Abstract
Phosphatidylinositol-3-kinase (PI3K)/AKT signaling is essential for growth and metabolism and is elevated in many cancers. Enzymatic activity of AKT has been shown to depend on phosphorylation of two conserved sites by PDK1 and TOR (target of rapamycin) complex 2 (TORC2) in a PI3K-dependent manner. Here we analyze the role of TORC2-mediated AKT phosphorylation in Drosophila. Mutants removing critical TORC2 components, rictor and sin1, strongly reduced AKT hydrophobic motif (HM) phosphorylation and AKT activity, but showed only minor growth impairment. A mutant form of AKT lacking the HM phosphorylation site displayed comparable activity. In contrast to the mild effects of removing HM site phosphorylation at normal levels of PI3K activity, loss of TORC2 activity strongly inhibited hyperplasia caused by elevated pathway activity, as in mutants of the tumor suppressor PTEN. Thus, TORC2 acts as a rheostat to broaden the range of AKT signaling at the high end of its range.
Keywords: AKT, PI3K, growth control, phosphorylation
AKT activity is principally determined by the level of phosphatidylinositol-3,4,5-triphosphate (PIP3) in the plasma membrane of cells. PIP3 is generated by phosphatidylinositol-3-kinase (PI3K) upon stimulation of receptor tyrosine kinases. PI3K is counteracted by the lipid-phosphatase, PTEN, a tumor suppressor inactivated in numerous cancers, which converts PIP3 back to PIP2 (for review, see Hay 2005). When PIP3 levels are elevated, AKT is recruited to the plasma membrane and phosphorylated in the “activation loop” by PDK1. In addition, AKT contains a highly conserved C-terminal hydrophobic motif (HM) that must also be phosphorylated for AKT activation (Alessi et al. 1996). Recent studies in both mammalian and Drosophila cell culture have indicated that TOR (target of rapamycin) complex 2 (TORC2) is responsible for HM phosphorylation (Sarbassov et al. 2005; Yang et al. 2006), implying a critical role for TORC2 in regulating AKT signaling and tissue growth. The kinase activity of TORC2 is provided by the protein kinase TOR, which is also found in a second complex, TORC1. These complexes have distinct properties. The rapamycin-sensitive TORC1 is essential for growth in yeast, Drosophila, and mammals (Wullschleger et al. 2006). Less is known about the in vivo role of TORC2, which contains at least two essential members, the “rapamycin-insensitive companion of mTOR,” Rictor, as well as SAPK-interacting protein 1, Sin1. Studies in yeast and mammalian cells have implicated TORC2 in regulating the actin cytoskeleton (Loewith et al. 2002; Jacinto et al. 2004; Sarbassov et al. 2004). It was recently reported that loss of TORC2 leads to loss of AKT HM phosphorylation and embryonic lethality in mouse (Shiota et al. 2006, Yang et al. 2006).
Results and Discussion
To study the role of TORC2 in Drosophila we generated mutants of rictor by imprecise excision of a P element in the first intron of the rictor gene (CG8002) (Fig. 1A). Two large deletions that are likely to eliminate rictor activity were recovered (rictorΔ1 and rictorΔ2) (see Supplementary Fig. S1 for detailed characterization). Unexpectedly, both mutants were homozygous viable, normal in appearance, and fertile (Fig. 1B). Recently, a novel member of TORC2, Sin1, was identified (Frias et al. 2006; Jacinto et al. 2006; Yang et al. 2006). A sin1 mutant was obtained from the Drosophila gene disruption project. This mutant is due to insertion of a PiggyBac transposon in the single exon of sin1 gene (CG10105), and is therefore likely to eliminate Sin1 activity (Fig. 1C; Supplementary Fig. S1). As observed for rictor mutants, Sin1 mutants were viable, fertile, normal in appearance, and displayed a very modest delay of development (<1 d) (data not shown).
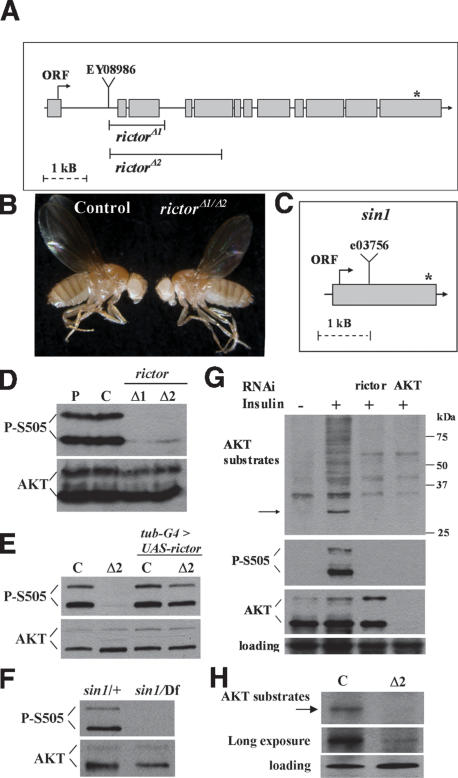
Figure 1.
rictor and sin1 mutant flies are viable despite strongly reduced AKT phosphorylation and compromised AKT activity. (A) Schematic representation of the rictor gene and the deletions induced by imprecise excision of the EY08986 P element. The coding region starts from exon 1. No alternative splice variants have been reported. The deletions remove exons 2 and 3 (Δ1) or exons 2–4 and part of exon 5 (Δ2) and destroy the ORF. A precise excision was also recovered as a control for all phenotypic analyses. (B) rictor mutant flies are viable and normal in appearance. (C) Schematic representation of the sin1 gene and the PiggyBac element e03756 inserted into the only exon of the gene. (D) Phosphorylation of the AKT HM S505 in control and rictor mutant flies. The EY08986 P-element line (P) and the precise excision line (denoted as C) were used as controls. Third instar larvae were homogenized in sample buffer, boiled, and analyzed by immunoblotting with the indicated antibodies. Quantification of band intensity suggests that S505 phosphorylation was reduced >#x003E;95% in the rictor mutant. (E) Lack of S505 phosphorylation was rescued by expression of a UAS-rictor driven by tubulin promoter-regulated GAL4 in control and rictor mutant (Δ2) background. Adult flies were homogenized in sample buffer, boiled, and analyzed by immunoblotting. (F) S505 phosphorylation in sin1 mutants |P[adult flies heterozygous for the e03756 PiggyBac insertion in sin1 over a deletion [Df(2R)BSC11] uncovering the sin1 gene|P]. sin1 heterozygotes were used as control flies. Analysis was done as in E. (G) Loss of TORC2 leads to reduced AKT substrate phosphorylation. S2 cells were treated for 4 d with dsRNA targeted against rictor or dAKT. Cells were treated with insulin (10 μg/mL for 30 min) or left untreated, homogenized in sample buffer, boiled, and analyzed by immunoblotting using antibodies against phosphorylated AKT target motif RxRxxS/T, AKT phospho-S505, or total AKT. An unspecific band was used as loading control. The most abundant AKT substrate is marked by an arrow (∼30 kDa). Comparable results were obtained using independent dsRNAs against rictor and dAKT (Supplementary Fig. S2). (H) Phosphorylation of the ∼30 kDa AKT substrate in rictor mutant (Δ2) compared with wild type. Third instar larval samples were analyzed as in G.
