Abstract
Bulk milk samples from 220 dairy herds were collected at 9 public milk collection centres in the northeastern and northern Thailand, and a subset of 11 herds was selected for individual testing. The samples were tested for presence of antibodies to BVDV and BHV-1 using an indirect ELISA. The results from the bulk milk testing demonstrated a moderate level of exposure to BVDV and BHV-1 (73% and 67%, respectively). However, the low proportion of herds with high BVDV antibody-levels (13%) and the low within-herd seroprevalence of BVDV and BHV-1 in the 11 herds (24% and 5%, respectively), particularly among the young stock (15% and 0%, respectively), demonstrated a low prevalence of active BVDV infection and a low rate of reactivation of latent BHV-1. The presence of a self-clearance process was also indicated by the results from the individual testing. Moreover, a surprisingly low prevalence of BVDV and BHV-1 antibody-positive herds at one of the milk centres was found. This centre was established 5–10 years before the others. Our impression is that this reflects the self-clearance process, where consecutive replacement of imported infected animals without further spread has resulted in a nearly total elimination of the infections.
Based on our experiences and on these results we are convinced that this process can continue if there is awareness of herd biosecurity. This is especially important in the context of a future intensification of the dairy production.
Keywords: BVDV, BHV-1, bulk milk, prevalence, Thailand.
Introduction
Bovine viral diarrhoea virus (BVDV) and bovine herpesvirus type 1 (BHV-1) are well-known, important pathogens of cattle that give rise to substantial economic losses due to reproductive failures and increased calf mortality, as well as enteric and respiratory disease. These pathogens have a worldwide distribution and tend to be endemic in most populations, although national and regional variations occur (for BVDV review see [21,14,18]; for BHV-1 review see [11,17,31]).
Vaccination has been the conventional way to control or reduce losses caused by BVDV and BHV-1 for the last 4–5 decades [7,17]. The number of licensed vaccines on the market is vast and they are widely used. The use of vaccines may reduce economic losses caused by clinical disease, but does not appear to result in reduction of the prevalence of either BVDV or BHV-1 infections [34,25]. The introduction of gene-deleted vaccines was considered a breakthrough for the control of BHV-1 [31]. During 1998–1999 a live attenuated gE-deleted marker vaccine provided the basis for a compulsory control programme in the Netherlands. However, a severe outbreak of BVDV type 2 on several dairy farms, induced by contaminated gE-deleted marker vaccine, was a drawback that illustrated the potential risks with the use of live vaccines [3]. During the last decades eradication programmes against BVDV and BHV-1, without the use of vaccines, have been implemented in some European countries. These have been based on identification and elimination of carrier animals, together with increased herd biosecurity. The national BVD programmes in the Scandinavian countries, as well as the regional programmes in a few other countries in Europe, have had success with control of BVDV and are aiming towards eradication [37,19,23,32,5]. Eradication of BHV-1, i.e. official declaration of freedom by the EU or Efta, has already been achieved in Switzerland, Norway, Finland, Denmark, Sweden, Austria and the province of Bolzano in Italy [2,29].
Experiences from the Swedish BVDV programme have shown that self-clearance, i.e. the process whereby an infection is eliminated from a population without intervention, is an important phenomenon that works in favour of any BVDV control scheme. Self-clearance occurs when persistently infected (PI) animals are removed from the herd (due to death, trade or culling) before they succeed in establishing additional persistent infections, and seems to be more frequent in smaller herds. However, harder rearing conditions, as might be seen in larger herds with intensive production, may increase the risk for early death in PI animals and may consequently increase the probability for self-clearance [19]. Self-clearance has, to our knowledge, not been described for BHV-1 infections. During the Swedish BHV-1 programme, however, reports on herds changing BHV-1 status from positive to negative without intervention between consecutive samplings, indicated a self-clearing process. Replacement of animals latently infected with BHV-1 before any reactivation of latent virus will result in self-clearance as long as introduced or newborn animals are BHV-1 negative.
