Tropospheric ozone is increasing at a fast pace and causes large losses of production in cultivated plants and forests (UNECE, 2004). Ozone risk assessment has been based for a long time on exposure to external ozone concentration. Doses above a selected threshold (generally 40 nL L−1) have been considered injurious, and the cumulated exposure to these concentrations has been the basis of the level I approach to determine and predict ozone damage to vegetation (Fuhrer et al., 1997).
In the attempt to improve ozone dose/effect predictions, a more mechanistic approach has been recently developed. This approach is based on the flux of ozone through stomata and on the consequent stomatal uptake by leaves (Emberson et al., 2000). The flux-based level II approach has been recommended as a suitable indicator of ozone damage in Europe (UNECE, 2004). Yet, in only a few cases has a clear improvement of risk assessment for vegetation when estimating actual ozone flux into leaves been reported (e.g. Uddling et al., 2004).
In this correspondence, it is shown that ozone uptake is largely driven by stomatal opening, but evidence is also presented that ozone uptake is not necessarily related to ozone sensitivity, as isoprenoids, and probably other ozone scavenging molecules (e.g. ascorbate; Eller and Sparks, 2006), may contribute to increased ozone uptake by leaves. Because isoprenoids have an important antioxidant action (Loreto and Velikova, 2001; Affek and Yakir, 2002), it is concluded that a part of the ozone uptake may not be related to damage, at least in isoprenoid-emitting species, and may indicate the activation of defensive mechanisms scavenging reactive oxygen molecules and an overall reduced ozone damage in leaves.
OZONE UPTAKE BY LEAVES DEPENDS ON STOMATAL CONDUCTANCE
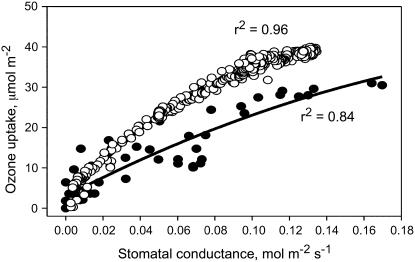
Estimation of ozone fluxes in ecosystems is problematic, especially because of the occurrence of nonstomatal deposition (Altimir et al., 2004). While recent models also consider deposition components (Emberson et al., 2000), the estimation of the stomatal component of the flux remains crucial, in that the ozone taken up through stomata directly impinges on and damages cellular structures (Pell et al., 1997). The stomatal flux is therefore considered the most reliable index of potential ozone damage. Resistances to ozone entry into leaves certainly play an important role in controlling the uptake. As shown in Figure 1, there is a robust relationship between ozone uptake and stomatal conductance in the sclerophyllous tree holm oak (Quercus ilex) and in the mesophyllous tree Populus nigra. The same relationship has been found in leaves of other trees such as Quercus robur and Quercus pubescens (data not shown). The uptake of ozone in darkened leaves in which stomata were allowed to close, and the uptake through the adaxial side of hypostomatous holm oak leaves, is less than 10% of that recorded in illuminated leaves and in both leaf surfaces (data not shown). This confirms that nonstomatal uptake plays a minor role in total ozone uptake, at least when the leaf surface is not wet (Altimir et al., 2006). A similar conclusion is indicated by the observation that very low ozone uptake is measured when stomatal conductance tends to zero (the best fits for the two plant species shown in Fig. 1 extrapolate at slightly less than 2 μmol m−2).
Figure 1.
Relationship between ozone uptake and stomatal conductance in leaves of holm oak (black symbols) and P. nigra (white symbols). The second-order best-fit lines (r2 = 0.84 and 0.96, respectively) were generated by the Sigmaplot 2002 software (Systat) and are not constrained through the origin.
However, ozone uptake through stomata is driven by the establishment of a continuous gradient between the external ozone concentration and the concentration of ozone inside leaves, assumed to be zero. The observed curvilinear relationship in Figure 1 suggests that, at high stomatal conductance, a saturation of the scavenging capacity of the pollutant inside leaves is reached and that ozone consequently accumulates inside leaves, reducing the gradient with external ozone concentration.