TORC2 has been reported to phosphorylate the HM site of AKT (Sarbassov et al. 2005). To evaluate the impact of TORC2 on AKT phosphorylation, we analyzed HM site phosphorylation (S505) in control and rictor mutant larvae. S505 phosphorylation was robust in wild-type larvae, but was barely detectable in the rictor mutants (Fig. 1D). AKT HM phosphorylation was also absent in the adult flies lacking either Rictor or Sin1 (Fig. 1E,F). HM phosphorylation in the rictor mutant could be restored by ubiquitous expression of a rictor transgene (Fig. 1E). Considering that AKT activity is essential during Drosophila development (Verdu et al. 1999) and that HM phosphorylation is considered to be essential for AKT activity (Alessi et al. 1996), we were surprised to find that S505 phosphorylation was apparently not required to support development.
This prompted us to ask whether HM phosphorylation is required for AKT activity. AKT activity was compared in S2 cells depleted of Rictor or AKT by RNA interference (RNAi). In control cells, insulin treatment led to phosphorylation of numerous AKT substrates, detected by an antibody to the phosphorylated AKT target motif RxRxxS/T (Fig. 1G). A majority of the insulin-induced bands were true AKT substrates, as they were not induced in cells depleted of AKT. Depletion of Rictor strongly reduced phosphorylation of AKT as well as the phosphorylation of the full spectrum of AKT substrates, comparable to cells depleted of AKT (Fig. 1G; Supplementary Fig. S2). This shows that TORC2-mediated HM phosphorylation is needed for full AKT activation, at least under conditions of strong insulin stimulation in vitro. We next examined AKT activity in a rictor mutant larval extract to assess the requirement for HM phosphorylation at a more physiological level of PI3K pathway activation. The signal was much lower than in insulin-treated S2 cells; however, in wild-type extracts we detected one prominent band corresponding to the most abundant band in S2 cells (Fig. 1H). Phosphorylation of this protein was strongly reduced in rictor mutants and was detectable only after the blot was overexposed (Fig. 1H, middle panel). This suggests that enzymatic activity of AKT was considerably reduced, but perhaps not completely eliminated, at physiological levels of PI3K pathway activity in the absence of HM site phosphorylation by TORC2.
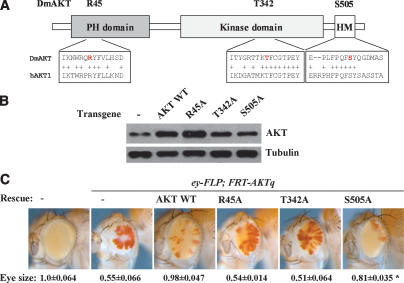
The possibility of residual AKT activity in the rictor mutant flies could indicate that they retain low levels of HM phosphorylation, sufficient to maintain the essential functions of AKT. Another possibility is that AKT lacking HM phosphorylation retains biological activity. To distinguish between these alternatives, we generated transgenic flies expressing wild-type and mutant forms of AKT under control of the tubulin promoter (Fig. 2A,B). To test their biological activity, we asked whether the mutant proteins could restore AKT activity in eye imaginal discs containing large regions of AKT mutant tissue. Using the eyeless-FLP recombinase system to produce clones of homozygous mutant cells (Newsome et al. 2000) typically leads to an eye composed of >80% of homozygous mutant tissue and <20% of heterozygous tissue if the mutation does not affect cell growth or survival. Because AKT mutant cells are severely growth impaired, a large portion of the AKT mosaic eyes were composed of heterozygous AKT/+ cells (Fig. 2C). Eye-specific loss of AKT led to severe reduction of eye size. Expression of the wild-type AKT transgene restored growth of the mutant tissue. AKT molecules bearing a mutation in the PH domain, which is essential for PIP3 binding (R45A), or in the activation loop site phosphorylated by PDK1 (T324A), did not show any rescue, indicating that these proteins lack AKT activity. In contrast, the AKT mutant lacking the HM phosphorylation site (S505A) rescued the AKT mutant. This was observed in the increased proportion of the eye consisting of AKT mutant cells (pale color) vs. the more darkly pigmented AKT/+ heterozygous tissue, as well as in terms of restoring eye size (Fig. 2C). Thus, in contrast to what might have been expected, AKT lacking HM phosphorylation retains considerable biological activity when assayed in vivo. Note that the in vivo assay for biological activity of AKT may be more sensitive than in vitro assays for kinase activity.
Figure 2.
AKT lacking HM phosphorylation retains biological activity. (A) Schematic representation of AKT domains and the mutants analyzed. R45 is a conserved amino acid in the PH domain essential for AKT membrane recruitment by PIP3 (Thomas et al. 2002). T342 is the PDK1 site in mammalian AKT molecules and is well conserved in Drosophila. The C-terminal HM (FxxFSY) containing the site (S505) is well conserved. (B) Expression of AKT mutants in the transgenic lines, visualized by immunoblotting for total AKT. Note that the transgenes were analyzed in the presence of wild-type AKT, so the bands display the sum of endogenous and transgenic proteins. (C) Adult eyes containing AKT mutant clones induced by eyeless promoter-driven expression FLP recombinase. To increase the relative amount of AKT mutant tissue, a cell-lethal mutation was used that eliminates the reciprocal products of the recombination event: cells homozygous for the chromosome with the wild-type copy of AKT. Cells expressing the red pigment are heterozygous AKT/+ cells that have not undergone recombination. Typically ∼20% of the cells remain heterozygous. Due to the severe undergrowth of AKT mutant tissue, the relative amount of AKT mutant tissue in the adult eye is only ∼50%, and the final size of the eye is severely reduced from wild type. Transgenic expression of wild-type AKT rescued eye size and leads to an eye with only small clones of heterozygous (pigmented) tissue. Expression of R45A and T324A did not rescue, but the S505A mutant rescued the tissue undergrowth. Total eye area was quantitated (N > 3). (*) Student’s t-test for the rescue by S505A <0.001.