Information on the epidemiology and impact of BVDV and BHV-1 in developing countries is limited, even though serological surveys have been performed in several countries in Asia, Africa and Latin America [28,4,36,24,30]. Given the assumption that presence of antibodies reflects exposure to a pathogen, these surveys show that BVDV and BHV-1 are widespread. However, considering the persistence of BVDV and BHV-1 specific antibodies and the phenomenon of self-clearance [16,10,19], serosurveys may give positive results years after last exposure to virus.
Dairy production in Thailand dates back to the 1960s when dairy cattle were imported to the central provinces of the country. The number of establishments increased rapidly during the following decades to meet the increasing demand. Since the early 1990s, smallholder dairy farming has been encouraged in other parts of the country, including the northeastern and northern provinces. Dairy cattle have been introduced to the new establishments mainly from the central provinces, but also through imports from other countries.
The purpose of this study was to estimate the prevalence of BVDV and BHV-1 in dairy cattle in the northeastern and northern parts of Thailand and to study the epidemiological patterns of the viruses. The purpose was also to estimate the prevalence of active or recent infection of BVDV and BHV-1, with the aim to make recommendations for disease control.
Materials and methods
Study population and survey design
This survey was carried out in 4 provinces in Thailand, 3 in northeast: Khon Kaen, Udorn Thani and Sakon Nakorn and one in north: Chiang Mai (see map, fig. 1). The average size of a dairy herd in the region is 10–20 milking cows, with mixed Holstein-Friesian and Sahival breeds, and with a daily milk production of approximately 9–11 kg/cow [9]. Most of the production in these provinces is commercialised through public milk collection centres.1
Figure 1.
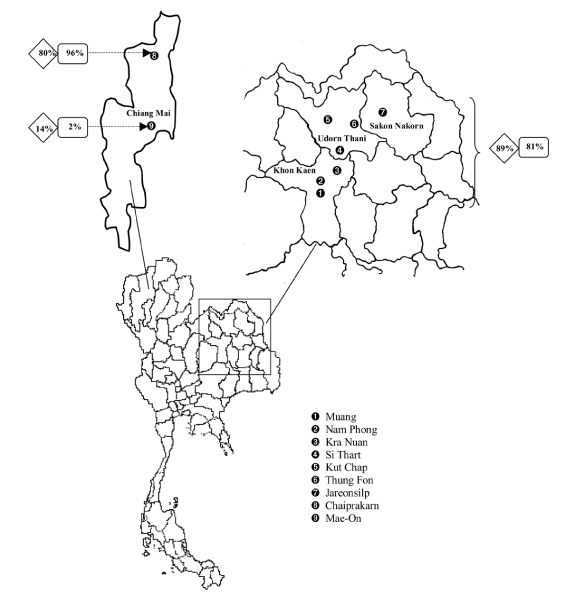
Map of Thailand showing the location of the 9 public milk centres from which the bulk milk samples were collected. The prevalence of BVDV ad BHV-1 seropositive herds among the 220 herds in milk centres 1–7, milk centre 8 and milk centre 9, are shown within diamonds and squares, respectively.
Two hundred and twenty dairy herds were randomly selected from 9 milk centres: 7 located in the northeastern provinces (milk centres 1–7) and 2 in one of the northern provinces (milk centres 8–9). These herds represented approximately 20% of the dairy herds in the 9 milk centres (range: 10%–36%). From this set of herds, a subset of 11 herds located in the Khon Kaen province (milk centres 1 and 2) was also selected. This selection was based on accessibility. Vaccination against BVDV or BHV-1 had not been practised in this part of the country.
Collection of samples
Bulk milk samples from all 220 herds were collected at the public milk collection centres: during May to August 2000 in the northeastern provinces (151 herds) and during January to June 2001 in the northern (69 herds). A second sampling was carried out in August 2001 on the 11 selected farms, where individual serum samples from all animals were collected. All samples were transported at 4–8°C to the laboratory on the day of the sampling and centrifuged at 1000 × g. The samples were then inactivated at 56°C for 30 min and stored at -20°C in 2.0 ml vials until analysed at the Faculty of Veterinary Medicine and Animal Science, Swedish University of Agricultural Sciences, Uppsala, Sweden.
