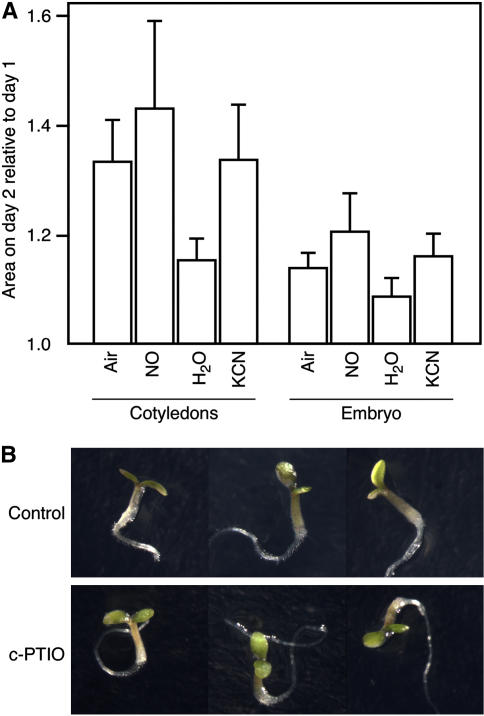
We compared the ability of embryos from dormant and nondormant C24 seeds to grow on agarose containing mannitol or polyethylene glycol (PEG) at concentrations up to 500 mOsmol. As expected, higher osmotic potentials slowed the rate of embryo growth, and this is shown in the photographs of embryos grown on mannitol in Figure 4A. Embryos from dormant seeds failed to grow at 400 mOsmol mannitol, while embryos from nondormant seeds were able to grow at 500 mOsmol mannitol (Fig. 4A). Embryo growth was quantified by measuring changes in surface area, and these data are plotted in Figure 4B for the embryo as a whole, and in Figure 4C for the cotyledons, hypocotyls, and roots individually. With increasing PEG or mannitol concentration, growth of dormant and nondormant embryos was slowed (Fig. 4B). Embryos from nondormant C24 seed had a greater capacity for growth at osmotic potentials above 200 mOsmol PEG than embryos from dormant seeds. At 200 mOsmol PEG, the embryos from nondormant seeds doubled in size over 2 d, while embryos from dormant seeds grew by less than 10%. Differences in growth rate on mannitol were largest at 300 mOsmol, where embryos from nondormant seeds grew twice as fast as embryos from dormant seeds. Differences in growth in response to 300 mOsmol mannitol and 300 mOsmol PEG were examined in more detail, as shown in Figure 4C. Differences in growth between embryos from dormant and nondormant seeds were particularly large for cotyledons and hypocotyls. These organs showed negligible growth in embryos from dormant seeds (<5%), but much more rapid growth was observed in embryos from nondormant seeds. The root, on the other hand, grew in embryos from dormant and nondormant seeds, but changes in root surface area in embryos from nondormant seeds were more than double those in dormant seeds.
Figure 4.
Embryos from nondormant seeds grow more rapidly than embryos from dormant seeds when imbibed on media containing mannitol or PEG as osmotica. A, Micrographs of embryos 7 d after removal from nondormant or dormant C24 Arabidopsis seeds. B, Surface area of isolated embryos growing on a substrate containing up to 500 mOsmol mannitol or PEG for 2 d relative to the surface area on day 0. C, Relative surface area of cotyledons, hypocotyls, and roots 1 or 2 d after placing freshly isolated embryos from dormant (D) or nondormant (ND) C24 Arabidopsis seeds on 300 mOsmol mannitol or 300 mOsmol PEG.
The difference in the ability of embryos from dormant and nondormant seeds to grow on plates containing an osmoticum raised the question of whether dormancy-breaking treatments increase the growth potential of the embryo. To test this hypothesis, dormant seeds were exposed to KCN vapors or to NO for 2 d using the same conditions described for testa-less seeds. Embryos isolated from these seeds were placed on agarose containing an osmoticum (300 mOsmol mannitol). The data presented in Figure 5A show that treatment of intact seeds with KCN vapors in an enclosed chamber or NO gas in a flowing gas stream resulted in increases in the mean growth of embryos from dormant seeds, but the rate of growth was not statistically different from controls treated with water vapor or air, respectively. A subset of embryos treated with NO gas or KCN vapor grew at more rapid rates than controls, however, and this might suggest that some embryos commit to germination and increased growth potential more readily than others. Differences between the water and air controls were unexpected but may be the result of decreased temperatures or decreased accumulation of volatile compounds in the flowing air controls relative to the enclosed water controls.
Figure 5.
The mean growth potential of embryos from dormant Arabidopsis seeds is not changed significantly following treatment of seeds with NO gas or cyanide vapors, or embryos with the NO scavenger c-PTIO. A, Change in relative surface area of the cotyledons or the whole embryo for embryos removed from seeds exposed to air, NO gas, water vapor, or cyanide vapor for 2 d. Isolated embryos were imbibed on agarose containing 300 mOsmol mannitol. Day 1 is 1 d after the end of the treatment period (n > 10). B, Embryos 5 d after isolation from dormant C24 Arabidopsis seeds. Embryos were placed on water agarose without (control) or with 100 μm c-PTIO. [See online article for color version of this figure.]
We also tested the hypothesis that NO is required for embryo growth by treating embryos isolated from dormant seeds with the NO scavenger c-PTIO. No significant reduction in growth was observed when embryos were placed on agarose containing c-PTIO compared to controls on agarose alone (Fig. 5B). These data indicate that the initiation and continuation of embryo growth do not require NO, and they are consistent with our previous observations showing that c-PTIO does not inhibit germination of nondormant Arabidopsis seeds (Bethke et al., 2004b).
The data showing that c-PTIO did not inhibit the growth of embryos isolated from dormant seeds is inconsistent with previous data demonstrating that c-PTIO strengthened seed dormancy for Arabidopsis and raised the possibility that the primary NO-dependent step that contributes to the loss of seed dormancy in Arabidopsis takes place in the aleurone layer. To test this hypothesis, we undertook a detailed investigation of the Arabidopsis aleurone layer that allowed us to examine the responses of isolated aleurone layers to c-PTIO, as well as to the plant growth regulators GA and ABA.
The Ultrastructure of Arabidopsis Aleurone Cells Is Similar to That of Cereal Aleurone Cells
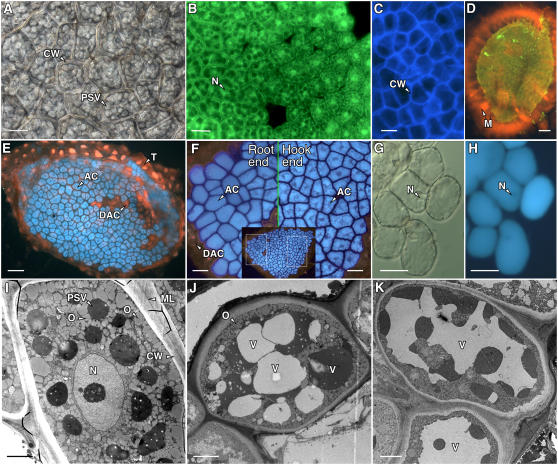
As illustrated by the light, fluorescence, and electron micrographs in Figure 6, the ultrastructure of mature Arabidopsis aleurone cells is very similar to that of the well-characterized cereal aleurone cell (Jones, 1969; Jones and Price, 1970). Each cell is typically an irregular polygon approximately 20 μm long and 10 to 20 μm wide. Arabidopsis aleurone cells are surrounded by thick cell walls (Fig. 6, A and I) that contain cellulose, as determined by staining with Calcofluor white M2R (Fig. 6C). Electron microscopy shows that the cell wall can be resolved into at least three regions: a middle lamella, a less densely stained region adjacent to the plasma membrane, and a darkly stained region that forms the bulk of the wall matrix (Fig. 6, I–K).
Figure 6.
Cells in the Arabidopsis endosperm are typical aleurone cells. Differential interference contrast (A and G), fluorescence (B–F, H), and electron microscopy (I–K) images of Arabidopsis aleurone layers. A, Region of an aleurone layer showing aleurone cells surrounded by a thick cell wall (CW) and containing numerous PSVs. B, Aleurone layer labeled with the vital fluorescent probe fluorescein diacetate showing green fluorescence from the cytosol and nucleus (N) of live cells. C, Cellulose in the Arabidopsis aleurone cell wall labeled with calcofluor white M2R. D, An extensive mucilage layer (M) that was labeled with orange fluorescent Congo red was retained by the testa cells associated with the isolated aleurone layer. E, Aleurone cells (AC) incubated with monochlorobimane accumulated blue fluorescent bimane in their PSV. The testa and nuclei in dead aleurone cells (DAC) in E fluoresced red after labeling with propidium iodide. F, Cells in Arabidopsis aleurone layers closest to the root became less angular in shape sooner than cells near the hypocotyl hook. Shown are higher magnification images that correspond to the boxed regions of the single aleurone layer shown in the inset. G, High magnification image of individual aleurone cells close to the site of root emergence. Cell walls had begun to separate, and individual cells had become nearly spherical. H, Cells in G are still alive, as indicated by the fluorescence from the vacuole and lack of propidium iodide fluorescence from the nucleus (N). I, Electron micrograph of an aleurone cell 1 h after imbibition showing the numerous PSVs and oleosomes (O). The aleurone cell wall and middle lamella (ML) are also indicated. J and K, Aleurone cells 24 h after imbibition. Note the enlargement of the vacuoles (V) and reduction in the number of oleosomes compared with those in I. The vacuoles in J are heterogeneous with regard to electron density. Scale bars are 2 μm in I to K, 10 μm in A, G, and H, 20 μm in B, C, and F, and 60 μm in D and E.