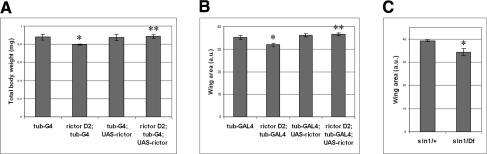
Although significant, rescue by the HM site mutant AKT was less complete than that obtained with wild-type AKT. This is consistent with the observation that flies lacking TORC2 activity showed reduced, but residual AKT activity (Fig. 1H). In view of the importance of AKT activity in tissue growth (Verdu et al. 1999), we asked whether there might be a more subtle growth defect associated with the reduced AKT activity in flies lacking TORC2 activity. Indeed, rictor mutants raised under controlled conditions on a rich diet displayed a modest (∼10%) reduction of body weight (Fig. 3A) and a modest reduction in tissue growth, indicated, for example, by smaller wings (Fig. 3B; Supplementary Fig. S1). Both were fully rescued by ubiquitous expression of a UAS-rictor transgene (Fig. 3A,B). Flies mutant for Sin1 displayed a similar modest growth reduction (Fig. 3C). Together these observations suggest that, at normal physiological levels of Insulin signaling, only minor growth impairment results from loss of AKT HM site phosphorylation. The residual level of AKT activity is thus nearly sufficient to support normal development.
Figure 3.
Tissue growth in rictor and sin1 mutants. (A) Reduced body size of rictor mutant flies. The flies were grown under controlled conditions and males were weighed 3 d after hatching. Measurements were performed in triplicate on batches of 10 flies. The data are representative of several independent experiments. (*) Student’s t-test <0.001. Ubiquitous expression of a UAS-rictor transgene driven by tubulin-GAL4 rescued the body-size reduction, but did not lead to increased body size. (**) Student’s t-test for the rescue <0.001 (B) Reduced wing size in rictor mutant flies. The flies were grown under controlled conditions. N > 15. (*) Student’s t-test <0.001. Expression of the rictor transgene rescued the reduced wing size. (**) Student’s t-test for the rescue <0.001 (C) Reduction of wing size in flies lacking sin1. As with sin1 loss-of-function mutants, we analyzed flies having the e03756 PiggyBac insertion over a larger deletion [Df(2R)BSC11] uncovering the sin1 gene. sin1/+ (w1118) heterozygotes were used as control flies. (*) Student’s t-test <0.001.
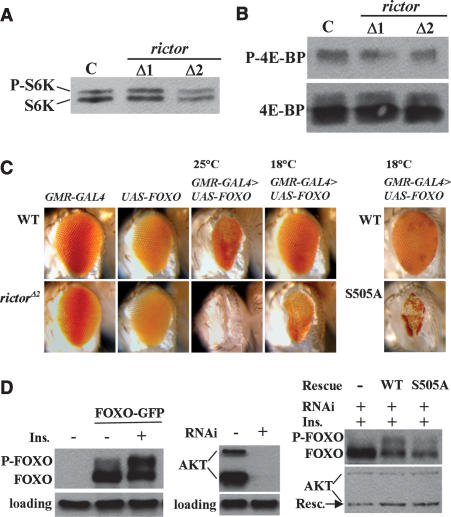
AKT signaling has been shown to regulate tissue growth via two effector routes: the TSC/Rheb/TORC1 pathway (Hay 2005), and by inhibiting the FOXO transcription factor (Junger et al. 2003; Puig et al. 2003). To assess the impact of reduced AKT activity without HM phosphorylation, we analyzed the phosphorylation of two TORC1 targets S6K and 4EBP in control and rictor mutant tissue samples and found no meaningful difference (Fig. 4A,B), consistent with the mild growth defects observed in rictor mutants. To ask whether rictor mutants affect FOXO activity, we used a sensitized assay in which FOXO overexpression in the eye imaginal disc using GMR-GAL4 causes a small rough eye phenotype (Junger et al. 2003). The eye was severely reduced in flies raised at 25°C, but at 18°C the eyes were nearly normal, indicating that the severity of this phenotype is sensitive to the level of excess FOXO activity (Fig. 4C). The phenotype caused by FOXO overexpression was strongly increased in the rictor mutant background at both temperatures.
Figure 4.
Increased FOXO activity in the absence of AKT HM phosphorylation. (A) Phosphorylation of S6K was not reduced in rictor mutant flies. Third instar larvae were homogenized in sample buffer, boiled, and analyzed by immunoblotting with anti-dS6K. The top band is the TORC1 phosphorylated product. (B) Phosphorylation of 4E-BP was not reduced in the fat body of the rictor mutant flies. Fat bodies were dissected from third instar larvae, homogenized in sample buffer, boiled, and analyzed by immunoblotting. (C) Loss of AKT HM phosphorylation enhanced the activity of FOXO. (Left panel) UAS-FOXO expression was induced in the developing eye with the GMR-GAL4 driver in wild-type or rictor mutant background. Flies were allowed to develop at 25°C or 18°C as indicated. GAL4 activity is lower at 18°C. (Right panel) Adult eyes containing AKT mutant clones were induced by eyeless promoter-driven expression FLP recombinase and rescued by expression of AKT WT or S505A transgenes, as described in Figure 2C. In this background, FOXO was overexpressed using GMR-GAL4. Flies were allowed to develop at 18°C. (D) Loss of AKT HM phosphorylation led to reduced FOXO phosphorylation. (Left panel) Insulin induces FOXO phosphorylation. S2 cells were transfected with dFOXO-GFP and treated with insulin or left untreated, and analyzed by immunoblotting using anti-GFP and anti-Kinesin (loading) antibodies. Insulin-induced FOXO phosphorylation causes a mobility shift on SDS-PAGE. (Middle panel) Knockdown of AKT. S2 cells were treated for 4 d with dsRNA targeted against 5′UTR of dAKT and analyzed by Western blotting using antibodies against AKT and Kinesin (loading). (Right panel) Loss of AKT HM phosphorylation led to reduced FOXO phosphorylation (AKT WT rescue: FOXO 33% in the slower migrating phosphorylated form; AKT S505A rescue: FOXO 19% phosphorylated). S2 cells were treated for 4 d with dsRNA targeted against 5′UTR of dAKT, transfected with FOXO-GFP and AKT WT or S505A as indicated. Cells were treated with insulin and analyzed by immunoblotting using antibodies against GFP and AKT. Equal transfection efficiency of AKT WT and S505A was verified by quantification of the AKT bands.