Serological testing and interpretation
Commercial indirect ELISA kits with antigen-coated microtitre plates were used for detection of antibodies to BVDV and BHV-1 in bulk milk and in serum, according to the instructions of the manufacturer (SVANOVA Biotech AB, Uppsala, Sweden). The corrected optical density (COD) level was calculated before interpretation of the results by subtracting the optical density (OD) for the control antigen from sample OD (OD sample - OD control = COD).
The Swedish system of classification and interpretation of the results of the BVDV antibody ELISA on bulk milk was used whereby herds are allocated into 4 different classes based on COD levels. Herds in classes 0 or 1 (CODs <0.05 or between 0.05–0.24, respectively) have a very low or low level of antibodies in the bulk milk and are unlikely to contain persistently infected animals, unless these have been recently introduced. Herds in classes 2 or 3 (CODs between 0.25–0.54 or ≥ 0.55 respectively) have a moderate or high level of antibodies. Herds with active BVDV infection, i.e. herds with PI animals are most likely to be found among class 3 herds, since the presence of a PI animal in a herd generally expresses itself as a high degree of seroconversion in the surrounding animals, resulting in high levels of antibodies in the bulk milk and, consequently, high CODs. These herds will also, in general, have a high seroprevalence among the young stock [19].
The results of the BHV-1 antibody ELISA on bulk milk were interpreted according to the Swedish IBR control programme, i.e. samples with CODs ≥ 0.05 were considered positive.
Sera from animals older than 6 months, in total 351 animals, were analysed for presence of antibodies to BVDV and BHV-1. The results were interpreted according to [15], i.e. sera with CODs ≥ 0.20 were considered positive. BHV-1 antibody-positive individuals are generally considered to be latently infected and to constitute a potential source of viral transmission [1]. BVDV antibody-positive individuals, on the other hand, have in general recovered from infection and developed a lifelong immunity [10]. Animals persistently infected with BVDV are in general antibody-negative (Coria et al .1972).
A commercial antigen ELISA (Herd Check BVDV Ag/Serum, Idexx laboratories, INC.) was used for detection of BVDV antigen in herds where the presence of PI animals could be suspected based on the results from the antibody ELISA testing. All seronegative animals older than 6 months and all animals younger than 6 months were tested in these herds. The analysis was performed according to the instructions of the manufacturer. The corrected optical density (COD) level was calculated before interpretation of the results by subtracting the mean OD for negative controls from sample OD (OD sample - mean OD negative = COD). COD values ≤ 0.300 were classified as being negative.
Statistical analysis
The Pearson Chi-square test was used to investigate a possible association between BVDV antibody status and BHV-1 status at the herd level and to analyse differences in prevalences of BVDV and BHV-1 antibody-positive herds between regions and between milk centres within the regions. The differences in mean bulk milk COD levels between the regions and between milk centres within the regions were analysed using the Wilcoxon rank sum-test (Mann-Whitney). On the individual level, the data was stratified into 4 categories based on age, and individual BVDV and BHV-1 seroprevalences within each age category were calculated. The Pearson Chi-square test was used for the analysis. P-values ≤ 0.05 were considered statistically significant. The statistical software, Stata, release 7.0 and 8.0 (StataCorp 2001, College Station, TX, USA) was used for all statistical analyses.
Results
Bulk milk testing
The distributions of bulk milk COD levels from BVDV and BHV-1 antibody assays are shown in fig. 2. The prevalence of BVDV antibody-positive herds (classes 1, 2 and 3) was 73% (160/220) and that of BHV-1 67% (147/220). Sixty-two per cent (136/220) of the herds had detectable levels of antibodies to both viruses in the bulk milk. Twenty-nine herds (13%) had BVDV COD levels above 0.55 and were classified as class 3 herds. There was a highly significant relationship between a herd's BVDV antibody status and its BHV-1 status (χ2 = 87.5, p = 0.000).
Figure 2.
