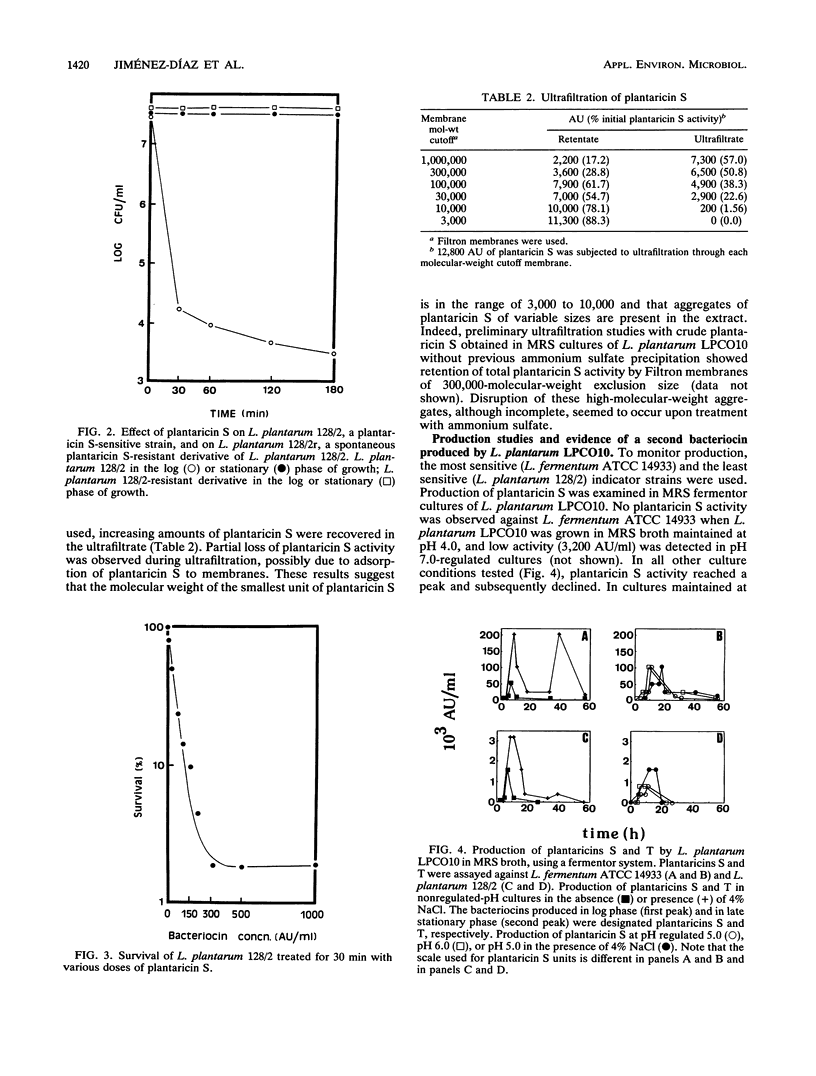
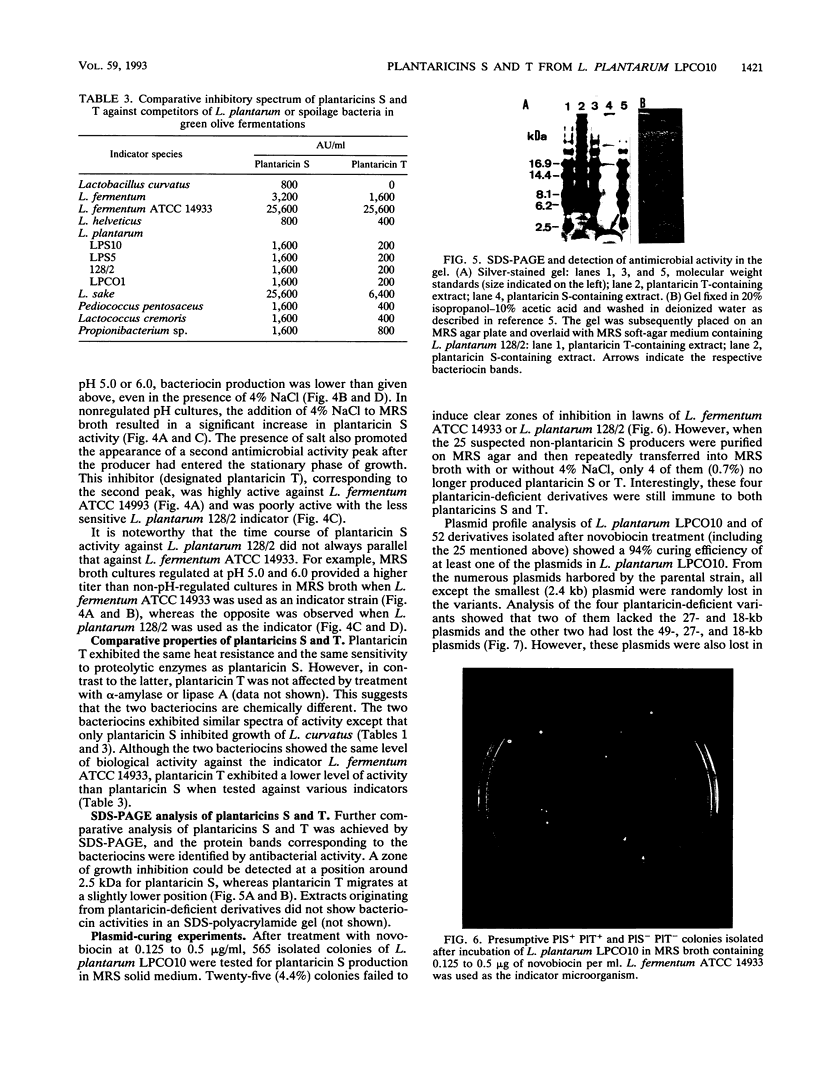
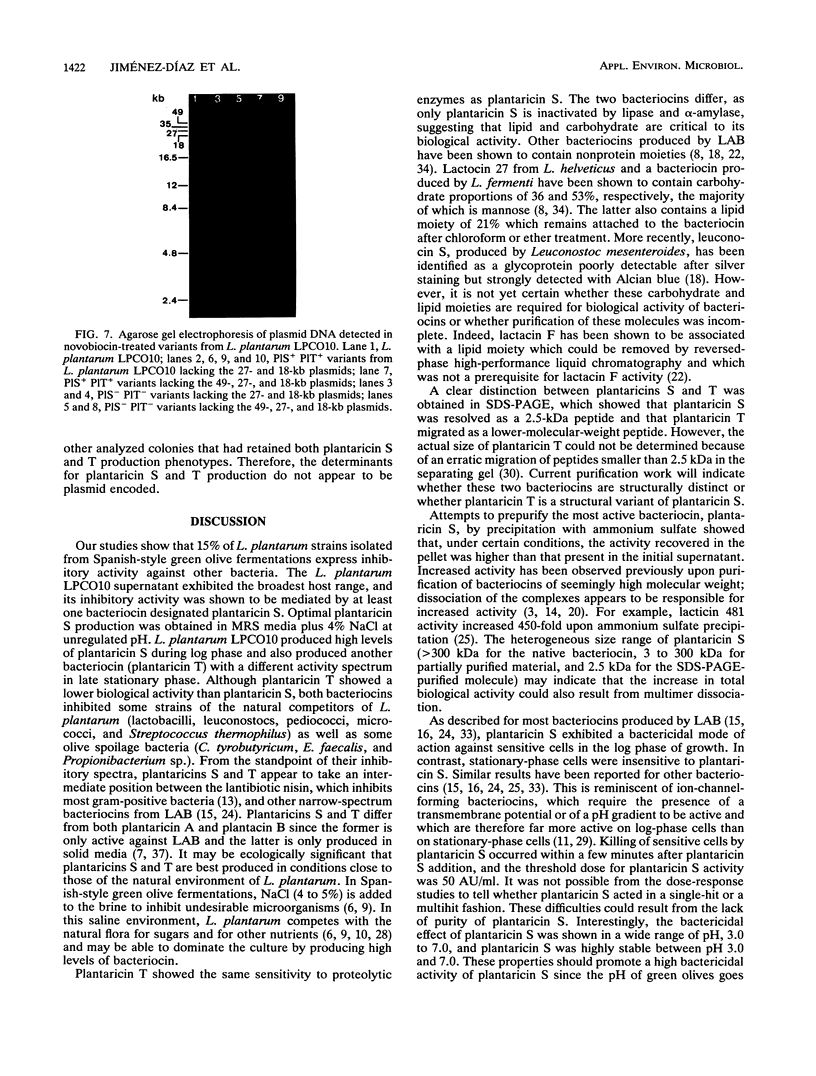
Abstract
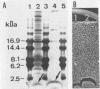
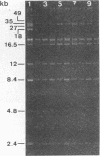
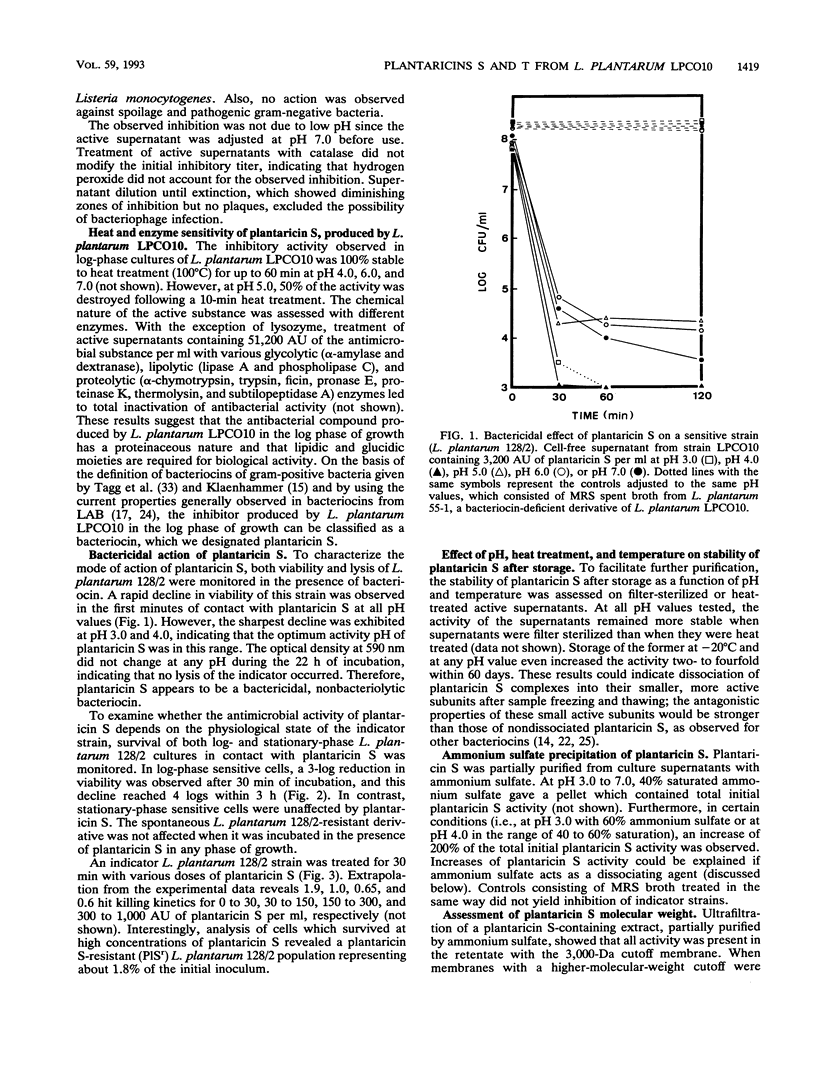
Twenty-six strains of Lactobacillus plantarum isolated from green olive fermentations were tested for cross-antagonistic activities in an agar drop diffusion test. Cell-free supernatants from four of these strains were shown to inhibit the growth of at least one of the L. plantarum indicator strains. L. plantarum LPCO10 provided the broadest spectrum of activity and was selected for further studies. The inhibitory compound from this strain was active against some gram-positive bacteria, including clostridia and propionibacteria as well as natural competitors of L. plantarum in olive fermentation brines. In contrast, no activity against gram-negative bacteria was detected. Inhibition due to the effect of organic acids, hydrogen peroxide, or bacteriophages was excluded. Since the inhibitory activity of the active supernatant was lost after treatment with various proteolytic enzymes, this substance could be classified as a bacteriocin, designated plantaricin S. Plantaricin S was also sensitive to glycolytic and lipolytic enzymes, suggesting that it was a glycolipoprotein. It exhibited a bactericidal and nonbacteriolytic mode of action against indicator cells. This bacteriocin was heat stable (60 min at 100°C), active in a pH range of 3.0 to 7.0, and also stable in crude culture supernatants