OZONE UPTAKE IS ALSO REGULATED BY THE PRESENCE OF ISOPRENOIDS IN THE LEAVES
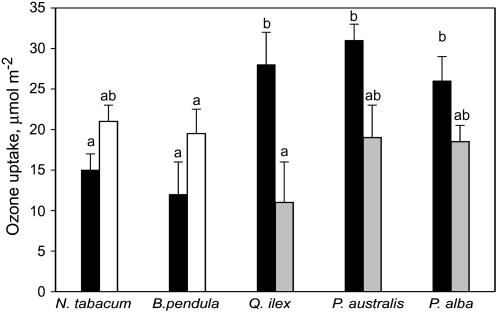
While ozone uptake of individual leaves is only minimally dependent on nonstomatal deposition, it may depend on active ozone removal inside leaves by antioxidant molecules. We have measured the rates of ozone uptake by leaves with the same stomatal conductance to cancel out this important resistance component in the flux. The ozone uptake by leaves of white poplar (Populus alba), common reed (Phragmites australis), and holm oak was statistically greater than by leaves of tobacco (Nicotiana tabacum) and birch (Betula pendula; Fig. 2). Poplar, reed, and oak species emit volatile isoprenoids (isoprene or monoterpenes) that have been demonstrated to act as powerful antioxidants, dramatically reducing oxidative damage in leaves (Loreto and Velikova, 2001; Affek and Yakir, 2002). In our experiment, isoprenoid emissions were measured by proton transfer reaction mass spectrometry (PTR-MS) and were in the range of 2 to 4 nmol m−2 s−1 for monoterpenes and 20 to 30 nmol m−2 s−1 for isoprene, but the concentration of these compounds inside leaves was calculated to be hundreds to thousands of nmol m−2, respectively (Loreto et al., 1998). Ozone flux into leaves may therefore be driven by the reaction with isoprenoids and other antioxidant molecules and by the consequently high rates of ozone transformation and scavenging.
Figure 2.
Ozone uptake (black bars) by leaves of two plant species that do not emit isoprenoids, tobacco and birch, by a species emitting monoterpenes, holm oak, and by two species emitting isoprene, common reed and white poplar. The ozone uptake was also measured in leaves of tobacco and birch fumigated with 3 μL L−1 of exogenous isoprene (white bar), and in leaves of holm oak, reed, and white poplar in which isoprenoid emission was previously inhibited by fosmidomycin (30 μm) feeding for 60 min (gray bars). Measurements were made on leaves showing the same stomatal conductance (0.1 mol m−2 s−1) and at the same environmental conditions (light intensity, 1,000 μmol m−2 s−1; leaf temperature, 30°C; relative humidity, 40%; and vapor pressure difference between leaf and air, 15 mbar). Measurements were repeated on five different leaves per species. Means and ses are shown, and means are separated by ANOVA using a multiple range test. Means significantly different are separated by different letters (P = 0.01, single letter; P = 0.05, double letters).
Dangerous reactive oxygen species formed by ozone may also be quenched by secondary reactions with volatile isoprenoids. To further test the hypothesis that ozone uptake may also depend on its removal by volatile antioxidants, isoprene emissions by reed and white poplar leaves and monoterpene emissions by holm oak leaves, were chemically inhibited. The flux of ozone in these isoprenoid-inhibited leaves was significantly lower than that recorded in the same leaves before isoprenoid inhibition (Fig. 2). On the other hand, also as previously reported (Loreto et al., 2001), ozone uptake by tobacco leaves (that do not produce appreciable amounts of isoprenoid) was enhanced upon fumigation with exogenous isoprene (Fig. 2). Ozone uptake was also stimulated by exogenous fumigation of isoprene in birch, the other nonemitting species sampled, although the enhancement was only slightly significant (P < 0.10).
Whether the reaction between ozone and volatile isoprenoids occurs outside leaves, in the intercellular spaces, or at the membrane level is still an unresolved question. We argue that the rate coefficient of the reaction between ozone and isoprene (around 10 × 10−18 cm3 molecule−1 s−1 at 286°K; Karl et al., 2004) makes this reaction impossible to occur outside leaves in our system, in which the cuvette was continuously flushed with a fast stream of air and the residence time of gases in the cuvette was extremely short (<5 s). The rate coefficients of the reactions between ozone and monoterpenes are higher (Atkinson, 1997) but still incompatible with the short residence time of the molecules in the cuvette. It is therefore suggested that isoprenoids actually scavenge ozone inside leaves, although they may additionally and efficiently react with ozone in the atmosphere (Griffin et al., 1999).
It should be also mentioned that experiments have recently shown that isoprenoid emission can be either positively (Loreto et al., 2004; Valkama et al., 2007) or negatively (Velikova et al., 2005; Fares et al., 2006; Calfapietra et al., 2007) affected by long-term exposure to ozone. This may change the observed uptake, and the capacity of isoprenoid-emitting leaves to take up ozone, under natural conditions.
