Abstract
Contagious caprine pleuropneumonia (CCPP) is a major threat to goat farming in parts of Africa and Asia. It classically causes acute high morbidity and mortality early in infection, but little is known of its long term epizootiology and course. In this study, 10 goats were inoculated with Mycoplasma capricolum subsp. capripneumoniae (M. capripneumoniae) and then mixed with 15 goats for contact transmission. The disease course was monitored in each goat for 56–105 days, whereafter the goats were killed and necropsied. Varying features signifying infection occurred in altogether 17 goats (7 inoculated, 10 in-contact). Clinical signs were severe in 8 goats but no fatalities occurred. Only 6 goats had serum antibody titres against M. capripneumoniae in ELISA. Fourteen goats (5 inoculated, 9 in-contact) had chronic pleuropulmonary lesions compatible with CCPP at necropsy and 7 of those showed M. capripneumoniae antigen in the lung by immunohistochemistry. Neither cultivation nor PCR tests were positive for the agent in any goat. The results indicate that the clinical course of CCPP in a flock may be comparatively mild, M. capripneumoniae-associated lung lesions may be present at a late stage of infection, and chronic infection may occur without a significant serological response.
Keywords: goat, Mycoplasma, contagious pleuropneumonia, ELISA, immunohistochemistry, serology, pathology.
Introduction
Contagious caprine pleuropneumonia (CCPP) is one of the most severe infectious diseases of goats, causing major economic losses in goat farming in Africa and Asia [12]. It is caused by Mycoplasma capricolum subsp. capripneumoniae (M. capripneumoniae), formerly Mycoplasma strain F38 [14,16]. Clinical outbreaks in a flock often show a 100% morbidity and mortality rates of 60 to 70% with lesions of fibrinous pleuropneumonia in the acute stage [13,22].
Long term survivors of acute disease may display chronic pleuropneumonia or chronic pleuritis [13,28] but cultural recovery of the agent has not been demonstrated in such late stage pulmonary lesions [18,35]. Still, negative results of cultivation of M. capripneumoniae is not proof of freedom of infection [32] and the use of complementary techniques for microbial identification is indicated. Especially so, since field observations indicate that outbreaks may follow the introduction of apparently healthy goats to a flock, suggesting that subclinical carriers may occur.
Most studies on CCPP have concentrated on vaccination trials and the stage of acute fulminant disease in flocks. There is an obvious need of further studies to monitor features of the long term course of infection, including possible persistence of the agent as well as serological responses and pulmonary pathology. The present study was designed to elucidate these matters in experimental M. capripneumoniae infection of a large flock of goats.
Materials and methods
Animals and husbandry
Thirty goats, 21 castrated males and 9 females, all of the Galla breed, were used. They originated from a large farmers' cooperative, ranching mixed cattle, sheep and goats in the Eastern Province of Kenya with no history of CCPP. The goats were brought to the National Veterinary Research Centre at the age of 12–15 months. Polymerase chain reaction (PCR) tests and microbial cultivation on nasal, pharyngeal and ear canal swabs did not reveal M. capripneumoniae or other mycoplasmas in the 'Mycoplasma mycoides cluster', but Mycoplasma ovipneumoniae and Mycoplasma arginini were in general cultivated. The goats were housed in pens with an adjoining fenced enclosure of approximately 20 × 30 m in which they were freed for feeding. They were dewormed with Nilzan plus cobalt® (Cooper, Nairobi, Kenya) directly upon arrival and 3 months later. They were fed on hay and mineral lick ad libitum and on concentrates (49.5% grain, 36.3% wheat and maize bran, 10.7% cotton seed cake, 3.5% mineral supplement) at 26 g/kg bw. every second day.
Experimental design
The goats were observed for 3 months, during which no signs of disease were seen. Complement fixation tests for serum antibodies to M. capripneumoniae [23] at arrival and 1 month before the start of the experiment, were negative in all goats (titers ≤ 1/16 at both occasions). They were then randomly allocated to either of 3 groups (A-C). Group A goats (n = 10), housed approximately 1 km away from the other goats, were inoculated intratracheally (i.t.) with 20 ml of inoculum (see below) containing a mixture of a freshly ground suspension (5 ml) of an infected lung and 15 ml of M. capripneumoniae culture. Seven goats were inoculated on day 0 and 3 goats on day 17. Group B goats (n = 15) were mixed with the A goats on day 18 for contact transmission, and group C goats (n = 5) were non-exposed controls. The course of infection was monitored by clinical examinations, serology and microbiology. Starting day 74, two group A, three group B and one of group C goats were killed by electrocution and exsanguination and necropsied each of 5 consecutive weeks.