during storage. Ultrafiltration studies indicated that plantaricin S occurred as multimolecular aggregates and that the size of the smallest active form is between 3 and 10 kDa. In sodium dodecyl sulfate-polyacrylamide gels, plantaricin S migrated as a peptide of ca. 2.5 kDa. Maximum production of plantaricin S was obtained in a fermentor system in unregulated pH and log-phase cultures of L. plantarum LPCO10 in MRS broth plus 4% NaCl. In these culture conditions, a second bacteriocin (designated plantaricin T) was produced in late-stationary-phase cultures of L. plantarum LPCO10. On the basis of its biological activity, its sensitivity to various enzymes, and its molecular weight (lower than that of plantaricin S) as assessed in sodium dodecyl sulfate-polyacrylamide gel electrophoresis, plantaricin T appeared different from plantaricin S. Curing experiments with L. plantarum LPCO10 resulted in the appearance of variants that no longer produced either of the two bacteriocins but that were still immune to both of them.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. G., McKay L. L. Simple and rapid method for isolating large plasmid DNA from lactic streptococci. Appl Environ Microbiol. 1983 Sep;46(3):549–552. doi: 10.1128/aem.46.3.549-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barefoot S. F., Klaenhammer T. R. Purification and characterization of the Lactobacillus acidophilus bacteriocin lactacin B. Antimicrob Agents Chemother. 1984 Sep;26(3):328–334. doi: 10.1128/aac.26.