Numerous protein storage vacuoles (PSVs; Fig. 6, A and I) and oleosomes (Fig. 6I) occupy most of the mature aleurone cell volume. Note that PSVs, but not oleosomes, can be resolved by light microscopy. In seeds imbibed for 1 h, the PSVs are approximately 2 to 4 μm in diameter, and the oleosomes are approximately 0.5 μm in diameter. The nucleus is approximately 4 μm in diameter and is centrally located in a mature aleurone cell (Fig. 6, B and I) but following imbibition is pushed to the periphery by the expanding vacuole (Fig. 6, G and H). The coalescence of smaller PSV into one large central vacuole is illustrated in Figure 6, I to K. A thick layer of mucilage (Fig. 6D) remains attached to the testa cells of the isolated aleurone layer (Fig. 6E). Like barley (Hordeum vulgare) aleurone cells (Swanson et al., 1998), Arabidopsis aleurone cells accumulate monochlorobimane in their PSVs (Fig. 6, E and F). Dead cells within Arabidopsis aleurone layers were readily identified because dead cells did not retain autofluorescent pigments within the PSVs, did not accumulate monochlorobimane, and the nuclei of dead cells stained readily with propidium iodide (Fig. 6, E and F).
Arabidopsis Aleurone Cells Undergo Characteristic Changes in Ultrastructure in Seeds That Will Germinate
Cereal aleurone cells have been characterized in depth at the molecular, biochemical, and ultrastructural levels, but little is known about structure, function, and regulation of mature Arabidopsis aleurone cells. To learn more about the potential role of the Arabidopsis aleurone layer in seed dormancy and germination, we looked for responses in Arabidopsis aleurone layers that were similar to those in cereal aleurone cells. For example, cereal aleurone cells secrete enzymes that digest their cells' walls. Fluorescence microscopy was used to observe changes in Arabidopsis aleurone cells following imbibition of nondormant seeds. These observations showed that cells near the radicle tip became less angular in seeds at or after the time of germination (compare root end to hypocotyl hook end in Fig. 6F), suggesting that thinning and weakening of the cell walls was occurring. After several days of imbibition, some cells near the radicle tip became almost completely spherical, and only a thin band of cell wall remained (Fig. 6, G and H).
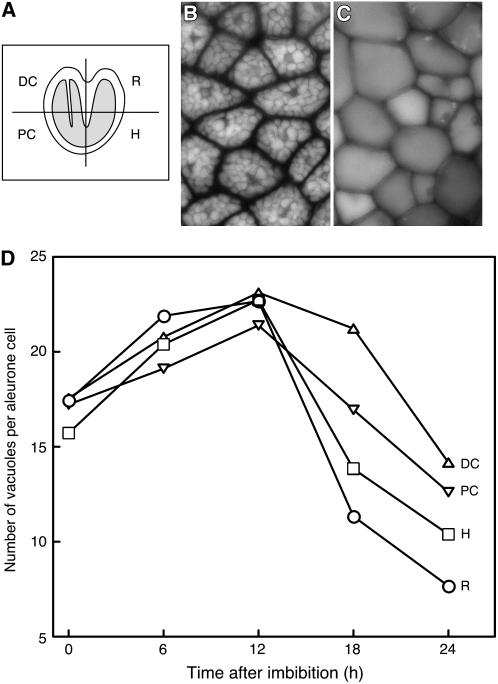
Cereal aleurone cells also undergo a process of increasing vacuolation whereby smaller protein-filled PSVs coalesce to form a single large vacuole. This process of vacuolation has been used as a semiquantitative marker for GA-dependent events (Bush et al., 1986; Ritchie et al., 1999). We quantified the number of vacuoles per aleurone cell at various times after imbibition of Arabidopsis seeds to determine if a similar vacuolation process occurs in the Arabidopsis aleurone cell. Nondormant Col seeds begin to germinate approximately 24 h after imbibition. We removed aleurone layers from Col seeds that had been imbibed for up to 24 h and determined the average number of vacuoles per cell in each of the four regions indicated in Fig. 7A. Vacuoles were easily observed by fluorescence microscopy, because endogenous pigments in the vacuole fluoresce when illuminated with UV light, as seen in Figure 7, B and C. Aleurone cells in seeds imbibed for 1 h had many PSV per cell (Fig. 7B), while those imbibed for extended times had one or a few larger vacuoles per cell (Fig. 7C). As plotted in Figure 7D, the number of PSV per cell was a dynamic parameter. Additional PSV were formed during the first 12 h after imbibition, and the number of PSV per cell increased from approximately 16 to 22. At later times, there was a progressive reduction in the number of PSV, but the rate of vacuolation depended on the position of the aleurone cell relative to the embryo. The rate of PSV loss was greatest for those cells proximal to the root tip (Fig. 7D). This is the region of the aleurone layer that ruptures first during germination. Cells adjacent to the hypocotyl vacuolated more slowly and the cells adjacent to the cotyledons more slowly still. The cells adjacent to the distal end of the cotyledons lost PSVs least rapidly. At 18 and 24 h after imbibition, there were significantly fewer vacuoles per cell in cells adjacent to the root than in the other three regions of the aleurone layer (P < 0.05 in Student's t test).
Figure 7.
Aleurone cells in nondormant Col Arabidopsis seeds vacuolate prior to germination. A, Schematic of an Arabidopsis seed illustrating the four regions of the aleurone layer where the number of PSVs per cell was quantified. In this illustration, the embryo is shaded in gray. Typical images of aleurone cells shortly after imbibition (B) and shortly after germination (C), where cells in B have many PSVs per cell but cells in C have only one. D, Quantification of PSV number 1, 6, 12, 18, and 24 h after seed imbibition. R, Root; H, hypocotyl; PC, proximal end of cotyledons; DC, distal end of cotyledons. Data in D are means of 32 to 266 cells. sds in D range from 4.21 to 7.19, and error bars have been omitted for clarity.
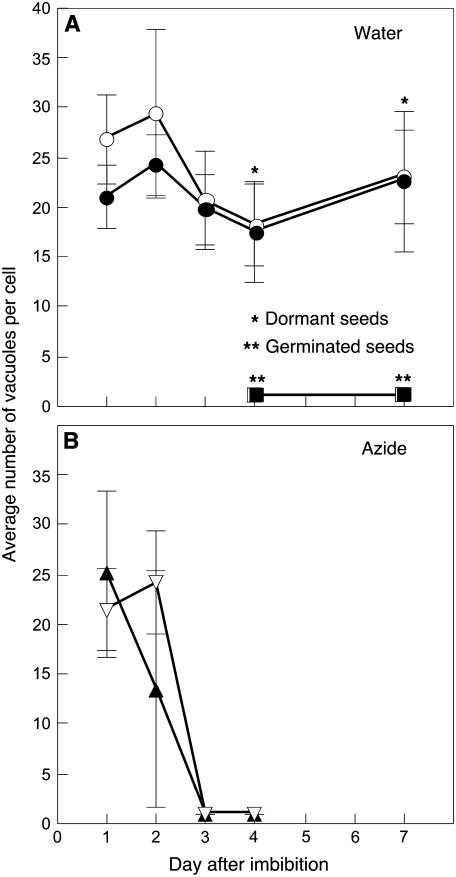
The change in the number of PSVs per cell was very tightly correlated with the dormancy status of the seed. An example is seen in Figure 8, where NaN3 was used to remove dormancy from mildly dormant C24 Arabidopsis seeds. These seeds gave final germination percentages of 12% when imbibed with water alone but over 95% when imbibed on 10 μm NaN3 for 4 d. There was essentially no reduction in the number of PSV per aleurone cell in layers dissected from dormant seeds imbibed in water (Fig. 8A). Seeds imbibed in water that germinated, however, had extensive vacuolation, and 96 h after imbibition, there was one large vacuole per cell. When dormant seeds were treated with 10 μm NaN3 to break dormancy, a reduction in the number of PSV per aleurone cell occurred prior to germination (Fig. 8B). Aleurone layers dissected from NaN3-treated seeds 72 h after imbibition had one large, central vacuole per cell.