Microbiological culture, PCR and serology procedures
Inoculum
The lung suspension was from a pneumonic lung of a goat surviving a field outbreak of CCPP in Western Kenya, and was positive for M. capripneumoniae in PCR test up to dilution 104 but on culture only when undiluted. M. ovipneumoniae was also found, but no bacteria or other mycoplasmas. As the goats harboured this mycoplasma in their normal flora, no attempt was made to exclude it from the inoculum. The culture given to the first 7 goats was passage 6 of a field isolate and that given to the additional 3 goats was passage 2 of another field isolate of M. capripneumoniae, Both cultures contained 109 color changing units (c.c.u.) of M. capripneumoniae per ml.
Sampling and procedures for microbial isolation
Nasal, pharyngeal and ear canal swab samples for culture of Mycoplasma and bacteria, and for PCR, were taken from each animal once every 2 weeks of the experiment and on the day of killing, tracheal lavage samples were taken immediately after killing, and lung samples were taken at necropsy at sites also sampled for histopathology (see below). M. capripneumoniae was cultured on modified Newing's broth [10] with 0.2% sodium pyruvate [34] and on Medium F with 0.2% sodium pyruvate [2,3]. Mycoplasma isolates were identified with PCR, growth inhibition (GI) [1] and immunofluorescence (IF) [27] tests. The GI and IF tests employed polyclonal rabbit antisera to the type strains of M. arginini, M. capricolum subsp. capricolum (M. capricolum), M. capripneumoniae, M. mycoides subsp. capri, M. mycoides subsp. mycoides LC and M. ovipneumoniae. Polymerase chain reaction and restriction enzyme analysis (REA) procedures were carried out according to [5]. Bacterial culture was carried out on blood agar plates with sheep red blood cells.
Serology
Blood samples during the trial were investigated at the National Veterinary Institute for serum antibodies to M. capripneumoniae by a modification of an i-ELISA test used in M. mycoides subsp. mycoides SC infection [6]. Samples were taken fortnightly in all goats but due to storage problems collected pre-infection sera were only rarely available for testing with ELISA and a few goats could not be tested throughout the entire period of infection. The test employed a sodium dodecyl sulfate (SDS)-solubilized antigen of M. capripneumoniae (strain F38), grown in modified F-medium with gamma globulin-free horse serum and 0.2% sodium pyruvate [3,24]. The sera were tested at dilution 1:100 and applied in duplicate wells in the microtiter plates (M129A, Dynatech, Chantilly, USA). The conjugate was a rabbit anti-goat Ig-HRP (P160, Dako, Glostrup, Denmark). Positive control sera were from one goat immunized at CIRAD-EMVT and from a pool of sera from an outbreak of CCPP in Uganda [4]. A cut off optical density (OD) value of 0.8 was established from the mean +3 SD of sera of 140 goats from Sweden or New Zealand. Blocking ELISA (b-ELISA), employing a cut off value at 20% inhibition [33], was carried out to check the validity of the i-ELISA results in 15 of the goats.
Clinical examination
Rectal temperature, cough, and respiratory rates and sounds were recorded daily between 8.00 and 10.00 am throughout the study period. Clinically normal goats showed rectal temperatures of 37.0°C to 39.3°C. Fever was recorded when the temperature was 39.5°C and above.
Necropsy and histopathology
A complete necropsy was performed on all goats and tissue samples were fixed in 10% phosphate buffered formalin. Samples were taken from lung lobes with and without gross lesions, and from the heart, liver, kidney, spleen, thoracic lymph nodes and small intestine. Paraffin sections were stained with hematoxylin and eosin (H&E) and, when appropriate, also with Periodic acid-Schiff (PAS), Masson's trichrome connective tissue stain and Martius-Scarlet-Blue (MSB) for fibrin.
Immunohistochemistry
The paraffin-embedded lung tissues were investigated for M. capripneumoniae antigen by immunohistochemistry, employing polyclonal anti-M. capripneumoniae antibodies.
Preparation of antibodies
A whole-cell antigen was used to inoculate rabbits for antibody production. M. capripneumoniae (strain F38), grown in rabbit meat infusion broth with 20% rabbit serum, 4% yeast extract, 40% Hanks' balanced salt solution and 0.2% sodium pyruvate, was harvested in late log phase growth, centrifuged at 12,000 g for 30 min and washed with PBS 3 times. Inoculation of rabbits was performed according to [24]. The rabbit antiserum was tested in GI [1] and IF [27] tests against type strains of Mycoplasma sp. Group 7, M. arginini, M. capricolum subsp. capricolum, M. capripneumoniae, M. mycoides subsp. capri, M. mycoides subsp. mycoides LC and M. ovipneumoniae. In both tests, it reacted strongly against M. capripneumoniae and Mycoplasma sp. Group 7, but no reaction against the other mycoplasmas was observed.
Immunohistochemical method
The streptavidin biotin complex-horse radish peroxidase method (StreptABC/HRP) was used. Non-specific protein binding was minimized by pre-treatment with 20% normal swine serum (Dako, Glostrup, Denmark) and endogenous peroxidase was quenched with 0.3% hydrogen peroxide. The primary antibody was diluted 1:100, and biotinylated swine-anti-rabbit immunoglobulins 1:200. The chromogen was amino-ethyl-carbazole, the counterstain hematoxylin, and tris-buffered saline (TBS), pH 7.6, was used for dilutions.
