Abstract
The effect of cereal non-starch polysaccharides (NSP) on the gut microbial populations was studied in 5 growing pigs between 39–116 kg body weight according to a Latin square design. The diets were composed to contain different NSP levels. The control diet had a normal NSP content (139 g/kg dry matter (DM)), 2 diets had a low total amount of NSP (95 and 107 g/kg DM) and 2 diets had a high amount of total NSP (191 and 199 g/kg DM). Furthermore, one of the diets within each category had a content of insoluble NSP similar to the control diet and one had a high content of insoluble NSP. Samples were collected from the ileum, via intestinal post valve T-caecum (PVTC) cannulas surgically inserted at the ileo-caecal ostium, and from the rectum. The total microbial flora of the ileal samples were analysed for by defining base pair length with terminal restriction fraction length polymorphism (T-RFLP). The microbial diversity of the coliform flora of the ileal and rectal samples were defined by biochemical fingerprinting. It was observed that many terminal restriction fragments (TRFs) disappeared when new diets were introduced and that some characteristic TRFs were found in the high and low NSP diets, respectively. Both the total gut microflora and the coliform flora were influenced by the dietary NSP content.
Keywords: pig, microbial diversity, coliform, ileum, rectum, cereal, non-starch polysaccharides Short title: Cereal NSP and gut microflora
Introduction
Gastro-intestinal disturbances constitute a common problem in pig production [11], causing both animal welfare problems as well as economical losses. Instead of curing an already existing outbreak of diarrhoea with antimicrobials, the focus has today shifted to search ways to prevent these disturbances. Further, the routine administration of antimicrobials to the pig feed is under consideration today [6] in order to avoid the development of microbial resistance towards antimicrobials, and is already prohibited in some countries.
Feed influences the intestinal microflora [36,8]. To exemplify this, high concentrations of protein in the feed stimulate growth of the pig, but may disturb the intestinal microflora and induce outbreaks of diarrhoea [7]. Consequently feed composition is of interest and as pig diets consist mainly of carbohydrates this ingredient has been focused [15,10,26,19,25]. Carbohydrates constitute a diverse nutrient category ranging from sugars easily digested by the pig in the small intestine to dietary fibre fermented by microbes in the large intestine [5]. Previous studies have shown that both type and inclusion level of dietary fibre in pig diets influences the bacterial density and composition [15,10,9]. In this context, non-starch polysaccharides (NSP) constitute fibre fractions of special interest. Carbohydrates, and especially NSP, are the main energy source for microbial fermentation in the large intestine [3], and it has been shown that the NSP content in the feed is related to the clinical expression of swine dysentery [33]. Moreover, NSP can be divided into soluble and insoluble fractions, and it has been shown that also the soluble fraction of NSP can predispose pigs to swine dysentery [33,32]. Thus, both the total content of NSP and the amount of soluble NSP in a diet appear to have the potential to influence the intestinal environment, and hence the gastro-intestinal health in pigs.
The aim of this study was to examine the impact of cereal NSP on the intestinal microbial populations of growing pigs. The influence of different feed compositions was monitored by scrutinising the whole microbial flora at the ileum with terminal restriction fraction length polymorphism (T-RFLP) and by defining the diversity of the coliform populations at the ileum and in the rectum with biochemical fingerprinting.
Materials and methods
Animals
The study included 5 Swedish Yorkshire castrates from a conventional herd free from diseases according to the A-list of the International Office of Epizootics http://www.oie.int and from Aujeszky's disease, atrophic rhinitis, Brachyspira spp., transmissible gastro-enteritis, porcine epidemic diarrhoea, porcine reproductive and respiratory syndrome and salmonellosis.
The pigs originated from different litters and were weaned at 5 weeks of age. At the start of the experiment they were aged 14–15 weeks and weighed 38.0–43.6 kg. Before the experiment started, the pigs had been fed a standard pig feed (Singel Flex, Lantmännen, Svalöv, Sweden). When the study was completed they were 26–27 weeks old and weighed 112–121 kg.
Housing and feeding
The pigs were kept in separate pens without straw. All pens were equipped with a rubber mat to lie on and a stone and a chain for the pigs to play with. Feed was offered twice daily at 7.30 and 15.30 at a level of 4% of the group mean live weight until 70 kg. Thereafter the pigs were offered 2.8 kg feed per day. Water was available ad libitum.