Figure 8.
Aleurone cells vacuolate in seeds that germinate. Mildly dormant C24 Arabidopsis seeds were imbibed in water (A) or exposed to 10 μm NaN3 (B), and the number of PSV per aleurone cell was determined. Most of the seeds in A were dormant, but some were not and germinated by 96 h. All of the seeds in B germinated by 96 h. White and black symbols represent the aleurone layer adjacent to the root/hypocotyl and cotyledons, respectively. Note that vacuolation of aleurone cells was tightly correlated with dormancy loss.
Changes in PSV Occur after Aleurone Layers Are Isolated from the Seed and These Changes Are Ecotype Dependent
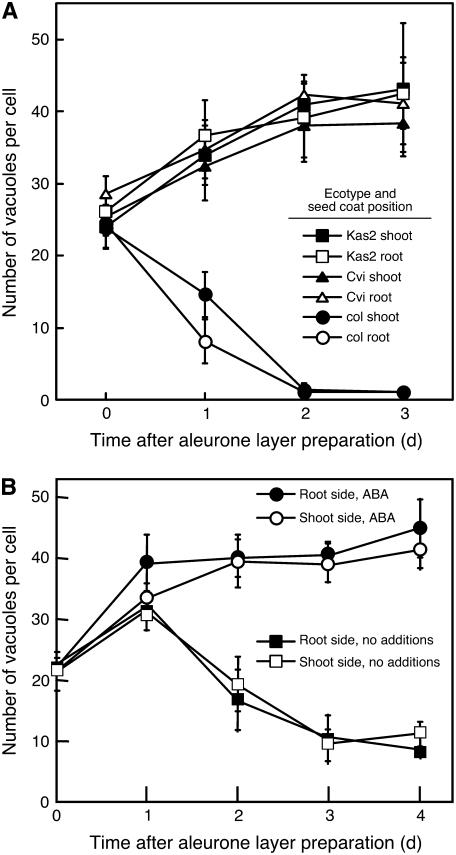
When aleurone layers were removed from seeds that had been imbibed for 1 to 3 h and the isolated layers were incubated on agarose, the aleurone cells in them showed time-dependent changes in PSV size and number. This process, as seen in Figure 9, was highly dependent on the ecotype used. The number of PSV declined in aleurone cells from Col seeds during the first 48 h after isolation with kinetics that were only a little slower than observed in aleurone cells of the intact seed (Fig. 9A). As in intact Col seeds, PSVs in cells closest to the root/hypocotyl vacuolated more rapidly than in cells adjacent to cotyledons. Cells in aleurone layers isolated from highly dormant Kas2 and Cvi ecotype seeds, however, showed a very different response. In this case, new PSV were formed, and the number of PSV per cell increased with time from approximately 25 in the mature seed to approximately 45 after 3 d of imbibition (Fig. 9A). The cells in aleurone layers from dormant C24 seeds showed yet another pattern of vacuolation (Fig. 9B). The number of PSV per cell increased for the first day but decreased thereafter, such that by day 4, there were approximately 10 vacuoles per cell.
Figure 9.
Vacuole number per cell is a dynamic parameter in aleurone layers incubated in vitro. The average number of vacuoles per cell between 0 and 4 d after removal of the aleurone layer from an imbibed seed is shown in A for the Col, Kas2, and Cvi ecotypes, and in B for the C24 ecotype of Arabidopsis. The Col ecotype seeds were nondormant, and the Kas2, Cvi, and C24 ecotype seeds were dormant. Aleurone layers in B were incubated on agarose with and without 10 nm ABA. In both A and B, data are presented for that part of the aleurone layer proximal to the root/hypocotyl (root side) and that part proximal to the cotyledons (shoot side).
Arabidopsis Aleurone Cells Are Extremely Sensitive to ABA
It was hypothesized that cellular ABA content might affect the vacuolation response of cells in isolated aleurone layers and that this might account for some of the differences observed between the four ecotypes examined in Figure 9. To test this hypothesis, we determined if vacuolation could be prevented by incorporating ABA into the agarose substrate on which isolated aleurone layers were incubated. The result of this experiment is shown in Table I. ABA at concentrations of 1 nm or greater strongly inhibited the vacuolation of C24 aleurone cells. A time course for cells in aleurone layers isolated from dormant C24 Arabidopsis seeds and treated with 10 nm ABA is shown in Figure 9B. It is clear from these data that ABA treatment results in an increase in the number of PSV per cell after 4 d compared to the number per cell in freshly isolated layers or to the number per cell in isolated layers not treated with ABA.
Table I.
ABA prevents the vacuolation of cells in isolated Arabidopsis aleurone layers
Data are means from a single representative experiment.
| ABA Concentration | Layers Showing Vacuolation after 3 d |
|---|---|
| nm | % |
| 0 | 100 |
| 0.1 | 88 |
| 1 | 0 |
| 10 | 0 |
c-PTIO Inhibits Aleurone Cell Vacuolation and GA Counteracts the Effect of c-PTIO
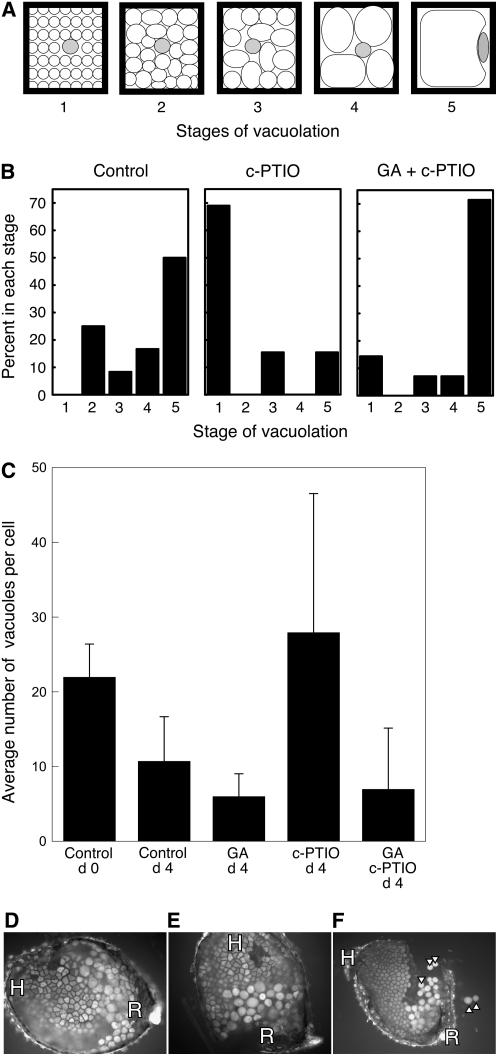
We used the vacuolation of aleurone cells in isolated aleurone layers as an assay to see if this tissue was sensitive to the NO scavenger c-PTIO. For this analysis, we used the semiquantitative assay diagrammed in Figure 10A. Cells with different degrees of vacuolation were assigned to stages on a scale of 1 to 5, with stage 1 being many small vacuoles per cell and stage 5 being one large vacuole that nearly fills the cell. Because vacuolation in isolated layers is relatively uniform, layers were scored from 1 to 5 based on the stage of representative cells within them. Cells in control aleurone layers vacuolated such that 3 d after isolation, approximately 50% of the layers contained mostly stage 5 cells, with the remaining layers having cells distributed in stages 2 to 4 (Fig. 10B). Treatment with 50 μm c-PTIO, however, inhibited the vacuolation process, and 70% of the layers had cells that were predominantly in stage 1. We consistently observed some cells that vacuolated in the presence of 50 μm c-PTIO, however, and these tended to be on the root side of the seed (Fig. 10B; data not shown). Note that the C24 seeds used for this experiment were less dormant than those used for the experiment described in Figure 9. Coincubation of isolated aleurone layers with 10 μm gibberellic acid (GA3) reversed the effects of c-PTIO and resulted in a reduction in the number of PSVs per cell (Fig. 10B). These data suggest that both NO and GA promote the vacuolation of aleurone cells and that NO is upstream of GA in a signaling pathway that leads to vacuolation.
Figure 10.
The NO scavenger c-PTIO inhibits vacuolation of aleurone cells in isolated, wild-type Arabidopsis aleurone layers but not in layers from the Spy-1 mutant. A, The extent of vacuolation for each layer in B was scored based on the stage (1–5) of vacuolation typical for the cells in the layer. A single cell is diagrammed for each stage. B, Vacuolation was delayed in C24 aleurone layers treated with 50 μm c-PTIO, and the effect of c-PTIO was reversed by 10 μm GA3. The data are from a single, representative experiment. C, Vacuolation of cells in aleurone layers from nondormant Ler seeds was stimulated by 10 μm GA3 and inhibited by c-PTIO. Data are means ± sd. D to F, Micrographs of isolated aleurone layers from the root/hypocotyl side of Spy-1 mutant seed showing that cells originally near the root (R) but not the hypocotyl (H) exhibit evidence of vacuolation and wall weakening in the presence of no additions (D) or 100 μm c-PTIO (E and F). Note that the walls of some cells in F were weakened enough that individual cells were released from the layer (arrowheads).