Specificity and methodological controls
Paraffin sections of the lungs of 6 goats that died with acute fibrinous pneumonia in the early phase of CCPP and with M. capripneumoniae confirmed on culture [36,35] were positive control tissues. For control of antigenic specificity of the primary antiserum, the following procedures were performed: the antiserum was (i) absorbed with M. capripneumoniae antigen (2 ml antiserum mixed with 0.2 mg antigen, kept at +4°C overnight and centrifuged) and then applied diluted 1:100 and 1:200; (ii) substituted by normal rabbit serum (Dako); (iii) applied on sections of pneumonic goat lungs with M. ovipneumoniae, M. capricolum, M. mycoides subsp. mycoides (LC) or Pasteurella multocida infection on culture; (iv) applied on sections of goat lung with schistosome egg granulomas, i.e., a completely irrelevant infection. Sections were incubated with TBS instead of primary antiserum for method controls.
Results
Clinical signs
See Table 1. Eight goats showed an episode of fever (40.0–41.4°C), as a rule accompanied by intense cough and harsh respirations. Respiratory signs only, in general with intermittent cough, at times with harsh breathing, were observed in some goats. In addition, all i.t.-inoculated goats showed a rise in temperature on days 1 and 2 after inoculation only. Group C goats remained healthy throughout the study period.
Table 1.
Goats with signs of M. capripneumoniae infection (n = 17). Data include serology (i-ELISA), immunohistochemistry (IHC) of lungs for M. capripneumoniae, pleuropulmonary lesions at necropsy, time periods of clinical signs, and timepoint of necropsy.
| Group | Goat no. | i-ELISA1 | IHC2 | Lesions3 | Time period of fever4 | Time period(s) of respiratory signs4 | Timepoint of necropsy4 |
| A | 130 | - | + | + | - | 28 | 80 |
| A | 131 | + | - | - | 27–30 | 27–34 | 75 |
| A | 139* | + | - | + | 5–6 | 5–11 | 82 |
| A | 142 | - | + | + | 16–18 | 16–79 | 80 |
| A | 145* | - | - | + | - | 11 | 88 |
| A | 174 | + | - | - | - | 2 | 88 |
| A | 188 | + | + | + | 28–32 | 28–73 | 74 |
| B | 124 | - | - | + | - | - | 56 |
| B | 127 | - | - | + | - | 40 | 63 |
| B | 129 | - | + | + | - | 16–67 | 70 |
| B | 133 | - | - | + | 15 | 14–15, 50 | 70 |
| B | 134 | - | + | + | - | 11–59 | 81 |
| B | 144 | - | - | - | 13–18 | 34, 53 | 63 |
| B | 151 | + | + | + | - | 6–7, 36–39 | 87 |
| B | 160 | - | + | + | - | 44–74 | 81 |
| B | 172 | - | - | + | 36–37 | 6–7, 36–39 | 87 |
| B | 191 | + | - | + | 21–26 | 19–56 | 56 |
1Pertinent antibody titre present: +, not present: -. 2Antigen present: +, not present: -.
3Lesions compatible with M. capripneumoniae infection: +, no lesions: -.
4Group A: day(s) post inoculation, group B: day(s) post time-point of contact transmission; no symptoms: -.
* Inoculated day 17
Serology
On i-ELISA (Table 1), 4 group A goats showed clear-cut seroconversion to M. capripneumoniae infection. One goat of group B had OD values above the cut-off value and another showed an antibody rise approaching the cutoff value. The OD values of the above goats are shown together with other variables of infection in Figs. 1 and 2. Three seropositive goats (nos. 131 and 188 of group A, and no. 151 of group B) were confirmed seropositive on b-ELISA, with inhibition values of 33%, 22% and 34%, respectively, and goat no. 139 had inhibition value 17%, just below cut-off. The other goats tested with b-ELISA were negative. Serocon-version was not observed in any goat of group C (OD values 0.17–0.62, mean 0.33).
Figure 1.
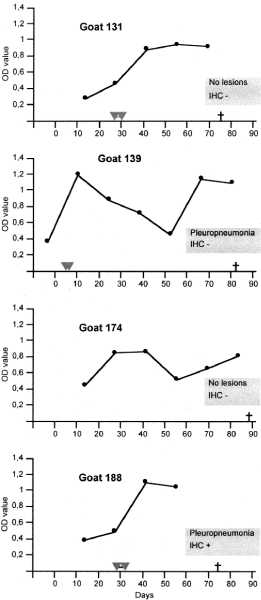
Serum antibody response (OD value of i-ELISA) in relation to period of fever and pleuropulmonary lesions and immunohistochemistry (IHC) for M. capripneumoniae at necropsy in sero-reactive group A (inoculated) goats. Days = days post inoculation.  -
-  : Period of fever; † : Timepoint of necropsy.