Surgery
At 11–12 weeks of age (27.5–33.0 kg body weight (bw)) the castrates were surgically fitted with a post valve T-caecum (PVTC) cannula according to the procedure previously described [35]. In connection to the surgery all pigs were intramuscularily injected once with oxytetracycline (20 mg per kg bw; Tetramycin vet. Prolongatum, Pfizer, New York, NY, USA), a long acting antibiotic against infections. To prevent post surgical pain the pigs were given 0.3 mg Buprenorphinum (Temgesic®, Reckitt & Coleman, Hull, UK) intramuscularily. Lanolin-based zinc oxide cream (Zinkpasta, Aco hud AB, Stockholm, Sweden) was used to avoid irritation of the skin close to the cannula.
Experimental diets
The diets were based on cereals and cereal byproducts (Table 1). One diet had a composition similar to a conventional feed for growing pigs and served as a control (C). This diet had a content of insoluble NSP referred to as normal (Table 2). Two of the experimental diets had a low content of total NSP, referred to as the Low NSP diets. The remaining 2 diets had a high content of total NSP, and are referred to as the High NSP diets. For each category, one of the diets had a normal content of insoluble NSP (the normal diets) and the other one had a high content of insoluble NSP (the insoluble diets). A more detailed description of the diets can be found in [13].
Table 1.
Main ingredients (% DM) in the experimental diets.1
| NSP content | |||||
| Control diet (C) | Low, insoluble (Li) | Low, normal (L) | High, insoluble (Hi) | High, normal (H) | |
| Barley | 51.6 | 1.1 | - | 2.4 | - |
| Oats | - | 4.9 | - | 10.0 | - |
| Oat meal | - | - | 24.2 | - | 48.3 |
| Oat bran | - | - | 10.8 | - | 21.5 |
| Rye bran | - | - | 8.3 | - | 16.5 |
| Triticale | 21.8 | 17.3 | - | 34.6 | - |
| Wheat | 20.9 | 8.2 | - | 15.8 | - |
| Wheat bran | - | 14.4 | 4.6 | 28.7 | 9.2 |
| Starch | - | 43.0 | 41.4 | - | - |
| Rape seed oil | - | - | - | 5.0 | 1.3 |
| Fish meal | 2.6 | 8.9 | 8.7 | 0.6 | 0.4 |
1 Control diet, Low (low total content of Non-Starch Polysaccharides, NSP), High (high total content of NSP), normal (similar amount of insoluble NSP as the control diet) and insoluble (high amount of insoluble NSP).
Table 2.
Analysed chemical composition (g/kg DM) of the standard pig feed and the experimental diets.1
| NSP content | ||||||
| Singel Flex (SF) | Control diet (C) | Low, insoluble (Li) | Low, normal (L) | High, insoluble (Hi) | High, normal (H) | |
| Crude protein | 155 | 146 | 145 | 158 | 144 | 158 |
| Fat | 38 | 26 | 27 | 49 | 71 | 77 |
| Starch2 | 436 | 575 | 622 | 593 | 428 | 434 |
| Total NSP | 176 | 139 | 107 | 95 | 199 | 191 |
| Insoluble NSP | 144 | 101 | 86 | 64 | 175 | 134 |
| Soluble NSP | 32 | 38 | 21 | 31 | 24 | 57 |
1 Diet abbreviations as in Table 1.
2 Sum of starch and maltodextrins.
Experimental design and collection of samples
The study had a 5 × 5 Latin square design comprising 5 pigs, 5 diets and 5 periods of 17 days. Thus, the total experiment lasted for 85 days. Each pig was offered each diet for a period of 17 days in dissimilar order and no pigs were offered identical diets during any of these periods. Thus, the design aimed to neutralise the influence of aging of the pigs.
Collection of all samples was performed on day 0, 9 and 17 in the first feeding period. During the subsequent periods samples were collected on day 9 and day 17. Digesta samples were collected from the cannula, immediately frozen at -20°C and subsequently used for T-RFLP analysis of microbial DNA. Coliform diversity samples were collected by cotton swabs through the PVTC cannula in the ileo-caecal ostium and from the rectum.
Chemical analysis
All feed samples were milled through a 1-mm mesh screen. The determination of dry matter (DM) was performed by drying at 103°C for 16 h and crude protein (CP) was estimated as Kjeldahl N × 6.25 [29]. Crude fat was determined according to [30], whereas starch and sugars were analysed by an enzymatic method previously described by [22]. The determination of total, soluble and insoluble NSP were performed after [2], and has previously been described in [13].