We investigated further the GA responsiveness of Arabidopsis aleurone cells to see if GA could stimulate vacuolation in cells that had not been treated with c-PTIO. The number of PSV per aleurone cell in aleurone layers isolated from nondormant Ler ecotype seeds declined slowly, as is seen in Figure 10C, and approximately 11 PSVs/cell were present after 4 d of incubation. The rate of vacuolation of these cells was stimulated by GA3, such that 4 d after incubation, GA-treated cells had an average of six PSVs/cell. The average number of PSVs per cell on day 4 was significantly less with GA treatment than in controls, as judged by a Student's t test (P < 0.05). As was the case in Figure 10B, vacuolation of Ler aleurone cells was inhibited by c-PTIO, and this could be prevented by treatment with GA (Fig. 10C).
Seeds of the Spy-1 mutant were used to determine if the SPY protein is a component of the GA signaling pathway leading to vacuolation of aleurone cells. The Arabidopsis SPY protein is an O-GlcNAc transferase, and Spy-1 mutant plants have phenotypes similar to wild-type plants that are treated with GA (Jacobsen and Olszewski, 1993). Spy mutant phenotype data suggest that SPY functions as a negative regulator of GA signal transduction (Thornton et al., 1999). Aleurone layers from Spy-1 seeds were indistinguishable from wild type when freshly isolated (data not shown). When Spy-1 aleurone layers were incubated for 4 d in vitro, however, aleurone cells near the root were highly vacuolate and were often enlarged and nearly spherical (Fig. 10D), indicating extensive wall weakening. This process of vacuolation and wall weakening occurred in the absence of added GA and in the presence of 100 μm c-PTIO (Fig. 10, E and F). Cells near the hypocotyl and cotyledons did not show the rapid wall weakening observed near the root (Fig. 10, D and F; data not shown).
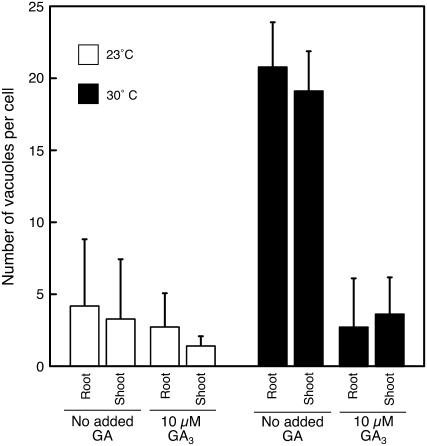
Seeds of Arabidopsis may be increasingly dormant as temperatures increase above 15°C (Baskin and Baskin, 1983). When isolated, Arabidopsis aleurone layers, Col ecotype, were incubated at 30°C, the number of PSV per aleurone cell did not decline, even though it declined for layers incubated at 23°C. These data are plotted in Figure 11. Vacuolation at 30°C was promoted by GA, however, such that cells treated with GA had an average of three to four PSVs/cell after 4 d at 30°C compared with 18 to 20 PSVs/cell for layers not treated with GA (Fig. 11).
Figure 11.
GA promotes the vacuolation of cells in isolated Col aleurone layers incubated at 30°C. Layers were incubated for 2 d in vitro on agarose with or without 10 μm GA3 and at 23°C or 30°C. Note that cells did not vacuolate at 30°C in the absence of GA but did vacuolate at 30°C in the presence of GA. Data are presented for that part of the aleurone layer proximal to the root/hypocotyl (root) and that part proximal to the cotyledons (shoot). Bars are means ± sd.
Changes in Gene Expression Associated with Dormancy and Germination in Arabidopsis Embryos and Aleurone Layers
We used quantitative PCR (qPCR) to monitor changes in gene expression within embryos and aleurone layers isolated from Arabidopsis seeds treated with no additions, KCN vapors, c-PTIO, or KCN vapors and c-PTIO (Fig. 12). For these seeds, expected germination percentages were 0%, 90%, 0%, and 27%, respectively. Because the C24 ecotype seeds that we used for these experiments began to germinate 72 h after treatment with KCN vapors, we isolated embryos and aleurone layers from seeds 1, 24, and 48 h after imbibition.
Figure 12.
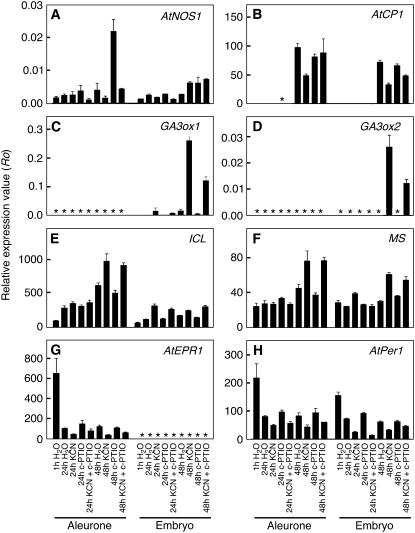
Quantification of relative mRNA abundance for genes associated with dormancy or germination in the aleurone layer and embryo of Arabidopsis seeds. Dormant seeds of C24 Arabidopsis were imbibed on water agarose supplemented with or without 200 μm c-PTIO and exposed to water vapors or KCN (300 μm) vapors. RNA abundance in A to H was quantified using qPCR for samples isolated 1, 24, or 48 h after imbibition and is expressed relative to the abundance of reference genes. Asterisks indicate samples where the gene product was not detected.
To demonstrate that our embryo samples were not contaminated with pieces of the aleurone layer, we looked at the expression of an extensin-like gene, AtEPR1, which had been shown previously to be specifically expressed in the aleurone layer of Arabidopsis (Dubreucq et al., 2000). The data presented in Figure 12G confirm that this gene was expressed in aleurone cells and demonstrate that expression was highest in aleurone layers from seeds 1 h after imbibition, but reduced afterward regardless of treatment (Fig. 12G). AtEPR1 mRNA was not detected in any of the embryo samples.
NO action is required for the loss of Arabidopsis seed dormancy triggered by CN−, nitrate, or nitrite (Bethke et al., 2006b). NO can be synthesized both enzymatically and nonenzymatically (Guo et al., 2003; Bethke et al., 2004a). We quantified the expression of an Arabidopsis gene associated with NO synthesis, AtNOS1 (Guo et al., 2003), in embryos and aleurone layers and found that it was expressed at low levels in both samples (Fig. 12A). Expression was 5 times higher in aleurone layers from seeds treated with c-PTIO for 48 h than in all other samples. AtNOS1 mRNA was at control levels, however, in aleurone layers treated with c-PTIO plus KCN.
Because isolated aleurone layers were responsive to GA (Figs. 10 and 11), and GA is associated with germination, we examined the expression of the GA3ox1 and GA3ox2 genes. These genes encode the final steps in the biosynthesis of active GA in Arabidopsis seeds (Yamaguchi et al., 2001; Ogawa et al., 2003). The data in Figure 12, C and D show that neither gene was expressed at detectable amounts in aleurone layers, but both genes were expressed in the embryo. Expression of GA3ox1 was very low in the embryo in 1-h imbibed seeds, but increased significantly at 24 h in KCN-treated seeds. By 48 h, the GA3ox1 transcript in embryos from KCN-treated seeds was 15 times higher than that in embryos from control seeds. Seeds treated with both KCN and c-PTIO had one-half the amount of GA3ox1 transcript as seeds treated with KCN alone. GA3ox2 expression was detected only in embryos 48 h after imbibition and only in those samples treated with KCN or KCN plus c-PTIO. As for GA3ox1, c-PTIO reduced GA3ox2 expression by 50% compared with KCN alone.
Although aleurone layers did not express either GA3 oxidase gene, this tissue showed strong stimulation of transcription for a putative GA-dependent Cys protease (Fig. 12B; Ogawa et al., 2003). The expression of this gene was similar to that of the GA3 oxidases, with low expression in 1- and 24-h-imbibed seed and high expression at 48 h. There was no clear correlation between mRNA abundance for this Cys protease at 48 h and the dormancy status of the seeds. Indeed, whereas expression of GA3 oxidases in 48-h c-PTIO-treated samples was low, expression of the Cys protease was comparable to controls.
Lipid metabolism is an important contributor to early seedling growth, and links have been made between the regulation of lipid metabolism and seed dormancy (Footitt et al., 2002; Eastmond and Jones, 2005). In Arabidopsis, where both the embryo and aleurone layer have extensive reserves of stored lipid, some mutants with lesions in lipid metabolism also have germination phenotypes (Hooks et al., 2004; Pinfield-Wells et al., 2005). Genes for both isocitrate lyase (ICL; Fig. 12E) and malate synthase (MS; Fig. 12F) were expressed in the embryo and the aleurone layer, and ICL expression was approximately 10 times greater than MS expression in either sample. Transcript abundance varied little with time or treatment, but the amount at 48 h was 1.5 to 2 times as high in seeds treated with KCN alone or KCN and c-PTIO than in seeds with water or c-PTIO alone.