: Period of fever; † : Timepoint of necropsy.
Figure 2.
Serum antibody response (OD value of i-ELISA) in relation to period of fever and pleuropulmonary lesions and immunohistochemistry (IHC) for M. capripneumoniae at necropsy in sero-reactive group B (in-contact exposed) goats. Days = days post initial contact exposure.  -
-  : Period of fever; † : Timepoint of necropsy.
: Period of fever; † : Timepoint of necropsy.
Gross pathology
Non-exposed goats
No gross changes were observed in the non-exposed goats.
Goats exposed to M. capripneumoniae
Gross lesions of lung and pleura (see Table 2) were observed in 14 (5 group A and 9 group B) goats. Unilateral lung and pleural involvement was the rule; bilateral affection was seen only in 2 goats. Lesions of the lungs were classified as focal, multifocal, or extensive (affecting a substantial part of each lobe of one lung). Goats showing pulmonary consolidation also showed firm enlargement of regional lymph nodes. Goats no. 142, 188 and 191 had marked accumulation of serous fluid in one of the pleural cavities.
Table 2.
Gross and light microscopic characterization of pulmonary lesions in goats with thoracic pathology at necropsy (n = 14).
| Gross pathology | Light microscopy | |||||||||||
| Goat, group and no. | Consolidation | Pleural adhesions | Pleural fibrin deposits | Distribution of gross changes1 | Bronchitis/bronchiolitis | Alveolar exudate | Alveolar fibrosis | Septal/peribronchial fibrosis | Abscessation | Lymphoid cell cuffs at airways | Acute fibrinous pleuritis | Chronic fibrous pleuritis |
| A 130 | + | - | - | Mf | + | + | - | + | - | + | - | + |
| A 139 | + | - | - | Mf | + | + | - | + | - | + | - | + |
| A 142 | + | + | - | Ext | + | + | + | + | - | + | - | - |
| A 145 | + | - | - | Mf | - | + | - | + | - | + | - | - |
| A 188 | + | + | - | Ext | + | + | + | - | - | + | - | + |
| B 124 | + | - | - | F | + | + | + | + | - | + | - | - |
| B 127 | - | + | - | Ext | - | - | - | + | - | - | - | + |
| B 129 | + | + | + | Mf | + | + | - | + | - | + | - | + |
| B 133 | - | + | - | Ext | + | + | - | + | - | + | - | + |
| B 134 | + | + | - | Ext | + | + | - | + | - | + | - | - |
| B 151 | - | + | - | F | - | + | - | + | - | + | - | + |
| B 160 | + | - | - | F | + | + | + | + | - | - | - | + |
| B 172 | - | + | - | Mf | - | + | - | - | - | + | - | + |
| B 191 | + | - | + | Ext | - | - | - | + | + | + | + | + |
1Ext = extensive; F = focal; Mf = multifocal.
Histopathology
Non-exposed goats
Slight infiltrates of macrophages and lymphocytes in the alveolar septa were regularly observed.
Goats exposed to M. capripneumoniae
Only goats with gross lesions showed significant histological changes (Table 2). Most of them had pneumonia characterized by mucopurulent to fibrinopurulent exudate, sometimes with necrosis, in dilated hyperplastic bronchi, alveolar exudates dominated by macrophages and with a variable component of neutrophils (Fig. 3), and pulmonary fibrosis. Alveolar fibrin deposits were found in occasional goats. Fibrosis was most often septal and peribronchial and was sometimes marked. Large strands of fibrous granulation tissue in alveoli (Fig. 4) was seen in some cases, and chronic fibrous pleuritis was often prominent. In a few goats, chronic pleuritis and septal fibrosis predominated markedly, whereas active inflammation of airways was not evident. Lymphoid nodules and follicles (cuffs) around airways were abundant in almost all goats. Sometimes, when combined with mononuclear alveolitis, such changes were compatible with bronchointerstitial pneumonia. Grossly enlarged bronchial lymph nodes displayed lymphoid hyperplasia with markedly enlarged follicle germinal centres. All other organs examined were normal. Goats without gross lesions showed pulmonary histology similar to controls.
Figure 3.
Alveolar exudate with prominence of alveolar macrophages, admixed with neutrophils. Goat no. 160. H&E, ×123.
Figure 4.
Collagen-rich granulation tissue in the alveolar tissue. Goat no. 142. Masson's trichrome, ×123.