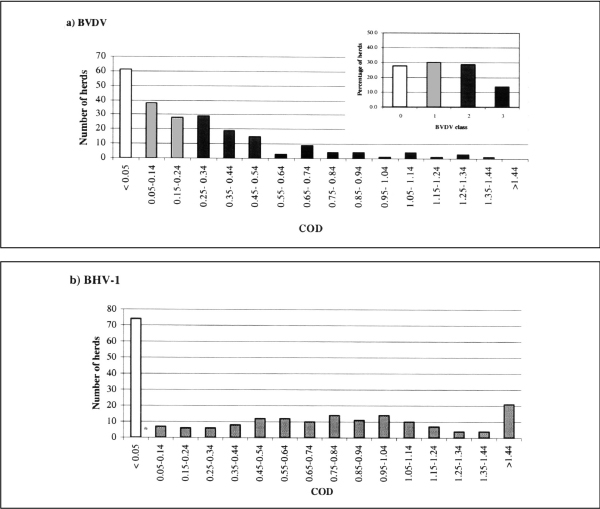
Frequency distribution of bulk milk corrected optical density (COD) values from the BVDV antibody assay (a) with an inserted histogram showing the percentage of herds in each BVDV class derived from the CODs, for 220 dairy herds in Thailand. (b) The frequency distribution of bulk milk COD from the BHV-1 antibody assay.
The mean prevalences of BVDV and BHV-1 antibody-positive herds were significantly higher in milk centres 1–7 in the northeastern provinces (mean prevalences: 89% and 81%, respectively) than in milk centres 8–9 in the northern province (mean prevalences: 34% and 36%, respectively; table 1). Milk centre 9 differed from all the other milk centres, with a very low prevalence of BVDV and BHV-1 antibody-positive herds (fig. 1), and significantly lower mean CODs (fig. 3). Antibodies to BVDV were detected in bulk milk from 6 out of 44 herds (14%) at milk centre 9, and antibodies to BHV-1 only in bulk milk from one herd (2%).
Table 1.
Results from BVDV and BHV-1 antibody analysis by indirect ELISA on 220 bulk milk samples collected at 9 public milk centres in northeastern (1–7) and northern (8–9) Thailand, 2000–2001.
| Milk centre | |||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Number of herds | 11 | 40 | 24 | 20 | 20 | 19 | 17 | 25 | 44 |
| No. of BVDV positive* | 11 | 36 | 22 | 19 | 14 | 17 | 15 | 20 | 6 |
| Prevalence (%) | 100 | 90 | 92 | 95 | 70 | 90 | 88 | 80 | 14 |
| No. of BHV1 positive* | 8 | 35 | 22 | 17 | 13 | 10 | 17 | 24 | 1 |
| Prevalence (%) | 73 | 88 | 92 | 85 | 65 | 53 | 100 | 96 | 2 |
* Herds with CODs ≥ 0.05 were considered antibody positive according to the Swedish BVDV and BHV-1 control programmes.
Figure 3.
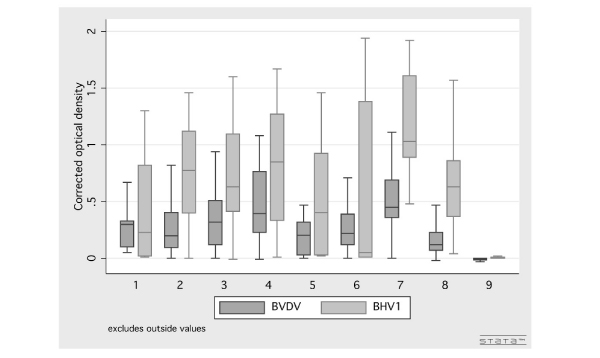
Box plot showing the distribution of bulk milk corrected optical densitiy (COD) values for BVDV and BHV-1 from dairy herds in nine public milk centres in Thailand, 2000–2001. Boxes represent the first quartile (q1), median and third quartile (q3) of the COD values from each milk centre, and lower (L) and upper (U) limits are defined as: L = q1 - 1.5 × IQR and U = q3 + 1.5 × IQR, where IQR = q3 - q1.