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F. H., Abee T., Konings W. N. Mechanism of action of the peptide antibiotic nisin in liposomes and cytochrome c oxidase-containing proteoliposomes. Appl Environ Microbiol. 1991 Aug;57(8):2164–2170. doi: 10.1128/aem.57.8.2164-2170.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C. F., Kunka B. S. Plasmid-Associated Bacteriocin Production and Sucrose Fermentation in Pediococcus acidilactici. Appl Environ Microbiol. 1987 Oct;53(10):2534–2538. doi: 10.1128/aem.53.10.2534-2538.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joerger M. C., Klaenhammer T. R. Characterization and purification of helveticin J and evidence for a chromosomally determined bacteriocin produced by Lactobacillus helveticus 481. J Bacteriol. 1986 Aug;167(2):439–446. doi: 10.1128/jb.167.2.439-446.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaenhammer T. R. Bacteriocins of lactic acid bacteria. Biochimie. 1988 Mar;70(3):337–349. doi: 10.1016/0300-9084(88)90206-4. [DOI] [PubMed] [Google Scholar]
- Lewus C. B., Sun S., Montville T. J. Production of an Amylase-Sensitive Bacteriocin by an Atypical Leuconostoc paramesenteroides Strain. Appl Environ Microbiol. 1992 Jan;58(1):143–149. doi: 10.1128/aem.58.1.143-149.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Muriana P. M., Klaenhammer T. R. Cloning, phenotypic expression, and DNA sequence of the gene for lactacin F, an antimicrobial peptide produced by Lactobacillus spp. J Bacteriol. 1991 Mar;173(5):1779–1788. doi: 10.1128/jb.173.5.1779-1788.