Gene expression was also quantified for the peroxiredoxin AtPer1 (Fig. 12H). Peroxiredoxins have been associated with seed dormancy or an inhibition of germination in several species, including Arabidopsis (Haslekas et al., 2003). This gene showed little regulation with time or treatment in either the aleurone layer or the embryo, except that expression was highest in both samples at 1 h. In both the embryo and the aleurone layer, expression was reduced in samples treated with KCN relative to samples treated without KCN. This same pattern was observed for the extensin-like gene, and this suggests that KCN treatment may further down-regulate some genes that have decreased expression when imbibed with water alone.
DISCUSSION
Seed dormancy is a complex phenomenon that remains poorly understood despite a century of research. The data presented here further our understanding of seed dormancy and seed physiology. In particular, the data show that: (1) the Arabidopsis aleurone layer is sufficient and necessary for the sustained dormancy of imbibed seeds (Figs. 1 and 2); (2) the Arabidopsis aleurone layer perceives and responds to NO during the process of dormancy release (Figs. 1, 2, 10, and 11); (3) NO signaling is upstream of GA signaling in a control pathway that leads to the vacuolation of aleurone cells (Fig. 10); (4) imbibition of intact seeds or isolated aleurone layers results, initially, in the formation of additional PSV (Figs. 7–9); and (5) the accumulation of transcripts for GA biosynthetic enzymes in the embryo (Fig. 12) and the vacuolation of cells in the aleurone layer (Figs. 7 and 8) are early markers for dormancy loss.
Dormancy is a state of an individual seed, and multiple components within each seed can contribute to seed dormancy. The data presented here, and prior work by others (Debeaujon and Koornneef, 2000; Debeaujon et al., 2000), suggest that determinants of Arabidopsis seed dormancy reside within the single-cell layered aleurone, the embryo, and the testa. The contribution of the aleurone layer was paramount under the experimental conditions employed here. Removal of the testa did not render seeds nondormant for up to 28 d (Fig. 1), but removal of the aleurone layer or damage to the aleurone layer resulted in embryo growth. These data demonstrate clearly that one function of the Arabidopsis aleurone layer is to maintain the dormancy of imbibed seeds.
Accumulating evidence indicates that NO is a key player in reducing seed dormancy and promoting germination (Bethke et al., 2004b, 2006b, and refs. therein). A site for NO perception leading to dormancy loss, however, has not been identified. Seeds lacking a testa responded to NO or KCN vapors by germinating (Figs. 1 and 2), and this indicated that the site of NO perception was in the aleurone cells or in the embryo. Growth of isolated embryos was insensitive to NO scavenger c-PTIO (Fig. 5B), and neither NO gas nor KCN vapors increased significantly the growth potential of embryos in dormant seeds (Fig. 5A). Experiments with the NO scavenger and isolated aleurone layers, on the other hand, demonstrated that the aleurone layer perceives and responds to NO because vacuolation of isolated aleurone layers was inhibited by the NO scavenger (Fig. 10). Because NO is a volatile gas, these data also suggest strongly that Arabidopsis aleurone cells synthesize NO.
ABA and GA are central to seed dormancy and germination. In a previous report, we showed that cyanide vapors reduced the sensitivity of Arabidopsis seeds to ABA (Bethke et al., 2006a). A link between GA and NO, however, had not been identified. The data in Figure 12 show that NO is required for the transcription of GA3ox1 and GA3ox2, two key biosynthetic enzymes for active GA. The data in Figure 10 show that GA is required for the vacuolation of cells in isolated aleurone layers in the absence of NO. When considered together, these three pieces of data suggest that NO may coordinate a reduction in ABA-imposed dormancy with the onset of GA-stimulated germination.
The vacuolation of aleurone cells is regulated by NO, GA, ABA, azide, and temperature (Figs. 9–11) in ways that are completely consistent with the physiology of dormancy and germination for the intact seed. Aleurone cells vacuolated in seeds that would germinate but did not vacuolate in dormant seeds (Fig. 8). We therefore used the vacuolation of aleurone cells as a marker for dormancy loss that could be scored before germination. Our quantification of vacuole number revealed novel aspects of the response of aleurone cells to ABA. In particular, the data from intact seeds and from isolated aleurone layers show that imbibition results, initially, in the formation of additional PSVs (Figs. 7–9). This increase was transient in nondormant Col seeds but sustained in the highly dormant ecotypes Cvi and Kas2. Indeed, the number of vacuoles per cell increased in Cvi and Kas2 layers such that aleurone cells 4 d after imbibition had approximately 60% more vacuoles per cell than aleurone cells in the mature seed. A sustained increase in vacuole number could be brought about in the moderately dormant C24 ecotype by treatment with extremely low concentrations of ABA (Fig. 9). This response suggests that the layers from highly dormant seeds may have higher amounts of ABA than those found in Col, Ler, and C24 seeds. PSV formation is a process that is associated with ABA and occurs during normal seed maturation. A resumption of that process following imbibition has not been documented previously for aleurone cells. The data presented here indicate that seed maturation programs leading to PSV formation are resumed in Arabidopsis aleurone cells upon imbibition and that ABA can stabilize these processes in imbibed, dormant seeds. Consistent with this is the observation that vacuole number begins to decrease between 24 and 48 h after dormant C24 seeds are treated with KCN vapors (Fig. 9), at the time when transcription of GA biosynthetic enzymes is rapidly increasing (Fig. 12).
A key role for the seed coats in regulating germination of a wide variety of other seeds has been reported (Kelly et al., 1992; Müller et al., 2006). Seed coats can affect germination by imposing a physical constraint, containing chemical signals, or contributing chemical signals. For example, it has been demonstrated conclusively in lettuce (Lactuca sativa) that photodormancy is regulated by the mechanical restraint imposed by seed coats (pericarp, testa, and aleurone; Nabors and Lang, 1971). Many reports show that polysaccharide-hydrolyzing enzymes are secreted from endosperm cells, and these enzymes facilitate germination by weakening endosperm cell walls in the vicinity of the radicle (e.g. Leung and Bewley, 1983; Ouellette and Bewley, 1986; Groot and Karssen, 1987; Sanchez et al., 1990; Nonogaki et al., 2000). More recently reactive oxygen intermediates have been shown to be synthesized by seed coats of Raphanus sativus in response to germination stimuli (Schopfer et al., 2001), and there is growing evidence that these molecules can bring about weakening of the cell wall (Schopfer, 2001). Aleurone cell walls in Arabidopsis show clear signs of weakening or modification (Figs. 6, G and H, and 10, D–F) and this is likely to facilitate embryo outgrowth by reducing the physical constraint imposed by the aleurone layer. Significantly, vacuolation and wall modification occur most rapidly in aleurone cells in the region where radicle outgrowth occurs (Figs. 6F, 7, and 10). Reactive oxygen intermediates are attractive candidates for wall modifying agents in the Arabidopsis aleurone. This tissue stores a large amount of lipid that is converted to sugars via the reactions of β-oxidation and the glyoxylate cycle (Penfield et al., 2004). Large amounts of hydrogen peroxide are produced during β-oxidation of fatty acyl chains, and although the glyoxysome contains catalase, considerable hydrogen peroxide is released from the embryo of germinating Arabidopsis seeds (T. Myint and R. Jones, unpublished data).
Seed coats may also function as permeability barriers to exclude or contain substances. The testa may play a particularly important role as a barrier that prevents small molecules from entering or leaving Arabidopsis seeds. Previous work has shown that Arabidopsis seeds from mutants that do not accumulate proanthocyanidins in the testa are less dormant and less able to exclude tetrazolium salts than wild-type seeds (Debeaujon et al., 2000). Data presented here suggest that the testa is also a barrier for NO. Application of NO gas to dormant seeds resulted in approximately 20% germination (Fig. 2B), but application of NO gas to seeds lacking the testa resulted in nearly 100% germination (Fig. 2A). A role for the seed coats in preventing the entry of ABA or in facilitating the degradation of ABA is indicated by data showing that ABA at 1 nm prevented vacuolation of cells in isolated aleurone layers (Table I), but ABA at concentrations of 1 μm or more is required to prevent vacuolation of aleurone cells in intact seeds (data not shown).