Individual testing
The results from the individual testing of animals older than 6 months in the 11 herds are given in table 2. The within-herd seroprevalence of BVDV was low to moderate in all herds (mean 24%; range: 6%–55%). The within-herd seroprevalence of BHV-1 was low in all herds (range: 70%–14%) and in total, only 18 seropositive animals were found. Fifteen out of these were also seropositive to BVDV.
Table 2.
Results from BVDV and BHV-1 antibody analysis by indirect ELISA on sera from all animals older than 6 months in 11 dairy herds in the Khon Kaen province, Thailand, 2000–2001.
| Herd | Total | |||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||
| Number of animals | 34 | 19 | 37 | 31 | 23 | 26 | 29 | 40 | 41 | 38 | 33 | 351 |
| No. of BVDV positive* | 4 | 2 | 5 | 4 | 3 | 8 | 2 | 22 | 11 | 20 | 2 | 83 |
| Prevalence (%) | 12 | 11 | 14 | 13 | 13 | 31 | 7 | 55 | 27 | 53 | 6 | 24** |
| No. of BHV-1 positive* | 2 | 2 | 5 | 3 | 3 | 1 | 0 | 2 | 0 | 0 | 0 | 18 |
| Prevalence (%) | 6 | 11 | 14 | 10 | 13 | 4 | 0 | 5 | 0 | 0 | 0 | 5** |
* Sera with CODs ≥ 0.20 were interpreted as seropositive ** i.e. mean prevalence within the eleven herds
All eleven herds were BVDV positive based on bulk milk testing (mean COD: 0.13; range: 0.05–0.23) and 6 out of 11 were BHV-1 positive (mean COD: 0.20; range: 0–0.56). The 5 herds that were BHV-1 negative based on bulk milk testing were also free from seropositive individuals among the lactating cows (data not shown). In herd 8, however, 2 seropositive cows were detected among the non-lactating cows.
The distribution of seropositive animals by age is given in table 3. We found significantly higher seroprevalence of BVDV and BHV-1 among cows older than 6 years (50% and 21% respectively) compared with that among animals younger than 6 years (17% and 1% respectively; table 3). The mean age for the BHV-1 seropositive animals was 8.2 years and none of the 224 animals between 6 months and 4 years had seroconverted to BHV-1. Only 15 of the 114 animals below 2 years had seroconverted to BVDV and the seroprevalence in this group was very low in 9 herds (herds 1–7, 9 and 11) and moderate to high in herds 8 and 10 (21% and 64% respectively; data not shown). No BVDV antigen-positive animals were detected.
Table 3.
Distribution by age of BVDV and BHV-1 seropositive individuals among animals older than 6 months, in 11 dairy herds in the Khon Kaen province, Thailand, 2001.
| Age (years) | No. of animals | BVDV* | BHV-1* | ||
| No. of positive | Prevalence (%) | No. of positive | Prevalence (%) | ||
| > 6 | 70 | 35 | 50 | 15 | 21 |
| 4–6 | 57 | 11 | 19 | 3 | 5 |
| 2–4 | 110 | 22 | 20 | 0 | 0 |
| 0.5–2 | 114 | 15 | 13 | 0 | 0 |
| Total | 351 | 83 | 24 | 18 | 5 |
* Sera with CODs ≥ 0.20 were interpreted as seropositive.
Discussion
The results demonstrate a moderate level of exposure to BVDV and BHV-1 in the studied dairy population with prevalences of antibody-positive herds of 73% and 67%, respectively. These prevalences are similar to those reported previously from northeastern Thailand [36], and do not differ greatly from other parts of the world [22,13,26,31].
The proportion of BVDV class 3 herds was 13%, indicating a low prevalence of active BVDV infection in the studied population. Results from previous publications demonstrate that the proportion of herds potentially having active BVDV infection shows a wide range of variation between countries and between regions within countries [6,26,23,12,20,30,35]. This variation may in part be explained by differences in demography, such as herd size and population density, and management factors such as the pattern and frequency of animal movements [13].