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muriana P. M., Klaenhammer T. R. Conjugal Transfer of Plasmid-Encoded Determinants for Bacteriocin Production and Immunity in Lactobacillus acidophilus 88. Appl Environ Microbiol. 1987 Mar;53(3):553–560. doi: 10.1128/aem.53.3.553-560.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mørtvedt C. I., Nissen-Meyer J., Sletten K., Nes I. F. Purification and amino acid sequence of lactocin S, a bacteriocin produced by Lactobacillus sake L45. Appl Environ Microbiol. 1991 Jun;57(6):1829–1834. doi: 10.1128/aem.57.6.1829-1834.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piard J. C., Muriana P. M., Desmazeaud M. J., Klaenhammer T. R. Purification and Partial Characterization of Lacticin 481, a Lanthionine-Containing Bacteriocin Produced by Lactococcus lactis subsp. lactis CNRZ 481. Appl Environ Microbiol. 1992 Jan;58(1):279–284. doi: 10.1128/aem.58.1.279-284.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Barba J. L., Piard J. C., Jiménez-Díaz R. Plasmid profiles and curing of plasmids in Lactobacillus plantarum strains isolated from green olive fermentations. J Appl Bacteriol. 1991 Nov;71(5):417–421. doi: 10.1111/j.1365-2672.1991.tb03810.x. [DOI] [PubMed] [Google Scholar]
- Sahl H. G., Kordel M., Benz R. Voltage-dependent depolarization of bacterial membranes and artificial lipid bilayers by the peptide antibiotic nisin. Arch Microbiol. 1987;149(2):120–124. doi: 10.1007/BF00425076. [DOI] [PubMed] [Google Scholar]