Terminal Restriction Fraction Length Polymorphism (T-RFLP)
T-RFLP were analysed according to the procedure previously described by Leser et al. (2000). Essentially, intestinal samples containing 200 mg was suspended in 600 μl of phosphate-buffered saline. The samples were centrifuged at 200 × g for 2 min followed by another centrifugation at 12000 × g for 5 min. Then the cells were lysed by shaking for 4 min on a mini bead beater (Biospec Products Inc., Bartlesville, OK, USA) on high speed. The DNA was then purified by the cetyltrimethy-lammonium bromide method. The DNA concentrations were measured on a GeneQuant RNA-DNA calculator (Pharmacia LKB Biochrom Ltd., Cambridge, UK) and adjusted to a concentration of 5 μg of DNA/ml. The extraction and purification of DNA was then followed by PCR amplification using bacteria specific primers, purification of the PCR products and digestion with a restriction enzyme (CfoI: Boehringer, Mannheim, Germany). The DNA fragments were analysed by electrophoresis on an automatic sequence analyser (ABI PRISM 373 DNA Sequencer, PE Biosystems, Foster City, California, USA). The lengths of the terminal restriction fragments (TRFs) were determined by comparison with the internal size standard using GeneScan software (PE Biosystems, Foster City, California, USA).
Biochemical fingerprinting
Ileo-caecal and rectal samples were spread on blood agar plates (blood agar base No.2; LabM, Salford, UK +5% horse blood) within 4 h after collection. They were incubated for 24 h at 37°C before being homogenised and dispersed in broth and frozen at -20°C until analysed. Samples were then spread on McConkey agar and incubated again for 24 h at 37°C. Analyses were performed using the metabolic finger-printing technique, the Phene Plate (PhP) system (Ph Plate AB, Stockholm, Sweden), a system for measuring the kinetics of bacterial growth in liquid medium in microtitre plates [20,27].
The samples were inoculated on PhP-RS plates (Ph Plate, Stockholm, Sweden). Each microtitre plate comprises 11 dehydrated reagents, chosen to differentiate between coliforms [21]. The metabolic response of each bacterial isolate to every substrate was measured at 620 nm using a microplate reader (Titertek Multiscan MCC/340, Labsystems OY, Helsinki, Finland) after 4, 7, 24 and 48 h of incubation at 37°C. The metabolic fingerprint consisted of the mean value of all readings for each isolate. After pair-wise comparisons of biochemical fingerprints a dendrogram was constructed [17]. The identity level was set at 97.5% for assigning strains to the same biochemical phenotype (BPT).
Simpson's index of diversity [12] was used to measure the phenotypic diversity of the coliforms. When only one BPT is present, the diversity is considered to be low, with a minimum value of 0. In a population containing different BPTs, the diversity is considered to be higher, with a maximum value of one.
Results
Health status and feed intake
All pigs remained healthy throughout the experiment. Occasionally, feed residues were noted. Each of these were dried and weighed in order to make corrections for feed intake. Due to problems with the cannula, one of the experimental pigs was replaced with one reserve pig after the first experimental period.
T-RFLP profiles
The T-RFLP detected TRFs between 34 and 672 base pairs of length, out of which 118 were found in samples collected from the ileo-caecal ostium after feeding a standard feed (SF) at experimental start (day 0) and following 17 days of feeding the experimental diets. However, the results obtained indicated a large variation between pigs, and only 41 base pairs were found in at least 3 pigs given the same diet. These TRFs were considered to be characteristic and are shown in Table 3. The number of characteristic TRFs was highest in the diet with high total NSP and a normal content of insoluble NSP (n = 20) and lowest in the control diet and the diet with high total NSP and a high content of insoluble NSP (n = 11) (Table 3). There were 7 TRFs (102, 175, 201, 271, 278, 380 and 565 base pairs of length) present only on day 0, i.e. when the pigs were aged 14–15 weeks and had been offered SF for several weeks.
Table 3.