To directly assess the effect of AKT HM phosphorylation on FOXO regulation in vivo, we overexpressed FOXO in an eye in which the endogenous AKT gene was mutant and AKT activity was instead provided by a transgene expressing the S505A mutant. Removing the possibility of AKT HM phosphorylation strongly enhanced the phenotype caused by FOXO overexpression (Fig. 4C, right panel), producing a phenotype very similar to that in the rictor mutant background. We also analyzed FOXO phosphorylation in S2 cells, in which endogenous AKT was depleted by RNAi and replaced by expression of the S505A AKT transgene. FOXO phosphorylation, visualized by a mobility shift on SDS-PAGE (Puig et al. 2003), was clearly compromised in the absence of AKT HM phosphorylation, compared with similarly treated cells expressing the wild-type AKT transgene (Fig. 4D). Thus, TORC2-mediated regulation of AKT is needed to limit FOXO activity by controlling its phosphorylation level in vivo.
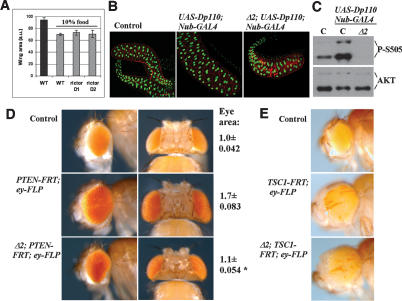
The results presented thus far indicate that loss of HM phosphorylation reduced AKT activity and that this had only a modest effect on tissue growth under normal physiological conditions of Insulin/PI3K activity. To test whether TORC2 would have a greater impact on tissue growth when PI3K signaling is compromised, we made use of the finding that PI3K signaling levels can be modulated by controlling the nutritional status of the animal (Junger et al. 2003; Teleman et al. 2005). We compared growth of flies raised on normal nutrient-rich food vs. food with 10% of the nutritional value. Nutrient-reduced food caused a developmental delay of several days and ∼25% reduction in growth of control flies. Surprisingly, instead of an enhanced phenotype, rictor mutants raised on nutrient-reduced food were indistinguishable from the control flies (Fig. 5A). Thus, TORC2 activity is not required for growth when PI3K signaling is low.
Figure 5.
TORC2 is needed for hyperplasia upon high PI3K signaling. (A) Histogram showing wing area of wild-type or rictor mutant flies reared under normal or nutrient-limited conditions. Larvae were grown under controlled conditions on normal food or 10% normal food. N > 16. (B) Ectopic expression of the catalytic subunit of PI3K Dp110 in the developing salivary gland was induced using nubbin-GAL4 in wild type of rictor mutant larvae. nubbin-GAL4 flies were used as control. Fixed salivary glands were stained with phalloidin (red) and DAPI (green) and visualized by confocal microscopy. (C) Immunoblot analysis of AKT S505 phosphorylation from isolated control salivary glands (C), and salivary glands overexpressing Dp110 in the presence (C) or absence of Rictor (Δ2). (D) Lateral and dorsal views of adult fly heads (top panel, control flies). (Middle panel) PTEN mutant clones were induced to the eye imaginal disc using eyeless-FLP and caused strong overgrowth of the eye. (Bottom panel) This overgrowth was efficiently suppressed in the rictorΔ2 mutant. Total eye area was quantitated (N = 5). (*) Student’s t-test for the suppression by rictorΔ2 <0.001. (E) Lateral view of adult fly heads. (Top panel) control flies. (Middle panel) Overgrowth caused by TSC1 mutant clones induced in the eye imaginal disc using eyeless-FLP. (Bottom panel) Overgrowth caused by TSC1 mutant clones was not suppressed in the rictorΔ2 mutant.
This suggested that TORC2 activity might be needed in vivo to permit high-level PI3K/AKT signaling. To test this, we compared the effects of genetically elevated PI3K activity in control and rictor mutant flies. Overexpressing the catalytic subunit of PI3K caused tissue overgrowth (as described previously; Leevers et al. 1996) and increased phosphorylation of AKT on S505 (Fig. 5B,C). In rictor mutants, S505 phosphorylation was undetectable, and intriguingly, overgrowth was efficiently suppressed despite elevated PI3K activity (Fig. 5B,C). Removal of PTEN provides an independent means to elevate PIP3 levels and increase AKT activity. PTEN mutant clones cause dramatic hyperplasia in the developing eye by increasing both cell number and cell size (Fig. 5D; Goberdhan et al. 1999; Gao et al. 2000). Removing rictor in this background efficiently suppressed the tissue overgrowth caused by loss of PTEN, leading to a nearly normal-sized eye (Fig. 5D). The observed suppression was likely to be due to abolished AKT HM phosphorylation, as PI3K-induced hyperplasia was also efficiently suppressed in the eye tissue in which endogenous AKT was replaced by the S505A mutant (Supplementary Fig. S3). We next assessed the ability of rictor to suppress overgrowth of the eye caused by clones mutant for TSC1, which activates TORC1 signaling downstream from AKT, and causes massive tissue overgrowth (Gao and Pan 2001; Potter et al. 2001; Tapon et al. 2001). Loss of TSC1 caused robust overgrowth of the eye, but this was not suppressed by simultaneously removing rictor (Fig. 5E). Thus, the growth-limiting effects of rictor are specific to activation of the pathway at or above the level of AKT.