- Schillinger U., Lücke F. K. Antibacterial activity of Lactobacillus sake isolated from meat. Appl Environ Microbiol. 1989 Aug;55(8):1901–1906. doi: 10.1128/aem.55.8.1901-1906.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel R., Slade H. D. Properties of a Streptococcus sanguis (group H) bacteriocin and its separation from the competence factor of transformation. J Bacteriol. 1973 Aug;115(2):655–661. doi: 10.1128/jb.115.2.655-661.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Tagg J. R., Dajani A. S., Wannamaker L. W. Bacteriocins of gram-positive bacteria. Bacteriol Rev. 1976 Sep;40(3):722–756. doi: 10.1128/br.40.3.722-756.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upreti G. C., Hinsdill R. D. Isolation and characterization of a bacteriocin from a homofermentative Lactobacillus. Antimicrob Agents Chemother. 1973 Oct;4(4):487–494. doi: 10.1128/aac.4.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Klerk H. C., Smit J. A. Properties of a Lactobacillus fermenti bacteriocin. J Gen Microbiol. 1967 Aug;48(2):309–316. doi: 10.1099/00221287-48-2-309. [DOI] [PubMed] [Google Scholar]
- van Belkum M. J., Hayema B. J., Jeeninga R. E., Kok J., Venema G. Organization and nucleotide sequences of two lactococcal bacteriocin operons. Appl Environ Microbiol. 1991 Feb;57(2):492–498. doi: 10.1128/aem.57.2.492-498.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Belkum M. J., Kok J., Venema G. Cloning, sequencing, and expression in Escherichia coli of lcnB, a third bacteriocin determinant from the lactococcal bacteriocin plasmid p9B4-6. Appl Environ Microbiol. 1992 Feb;58(2):572–577. doi: 10.1128/aem.58.2.572-577.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]