Base pair length (bp) of characteristic terminal restriction fragments (TRFs), i.e. TRFs demonstrated in at least 3 out of 5 pigs in samples collected at the ileo-caecal ostium. Samples were collected following feeding a standard feed (SF) as the trial was initiated (day 0) and after offering feed with different contents of NSP for 17 days.1
| TRF (bp) | Diets | Comments | |||||
| SF | C | Li | L | Hi | H | ||
| 38 | 1 | 2 | 2 | 2 | 0 | 3 | |
| 43 | 1 | 1 | 2 | 1 | 3 | 1 | |
| 55 | 0 | 5 | 0 | 0 | 1 | 0 | |
| 60 | 3 | 0 | 4 | 4 | 2 | 5 | |
| 83 | 3 | 0 | 0 | 0 | 0 | 0 | Present only day 0 |
| 94 | 0 | 3 | 4 | 4 | 0 | 5 | |
| 102 | 3 | 0 | 1 | 0 | 1 | 2 | Present only day 0 |
| 113 | 0 | 2 | 2 | 3 | 0 | 4 | |
| 151 | 0 | 0 | 2 | 0 | 0 | 3 | |
| 175 | 4 | 0 | 0 | 0 | 2 | 0 | Present only day 0 |
| 189 | 5 | 4 | 5 | 2 | 4 | 4 | |
| 201 | 4 | 0 | 1 | 0 | 2 | 0 | Present only day 0 |
| 232 | 0 | 0 | 3 | 0 | 1 | 0 | |
| 237 | 0 | 1 | 4 | 3 | 0 | 4 | |
| 271 | 3 | 1 | 1 | 0 | 1 | 1 | Present only day 0 |
| 272 | 0 | 3 | 4 | 4 | 2 | 3 | |
| 278 | 4 | 1 | 2 | 1 | 1 | 0 | Present only day 0 |
| 285 | 0 | 0 | 1 | 1 | 0 | 4 | |
| 287 | 5 | 5 | 1 | 0 | 4 | 1 | |
| 298 | 2 | 4 | 1 | 1 | 3 | 5 | High NSP diets and C |
| 340 | 0 | 0 | 2 | 1 | 0 | 3 | |
| 341 | 0 | 2 | 2 | 3 | 0 | 1 | |
| 380 | 3 | 0 | 0 | 0 | 0 | 0 | Present only day 0 |
| 394 | 0 | 2 | 1 | 1 | 0 | 4 | |
| 396 | 2 | 2 | 0 | 0 | 3 | 0 | |
| 399 | 0 | 1 | 3 | 3 | 0 | 3 | |
| 408 | 2 | 4 | 2 | 2 | 3 | 5 | High NSP diets and C |
| 416 | 2 | 2 | 0 | 0 | 3 | 0 | |
| 429 | 0 | 2 | 4 | 4 | 0 | 4 | |
| 478 | 0 | 3 | 4 | 4 | 2 | 5 | |
| 490 | 4 | 3 | 5 | 4 | 3 | 5 | Always present |
| 519 | 0 | 0 | 4 | 3 | 0 | 2 | Low NSP diets |
| 565 | 4 | 1 | 1 | 0 | 2 | 0 | Present only day 0 |
| 571 | 0 | 1 | 4 | 4 | 0 | 2 | Low NSP diets |
| 583 | 4 | 2 | 4 | 0 | 0 | 5 | |
| 584 | 1 | 0 | 0 | 5 | 3 | 0 | |
| 592 | 0 | 0 | 3 | 2 | 0 | 0 | |
| 597 | 0 | 1 | 2 | 2 | 0 | 3 | |
| 598 | 4 | 4 | 1 | 1 | 4 | 0 | |
| 609 | 1 | 3 | 2 | 1 | 3 | 2 | |
| 610 | 0 | 1 | 1 | 1 | 0 | 3 | |
1Diet abbreviations as in Table 1.
0–5 Number of pigs showing the TRF.
Many TRFs disappeared when new diets were introduced, i.e. from day 0 to day 9 (data for day 9 not shown). There was only one TRF represented both on day 0 and following all dietary treatments, and that fragment had a base pair length of 490 (Table 3). The TRFs of 298 and 408 base pairs of length were represented following the control diet and the High NSP diets, but were not following the Low NSP diets. In contrast, the TRFs of 519 and 571 base pairs of length were only demonstrated following the Low NSP diets.
Coliform diversity
The pattern of microbial diversity of the coliforms collected from the ileo-caecal ostium decreased after consumption of the High NSP diets for 9 days, but the initial values were restored at the end of the feeding period, i.e. day 17 (Fig. 1). In contrast, feed containing low levels of NSP affected the coliform populations at the ileo-caecal ostium less during the first 9 days on the diet. However, a decrease in diversity pattern was observed towards the end of the experimental period (day 17). This observation was most clear in pigs offered the diet with a low total NSP content and a normal content of insoluble NSP.
Figure 1.