An underappreciated function of the seed coats in seed dormancy may be to contain solutes within the apoplast of the seed. In this way, the osmotic potential of apoplastic water can be maintained following imbibition, and seeds whose embryos have insufficient growth potential can remain dormant. In this context, the biophysical properties of the embryo profoundly affect the dormancy status of the seed. The ability of intact seeds to germinate reflects, in part, the ability of the embryo to develop sufficient force to push through the seed coverings. The growth of isolated embryos is likely to reflect a similar capacity for the generation of turgor. We show that isolated embryos from the nondormant C24 ecotype of Arabidopsis have a higher growth potential than embryos from dormant seeds. Isolated embryos imbibed on media having a water potential close to zero grew readily, regardless of seed dormancy status or ecotype (Fig. 3). At lower water potentials, however, embryos from nondormant C24 Arabidopsis grew more rapidly than embryos from dormant seeds, and the water potential at which growth stopped was lower for embryos from nondormant seeds than for dormant seeds (Fig. 4). A reduced capacity for growth at low water potentials, therefore, may be one component of seed dormancy that resides in the Arabidopsis embryo. In their study of the growth biophysics of lettuce embryos, Nabors and Lang (1971) found that PEG was a more effective osmoticum than mannitol, because mannitol penetrated more readily into embryos than PEG. Our data showing that PEG was more effective than mannitol in reducing embryo growth (Fig. 4) may reflect this difference in permeability.
We have used the process of vacuole expansion as an assay for responses associated with dormancy loss in Arabidopsis. Changes in PSVs have been reported in the aleurone or endosperm of cereals (Bethke et al., 1998), dicots (Nonogaki et al., 1998), and in protein-storing cotyledons (Gifford et al., 1983). In cereal aleurone cells, vacuolation has been shown to be a reliable assay for the response of this tissue to GA (Bush et al., 1986; Bethke et al., 1998; Ritchie et al., 1999). There is general agreement that vacuolar storage proteins are proteolyzed and that the resulting amino acids and peptides are used for de novo protein synthesis. In the cereal aleurone layer, proteolysis of stored protein gives rise to secretory proteins such as α-amylases and nucleases (Filner and Varner, 1967; Chrispeels and Varner, 1973). It is probable that the changes in Arabidopsis aleurone PSV that we observed were also linked to the synthesis of secretory proteins, a conclusion that is supported by the changes in vacuoles at the electron microscope level. Some of the proteins secreted from the aleurone are likely to modify the cell wall and facilitate radicle protrusion. This is seen most clearly in aleurone layers from the Spy-1 mutant. Aleurone cells positioned near the root tip of the embryo show wall weakening, and in some cases, individual cells separate from the aleurone layer (Fig. 10, D–F). Significantly, this position-specific response occurs in isolated aleurone layers after they have been separated from the embryo.
We used qPCR to identify molecular markers in the embryo or aleurone layers that are associated with a loss of dormancy and that change in abundance prior to germination. The genes selected for this analysis encode enzymes that are participants in hormone signaling or response pathways (AtNOS1, GA3ox1, GA3ox2, and AtCP1), lipid mobilization (ICL and MS), or other processes associated with germination or dormancy (AtEPR1 and AtPer1). The data in Figure 12 show clearly that there is strong up and down-regulation of gene expression in both the aleurone layer and embryo within 24 h of imbibition (Fig. 12). The effect of KCN on the expression of GA biosynthetic genes is especially noteworthy. KCN treatment caused a 15-fold increase in GA3ox1 and GA3ox2 expression relative to water treated controls at 48 h. KCN breaks dormancy in a wide range of seeds, and we speculate that it does so by increasing GA biosynthesis. This aspect of the action of KCN is being explored further. The GA biosynthetic genes were not expressed in the Arabidopsis aleurone layer, an observation consistent with reports by others (Yamaguchi et al., 2001; Ogawa et al., 2003; Mitchum et al., 2006).
The regulation of AtNOS1 in the aleurone layer is intriguing in that expression was up-regulated in samples treated with c-PTIO for 48 h relative to controls and samples treated with KCN. This suggests that this NO-associated gene is under feedback regulation, and this hypothesis warrants further consideration. ICL and MS exhibited relatively small changes in mRNA abundance with treatment or time, except for ICL between 1 and 24 h where there was a moderate increase in the amount of ICL transcript. Of the other genes examined, AtEPR1 is notable for showing strong down-regulation between 1 and 24 h and for being specifically expressed in the aleurone layer.
The data presented in Figures 6, 7, 10, and 11 and Table I illustrate the striking similarities between Arabidopsis and barley aleurone layers. The cells in these two tissues are remarkably similar at the level of ultrastructure. In both species, the mature aleurone cell is surrounded by a thick cell wall, contains large reserves of lipid in oleosomes, and has numerous PSVs (Fig. 6). Aleurone layers from Arabidopsis and barley both metabolize stored reserves to support growth of the embryo, and both undergo a process of vacuolation that results in a large vacuole occupying most of the volume of the cell. Both Arabidopsis and barley aleurone cells respond to GA and ABA, with GA promoting vacuolation and ABA inhibiting vacuolation. Neither the barley aleurone layer nor the Arabidopsis aleurone layer, however, expresses genes for the synthesis of active GAs. The aleurone layer of Arabidopsis also responds to NO as part of the dormancy loss process, but it is not known if cereal aleurone layers respond likewise. These similarities in structure and regulation suggest that there has been conservation of aleurone cell functions as Arabidopsis and the small grain cereals diverged. It is clear that cereal aleurone cells are specialized secretory cells that synthesize and secrete the hydrolytic enzymes that break down the massive reserves stored in the dead starchy endosperm. The data presented here are consistent with the hypothesis that Arabidopsis aleurone cells are also secretory cells and that they secrete cell wall degrading enzymes that hydrolyze aleurone cell walls and result in dormancy loss.
MATERIALS AND METHODS
Plant Material
Dormant and nondormant seeds of the C24 ecotype of Arabidopsis (Arabidopsis thaliana) were harvested from plants grown in a phytotron at Commonwealth Scientific and Industrial Research Organization Canberra with 17°C temperatures and an 18-h-light (300 μmol m−2 s−1), 8-h-dark photoperiod. Dormant seeds were stored at −80°C after harvest, and nondormant seeds were generated by after ripening at room temperature (RT). Col seeds were obtained from plants grown under fluorescent lights at 21°C to 23°C with an 18-h-light (75 μmol m−2 s−1), 8-h-dark photoperiod, and seeds were after ripened at RT for over 6 months. Seeds of the Cvi and Kas2 ecotypes were harvested from greenhouse-grown plants and were stored at −80°C. Seeds of the Ler ecotype and of the Spy-1 mutant were either obtained from the Arabidopsis Biological Resource Center or were harvested from plants grown under fluorescent lights at 21°C to 23°C with an 18-h-light (75 μmol m−2 s−1), 8-h-dark photoperiod. Ler and Spy-1 seeds were after ripened at RT for at least 1 month.
Micro Dissections
The testa was removed from some seeds by imbibing them with distilled water on filter paper for 1 to 3 h and then gently tearing and peeling away the testa with a fine metal probe. For experiments with isolated embryos, the testa and endosperm of seeds imbibed for 1 to 3 h were ruptured with a fine probe or razor blade, and the embryo was gently pushed through the opening. For experiments with isolated aleurone layers, seeds were imbibed 1 to 3 h and bisected between the root/hypocotyl and cotyledons with a razor blade. The embryo halves were discarded, leaving two intact, half aleurone layers. For experiments using real-time reverse transcription qPCR, seeds were imbibed and treated for 1, 24, or 48 h and then individual seeds separated into the embryo and aleurone later by making a small incision through the testa and aleurone layer and gently pushing the intact embryo through the incision.
Germination and Incubation
Unless indicated otherwise, seeds, seeds with the testa removed, embryos, and aleurone layers were incubated in 3.5-cm plastic petri dishes containing 3 mL of 0.6% or 1% agarose, with or without additions as described in the text, and dishes were sealed with Parafilm. Germination was scored as emergence of the radicle from the seed coats. Isolated embryos or isolated aleurone layers were placed directly on 0.6% or 1% agarose with additions as indicated in the text. Embryos were positioned laterally and photographed. The surface area of each embryo (maximum projection) or distinct region of the embryo was determined by tracing with a touchpad and computing the surface area using ImageJ version 1.36b (http://rsb.info.nih.gov/ij). Isolated aleurone layers were placed on agarose (0.6%) with the testa side down, taking care that the top of the layer was moistened with a drop of water. Seeds or embryos were exposed to vapors from KCN, as described in Bethke et al. (2006b). Briefly, one application dish containing 3 mL of 200 μm KCN was enclosed in a sealed glass petri dish with a sample dish containing 3 mL agarose on which seeds and testa-less seeds were placed. Cyanide equilibrates quickly between the two dishes under these conditions, resulting in an effective application of 100 μm CN. NO gas (15% O2, 50 ppm NO, and 85% N2 at a flow rate of 50 standard cubic cm/min) was applied to seeds, seeds lacking the testa, or isolated embryos, as described in Libourel et al. (2006), except that the gas stream was configured such that dry NO was mixed with humidified air immediately before passing into a dummy sample bottle containing water to adjust the humidity, a sample bottle containing seeds, and then a bottle containing seeds lacking the testa or isolated embryos.