There was a significant association between being antibody-positive to BVDV and being BHV-1-positive at the herd level. This corresponds with the results reported by [26], suggesting that similar factors affect the risk of being infected with either virus. One major risk factor is the introduction of purchased animals with insufficient health documentation [33]. In this context, we found the results from milk centre 9 interesting. The prevalence of BVDV and BHV-1 antibody-positive herds at milk centre 9 was very low (14% and 2%, respectively) compared with the other milk centres and in sharp contrast with the neighbouring herds at milk centre 8. It was beyond the scope of this study to explain this finding as demography, and management practices were not studied in detail. However, we know that most of the herds at milk centre 9 were established in the late 1980s, whereas most of the herds at milk centres 1 to 8 were established around 1995. One might thus speculate that one reason for the aberrant finding is the difference in time that has passed since the milk centres were established; that the higher prevalence in the herds at milk centres 1 to 8 to a large extent represents historical infections (for BVDV) or latent infections (for BHV-1) in introduced, i.e. imported, cattle. At milk centre 9, on the other hand, the process has gone further and replacement of infected, imported animals most probably has occurred over the years, resulting in a nearly total self-clearance of both BVDV and BHV-1. This hypothesis, however, needs to be further investigated.
The selection of the 11 herds used for the individual testing was not based on a random procedure and represents only 2 of the milk centres. Therefore, these results cannot be generalised to the entire population. Nevertheless, they do provide new knowledge on the regional BVDV and BHV-1 situation. The age distribution of BVDV seropositive animals in 9 of the herds clearly demonstrates 2 things: firstly, absence of PI animals and secondly that the herds have been exposed to BVDV in the past. According to our experiences from the Swedish BVD programme this indicates that self-clearance has occurred. The corresponding results from herd 8 and especially herd 10, on the other hand indicated, possible presence of PI animals. To investigate these findings and to identify PI animals, sera from all seronegative cows and all calves (including calves younger than 6 months) in herds 8 and 10 were analysed by antigen ELISA, with negative results. It is possible that PI animals have been present in the herds recently but that they have died or been traded before our sampling. But if they succeeded to transmit the virus to susceptible dams in early pregnancy, new PI's will be born, and the infection will be maintained within the herd. Therefore, these 2 herds should be kept under surveillance.
The results of the individual testing also suggest an absence of active BHV-1 infection in the 11 herds, with the lack of seroreactors among cows younger than 4 years. Moreover, we found evidence that at least 14 out of 18 seropositive cows had in fact been imported, indicating that no or very limited virus transmission had occurred following their introduction. This corresponds with the results reported by [27], suggesting that the risk for reactivation of latent BHV-1 infections under natural conditions has been overestimated in the past and that self-clearance may occur. This is probably particularly true in regions with low-intensive production systems, i.e. with low levels of stress to the animals.
Finally, the results suggest a low rate of reactivation of latent BHV-1 infections and indicate a progressive self-clearance of BVDV as well as BHV-1. Based on our experiences from Sweden and on these results we are convinced that this process can continue as long as introductions of infection to non-infected herds are prevented. Farmers and local authorities must be made aware of the improvements in animal health and, thus, on production that can be gained through the control of these infections. The importance of herd biosecurity must be stressed, and the information necessary to achieve a sufficient level of biosecurity must be provided. This is especially important in the context of a future intensification of the dairy production. We conclude, based on our results, that the BVDV and BHV-1 situation in the region is favourable
Note
1 "Milk centre", when used further on in the text, refers to the population of herds delivering milk for commercialisation to a specific public milk collection centre.
Acknowledgments
Acknowledgements
The authors thank Dr. Suvichai Rojanasthein and his colleagues for bulk tank milk and data collection from dairy herds in the Chiangmai province. We are also grateful to the staff of the Dairy Farming Promotion Organisation for collection of bulk tank milk from milk centres 3–7. Jaruwan Kampa is a holder of a scholarship from the Swedish Foundation for International Cooperation in Research and Higher Education (STINT).