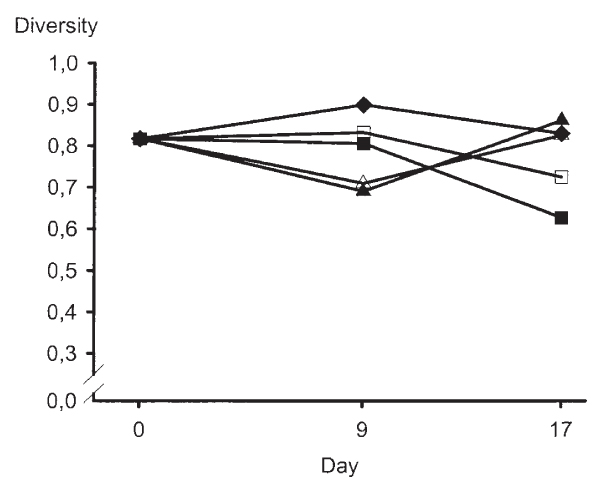
Median values of coliform diversity in the ileo-caecal ostium in pigs fed different diets. Filled diamond = control diet, open square = diet Li, filled square = diet L, open triangle = diet Hi and filled triangle = diet H. Diet abbreviations as in Table 1.
When the pattern of coliform populations of the rectum was scrutinised, it was difficult to interpret the alterations in diversity in connection to the dietary changes (Fig. 2).
Figure 2.
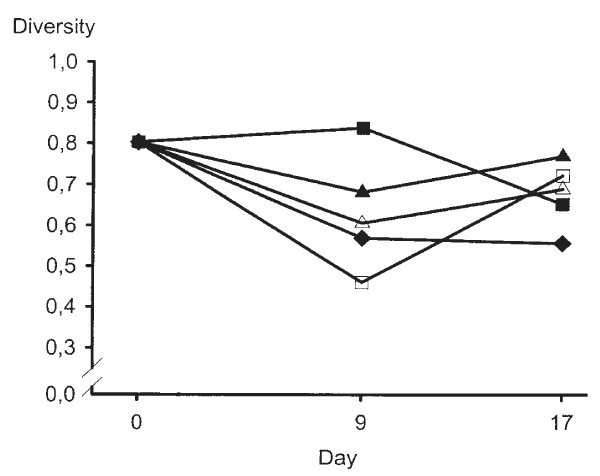
Median values of coliform diversity in rectum of pigs fed different diets. Filled diamond = control diet, open square = diet Li, filled square = diet L, open triangle = diet Hi and filled triangle = diet H. Diet abbreviations as in Table 1.
Discussion
The PVTC cannulation technique provides excellent possibilities to study intestinal processes at the ileo-caecal ostium in living pigs. This technique has previously been shown not to interfere with either the digestibility of nutrients [24] or the gut coliform microflora [14]. Therefore the results presented in this study were considered to mirror the intestinal gut microflora in intact pigs.
The T-RFLP analysis indicated that there were no dramatic changes in the major composition of the intestinal microbial flora in relation to the dietary NSP content. Some terminal restriction fractions (TRFs) were only present on day 0 and disappeared when the experimental diets were introduced. This may be an effect of dietary change, but may also be related to the younger age of the pigs at the time of sampling. On the other hand, there were changes influenced by the different NSP content even if the pattern was not uniform within similar treatments. The TRFs of 298 and 408 base pairs of length were observed in the control diet and the High NSP diets but not in the Low NSP diets, whereas the reverse was true for the terminal restriction fractions of 519 and 571 base pairs of length. Taken together, these differences implied that varying NSP content affects the intestinal microflora in different ways. However, as the analyses were carried out on digesta and not from isolated colony forming units (CFU) the TRFs demonstrated may have emanated from several different known and/or unknown bacterial species. Therefore the present results do not give an explicit explanation about the bacterial origin of the TRFs. To achieve such information single CFU have to be isolated, DNA sequenced and compared to information available from base pair libraries.
The NSP content has previously been shown to alter the proportion of short chain fatty acids [13]. As the control diet and the High NSP diets induced a higher proportion of propionic acid, while the Low NSP diets induced a higher proportion of acetic acid, this suggests that the dietary content of total NSP influenced the amount of acetic and propionic acid producing microbes in the ileo-caecal ostium. The current study therefore demonstrated that the dietary carbohydrate composition has an impact on the total microbial flora at the ileo-caecal ostium of pigs.
The diversity of the coliform populations collected at the ileo-caecal ostium of the pigs fed the control diet was high throughout the experiment (Table 3), which indicated that the coliform microflora of the small intestine was stable in pigs given this diet. As a high intestinal microbial diversity is believed to prevent invasion by pathogens [17,18,21], for instance by competing for nutrients by so called competitive exclusion [1,11], the control diet was concluded to not be provocative by itself. Still, the diversity of the rectal coliform populations was clearly influenced when the control diet was introduced. Such alterations are, however, often seen when diets to pigs are altered [36,4,15] and presumably mirror altered nutritional circumstances for the intestinal microbial flora.