The PI3K/AKT signaling pathway is conserved between Drosophila and mammalian species. The lack of genetic redundancy among pathway components makes Drosophila a useful system in which to dissect the roles of the individual pathway members in vivo. Earlier analyses of other pathway members have shown that the Insulin receptor, PI3K, PDK1, and AKT are each essential for viability, and that mutant tissue displays severe undergrowth (Supplementary Fig. S4; Verdu et al. 1999; Weinkove et al. 1999; Brogiolo et al. 2001; Rintelen et al. 2001). Mutants of Drosophila insulin receptor substrate homolog, chico, are semiviable but severely growth impaired (Böhni et al. 1999). Although individual AKT mutants are viable in mouse, the essential nature of AKT is likely to be masked by genetic redundancy among the three AKT genes (Chen et al. 2001; Cho et al. 2001a, b; Peng et al. 2003). Previous studies in cultured cells have suggested that TORC2 is an important regulator of AKT phosphorylation and activity, and that this phosphorylation event is required for AKT kinase activity (Alessi et al. 1996; Sarbassov et al. 2005). It was recently shown that loss of TORC2 activity in rictor mutant mice leads to loss of AKT HM phosphorylation and to embryonic lethality (Shiota et al. 2006; Yang et al. 2006), suggesting that HM phosphorylation is essential for AKT activity in the mouse. In contrast, our findings show that TORC2-mediated phosphorylation on the HM site is not essential for AKT activity in vivo. Indeed, although AKT activity was reduced, considerable residual activity was found in flies lacking TORC2 activity. Flies expressing a mutant form of AKT lacking the HM phosphorylation site also showed considerable AKT activity in vivo. Our findings indicate that the maximal level of AKT activity is limited in the absence of HM phosphorylation. Under normal physiological conditions in Drosophila, this reduced level of AKT activity is almost sufficient to support normal growth. But without HM phosphorylation, AKT cannot transduce the higher-than-normal levels of PI3K pathway activity that result from mutation of the tumor suppressor PTEN or increased insulin stimulation. When considered in this context, the lethality of rictor mutant mice could reflect a higher threshold in the requirement for AKT activity in some biological process in mouse than in fly, but the possibility of essential TORC2 targets other than AKT cannot be excluded.
Perhaps the most intriguing implication of this study lies in the area of cancer biology. Elevated AKT activity is a hallmark of human cancer, with a substantial proportion of human tumors depending on AKT pathway activation, for example, due to PTEN mutations (Hay 2005). Our findings suggest that inhibiting TORC2 activity, rather than AKT itself, may prove to be a promising strategy for cancer therapy.
Materials and methods
Fly strains
EY08986, e03756, Df(1)JA27, Df(2R)BSC11, and GMR-GAL4 flies were obtained from the Bloomington Stock Center. The PTEN3 mutant is described in Goberdhan et al. (1999). The TSC1R453X mutant is described in Tapon et al. (2001). UAS-Dp110 WT and UAS-Dp110 CAAX are described in Leevers et al. (1996). The AKTq mutant is described in Staveley et al. (1998). The generation of pUAST-FOXO-GFP plasmid is described in Teleman et al. (2005). The pUAST-FOXO-GFP transgenic flies were provided by Aurelio Teleman. The rictor coding region was subcloned into pUAST vector using BglII and XhoI sites. The coding region of AKT (530 AA isoform) cDNA was amplified by PCR and subcloned into pCaSpeR-tubulin (EcoRI/KpnI fragment of tubulin-1 promoter in pCaSpeR-4) (Basler and Struhl 1994) using NotI and XbaI sites. The point mutations were made by QuikChange site-directed mutagenesis kit (Stratagene) and confirmed by sequencing.
Cell culture and treatments
The S2 cells were grown in 25°C in SFM (Gibco) supplemented with L-glutamine. Double-stranded RNA (dsRNA) was prepared using the following templates: Rictor1, nucleotides 1878–2342 of rictor coding sequence; Rictor2, 1457–1874; dAKT1, nucleotides 475–1008 of AKT (530 AA isoform) coding sequence; and dAKT2, nucleotides −525 to −2 in the 5′ untranslated region (UTR) of AKT 530 AA isoform. S2 cells were treated with 37 nM dsRNA for 4 d. To activate AKT, cells were treated with 10 μg/mL bovine insulin (Sigma) for 30 min.
Generation of clones
Clones of homozygous mutant cells were generated by using the following genotypes:
w, ey-flp; tub-AKT (wild type or mutants), w+; FRT82B, cell lethal, w+/FRT82B, AKTq (adult eye).
w, ey-flp/UAS-FOXO, w+; tub-AKT (wild type or S505A), w+/GMR-GAL4, w+; FRT82B, cell lethal, w+/FRT82B, AKTq (adult eye).
yw; FRT42,PTEN3/FRT42; ey-flp (adult eye), males with or without rictorΔ2 on X chromosome (adult eye).
w, ey-flp/UAS-Dp110-CAAX, w+; tub-AKT (wild type or S505A), w+/GMR-GAL4, w+; FRT82B, cell lethal, w+/FRT82B, AKTq (adult eye).
yw; ey-flp; FRT82B, TSC1R453X/FRT82B (adult eye), males with or without rictorΔ2 on X chromosome (adult eye).
Immunoblotting
Antibodies to phospho-S505-dAKT, AKT, phospho-S475-hAKT, phospho-Thr37/46–4E-BP, and phospho-AKT substrate were from Cell Signaling Technology. Anti-Tubulin was from Sigma, anti-GFP was from Torrey Pines Biolabs, and anti-Kinesin was from Cytoskeleton. Anti-dS6K is described in Stewart et al. (1996). Anti-d4E-BP is described in Miron et al. (2001).
Measurements
Weight and wing area measurements were done on flies grown under identical conditions. Fifty newly hatched first instar larvae were seeded per vial. After hatching, adults were aged for 2 or 3 d before analysis. Wing and eye areas as well as band intensities were quantified by using ImageJ software (NIH).
Acknowledgments
We thank Iswar Hariharan, Sally Leevers, Armen Manoukian, Mary Stewart, Nahum Sonenberg, and Clive Wilson for reagents. Barry Thompson, Sebastien Szuplewski, Aurelio Teleman, David Hipfner, and Minna Poukkula gave practical advice and helpful discussions. V.H. was supported by the Academy of Finland, “Helsingin Sanomain 100-vuotissäätiö” (Finland), and Marie Curie Intra-European Fellowship.