OZONE UPTAKE IS NOT ALWAYS A SUITABLE DAMAGE INDEX AND MAY ALSO INCLUDE A DETOXIFICATION COMPONENT
Because ozone uptake is also driven by the presence of natural antioxidants such as the volatile isoprenoids, the notion that ozone uptake produces ozone damage may not be always correct.
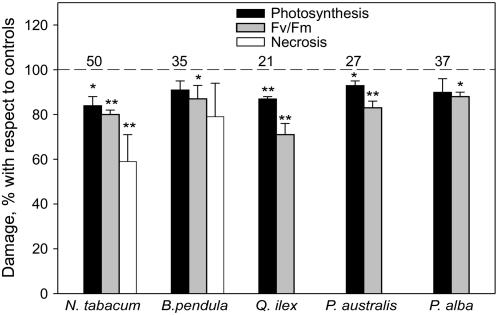
We have monitored photosynthesis, chlorophyll fluorescence, and visible necrosis as indicators of ozone damage. As expected, all parameters were negatively affected by ozone, with photosynthesis and maximal quantum yield of fluorescence reaching 50% of the values of intact leaves in tobacco, the most sensitive species (data not shown). However, in all plant species, at least one of these parameters was significantly less inhibited by ozone when naturally emitting isoprenoids (holm oak, white poplar, and reed) or when supplied exogenous isoprene (tobacco and birch) in comparison to the same plants in which isoprenoid emission was inhibited or exogenous isoprene was not supplied (Fig. 3). The effect was very evident when the damage was inherently high, such as in tobacco, as also previously observed (Loreto et al., 2001), and in the monoterpene-emitting species holm oak, in which it may be associated with the very efficient action of monoterpenes as ozone scavengers (also indicated by Fig. 2). Interestingly, an ozone-sensitive clone of Trifolium repens cv Regal (Postiglione et al., 2000) was also found to take up less ozone than a resistant clone of the same cultivar when measured at similar stomatal conductance, again suggesting that ozone uptake and ozone damage are not necessarily directly correlated (data not shown). T. repens is not known as an isoprenoid-emitting species, and no emission was detected in our experiment. Other compounds such as ascorbate, a major player in apoplastic ozone scavenging processes (Burkey and Eason, 2002), might have been responsible for the larger ozone uptake of the resistant clone. In a recent experiment, ascorbate levels of Catharanthus roseus explained part of the variation in ozone flux that stomatal conductance did not account for (Eller and Sparks, 2006). Ascorbate was detected at a level 50% to 70% higher in the ozone resistant clone of T. repens cv Regal with respect to the sensitive one (Ferreira Severino et al., 2007), although this finding is still controversial (D'Haese et al., 2005). Ozone may apparently also induce biosynthesis and emission of volatile isoprenoids, mainly sesquiterpenes, even in plants that do not naturally emit these compounds (Heiden et al., 1999). Whether any of these compounds might also be responsible for the larger ozone uptake by ozone resistant plant species and cultivars needs to be studied.
Figure 3.
Ozone damage in leaves naturally emitting volatile isoprenoids (holm oak, common reed, and white poplar) and in leaves of plants that do not emit isoprenoids but were fumigated with 3 μL L−1 of exogenous isoprene during the ozone treatment (reed and birch). Ozone (100 nL L−1) was fumigated consecutively for different times to illuminated leaves, as indicated in the text. Damage indicators were the reduction of net photosynthesis and of maximal quantum yield of chlorophyll fluorescence in dark-adapted leaves (Fv/Fm), measured 12 h after the ozone treatment, and the reduced appearance of visible necrotic areas in the leaf lamina, measured 7 d after the treatment. Visible necrotic damage was assessed only in intact leaves of tobacco and birch, not in those leaves that were cut to feed the isoprenoid inhibitor. All damage indicators are shown as percentage of the level measured in leaves that do not emit naturally isoprene or in which isoprenoid emission was previously inhibited by fosmidomycin, as indicated in the text (dotted horizontal line). The numbers above the dotted line indicate the reduction of photosynthesis (%) caused by the ozone treatment in leaves that do not naturally emit isoprene or in which isoprenoid emission was previously inhibited by fosmidomycin, with respect to the nonfumigated controls. Measurements were repeated on five different leaves per species. Means significantly different from 100% (the damage measured in leaves without endogenous or exogenous volatile isoprenoids) are separated by ANOVA using a multiple range test (P = 0.01, double asterisk; P = 0.05, single asterisk).