Compared to day 0, a pattern of decreased diversity values was obtained among rectal coliform populations on day 9 of the pigs fed the control diet and remained at the lower level also on day 17. However, the diversity values obtained in coliform populations at the end of each experimental period (day 17) were fairly similar in all treatments, possibly indicating a stabilisation over time following a change in diet. The influence of the different diets on the coliform diversity was less clear in the rectum than at the ileo-caecal ostium. This could be due to the higher density of bacteria in the rectum compared to at the ileo-caecal ostium [37]. It may be more difficult to manipulate a dense microbial population, and this might have contributed to the different observations made in the coliform populations located at the ileo-caecal ostium and in the rectum. However, intestinal disorders induced by coliforms generally emanate from the small intestine [11]. Therefore, the coliform diversity values obtained at the ileo-caecal ostium might be of higher significance than those obtained in rectal populations. Consequently, the diversity values obtained at the ileo-caecal ostium are focused in the discussion below.
The pattern of decreasing coliform diversity at the ileo-caecal ostium during the first week of feeding the High NSP diets was probably an effect of dietary change (Fig. 2). It is well documented that increased dietary fibre content may decrease the digestibility of dietary nutrients and energy for the pig [16,28,31,5]. Depending on fibre source, this may also be true for the substrate available for microbial growth, and can lead to a decreased diversity, which in turn may indicate a decreased resistance to "invading microbes". If the diversity already is low due to low substrate availability for the microbes, the establishment of a dominant microorganism within the intestinal flora may be facilitated [21]. However, the diversity pattern increased during the second week and resumed a diversity value similar to day 0 (Fig. 2), indicating that more microbial clones had adapted to the feed at this time. The high availability of numerous nutrients for the intestinal microbes probably provided good conditions for a high diversity once the microbes had adjusted to them.
Low NSP diets are more digestible to the pig than high NSP diets due to lower fibre content [13], and the pattern of coliform diversity was maintained at the ileo-caecal ostium during the first week following feeding the Low NSP diets. However, the coliform diversity decreased during the second week (Fig. 1).
In this study, the ratio of soluble and insoluble NSP did not alter the coliform populations at the ileo-caecal ostium. This observation is important since earlier studies suggest that the soluble fraction of NSP facilitates proliferation of enterotoxigenic E. coli in the small intestine [8,26]. We found indications that the ratio of soluble and insoluble NSP influenced the coliform diversity in the large intestine. In the Low NSP diets, the pattern of coliform diversity was increased in the diet with a higher proportion of soluble NSP on day 9. Previous findings suggest that it is the soluble fraction of NSP that predisposes pigs to swine dysentery. Diets based on cooked rice and animal protein reduced the clinical expression of Brachyspira hyodysenteriae, presumably by limiting the amount of fermentable substrates entering the large intestine [33,32]. However, we found no difference in coliform diversity between the Low NSP diets on day 17, possibly indicating a stabilisation over time. Further, we found no difference in the mean coliform diversity in connection to varying total NSP level measured in the rectal samples. Indeed, other researchers have failed to prevent development of swine dysentery with low fibre diets based on cooked rice and animal protein [19,25]. Consequently, also the balance of the intestinal flora, as well as pig genotype and microbial environment, might be factors of significance for the development of swine dysentery.
The different diets used in this study resulted in alterations of the total gut flora at the ileo-caecal ostium and in the diversity of both the ileo-caecal and the rectal coliform flora. Therefore we conclude that dietary carbohydrate composition seems to have a potential in preventing or provoking intestinal disorders in pigs.
Acknowledgments
Acknowledgements
We gratefully acknowledge the excellent technical assistance of Bengt Pettersson, Anna-Greta Haglund and Sigbrit Mattsson. Further, we are thankful to Kaare Johnsen for valuable comments on the manuscript. This study was supported by grants from the Swedish Meat Producing Farmers R&D Program, the Swedish Pig Producers Research Foundation and the Nordic research network AFAC (Alternatives to Feed Antibiotics and Anticoccidials in Pig and Poultry Meat Production).