References
- Ackermann M, Peterhans E, Wyler R. DNA of bovine herpesvirus type 1 in the trigeminal ganglia of latently infected calves. Am J Vet Res. 1982;43(1):36–40. [PubMed] [Google Scholar]
- Ackermann M, Müller HK, Bruckner L, Kihm U. Eradication of infectious bovine rhinotracheitis in Switzerland: review and prospects. Vet Microbiol. 1990;23(1–4):365–370. doi: 10.1016/0378-1135(90)90168-U. [DOI] [PubMed] [Google Scholar]
- Barkema HW, Bartels CJ, van Wuijckhuise L, Hesselink JW, Holzhauer M, Weber MF, Franken P, Kock PA, Bruschke CJ, Zimmer GM. Outbreak of bovine virus diarrhea on Dutch dairy farms induced by a bovine herpesvirus 1 marker vaccine contaminated with bovine virus diarrhea virus type 2. [Uitbraak Van Bovine Virus Diarree Op Nederlandse Rundveebedrijven Na Vaccinatie Met Een Met BVDV Type 2 Gecontamineerd BHV1 Markervaccin.] (In Dutch) Tijdschr Diergeneeskd. 2001;126(6):158–165. [PubMed] [Google Scholar]
- Baule C, van Vuuren M, Lowings JP, Belák S. Genetic heterogeneity of bovine viral diarrhoea viruses isolated in Southern Africa. Virus Res. 1997;52(2):205–220. doi: 10.1016/S0168-1702(97)00119-6. [DOI] [PubMed] [Google Scholar]
- Bitsch V, Hansen KE, Rønsholt L. Experiences from the Danish programme for eradication of bovine virus diarrhoea (BVD) 1994–1998 with special reference to legislation and causes of infection. Vet Microbiol. 2000;77(1–2):137–143. doi: 10.1016/S0378-1135(00)00270-4. [DOI] [PubMed] [Google Scholar]
- Bitsch V, Ronshølt L. Control of bovine viral diarrhea virus infection without vaccines. Vet Clin North Am Food Anim Pract. 1995;11(3):627–640. doi: 10.1016/s0749-0720(15)30471-0. [DOI] [PubMed] [Google Scholar]
- Brock KV. Strategies for the control and prevention of bovine viral diarrhea virus. Vet Clin North Am Food Anim Pract. 2004;20:171–180. doi: 10.1016/j.cvfa.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Coria MF, McClurkin AW. Specific immune tolerance in an apparently healthy bull persistently infected with bovine viral diarrhea virus. J Am Vet Med Ass. 1978;172(4):449–451. [PubMed] [Google Scholar]
- Department of Livestock Development Statistics of dairy cattle on 1st Jaunary 2002. Satistics branch, Planning division, Department of Livestock Development, Thailand.
- Fredriksen B, Sandvik T, Løken T, Ødegaard SA. Level and duration of serum antibodies in cattle infected experimentally and naturally with bovine virus diarrhoea virus. Vet Rec. 1999;144(5):111–114. doi: 10.1136/vr.144.5.111. [DOI] [PubMed] [Google Scholar]
- Gibbs EPJ, Rweyemam MM. Bovine herpesvirus 1. Vet Bull. 1977;47:317–343. [Google Scholar]
- Graham DA, German A, McLaren IE, Fitzpatrick DA. Testing of bulk tank milk from Northern Ireland dairy herds for viral RNA and antibody to bovine viral diarrhoea virus. Vet Rec. 2001;149(9):261–265. doi: 10.1136/vr.149.9.261. [DOI] [PubMed] [Google Scholar]
- Houe H. Epidemiology of bovine viral diarrhea virus. Vet Clin North Am Food Anim Pract. 1995;11(3):521–547. doi: 10.1016/s0749-0720(15)30465-5. [DOI] [PubMed] [Google Scholar]
- Houe H. Epidemiological features and economical importance of bovine virus diarrhoea virus (BVDV) infections. Vet Microbiol. 1999;64(2–3):89–107. doi: 10.1016/S0378-1135(98)00262-4. [DOI] [PubMed] [Google Scholar]