Culture and PCR for mycoplasma and bacteria M. capripneumoniae was not isolated on culture and was neither identified on PCR in any sample of any one goat. Nasal swabs during the course of the experiment yielded M. ovipneumoniae in 8 goats (nos. 128, 140, 156 and 188 of group A and nos. 126, 134, 144 and 160 of group B). This microorganism was isolated from the lungs and/or tracheal lavage samples at necropsy of all the above goats except no.160, and in addition in 1 group C goat and in goat 145 of group A. Pharyngeal swabs yielded M. arginini in 2 group A (nos. 140 and 188) and 2 group B (nos. 129 and 144) goats. From lung samples, non-haemolytic streptococci were grown in 4 group A (nos. 130, 139, 142 and 145) and 2 group B goats (nos. 134 and 160) goats and Staphylococcus sp. in 1 group A (no. 145) and 3 group B goats (nos. 134, 143 and 172).
Immunohistochemistry
Control materials
The positive control lung tissues showed distinct immunostaining as granular material in free form in fibrinous deposits and granular or large, dense material in the cytoplasm of alveolar macrophages (Fig. 5). No staining was seen with antigen-absorbed antiserum, normal sera or TBS. Lung tissue with M. mycoides subsp. mycoides LC infection showed weak immunoreactivity. Lungs in which other mycoplasmas were isolated, or with schistosome or bacterial infection, were all negative.
Figure 5.
Immunostained M. capripneumoniae antigen in alveolar macrophages (one is arrowed) and free in alveoli (arrow-head). Control goat (culture positive) for immunohistochemistry. StreptABC/HRP, ×504.
Non-exposed goats
No immunostaining was detected in the lung of any goat.
Goats exposed to M. capripneumoniae
Immunostaining was seen in 3 group A and 4 group B goats (Table 1). In all cases, immunoreactivity was distinct and only intracellular, appearing in the cytoplasm of alveolar macrophages (Fig. 6). Cells with positive staining appeared multifocally, sometimes in abundance.
Figure 6.
Immunostained M. capripneumoniae antigen in alveolar macrophages (arrows) of an inoculated goat (no. 188). StreptABC/HRP, ×318.
Discussion
Contagious caprine pleuropneumonia (CCPP) is generally recognized as a disease with a fulminant clinical course and high mortality from fibrinous pleuropneumonia shortly after infection [20]. The present experimental reproduction of CCPP displayed another pattern with a milder course and no acute mortality. [19] described a similar course in a study which however comprised only a few goats. In our study, true reproduction of infection was indicated by cases with sero-conversion and M. capripneumoniae antigen in the lungs on immunohistochemistry (IHC). However, neither cultural nor molecular identification of the agent was successful and only a minority of goats seroconverted. Altogether, these features suggest that the diagnosis of CCPP also under field conditions may not be straightforward.
One possible reason to consider for the mild disease course is a low virulence of the organisms in the inoculum, since repeated subcultures of laboratory strains may lower their virulence. Further, the lung suspension with M. capripneumoniae used was derived from a surviving goat at the end of a CCPP outbreak. It is argued that M. mycoides (SC) strains isolated at the end of contagious bovine pleuropneumonia (CBPP) outbreaks are less virulent than those from early cases [26] and this possibly applies to CCPP as well.
Severe CCPP is characterized by appearance of fever at the end of the incubation period [17]. The wide variation in the incubation period of inoculated goats in our study was a striking finding. Fever was generally delayed, up to 28 days post inoculation (p.i.). This surprising lack of early clinical signs was the reason for the later inoculation of additionally 3 goats with another strain, which actually produced in one goat an episode of fever starting within the commonly observed 3–9 days p.i. [17]. In-contact goats became febrile within a previously recorded time range of 11–41 days [17]. The observed fever on days 1 and 2 p.i. was possibly a response to components of the inoculation medium since goats receiving medium only (Wesonga, unpublished data) also responded in this way.
In the great majority of goats with lesions at necropsy, the lungs showed fibrosis combined with fibrinopurulent or mucopurulent airway exudates and active alveolar inflammation with macrophages predominating over neutrophils. These features are collectively consistent with chronic fibrinous bronchopneumonia [8,15] and thus compatible with poor resolution of pneumonia from M. capripneumoniae infection. However, the agent was not grown from the lungs, in accordance with previous experiences from attempts to cultivate M. capripneumoniae from pulmonary or pleural lesions late in experimental infection [18,28,35]. In our study, cultivation was negative despite use of pyruvate-containing media known to markedly support growth [4,22]. This makes it likely that living organisms were truly not present in the lungs.
On i-ELISA, clear-cut seroconversion was observed in 40% of the inoculated (A) goats. In most seroreactive A goats (Fig. 1), titres rose from about week 3 p.i., similar to previous findings [18,19]. Goat no. 131 showed fever, further confirming infection that, however, was overcome at the timepoint of necropsy. The slow development of the disease in this goat and goat no.188 was reflected by a slow and steady rise of antibody titres. In comparison, goats no. 139 and 174 showed early signs and a biphasic pattern of the antibody response after a steep rise to the initial peak. The biphasic pattern is similar to that observed in the antibody response to M. capripneumoniae capsular polysaccharide antigen by [19], who explained it as a possible effect of an 'eclipse' of antibodies by excess antigen in circulation. It can also be speculated that our goats with this pattern may have mounted a humoral response that was not protective to a first wave of infection, resulting in a second antibody response following intra-pulmonary mycoplasmal proliferation. Though goat no. 151 of the in contact-exposed group showed steadily significant antibody titres after infection, both on i-ELISA and b-ELISA, it was consistently afebrile and at necropsy 3 months post exposure M. capripneumoniae antigen was present in a focus of residual chronic pleuropneumonia. These features altogether suggests a low-grade chronic infection in this goat.