Electron Microscopy
Seed coats from Col Arabidopsis seeds imbibed for 1 and 24 h were fixed in 3% glutaraldehyde buffered with 0.1 m sodium cacodylate, pH 7.2, and post fixed with 1% osmium tetroxide and 1% uranyl acetate. Fixed tissue was dehydrated in a graded acetone series and embedded in Epon/Araldite. Sections were cut with a diamond knife, stained with uranyl acetate (1%), and observed in the electron microscope.
Light and Fluorescence Microscopy
Embryos were observed and photographed with a Zeiss Lumar stereo microscope and Qimaging micropublisher 3.5 camera. Aleurone layers were observed with a Zeiss Axiophot and 20× plan-neofluor objective. Fluorescence images were captured with a Qimaging micropublisher 5.0 camera. Unless specified otherwise, aleurone cells were observed using fluorescence from endogenous pigments with excitation at 365 nm and emission at 420 nm. When cells were labeled with fluorescent probes, the final concentrations were: propidium iodide, 1 μg/mL; monochlorobimane, 100 μm; Congo red, 10 μg/mL; fluorescein diacetate, 10 μg/mL; and calcofluor white M2R, 0.1 mg/mL.
Real-Time Reverse Transcription qPCR
Samples for real-time reverse transcription qPCR consisted of 20 isolated embryos or 20 aleurone layers. To minimize the effect of nuclease activity, embryos or aleurone layers were added to extraction buffer as they were prepared. Total RNA was extracted and treated with DNase I using the Ambion RNAqueous-Micro kit following the manufacturer's directions. RNA was checked for quantity, purity, and intactness with an Agilent Bioanalyzer RNA 6000 Pico assay according to the manufacturer's instructions, and only samples containing nondegraded RNA were used for analysis. Yields of total RNA averaged 79 ng from 20 embryos and 15 ng from 20 aleurone layers. Three independently extracted total RNA samples from each treatment were converted to cDNA immediately after quantification by using the Invitrogen Superscript III first-strand synthesis system according to the manufacturer's instructions. Then 25 ng of total RNA from embryo samples and 8 ng of total RNA from aleurone layer samples were used as template for cDNA synthesis with oligo(dT)20 primers. Real-time qPCR reactions (20 μL) contained 2 μL template cDNA, 10 μm of each primer, 1× Takara SYBR Premix Ex Taq, 1× ROX II reference dye, and 5% dimethyl sulfoxide. Amplification was performed using a Stratagene Mx3000P qPCR system. Each cDNA sample was assayed in triplicate. Thermal cycle conditions were optimized for each primer pair such that amplification efficiencies were 0.903 or greater, as listed in Supplemental Table S1. The primer sequences used are listed in Supplemental Table S2. DART-PCR version 1.0 software (http://www.gene-quantification.de/DART_PCR_version_1.0.xls; Peirson et al., 2003) was used to compute the amplification efficiency for each primer set and to compute the relative expression value. Relative expression values were normalized to the geometric mean of the relative expression values of three reference genes (http://medgen.ugent.be/∼jvdesomp/genorm/; Vandesompele et al., 2002), Actin2, TIP4, and YLS8, which have been described as stable reference genes (Czechowski et al., 2005). To check the specificity of each primer pair, the predicted PCR amplicon Tm was confirmed by dissociation curve analysis and amplicon size was determined by electrophoresis on agarose (2% [w/v]) or Metaphore (4% [w/v]) gels. All PCR amplicons were sequenced at the University of California Berkeley DNA sequencing facility and the correct product confirmed with the exception of GA3ox2, for which we were unable to generate enough product to get a sequence.
Statistics
Unless stated otherwise, error bars in figures signify se of the mean and statistical significance was determined using two-tailed Student's t tests assuming unequal variance. T tests were performed using Microsoft Excel.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Table S1. Amplification conditions used for qPCR and calculated amplification efficiencies.
Supplemental Table S2. Primer sequences used for qPCR.
Supplementary Material
Acknowledgments
The authors express their appreciation to Elaheh Karbassi, Krystal Vasoya, Amanda Marciel, Andrew Tarquinio, and Thomas Myint for their assistance in carrying out some of the experiments described here. The electron microscopy work was done in the UC Berkeley Electron Microscope Lab, with the assistance of Kent McDonald and Reena Zalpuri. The fluorescence microscopy was done in the College of Natural Resources Biological Imaging Facility.
This work was supported by the National Science Foundation (to R.L.J.), by the California Agricultural Research Initiative (to D.W.S.), by the Plant Signal Network Research Center of the Ministry of Science and Technology, and by the Biogreen 21 program of Rural Development Administration Republic of Korea.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Russell L. Jones (rjones@nature.berkeley.edu).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Baskin J, Baskin C (1983) Seasonal changes in germination responses of buried seeds of Arabidopsis thaliana and ecological interpretations. Bot Gaz 144 540–543 [Google Scholar]
- Bethke PC, Badger MR, Jones RL (2004. a) Apoplastic synthesis of nitric oxide by plant tissues. Plant Cell 16 332–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethke PC, Gubler F, Jacobsen JV, Jones RL (2004. b) Dormancy of Arabidopsis seeds and barley grains can be broken by nitric oxide. Planta 219 847–855 [DOI] [PubMed] [Google Scholar]
- Bethke PC, Libourel IGL, Jones RL (2006. a) Nitric oxide reduces seed dormancy in Arabidopsis. J Exp Bot 57 517–526 [DOI] [PubMed] [Google Scholar]
- Bethke PC, Libourel IGL, Reinohl V, Jones RL (2006. b) Sodium nitroprusside, cyanide, nitrite, and nitrate break Arabidopsis seed dormancy in a nitric oxide-dependent manner. Planta 223 805–812 [DOI] [PubMed] [Google Scholar]
- Bethke PC, Swanson SJ, Hillmer S, Jones RL (1998) From storage compartment to lytic organelle: the metamorphosis of the aleurone protein storage vacuole. Ann Bot (Lond) 82 399–412 [Google Scholar]
- Bewley JD (1997) Seed germination and dormancy. Plant Cell 9 1055–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley JD, Black M (1994) Seeds: Physiology of Development and Germination. Plenum Press, New York
- Bush DS, Cornejo M-J, Huang CN, Jones RL (1986) Ca2+-stimulated secretion of α-amylase during development in barley aleurone protoplasts. Plant Physiol 82 566–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels MJ, Varner JE (1973) A test for the de novo synthesis of enzymes in germinating seeds: density labeling with D2O. In MJ Chrispeels, ed, Molecular Techniques and Approaches in Developmental Biology. John Wiley, New York
- Clerkx EJM, El-Lithy ME, Vierling E, Ruys GJ, Blankestijin-De Vries H, Groot SPC, Vreugdenhil D, Koornneef M (2004) Analysis of natural allelic variation of Arabidopsis seed germination and seed longevity traits between the accessions Landsberg erecta and Shakdara, using a new recombinant inbred line population. Plant Physiol 135 432–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible W-R (2005) Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol 139 5–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahal P, Nevins DJ, Bradford KJ (1997) Relationship of endo-β-d-mannanase activity and cell wall hydrolysis in tomato endosperm to germination rates. Plant Physiol 113 1243–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debeaujon I, Koornneef M (2000) Gibberellin requirement for Arabidopsis seed germination is determined both by testa characteristics and embryonic abscisic acid. Plant Physiol 122 415–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debeaujon I, Leon-Kloosterziel KM, Koornneef M (2000) Influence of the testa on seed dormancy, germination, and longevity in Arabidopsis. Plant Physiol 122 403–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debeaujon I, Nesi N, Perez P, Devic M, Grandjean O, Caboche M, Lepiniec L (2003) Proanthocyanidin-accumulating cells in Arabidopsis testa: regulation of differentiation and role in seed development. Plant Cell 15 2514–2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debeaujon I, Peeters AJM, Leon-Kloosterziel KM, Koornneef M (2001) The TRANSPARENT TESTA12 gene of Arabidopsis encodes a multidrug secondary transporter-like protein required for flavonoid sequestration in vacuoles of the seed coat endothelium. Plant Cell 13 853–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Declercq A, Vandewiele M, Derycke R, Vandamme J, Vanmontagu M, Krebbers E, Vandekerckhove J (1990) Expression and processing of an Arabidopsis 2s albumin in transgenic tobacco. Plant Physiol 92 899–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreucq B, Berger N, Vincent E, Boisson M, Pelletier G, Caboche M, Lepiniec L (2000) The Arabidopsis AtEPR1 extensin-like gene is specifically expressed in endosperm during seed germination. Plant J 23 643–652 [DOI] [PubMed] [Google Scholar]