Footnotes
Supplemental material is available at http://www.genesdev.org.
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.416307
References
- Alessi D.R., Andjelkovic M., Caudwell B., Cron P., Morrice N., Cohen P., Hemmings B.A., Andjelkovic M., Caudwell B., Cron P., Morrice N., Cohen P., Hemmings B.A., Caudwell B., Cron P., Morrice N., Cohen P., Hemmings B.A., Cron P., Morrice N., Cohen P., Hemmings B.A., Morrice N., Cohen P., Hemmings B.A., Cohen P., Hemmings B.A., Hemmings B.A. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- Basler K., Struhl G., Struhl G. Compartment boundaries and the control of Drosophila limb pattern by hedgehog protein. Nature. 1994;368:208–214. doi: 10.1038/368208a0. [DOI] [PubMed] [Google Scholar]
- Böhni R., Riesgo-Escovar J., Oldham S., Brogiolo W., Stocker H., Andruss B.F., Beckingham K., Hafen E., Riesgo-Escovar J., Oldham S., Brogiolo W., Stocker H., Andruss B.F., Beckingham K., Hafen E., Oldham S., Brogiolo W., Stocker H., Andruss B.F., Beckingham K., Hafen E., Brogiolo W., Stocker H., Andruss B.F., Beckingham K., Hafen E., Stocker H., Andruss B.F., Beckingham K., Hafen E., Andruss B.F., Beckingham K., Hafen E., Beckingham K., Hafen E., Hafen E. Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1-4. Cell. 1999;97:865–875. doi: 10.1016/s0092-8674(00)80799-0. [DOI] [PubMed] [Google Scholar]
- Brogiolo W., Stocker H., Ikeya T., Rintelen F., Fernandez R., Hafen E., Stocker H., Ikeya T., Rintelen F., Fernandez R., Hafen E., Ikeya T., Rintelen F., Fernandez R., Hafen E., Rintelen F., Fernandez R., Hafen E., Fernandez R., Hafen E., Hafen E. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr. Biol. 2001;11:213–221. doi: 10.1016/s0960-9822(01)00068-9. [DOI] [PubMed] [Google Scholar]
- Chen W.S., Xu P.Z., Gottlob K., Chen M.L., Sokol K., Shiyanova T., Roninson I., Weng W., Suzuki R., Tobe K., Xu P.Z., Gottlob K., Chen M.L., Sokol K., Shiyanova T., Roninson I., Weng W., Suzuki R., Tobe K., Gottlob K., Chen M.L., Sokol K., Shiyanova T., Roninson I., Weng W., Suzuki R., Tobe K., Chen M.L., Sokol K., Shiyanova T., Roninson I., Weng W., Suzuki R., Tobe K., Sokol K., Shiyanova T., Roninson I., Weng W., Suzuki R., Tobe K., Shiyanova T., Roninson I., Weng W., Suzuki R., Tobe K., Roninson I., Weng W., Suzuki R., Tobe K., Weng W., Suzuki R., Tobe K., Suzuki R., Tobe K., Tobe K., et al. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes & Dev. 2001;15:2203–2208. doi: 10.1101/gad.913901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H., Mu J., Kim J.K., Thorvaldsen J.L., Chu Q., Crenshaw E.B., III, Kaestner K.H., Bartolomei M.S., Shulman G.I., Birnbaum M.J., Mu J., Kim J.K., Thorvaldsen J.L., Chu Q., Crenshaw E.B., III, Kaestner K.H., Bartolomei M.S., Shulman G.I., Birnbaum M.J., Kim J.K., Thorvaldsen J.L., Chu Q., Crenshaw E.B., III, Kaestner K.H., Bartolomei M.S., Shulman G.I., Birnbaum M.J., Thorvaldsen J.L., Chu Q., Crenshaw E.B., III, Kaestner K.H., Bartolomei M.S., Shulman G.I., Birnbaum M.J., Chu Q., Crenshaw E.B., III, Kaestner K.H., Bartolomei M.S., Shulman G.I., Birnbaum M.J., Crenshaw E.B., III, Kaestner K.H., Bartolomei M.S., Shulman G.I., Birnbaum M.J., Kaestner K.H., Bartolomei M.S., Shulman G.I., Birnbaum M.J., Bartolomei M.S., Shulman G.I., Birnbaum M.J., Shulman G.I., Birnbaum M.J., Birnbaum M.J. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB β) Science. 2001a;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- Cho H., Thorvaldsen J.L., Chu Q., Feng F., Birnbaum M.J., Thorvaldsen J.L., Chu Q., Feng F., Birnbaum M.J., Chu Q., Feng F., Birnbaum M.J., Feng F., Birnbaum M.J., Birnbaum M.J. Akt1/PKBα is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J. Biol. Chem. 2001b;276:38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- Frias M.A., Thoreen C.C., Jaffe J.D., Schroder W., Sculley T., Carr S.A., Sabatini D.M., Thoreen C.C., Jaffe J.D., Schroder W., Sculley T., Carr S.A., Sabatini D.M., Jaffe J.D., Schroder W., Sculley T., Carr S.A., Sabatini D.M., Schroder W., Sculley T., Carr S.A., Sabatini D.M., Sculley T., Carr S.A., Sabatini D.M., Carr S.A., Sabatini D.M., Sabatini D.M. mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr. Biol. 2006;16:1865–1870. doi: 10.1016/j.cub.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Gao X., Pan D., Pan D. TSC1 and TSC2 tumor suppressors antagonize insulin signaling in cell growth. Genes & Dev. 2001;15:1383–1392. doi: 10.1101/gad.901101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Neufeld T.P., Pan D., Neufeld T.P., Pan D., Pan D. Drosophila PTEN regulates cell growth and proliferation through PI3K-dependent and -independent pathways. Dev. Biol. 2000;221:404–418. doi: 10.1006/dbio.2000.9680. [DOI] [PubMed] [Google Scholar]