These experiments show that ozone flux into leaves may not necessarily be associated with damage. In fact, high ozone fluxes may indicate efficient protective mechanisms of detoxification and resistance to the stressor. This finding calls for a redefinition of the stomatal ozone uptake parameter to include a detoxification component, especially when using stomatal flux to assess ozone risk (UNECE, 2004). The experiments also indicate that ozone removal inside leaves may occur at different rates, depending on the presence of reactive compounds. In leaves exposed to an ozone concentration of 100 nL L−1, it may be calculated (following the formalism presented by Laisk et al., 1989) that the ozone concentration inside leaves emitting isoprenoids is close to 0 nL L−1, while that inside nonemitting leaves is around 30 nL L−1. Thus, the absence of volatile isoprenoids, and perhaps other antioxidants, may also challenge the notion that ozone inside leaves is instantaneously scavenged to a concentration close to zero (Laisk et al., 1989), in turn biasing current ozone flux estimates based on stomatal flux (Emberson et al., 2000). The concept that a relevant ozone uptake by plants may be related to protection mechanisms fosters more research to study the biochemistry of ozone reaction inside leaves and to exploit the capacity of vegetation to naturally scavenge ozone.
MATERIALS AND METHODS
The experiments were carried out with a cuvette made of glass and Teflon to minimize ozone adsorption by the cuvette material, as described in Thöll et al. (2006). The cuvette uptake was measured without the leaf and with a leaf replica made by paper, and the uptake was subtracted to the leaf uptake. These measurements were repeated before and after each measurement of ozone uptake by leaves. The cuvette was connected to the gas exchange system described by Loreto et al. (2001), which allowed simultaneous measurements of gas exchange (CO2, water, ozone, and isoprenoids) and chlorophyll fluorescence. In these experiments, however, isoprenoids were detected online by sensitive (detection limit <1 nL L−1) PTR-MS (Ionicon).
When measuring ozone uptake, intact leaves of holm oak (Quercus ilex) and Populus nigra and of two clones of Trifolium repens cv Regal were used. When testing the relationship between ozone uptake and isoprenoid emission, leaves of two plant species that do not emit appreciable levels of isoprenoids (i.e. with measured emission rates <0.1 nmol m−2 s−1), tobacco (Nicotiana tabacum) and birch (Betula pendula); a species emitting monoterpenes, holm oak; and two species emitting large quantities of isoprene, common reed (Phragmites australis) and white poplar (Populus alba); were used. In the second experiment, leaves were detached from the plants and maintained in a vial filled with water during measurements under a light intensity of 1,000 μmol photons m−2 s−1 and with a leaf temperature of 30°C. Details on the control of environmental parameters are reported in Loreto et al. (2001). Care was taken that leaf cutting did not affect physiological parameters (photosynthesis, stomatal conductance, and isoprenoid emission). Care was also taken to choose leaves with similar conductance (0.10 ± 0.02 mol m−2 s−1, without modulating environmental conditions) to cancel this important component in the diffusion of ozone inside leaves. A 5-cm2 leaf area was enclosed in the cuvette and exposed to a flux (0.4 L min−1) of synthetic air made by mixing N2, O2, and CO2 at atmospheric concentrations and containing no contaminants and pollutants other than a fixed concentration of ozone (100 nL L−1). Ozone was generated by diverting a part of the oxygen (20 mL min−1) inside a UV light source. Ozone concentration was continuously monitored with a UV Photometric O3 analyzer (1108 Dasibi Environmental). The uptake of ozone by the leaf was obtained measuring the difference between ozone concentration at the inlet and the outlet of the cuvette, after correcting for the chamber uptake.
Isoprenoid emission was inhibited by adding fosmidomycin to the water in the vial and by allowing the compound to travel through the transpiration stream. We used the minimal concentrations (30 μm) at which the effect was complete (isoprenoid inhibition was about 90%, as monitored online by PTR-MS). Exogenous isoprene was fumigated to the leaves of plants that do not emit isoprenoids (tobacco and birch) using the setup described by Loreto et al. (2001) to obtain a constant concentration of 3 μL L−1 of isoprene in the air and a concentration of isoprene inside leaves putatively similar to the endogenous one (Loreto et al., 1998). Isoprenoid inhibition and isoprene fumigation did not significantly affect the measured physiological parameters, as expected from previous experiments. Measurements of ozone uptake were carried out on the same leaves before and 30 min after isoprene inhibition or isoprene fumigation.