Whereas serum antibody levels indicating infection were observed in only 24% of the exposed goats, 56% had lesions compatible with M. capripneumoniae infection at necropsy, and 20% were serologically negative but positive for M. capripneumoniae antigen in lesions. Though local antibody secretion into the lungs and airways was not investigated, this may indicate a poor activation of antigen-specific Th 2 immune responses in many goats.
This is apparently the first report on the use of IHC to detect M. capripneumoniae. However, IHC has been applied for some other mycoplasmas in ruminants, including M. mycoides subsp. mycoides (SC) in CBPP, where it was considered useful for etiological diagnosis [9,30,29]. In our study, M. capripneumoniae antigen was demonstrated in 7 of the 14 goats with lung lesions, but in none of the goats which lacked pulmonary pathology at necropsy. The results provide strong evidence that M. capripneumoniae antigen is associated with late stage lesions of CCPP. Whereas in positive control cases from an acute CCPP outbreak the antigen was both in free form in alveolar spaces and in alveolar macrophages, similar to findings in MmmSC infection [9], the goats of our study displayed antigen only in alveolar macrophages.
The presence of antigen in alveolar macrophages indicates the importance of these cells in the defense of the goat lung against M. capripneumoniae. In vitro studies have indicated antibody-mediated phagocytosis of mycoplasmas by alveolar macrophages as an effective mechanism of defense against Mycoplasma pneumoniae as well as Mycoplasma pulmonis [25,31]. In mice with M. pulmonis, involvement of innate host defenses, such as surfactant protein A-dependent killing of organisms by IFNγ-activated alveolar macrophages, appears critical for the pulmonary defense [7,11], whereas humoral responses seem particularly important against systemic dissemination of the agent [7].
Surprisingly, the PCR did not detect any M. capripneumoniae DNA even in lungs amply displaying microbial antigen on IHC. This might be due to insufficient sensitivity or inhibition of the PCR reaction by substances in the samples [37] and warrants studies on optimization of sample pretreatment and use of internal amplification control for this PCR method.
M. ovipneumoniae, considered an ubiquitous mycoplasma of small ruminants world-wide [12], was isolated from the lungs or trachea of 8 experimental goats with or without pulmonary pathology, and one control goat. The organism was a common inhabitant of the upper respiratory tract of the goats prior to infection and was also cultivated from the lung suspension used as part of the inoculum. The possible contribution to lung disease by this agent is difficult to ascertain, but caprine M. ovipneumoniae infection is one possible cause of cuffing pneumonia [21], which commonly was a part of the pulmonary histopathology observed in our goats.
This study of long-term M. capripneumoniae infection has shown that the disease pattern and infection biology of CCPP may be a complex matter. Any chronic carrier state was not demonstrable. Our results indicate the need of further studies especially on immune mechanisms and interactions of M. capripneumoniae with host tissue cells in CCPP.
Acknowledgments
Acknowledgements
The authors are grateful to Christina Nilsson and Briitta Ojava, Department of Pathology, Swedish University of Agricultural Sciences, S. Jernstedt, Virginia Melys and Marianne Persson, National Veterinary Institute, Sweden, E. Gitonga and H. Ongaro, NVRC, KARI, and L. Ndirangu, Veterinary Laboratories, Kabete, Kenya, for technical assistance. Funds for carrying out studies in Kenya were provided by International Foundation for Science, Stockholm.