- Eastmond PJ, Jones RL (2005) Hormonal regulation of gluconeogenesis in cereal aleurone is strongly cultivar-dependent and gibberellin action involves SLENDER1 but not GAMYB. Plant J 44 483–493 [DOI] [PubMed] [Google Scholar]
- Eastmond PJ, Germain V, Lange PR, Bryce JH, Smith SM, Graham IA (2000) Postgerminative growth and lipid catabolism in oilseeds lacking the glyoxylate cycle. Proc Natl Acad Sci USA 97 5669–5674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filner P, Varner JE (1967) A test of de novo synthesis of enzymes: density labeling with H2O18 of barley α-amylase induced by gibberellic acid. Proc Natl Acad Sci USA 58 1520–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch-Savage WE, Leubner-Metzger G (2006) Seed dormancy and the control of germination. New Phytol 171 501–523 [DOI] [PubMed] [Google Scholar]
- Footitt S, Slocombe S, Larner V, Kurup S, Wu Y, Larson T, Graham IA, Baker A, Holdsworth M (2002) Control of germination and lipid mobilization by COMATOSE, the Arabidopsis homologue of human ALDP. EMBO J 21 2912–2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford DJ, Greenwood JS, Bewley JD (1983) Vacuolation and storage protein breakdown in the castor bean endosperm following imbibition. J Exp Bot 34 1433–1443 [Google Scholar]
- Gong XM, Bassel GW, Wang AX, Greenwood JS, Bewley JD (2005) The emergence of embryos from hard seeds is related to the structure of the cell walls of the micropylar endosperm, and not to endo-beta-mannanase activity. Ann Bot (Lond) 96 1165–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot SPC, Karssen CM (1987) Gibberellins regulate seed-germination in tomato by endosperm weakening: a study with gibberellin-deficient mutants. Planta 171 525–531 [DOI] [PubMed] [Google Scholar]
- Guo FQ, Okamoto M, Crawford NM (2003) Identification of a plant nitric oxide synthase gene involved in hormonal signaling. Science 302 100–103 [DOI] [PubMed] [Google Scholar]
- Haslekas C, Viken MK, Grini PE, Nygaard V, Nordgard SH, Meza TJ, Aalen RB (2003) Seed 1-cysteine peroxiredoxin antioxidants are not involved in dormancy, but contribute to inhibition of germination during stress. Plant Physiol 133 1148–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilhorst H, Karssen CM (1992) Seed dormancy and germination: the role of abscisic acid and gibberellins and the importance of hormone mutants. Plant Growth Regul 11 225–238 [Google Scholar]
- Hooks MA, Turner JE, Murphy EC, Graham IA (2004) Acetate non-utilizing mutants of Arabidopsis: evidence that organic acids influence carbohydrate perception in germinating seedlings. Mol Genet Genomics 271 249–256 [DOI] [PubMed] [Google Scholar]
- Jacobsen SE, Olszewski NE (1993) Mutations at the SPINDLY locus of Arabidopsis alter gibberellin signal-transduction. Plant Cell 5 887–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RL (1969) The fine structure of barley aleurone cells. Planta 85 359–374 [DOI] [PubMed] [Google Scholar]
- Jones RL, Price JM (1970) Gibberellic acid and the fine structure of barley aleurone cells. III. Vacuolation of the aleurone cell during the phase of ribonuclease release. Planta 94 191–202 [DOI] [PubMed] [Google Scholar]
- Jullien M, Bouinot D, Ali-Rachedi S, Sotta B, Grappin P (2000) Abscisic acid control of seed dormancy expression in Nicotiana plumbaginifolia and Arabidopsis thaliana. In J-D Viemont, J Crabbe, eds, Dormancy in Plants: From Whole Plant Behaviour to Cellular Control. CAB International, Wallingford, Oxon, UK
- Kelly K, van Staden J, Bell W (1992) Seed coat structure and dormancy. Plant Growth Regul 11 201–209 [Google Scholar]
- Koornneef M, Alonso-Blanco C, Bentsink L, Blankenstein-de Vries H, Debeaujon I, Hanhart CJ, Leon-Kloosterziel KM, Peeters AJM, Raz V (2000) The Genetics of Seed Dormancy. CAB International, Wallingford, Oxon, UK
- Kucera B, Cohn MA, Leubner-Metzger G (2005) Plant hormone interactions during seed dormancy release and germination. Seed Sci Res 15 281–307 [Google Scholar]
- Leung D, Bewley JD (1983) A role for α-galactosidase in the degradation of the endosperm cell walls of lettuce seeds, cv. Grand Rapids. Planta 157 274–277 [DOI] [PubMed] [Google Scholar]
- Libourel IGL, Bethke PC, Jones RL (2006) Nitric oxide gas stimulates germination of dormant Arabidopsis seeds: use of a flow-through apparatus for delivery of nitric oxide. Planta 223 813–820 [DOI] [PubMed] [Google Scholar]
- Mitchum MG, Yamaguchi S, Hanada A, Kuwahara A, Yoshioka Y, Kato T, Tabata S, Kamiya Y, Sun TP (2006) Distinct and overlapping roles of two gibberellin 3-oxidases in Arabidopsis development. Plant J 45 804–818 [DOI] [PubMed] [Google Scholar]
- Müller K, Tintelnot S, Leubner-Metzger G (2006) Endosperm-limited Brassicaceae seed germination: abscisic acid inhibits embryo-induced endosperm weakening of Lepidium sativum (cress) and endosperm rupture of cress and Arabidopsis thaliana. Plant Cell Physiol 47 864–877 [DOI] [PubMed] [Google Scholar]
- Nabors M, Lang A (1971) The growth physics and water relations of red-light induced germination in lettuce seeds. I. Embryos germinating in osmoticum. Planta 101 1–25 [DOI] [PubMed] [Google Scholar]
- Nonogaki H, Gee OH, Bradford KJ (2000) A germination-specific endo-β-mannanase gene is expressed in the micropylar endosperm cap of tomato seeds. Plant Physiol 123 1235–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonogaki H, Nomaguchi M, Okumoto N, Kaneko Y, Matsushima H, Morohashi Y (1998) Temporal and spatial pattern of the biochemical activation of the endosperm during and following imbibition of tomato seeds. Physiol Plant 102 236–242 [Google Scholar]
- Ogawa M, Hanada A, Yamauchi Y, Kuwahara A, Kamiya Y, Yamaguchi S (2003) Gibberellin biosynthesis and response during Arabidopsis seed germination. Plant Cell 15 1591–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette B, Bewley JD (1986) β-mannosidase mannohydrolase and the mobilization of the endosperm cell walls of lettuce seeds, cv. Grand Rapids. Planta 169 333–338 [DOI] [PubMed] [Google Scholar]
- Peirson S, Butler J, Foster R (2003) Experimental validation of novel and conventional approaches to quantitative real-time PCR data analysis. Nucl Acids Res 31 e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield S, Rylott EL, Gilday AD, Graham S, Larson TR, Graham IA (2004) Reserve mobilization in the Arabidopsis endosperm fuels hypocotyl elongation in the dark, is independent of abscisic acid, and requires PHOSPHOENOLPYRUVATE CARBOXYKINASE1. Plant Cell 16 2705–2718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinfield-Wells H, Rylott EL, Gilday AD, Graham S, Job K, Larson TR, Graham IA (2005) Sucrose rescues seedling establishment but not germination of Arabidopsis mutants disrupted in peroxisomal fatty acid catabolism. Plant J 43 861–872 [DOI] [PubMed] [Google Scholar]
- Ritchie S, McCubbin A, Ambrose G, Kao T-h, Gilroy S (1999) The sensitivity of barley aleurone tissue to gibberellin is heterogeneous and may be spatially determined. Plant Physiol 120 361–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez R, Sunel L, Labavitch JM, Bonner BA (1990) Changes in the endosperm walls of two Datura species before radical protrusion. Plant Physiol 93 89–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopfer P (2001) Hydroxyl radical-induced cell-wall loosening in vitro and in vivo: implications for the control of elongation growth. Plant J 28 679–688 [DOI] [PubMed] [Google Scholar]
- Schopfer P, Plachy C, Frahry G (2001) Release of reactive oxygen intermediates (superoxide radicals, hydrogen peroxide, and hydroxyl radicals) and peroxidase in germinating radish seeds controlled by light, gibberellin and abscisic acid. Plant Physiol 125 1591–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson S, Bethke PC, Jones RL (1998) Barley aleurone cells contain two types of vacuoles: characterization of lytic compartments using fluorescent probes. Plant Cell 10 685–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton TM, Swain SM, Olszewski NE (1999) Gibberellin signal transduction presents…the SPY who O-GlcNAc'd me. Trends Plant Sci 4 424–428 [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe AFS (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3 research00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windsor J, Symonds V, Mendenhall J, Lloyd A (2000) Arabidopsis seed coat development: morphological differentiation of the outer integument. Plant J 22 483–493 [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Kamiya Y, Sun TP (2001) Distinct cell-specific expression patterns of early and late gibberellin biosynthetic genes during Arabidopsis seed germination. Plant J 28 443–453 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.