References
- Asplund K, Hakkinen M, Björkroth J, Nuotio L, Nurmi E. Inhibition of the growth of Yersinia enterocolitica O:3 by the microflora of porcine caecum and ileum in an in vitro model. J Appl Bacteriol. 1996;81:217–222. doi: 10.1111/j.1365-2672.1996.tb04504.x. [DOI] [PubMed] [Google Scholar]
- Bach Knudsen KE. Carbohydrate and lignin contents of plant materials used in animal feeding. Anim Feed Sci Technol. 1997;67:319–338. doi: 10.1016/S0377-8401(97)00009-6. [DOI] [Google Scholar]
- Bach Knudsen KE, Jensen BB. Effect of source and level of dietary fibre on microbial fermentation in the large intestine of pigs. EAAP publication. 1991;54:389–394. [Google Scholar]
- Bach Knudsen KE, Jensen BB, Andersen JO, Hansen I. Gastrointestinal implications in pigs of wheat and oat fractions. 2. Microbial activity in the gastrointestinal tract. Br J Nutr. 1991;65:233–248. doi: 10.1079/BJN19910083. [DOI] [PubMed] [Google Scholar]
- Bach Knudsen KE, Jørgensen H. In: Intestinal degradation of dietary carbohydrates – from birth to maturity. Lindberg JE, Ogle B, editor. Digestive physiology of pigs. Wallingford. CABI publishing.; 2001. pp. 109–120. [Google Scholar]
- Baynes P, Varley M. Gut health: practical considerations. In: Varley MA, Wiseman J, editor. The weaner pig Nutrition and management. Walling-ford. CABI Publishing.; 2001. pp. 249–257. [Google Scholar]
- Bertschinger HU, Eggenberger E, Jucker H, Pfirter HP. Evaluation of low nutrient, high fibre diets for the prevention of porcine Escherichia coli enterotoxaemia. Vet Microbiol. 1978;3:218–290. [Google Scholar]
- Bolduan G, Jung H, Schnabel E, Schneider R. Recent advances in the nutrition of weaner piglets. Pig news inf. 1988;9:381–385. [Google Scholar]
- Durmic Z, Pethick DW, Mullan BP, Schulze H, Accioly JM, Hampson DJ. Extrusion of wheat or sorghum and/or addition of exogenous enzymes to pig diets influences the large intestinal micro-biota but does not prevent development of swine dysentery following experimental challenge. J Appl Microbiol. 2000;89:678–686. doi: 10.1046/j.1365-2672.2000.01166.x. [DOI] [PubMed] [Google Scholar]
- Durmic Z, Pethick DW, Pluske JR, Hampson DJ. Changes in bacterial populations in the colon of pigs fed different sources of dietary fibre, and the development of swine dysentery after experimental infection. J Appl Microbiol. 1998;85:574–582. doi: 10.1046/j.1365-2672.1998.853539.x. [DOI] [PubMed] [Google Scholar]
- Hampson DJ, Pluske JR, Pethick DW. In: Dietary manipulation of enteric disease. Lindberg JE, Ogle B, editor. Digestive physiology of pigs. Wallingford. CABI Publishing.; 2001. pp. 247–258. [Google Scholar]
- Hunter PR, Gaston MA. Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. J Clin Microbiol. 1988;26:2465–2466. doi: 10.1128/jcm.26.11.2465-2466.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Högberg A, Lindberg JE. Influence of cereal non-starch polysaccharides on digestion site and gut environment in growing pigs. Livest Prod Sci. 2004;87:121–130. doi: 10.1016/j.livprodsci.2003.10.002. [DOI] [Google Scholar]
- Högberg A, Lindberg JE, Wallgren P. Influence of ileo-caecal cannulation and oxytetracycline on ileo-caecal and rectal coliform populations in pigs. Acta Vet Scand. 2001;42:435–440. doi: 10.1186/1751-0147-42-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen BB, Jørgensen H. Effect of dietary fiber on microbial activity and microbial gas production in various regions of the gastrointestinal tract of pigs. Appl Environ Microbiol. 1994:1897–1904. doi: 10.1128/aem.60.6.1897-1904.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just A. The influence of crude fibre from cereals on the net energy value of diets for growth in pigs. Livest Prod Sci. 1982;9:569–580. doi: 10.1016/0301-6226(82)90004-5. [DOI] [Google Scholar]
- Katouli M, Erhardt-Bennet AS, Kühn I, Kollberg B, Möllby R. Metabolic capacity and pathogenic properties of the intestinal coliforms in patients with ulcerative colitis. Microb Ecol Health Diseas. 1992;5:245–255. [Google Scholar]
- Katouli M, Melin L, Jensen-Waern M, Wallgren P, Möllby R. The effect of zinc oxide supplementation on the stability of the intestinal flora with special reference to composition of coliforms in weaned pigs. J Appl Microbiol. 1999;87:564–573. doi: 10.1046/j.1365-2672.1999.00853.x. [DOI] [PubMed] [Google Scholar]