References
- Black FT. Modification of the growth inhibition test and application to human T-mycoplasma. Appl Microbiol. 1973;25:525–533. doi: 10.1128/am.25.4.528-533.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölske G. Survey of mycoplasma infections in cell cultures and a compatison of detection methods. Zentralbl Bacteriol Hyg A. 1988;269:331–340. doi: 10.1016/s0176-6724(88)80176-7. [DOI] [PubMed] [Google Scholar]
- Bölske G. Doctoral thesis. Swedish University of Agricultural Sciences, Uppsala, Sweden; 1995. Respiratory mycoplasmoses in goats, especially with regard to diagnosis of contagious caprine pleuropneumonia. [Google Scholar]
- Bölske G, Johansson KE, Heinonen R, Panvuga PA, Twinamasiko E. Contagious caprine pleuropneumonia in Uganda and isolation of Mycoplasma capricolum subspecies capripneumoniae from goats and sheep. Vet Rec. 1995;137:594. [PubMed] [Google Scholar]
- Bölske G, Mattsson JG, Ros Bascunana C, Bergström K, Wesonga H, Johansson KE. Diagnosis of contagious caprine pleuropneumonia by detection and identification of Mycoplasma capricolum subsp. capripneumoniae by PCR and restriction enzyme analysis. J Clin Microbiol. 1996;34:785–791. doi: 10.1128/jcm.34.4.785-791.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölske G, Msami HM, Gunnarsson A, Kapaga AM, Loomu PM. Contagious bovine pleuropneumonia in northern Tanzania, culture confirmation and serological studies. Trop Anim Health Prod. 1995;27:193–201. doi: 10.1007/BF02250690. [DOI] [PubMed] [Google Scholar]
- Cartner SC, Lindsey JR, Gibbs-Erwin J, Cassell GH, Simecka JW. Roles of innate and adaptive immunity in respiratory mycoplasmosis. Infect Immun. 1998;66:3485–3491. doi: 10.1128/iai.66.8.3485-3491.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dungworth DL. The respiratory system. In: Jubb KVF, Kennedy PC, Palmer N, editor. Pathology of Domestic Animals. 4. II. Academic Press, San Diego; 1993. pp. 539–699. [Google Scholar]
- Ferronha MH, Nunes Petisca LJ, Sousa Ferreira H, Machado M, Regalla J, Penha Goncalves A. Detection of Mycoplasma mycoides subsp. mycoides immunoreactive sites in pulmonary tissue and sequestra of bovines with contagious pleuropneumonia. In: Regalla J, editor. Contagious bovine pleuropneumonia Report EUR 12065 EN, Commission of the European Communities, Luxembourg. 1990. pp. 17–25. [Google Scholar]
- Gourlay RN. Antigenicity of M. mycoides I. Examination of body fluids from cases of CBPP. Res Vet Sci. 1964;50:59–67. [Google Scholar]
- Hickman-Davis JM, Lindsey JR, Zhu S, Matalon S. Surfactant protein A mediates mycoplasmacidal activity of alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 1998;274:L270–L277. doi: 10.1152/ajplung.1998.274.2.L270. [DOI] [PubMed] [Google Scholar]
- Jones GE. Contagious caprine pleuropneumonia. Technical Series no.9. Office International des Epizooties, Paris. 1989.
- Kaliner G, MacOwan KJ. The pathology of experimental and natural contagious caprine pleuropneumonia in Kenya. Zentralbl. Veterinarmed (B) 1976;23:652–661. doi: 10.1111/j.1439-0450.1976.tb00703.x. [DOI] [PubMed] [Google Scholar]
- Leach RH, Ernø H, MacOwan KJ. Proposal for designation of F38-type caprine mycoplasmas as Mycoplasma capricolum subsp. capripneumoniae subsp. nov. and consequent obligatory relegation of strains currently classified as M. capricolum (Tully, Barile, Edward, Theodore, and Ernø 1974) to an additional new subspecies, M. capricolum subsp. capricolum subsp. nov. Int J Syst Bacteriol. 1993;43:603–605. doi: 10.1099/00207713-43-3-603. [DOI] [PubMed] [Google Scholar]
- López A. Respiratory system, thoracic cavity, and pleura. In: McGavin MD, Carlton WW, Zachary JF, editor. Thomson's special veterinary pathology. 3. Mosby, St. Louis; 2001. pp. 125–195. [Google Scholar]
- MacOwan KJ, Minnette JE. A mycoplasma from acute contagious caprine pleuropneumonia in Kenya. Trop Anim Health Prod. 1976;8:91–96. doi: 10.1007/BF02383376. [DOI] [PubMed] [Google Scholar]
- MacOwan KJ, Minnette JE. The role of Mycoplasma strain F38 in contagious caprine pleuropneumonia (CCPP) in Kenya. Vet Rec. 1977;101:380–381. doi: 10.1136/vr.101.19.380. [DOI] [PubMed] [Google Scholar]
- MacOwan KJ, Minnette JE. The effect of high passage Mycoplasma strain F38 on the course of contagious caprine pleuropneumonia (CCPP) Trop Anim Health Prod. 1978;10:31–35. doi: 10.1007/BF02235300. [DOI] [PubMed] [Google Scholar]