Ozone damage to leaves was assessed by measuring photosynthesis and the maximal photochemical efficiency of dark-adapted leaves, as monitored by the ratio between variable and maximal fluorescence (Fv/Fm) 12 h after the exposure of illuminated leaves to 100 nL L−1 of ozone. The length of the ozone treatment carried out to assess the isoprenoid impact on ozone damage was different in the different plants. Fumigation lasted 12 h in the ozone-sensitive species that do not emit volatile isoprenoids (tobacco and birch), 24 h in the isoprene-emitting plants (white poplar and reed), and 48 h in the scherophyllous, ozone-resistant, and monoterpene-emitting holm oak. Measurements of the physiological parameters were carried out as reported in Loreto et al. (2001). Visible damage caused by ozone was also recorded, 7 d after the ozone treatment, by photographing the leaf lamina exposed to the pollutant with a digital camera (DC 120, Eastman-Kodak). Ozone-induced necroses of the leaf lamina in presence or in absence of isoprene were compared by separating the damaged areas with computer software (DS1D Scientific Imaging System, Kodak). Visible damage could be assessed only in intact leaves of tobacco and birch. Leaves of holm oak, reed, and white poplar were cut to infiltrate the isoprene inhibitor and could not be maintained alive for 7 d.
Measurements were repeated on at least five different leaves per species. The relationship between ozone uptake and stomatal conductance in holm oak and P. nigra is described by best-fit lines generated by the Sigmaplot 2002 software (Systat). When assessing isoprenoid impact on ozone uptake and ozone damage (ozone effect on photosynthesis, Fv/Fm, and visible necrosis) means and ses are shown and means are separated by ANOVA using a multiple range test. Means significantly different are separated by different letters or asterisks, as reported in the figure legends.
Acknowledgments
We thank Nuria Altimir, Peter Harley, and Nick Hewitt for critical reading of the manuscript and useful discussions. We also thank Marcello Vitale for supplying some of the plants used in the experiments and for stimulating discussions.
This work was supported by the European Commission Marie Curie program ISONET, by the European Science Foundation program VOCBAS, and by the Italian Ministry of University program PRIN Valutazione degli effetti dell'assorbimento fogliare dell'ozono troposferico in specie mediterranee.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Francesco Loreto (francesco.loreto@ibaf.cnr.it).
References
- Affek HP, Yakir D (2002) Protection by isoprene against singlet oxygen in leaves. Plant Physiol 129 269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altimir N, Kolari P, Tuovinen J-P, Vesala T, Back J, Suni T, Kulmala M, Hari P (2006) Foliage surface ozone deposition: a role for surface moisture? Biogeosciences 3 209–228 [Google Scholar]
- Altimir N, Tuovinen J-K, Vesala T, Kulmala M, Hari P (2004) Measurements of ozone removal by Scots pine shoots: calibration of a stomatal uptake model including the non-stomatal component. Atmos Environ 38 2387–2398 [Google Scholar]
- Atkinson R (1997) Gas-phase tropospheric chemistry of volatile organic compounds: 1, alkanes and alkenes. J Phys Chem 26 215–290 [Google Scholar]
- Burkey KO, Eason G (2002) Ozone tolerance in snap bean is associated with elevated ascorbic acid in the leaf apoplast. Physiol Plant 114 387–394 [DOI] [PubMed] [Google Scholar]
- Calfapietra C, Wiberley AE, Falbel TG, Linskey AR, Scarascia Mugnozza G, Karnosky DF, Loreto F, Sharkey TD (2007) Isoprene synthase expression and protein levels are reduced under elevated O3 but not under elevated CO2 in field-grown aspen trees. Plant Cell Environ (in press) [DOI] [PubMed]
- D'Haese D, Vandermeiren K, Asard H, Horemans N (2005) Other factors than apoplastic ascorbate contribute to the differential ozone tolerance of two clones of Trifolium repens L. Plant Cell Environ 28 623–632 [Google Scholar]
- Eller ASD, Sparks JP (2006) Predicting leaf-level fluxes of O3 and NO2: the relative roles of diffusion and biochemical processes. Plant Cell Environ 29 1742–1750 [DOI] [PubMed] [Google Scholar]