- Kirkwood RN, Huang SX, McFall M, Aherne FX. Dietary factors do not influence the clinical expression of swine dysentery. Swine Health Prod. 2000;8:73–76. [Google Scholar]
- Kühn I, Franklin A, Söderlind O, Möllby R. Phenotypic variations among enterotoxinigenic Escherichia coli from Swedish piglets with diarrhoea. Med Microbiol Immunol. 1985;174:119–130. doi: 10.1007/BF02298122. [DOI] [PubMed] [Google Scholar]
- Kühn I, Katouli M, Lund A, Wallgren P, Möllby R. Phenotypic diversity and stability of the intestinal coliform flora in piglets during the first 3 months of age. Microb Ecol Health Diseas. 1993;6:101–107. [Google Scholar]
- Larsson K, Bengtsson S. Bestämning av lättillgängliga kolhydrater i växtmaterial. (Determination of easily available carbohydrates in plant material) Metodrapport nr 22. 1983. (In Swedish)
- Leser TD, Lindecrona RH, Jensen TK, Jensen BB, Möller K. Changes in bacterial community structure in the colon of pigs fed different experimental diets and after infection with Brachyspira hyodysenteriae. Appl Environ Microbiol. 2000:3290–3296. doi: 10.1128/AEM.66.8.3290-3296.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg JE. In: A comparison of the total tract digestibility in intact and PVTC-cannulated pigs. Laplace JP, Fevrier C, Barbeau A, editor. Digestive physiology in pigs. Proceedings of the VII th international symposium, Saint Malo, France; pp. 404–407. May 26–28, 1997. [Google Scholar]
- Lindecrona RH, Jensen TK, Jensen BB, Leser TD, Jiufeng W, Møller K. The influence of diet on the development of swine dysentery upon experimental infection. Anim Sci. 2003;76:81–87. [Google Scholar]
- McDonald DE, Pethick DW, Pluske JR, Hampson DJ. Adverse effects of soluble non-starch polysac-charides (guar gum) on piglet growth and experimental colibacillosis immediately after weaning. Res Vet Sci. 1999;67:245–250. doi: 10.1053/rvsc.1999.0315. [DOI] [PubMed] [Google Scholar]
- Möllby R, Kühn I, Katouli M. Computerised biochemical fingerprinting -a new tool for typing of bacteria. Reviews in Medical Microbiology. 1993;4:231–241. [Google Scholar]
- Noblet J, Perez JM. Prediction of digestibility of nutrients and energy values of pig diets from chemical analysis. J Anim Sci. 1993;71:3389–3398. doi: 10.2527/1993.71123389x. [DOI] [PubMed] [Google Scholar]
- Nordic committee on food analysis . Determination in foods and feeds according to Kjeldahl. 3 1976. Nitrogen. [Google Scholar]
- Official Journal of the European Communities Determination of crude oils and fat. Method B. 1984. pp. 29–30.
- Pettersson Å, Lindberg JE, Thomke S. Nutritive value of oats of different composition evaluated by intact and fistulated pigs. Acta Agric Scand, Sect A, Animal Sci. 1997;47:247–253. [Google Scholar]
- Pluske JR, Durmic Z, Pethick DW, Mullan BP, Hampson DJ. Confirmation of the role of rapidly fermentable carbohydrates in the expression of swine dysentery in pigs after experimental infection. J Nutr. 1998;128:1737–1744. doi: 10.1093/jn/128.10.1737. [DOI] [PubMed] [Google Scholar]
- Pluske JR, Siba PM, Pethick DW, Durmic Z, Mullan BP, Hampson DJ. The incidence of swine dysentery in pigs can be reduced by feeding diets that limit the amount of fermentable substrate entering the large intestine. J Nutr. 1996;126:2920–2933. doi: 10.1093/jn/126.11.2920. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc . Version 612. Cary, NC, USA. SAS Institute Inc; 1989. SAS® User's guide I+II. [Google Scholar]
- van Leeuwen P, van Kleef DJ, van Kempen GJM, Huisman J, Verstegen MWA. The post valve T-caecum cannulation technique in pigs applicated to determine the digestibility of amino acid in maize, groundnut and sunflower meal. J Anim Physiol A Anim Nutr. 1991;65:183–193. [Google Scholar]
- Varel VH, Pond WG. Enumeration and activity of cellulolytic bacteria from gestating swine fed various levels of dietary fiber. Appl Environ Microbiol. 1985;49:858–862. doi: 10.1128/aem.49.4.858-862.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoric M, Arvidsson A, Melin L, Kühn I, Lindberg JE, Wallgren P. Comparison between coliform populations at different sites of the intestinal tract of pigs. Microb Ecol Health Diseas. 2002;14:174–178. doi: 10.1080/089106002320644366. [DOI] [Google Scholar]