- Goberdhan D.C., Paricio N., Goodman E.C., Mlodzik M., Wilson C., Paricio N., Goodman E.C., Mlodzik M., Wilson C., Goodman E.C., Mlodzik M., Wilson C., Mlodzik M., Wilson C., Wilson C. Drosophila tumor suppressor PTEN controls cell size and number by antagonizing the Chico/PI3-kinase signaling pathway. Genes & Dev. 1999;13:3244–3258. doi: 10.1101/gad.13.24.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell. 2005;8:179–183. doi: 10.1016/j.ccr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Jacinto E., Loewith R., Schmidt A., Lin S., Ruegg M.A., Hall A., Hall M.N., Loewith R., Schmidt A., Lin S., Ruegg M.A., Hall A., Hall M.N., Schmidt A., Lin S., Ruegg M.A., Hall A., Hall M.N., Lin S., Ruegg M.A., Hall A., Hall M.N., Ruegg M.A., Hall A., Hall M.N., Hall A., Hall M.N., Hall M.N. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat. Cell Biol. 2004;6:1122–1128. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- Jacinto E., Facchinetti V., Liu D., Soto N., Wei S., Jung S.Y., Huang Q., Qin J., Su B., Facchinetti V., Liu D., Soto N., Wei S., Jung S.Y., Huang Q., Qin J., Su B., Liu D., Soto N., Wei S., Jung S.Y., Huang Q., Qin J., Su B., Soto N., Wei S., Jung S.Y., Huang Q., Qin J., Su B., Wei S., Jung S.Y., Huang Q., Qin J., Su B., Jung S.Y., Huang Q., Qin J., Su B., Huang Q., Qin J., Su B., Qin J., Su B., Su B. SIN1/MIP1 maintains rictor–mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- Junger M.A., Rintelen F., Stocker H., Wasserman J.D., Vegh M., Radimerski T., Greenberg M.E., Hafen E., Rintelen F., Stocker H., Wasserman J.D., Vegh M., Radimerski T., Greenberg M.E., Hafen E., Stocker H., Wasserman J.D., Vegh M., Radimerski T., Greenberg M.E., Hafen E., Wasserman J.D., Vegh M., Radimerski T., Greenberg M.E., Hafen E., Vegh M., Radimerski T., Greenberg M.E., Hafen E., Radimerski T., Greenberg M.E., Hafen E., Greenberg M.E., Hafen E., Hafen E. The Drosophila forkhead transcription factor FOXO mediates the reduction in cell number associated with reduced insulin signaling. J. Biol. 2003;2:20. doi: 10.1186/1475-4924-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leevers S.J., Weinkove D., MacDougall L.K., Hafen E., Waterfield M.D., Weinkove D., MacDougall L.K., Hafen E., Waterfield M.D., MacDougall L.K., Hafen E., Waterfield M.D., Hafen E., Waterfield M.D., Waterfield M.D. The Drosophila phosphoinositide 3-kinase Dp110 promotes cell growth. EMBO J. 1996;15:6584–6594. [PMC free article] [PubMed] [Google Scholar]
- Loewith R., Jacinto E., Wullschleger S., Lorberg A., Crespo J.L., Bonenfant D., Oppliger W., Jenoe P., Hall M.N., Jacinto E., Wullschleger S., Lorberg A., Crespo J.L., Bonenfant D., Oppliger W., Jenoe P., Hall M.N., Wullschleger S., Lorberg A., Crespo J.L., Bonenfant D., Oppliger W., Jenoe P., Hall M.N., Lorberg A., Crespo J.L., Bonenfant D., Oppliger W., Jenoe P., Hall M.N., Crespo J.L., Bonenfant D., Oppliger W., Jenoe P., Hall M.N., Bonenfant D., Oppliger W., Jenoe P., Hall M.N., Oppliger W., Jenoe P., Hall M.N., Jenoe P., Hall M.N., Hall M.N. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol. Cell. 2002;10:457–468. doi: 10.1016/s1097-2765(02)00636-6. [DOI] [PubMed] [Google Scholar]
- Miron M., Verdu J., Lachance P.E., Birnbaum M.J., Lasko P.F., Sonenberg N., Verdu J., Lachance P.E., Birnbaum M.J., Lasko P.F., Sonenberg N., Lachance P.E., Birnbaum M.J., Lasko P.F., Sonenberg N., Birnbaum M.J., Lasko P.F., Sonenberg N., Lasko P.F., Sonenberg N., Sonenberg N. The translational inhibitor 4E-BP is an effector of PI(3)K/Akt signalling and cell growth in Drosophila. Nat. Cell Biol. 2001;3:596–601. doi: 10.1038/35078571. [DOI] [PubMed] [Google Scholar]
- Newsome T.P., Asling B., Dickson B.J., Asling B., Dickson B.J., Dickson B.J. Analysis of Drosophila photoreceptor axon guidance in eye-specific mosaics. Development. 2000;127:851–860. doi: 10.1242/dev.127.4.851. [DOI] [PubMed] [Google Scholar]
- Peng X.D., Xu P.Z., Chen M.L., Hahn-Windgassen A., Skeen J., Jacobs J., Sundararajan D., Chen W.S., Crawford S.E., Coleman K.G., Xu P.Z., Chen M.L., Hahn-Windgassen A., Skeen J., Jacobs J., Sundararajan D., Chen W.S., Crawford S.E., Coleman K.G., Chen M.L., Hahn-Windgassen A., Skeen J., Jacobs J., Sundararajan D., Chen W.S., Crawford S.E., Coleman K.G., Hahn-Windgassen A., Skeen J., Jacobs J., Sundararajan D., Chen W.S., Crawford S.E., Coleman K.G., Skeen J., Jacobs J., Sundararajan D., Chen W.S., Crawford S.E., Coleman K.G., Jacobs J., Sundararajan D., Chen W.S., Crawford S.E., Coleman K.G., Sundararajan D., Chen W.S., Crawford S.E., Coleman K.G., Chen W.S., Crawford S.E., Coleman K.G., Crawford S.E., Coleman K.G., Coleman K.G., et al. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes & Dev. 2003;17:1352–1365. doi: 10.1101/gad.1089403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter C.J., Huang H., Xu T., Huang H., Xu T., Xu T. Drosophila Tsc1 functions with Tsc2 to antagonize insulin signaling in regulating cell growth, cell proliferation, and organ size. Cell. 2001;105:357–368. doi: 10.1016/s0092-8674(01)00333-6. [DOI] [PubMed] [Google Scholar]