- Emberson LD, Ashmore MR, Cambridge HM, Simpson D, Tuovinen J (2000) Modelling stomatal ozone flux across Europe. Environ Pollut 109 403–413 [DOI] [PubMed] [Google Scholar]
- Fares S, Barta C, Brilli F, Centritto M, Ederli L, Ferranti F, Pasqualini S, Reale L, Tricoli D, Loreto F (2006) Impact of high ozone on isoprene emission, photosynthesis and histology of developing Populus alba leaves directly or indirectly exposed to the pollutant. Physiol Plant 128 456–465 [Google Scholar]
- Ferreira Severino J, Stich K, Soja G (2007) Ozone stress and antioxidant substances in Trifolium repens and Centaurea jacea leaves. Environ Pollut (in press) [DOI] [PubMed]
- Fuhrer J, Skärby L, Ashmore MR (1997) Critical levels for ozone pollution in Europe. Environ Pollut 97 91–106 [DOI] [PubMed] [Google Scholar]
- Griffin RJ, Cocker DR, Flagan RC, Seinfeld JH (1999) Organic aerosol formation from the oxidation of biogenic hydrocarbons. J Geophys Res 104 3555–3567 [Google Scholar]
- Heiden AC, Hoffmann T, Kahl J, Kley D, Klockow D, Langebartels C, Mehlhorn H, Sandermann H, Schraudner M, Schuh G, et al (1999) Emission of volatile organic compounds from ozone-exposed plants. Ecol Appl 9 1160–1167 [Google Scholar]
- Karl M, Brauers T, Dorn HP, Holland F, Komenda M, Poppe D, Rohrer F, Rupp L, Schaub A, Wahner A (2004) Kinetic study of the OH-isoprene and O3-isoprene reaction in the atmosphere simulation chamber, SAPHIR. Geophys Res Lett 31 L05117 [Google Scholar]
- Laisk A, Kull O, Moldau H (1989) Ozone concentration in leaf intercellular air spaces is close to zero. Plant Physiol 90 1163–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Ciccioli P, Brancaleoni E, Cucinato A, Frattoni M (1998) Measurement of isoprenoid content in leaves of Mediterranean Quercus spp. by a novel and sensitive method and estimation of the isoprenoid partition between liquid and gas phase inside the leaves. Plant Sci 136 25–30 [Google Scholar]
- Loreto F, Mannozzi M, Maris C, Nascetti P, Ferranti F, Pasqualini S (2001) Ozone quenching properties of isoprene and its antioxidant role in plants. Plant Physiol 126 993–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Pinelli P, Manes F, Kollist H (2004) Impact of ozone on monoterpene emissions and evidences for an isoprene-like antioxidant action of monoterpenes emitted by Quercus ilex (L.) leaves. Tree Physiol 24 361–367 [DOI] [PubMed] [Google Scholar]
- Loreto F, Velikova V (2001) Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol 127 1781–1787 [PMC free article] [PubMed] [Google Scholar]
- Pell EJ, Schlagnhaufer CD, Arteca RN (1997) Ozone induced oxidative stress: mechanisms of action and reaction. Physiol Plant 100 264–273 [Google Scholar]
- Postiglione L, Fagnano M, Merla G (2000) Response to ambient ozone of two white clover (Trifolium repens L.cv. “Regal”) clones, one resistant and one sensitive, grown in a Mediterranean environment. Environ Pollut 109 525–531 [DOI] [PubMed] [Google Scholar]
- Thöll D, Boland W, Hansel A, Loreto F, Roese USR, Schnitzler J-P (2006) Practical approaches to plant volatile analysis. Plant J 45 540–560 [DOI] [PubMed] [Google Scholar]
- Uddling J, Gunthardt-Goerg MS, Matyssek R, Oksanen E, Pleijel H, Sellden G, Karlsson PE (2004) Biomass reduction of juvenile birch is more strongly related to stomatal uptake of ozone than to indices. Atmos Environ 38 4709–4719 [Google Scholar]
- UNECE (2004) Revised manual on methodologies and criteria for mapping critical levels/loads and geographical areas where they are exceeded. www.icpmapping.org (February 12, 2006)
- Valkama E, Koricheva J, Oksanen E (2007) Effects of elevated O3, alone and in combination with elevated CO2, on tree leaf chemistry and insect herbivore performance: a meta-analysis. Glob Change Biol 13 184–201 [Google Scholar]
- Velikova V, Tsonev T, Pinelli P, Alessio GA, Loreto F (2005) Localized ozone fumigation system for studying ozone effects on photosynthesis, respiration, electron transport rate and isoprene emission in field-grown Mediterranean oak species. Tree Physiol 25 1523–1532 [DOI] [PubMed] [Google Scholar]