- March JB, Harrison JC, Borich SM. Humoral immune responses following experimental infection in goats with Mycoplasma capricolum subsp. capripneumoniae. Vet Microbiol. 2002;84:29–45. doi: 10.1016/S0378-1135(01)00434-5. [DOI] [PubMed] [Google Scholar]
- McMartin DA, MacOwan KJ, Swift LL. A century of classical contagious caprine pleuropneumonia: from original description to aetiology. Br Vet J. 1980;136:507–515. doi: 10.1016/s0007-1935(17)32196-6. [DOI] [PubMed] [Google Scholar]
- Mohan K, Obwolo MJ, Hill FWG. Mycoplasma ovipneumoniae infection in Zimbabwean goats and sheep. J Comp Pathol. 1992;107:73–79. doi: 10.1016/0021-9975(92)90097-E. [DOI] [PubMed] [Google Scholar]
- Msami HM, Kapaga AM, Heldtander M, Bölske G. Contagious caprine pleuropneumonia in Tanzania. Vet Rec. 2001;148:22–23. doi: 10.1136/vr.148.1.22. [DOI] [PubMed] [Google Scholar]
- Muthomi EK, Rurangirwa FR. Passive haemagglutination and complement fixation as diagnostic tests for contagious caprine pleuropneumonia caused by F38 strain of mycoplasma. Res Vet Sci. 1983;35:1–4. [PubMed] [Google Scholar]
- Olsson B, Bölske G, Bergström K, Johansson KE. Analysis of caprine mycoplasmas and mycoplasma infections in goats using two-dimensional electrophoresis and immunoblotting. Electrophoresis. 1990;11:861–869. doi: 10.1002/elps.1150111016. [DOI] [PubMed] [Google Scholar]
- Powell DA, Clyde WA., Jr Opsonin-reversible resistance of Mycoplasma pneumoniae to in vitro phagocytosis by alveolar macrophages. Infect Immun. 1975;11:540–550. doi: 10.1128/iai.11.3.540-550.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provost A, Perreau P, Breard A, Le Goff C, Martel JL, Cottew GS. Contagious bovine pleuropneumonia. Rev Sci Tech Off Int Epiz. 1987;6:625–679. doi: 10.20506/rst.6.3.306. [DOI] [PubMed] [Google Scholar]
- Rosendal S, Black FT. Direct and indirect immunofluorescence of unfixed and fixed mycoplasma colonies. Acta Pathol Microbiol Scand (B) 1972;80:615–622. doi: 10.1111/j.1699-0463.1972.tb00186.x. [DOI] [PubMed] [Google Scholar]
- Rurangirwa FR, McGuire TC, Mbai L, Ndung'u L, Wambugu A. Preliminary field tests of lyophilised contagious caprine pleuropneumonia vaccine. Res Vet Sci. 1991;50:240–241. doi: 10.1016/0034-5288(91)90114-4. [DOI] [PubMed] [Google Scholar]
- Scanziani E. Immunohistochemical staining of fixed tissues. In: Miles RJ, Nicholas RAJ, editor. Mycoplasma protocols. Vol. 104. Methods in Molecular Biology Humana Press, Totowa; 1998. pp. 133–140. [DOI] [PubMed] [Google Scholar]
- Scanziani E, Paltrinieri S, Boldini M, Grieco V, Monaci C, Giusti AM, Mandelli G. Histological and immunohistochemical findings in thoracic lymph nodes of cattle with contagious bovine pleuropneumonia. J Comp Pathol. 1997;117:127–136. doi: 10.1016/S0021-9975(97)80029-1. [DOI] [PubMed] [Google Scholar]
- Taylor G, Howard CJ. Protection of mice against Mycoplasma pulmonis infection using purified mouse immunoglobulins: comparison between protective effect and biological properties of immunoglobulin classes. Immunology. 1981;43:519–525. [PMC free article] [PubMed] [Google Scholar]
- Thiaucourt F, Bölske G. Contagious caprine pleuropneumonia and other pulmonary mycoplas-moses of sheep and goats. Rev Sci Tech Off Int Epiz. 1996;15:1397–1414. doi: 10.20506/rst.15.4.990. [DOI] [PubMed] [Google Scholar]
- Thiaucourt F, Bölske G, Libeau G, Le Goff C, Lefevre PC. The use of monoclonal antibodies in the diagnosis of contagious caprine pleuropneumonia (CCPP) Vet Microbiol. 1994;41:191–203. doi: 10.1016/0378-1135(94)90100-7. [DOI] [PubMed] [Google Scholar]
- Thiaucourt F, Guerin C, Mady V, Lefevre PC. Diagnosis of caprine contagious pleuropneumonia: recent improvements (In French) Rev Sci Tech Off Int Epiz. 1992;11:859–865. [PubMed] [Google Scholar]
- Wesonga HO, Lindberg R, Litamoi JK, Bölske G. Late lesions of experimental contagious caprine pleuropneumonia caused by Mycoplasma capricolum subsp. capripneumoniae J Vet Med B. 1998;45:105–114. doi: 10.1111/j.1439-0450.1998.tb00772.x. [DOI] [PubMed] [Google Scholar]
- Wesonga HO, Litamoi JK, Kagumba M, Wakhusama E. Relationship between clinical signs and early lesions of contagious caprine pleuropneumonia caused by Mycoplasma strain F38. Small Rumin Res. 1993;10:45–54. doi: 10.1016/0921-4488(93)90106-R. [DOI] [Google Scholar]
- Wilson IG. Inhibition and facilitation of nucleic acid amplification. Appl Environ Microbiol. 1997;63:3741–3751. doi: 10.1128/aem.63.10.3741-3751.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]