- Puig O., Marr M.T., Ruhf M.L., Tjian R., Marr M.T., Ruhf M.L., Tjian R., Ruhf M.L., Tjian R., Tjian R. Control of cell number by Drosophila FOXO: Downstream and feedback regulation of the insulin receptor pathway. Genes & Dev. 2003;17:2006–2020. doi: 10.1101/gad.1098703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rintelen F., Stocker H., Thomas G., Hafen E., Stocker H., Thomas G., Hafen E., Thomas G., Hafen E., Hafen E. PDK1 regulates growth through Akt and S6K in Drosophila. Proc. Natl. Acad. Sci. 2001;98:15020–15025. doi: 10.1073/pnas.011318098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov D.D., Ali S.M., Kim D.H., Guertin D.A., Latek R.R., Erdjument-Bromage H., Tempst P., Sabatini D.M., Ali S.M., Kim D.H., Guertin D.A., Latek R.R., Erdjument-Bromage H., Tempst P., Sabatini D.M., Kim D.H., Guertin D.A., Latek R.R., Erdjument-Bromage H., Tempst P., Sabatini D.M., Guertin D.A., Latek R.R., Erdjument-Bromage H., Tempst P., Sabatini D.M., Latek R.R., Erdjument-Bromage H., Tempst P., Sabatini D.M., Erdjument-Bromage H., Tempst P., Sabatini D.M., Tempst P., Sabatini D.M., Sabatini D.M. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- Sarbassov D.D., Guertin D.A., Ali S.M., Sabatini D.M., Guertin D.A., Ali S.M., Sabatini D.M., Ali S.M., Sabatini D.M., Sabatini D.M. Phosphorylation and regulation of Akt/PKB by the rictor–mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Shiota C., Woo J.T., Lindner J., Shelton K.D., Magnuson M.A., Woo J.T., Lindner J., Shelton K.D., Magnuson M.A., Lindner J., Shelton K.D., Magnuson M.A., Shelton K.D., Magnuson M.A., Magnuson M.A. Multiallelic disruption of the rictor gene in mice reveals that mTOR complex 2 is essential for fetal growth and viability. Dev. Cell. 2006;11:583–589. doi: 10.1016/j.devcel.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Staveley B.E., Ruel L., Jin J., Stambolic V., Mastronardi F.G., Heitzler P., Woodgett J.R., Manoukian A.S., Ruel L., Jin J., Stambolic V., Mastronardi F.G., Heitzler P., Woodgett J.R., Manoukian A.S., Jin J., Stambolic V., Mastronardi F.G., Heitzler P., Woodgett J.R., Manoukian A.S., Stambolic V., Mastronardi F.G., Heitzler P., Woodgett J.R., Manoukian A.S., Mastronardi F.G., Heitzler P., Woodgett J.R., Manoukian A.S., Heitzler P., Woodgett J.R., Manoukian A.S., Woodgett J.R., Manoukian A.S., Manoukian A.S. Genetic analysis of protein kinase B (AKT) in Drosophila. Curr. Biol. 1998;8:599–602. doi: 10.1016/s0960-9822(98)70231-3. [DOI] [PubMed] [Google Scholar]
- Stewart M.J., Berry C.O., Zilberman F., Thomas G., Kozma S.C., Berry C.O., Zilberman F., Thomas G., Kozma S.C., Zilberman F., Thomas G., Kozma S.C., Thomas G., Kozma S.C., Kozma S.C. The Drosophila p70s6k homolog exhibits conserved regulatory elements and rapamycin sensitivity. Proc. Natl. Acad. Sci. 1996;93:10791–10796. doi: 10.1073/pnas.93.20.10791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapon N., Ito N., Dickson B.J., Treisman J.E., Hariharan I.K., Ito N., Dickson B.J., Treisman J.E., Hariharan I.K., Dickson B.J., Treisman J.E., Hariharan I.K., Treisman J.E., Hariharan I.K., Hariharan I.K. The Drosophila tuberous sclerosis complex gene homologs restrict cell growth and cell proliferation. Cell. 2001;105:345–355. doi: 10.1016/s0092-8674(01)00332-4. [DOI] [PubMed] [Google Scholar]
- Teleman A.A., Chen Y.W., Cohen S.M., Chen Y.W., Cohen S.M., Cohen S.M. Drosophila melted modulates FOXO and TOR activity. Dev. Cell. 2005;9:271–281. doi: 10.1016/j.devcel.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Thomas C.C., Deak M., Alessi D.R., van Aalten D.M., Deak M., Alessi D.R., van Aalten D.M., Alessi D.R., van Aalten D.M., van Aalten D.M. High-resolution structure of the pleckstrin homology domain of protein kinase b/akt bound to phosphatidylinositol (3,4,5)-trisphosphate. Curr. Biol. 2002;12:1256–1262. doi: 10.1016/s0960-9822(02)00972-7. [DOI] [PubMed] [Google Scholar]
- Verdu J., Buratovich M.A., Wilder E.L., Birnbaum M.J., Buratovich M.A., Wilder E.L., Birnbaum M.J., Wilder E.L., Birnbaum M.J., Birnbaum M.J. Cell-autonomous regulation of cell and organ growth in Drosophila by Akt/PKB. Nat. Cell Biol. 1999;1:500–506. doi: 10.1038/70293. [DOI] [PubMed] [Google Scholar]
- Weinkove D., Neufeld T.P., Twardzik T., Waterfield M.D., Leevers S.J., Neufeld T.P., Twardzik T., Waterfield M.D., Leevers S.J., Twardzik T., Waterfield M.D., Leevers S.J., Waterfield M.D., Leevers S.J., Leevers S.J. Regulation of imaginal disc cell size, cell number and organ size by Drosophila class I(A) phosphoinositide 3-kinase and its adaptor. Erratum. Curr. Biol. Curr. Biol. 1999;99:1019–1029. R867. doi: 10.1016/s0960-9822(99)80450-3. [DOI] [PubMed] [Google Scholar]
- Wullschleger S., Loewith R., Hall M.N., Loewith R., Hall M.N., Hall M.N. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Yang Q., Inoki K., Ikenoue T., Guan K.L., Inoki K., Ikenoue T., Guan K.L., Ikenoue T., Guan K.L., Guan K.L. Identification of Sin1 as an essential TORC2 component required for complex formation and kinase activity. Genes & Dev. 2006;20:2820–2832. doi: 10.1101/gad.1461206. [DOI] [PMC free article] [PubMed] [Google Scholar]