- Juntti N, Larsson B, Fossum C. The use of monoclonal antibodies in enzyme linked immunosorbent assays for detection of antibodies to bovine viral diarrhoea virus. Zentralbl Veterinarmed B. 1987;34(5):356–363. doi: 10.1111/j.1439-0450.1987.tb00408.x. [DOI] [PubMed] [Google Scholar]
- Kaashoek MJ, Rijsewijk FA, van Oirschot JT. Persistence of antibodies against bovine herpesvirus 1 and virus reactivation two to three years after infection. Vet Microbiol. 1996;53(1–2):103–110. doi: 10.1016/S0378-1135(96)01238-2. [DOI] [PubMed] [Google Scholar]
- Kahrs RF. Viral diseases of cattle. 2. Iowa state university; 2001. Infectious bovine rhinotracheitis; pp. 159–170. [Google Scholar]
- Lindberg AL. Bovine viral diarrhoea virus infections and its control. A review Vet Q. 2003;25(1):1–16. doi: 10.1080/01652176.2003.9695140. [DOI] [PubMed] [Google Scholar]
- Lindberg AL, Alenius S. Principles for eradication of bovine viral diarrhoea virus (BVDV) infections in cattle populations. Vet Microbiol. 1999;64(2–3):197–222. doi: 10.1016/S0378-1135(98)00270-3. [DOI] [PubMed] [Google Scholar]
- Mainar-Jaime RC, Berzal-Herranz B, Arias P, Rojo-Vázquez FA. Epidemiological pattern and risk factors associated with bovine viral-diarrhoea virus (BVDV) infection in a non-vaccinated dairy-cattle population from the Asturias region of Spain. Prev Vet Med. 2001;52(1):63–73. doi: 10.1016/S0167-5877(01)00239-2. [DOI] [PubMed] [Google Scholar]
- Nettleton PF, Entrican G. Ruminant pestiviruses. Br Vet J. 1995;151(6):615–642. doi: 10.1016/S0007-1935(95)80145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niskanen R, Alenius S, Larsson B, Jacobsson SO. Determination of level of antibodies to bovine virus diarrhoea virus (BVDV) in bulk tank milk as a tool in the diagnosis and prophylaxis of BVDV infections in dairy herds. Arch Virol Suppl. 1991;3:245–251. doi: 10.1007/978-3-7091-9153-8_30. [DOI] [PubMed] [Google Scholar]
- Nuotio L, Juvonen M, Neuvonen E, Sihvonen L, Husu-Kallio J. Prevalence and geographic distribution of bovine viral diarrhoea (BVD) infection in Finland 1993–1997. Vet Microbiol. 1999;64(2–3):231–235. doi: 10.1016/S0378-1135(98)00272-7. [DOI] [PubMed] [Google Scholar]
- Obando RC, Hidalgo M, Merza M, Montoya A, Klingeborn B, Moreno-López J. Seroprevalence to bovine virus diarrhoea virus and other viruses of the bovine respiratory complex in Venezuela (Apure State) Prev Vet Med. 1999;41(4):271–278. doi: 10.1016/S0167-5877(99)00049-5. [DOI] [PubMed] [Google Scholar]
- O'Rourke K. BVDV: 40 years of effort and the disease still has a firm hold. J Am Vet Med Ass. 2002;220(12):1770–1773. [PubMed] [Google Scholar]
- Paton DJ, Christiansen KH, Alenius S, Cranwell MP, Pritchard GC, Drew TW. Prevalence of antibodies to bovine virus diarrhoea virus and other viruses in bulk tank milk in England and Wales. Vet Rec. 1998;142(15):385–391. doi: 10.1136/vr.142.15.385. [DOI] [PubMed] [Google Scholar]
- Pritchard GC. Epidemiology of BHV-1 infection in cattle breeding herds in Norfolk. In: Thrusfield MV, editor. Proceedings of the Society for Veterinary Epidemiology and Preventive Medicine, Edinburgh. pp. 169–185. April 1–3, 1992. [Google Scholar]
- Rweyemamu MM, Fernandez AA, Espinosa AM, Schudel AA, Lager IA, Mueller SB. Incidence, epidemiology and control of bovine virus diarrhoea virus in South America. Rev Sci Tech. 1990;9(1):207–221. doi: 10.20506/rst.9.1.479. [DOI] [PubMed] [Google Scholar]
- SCAHAW (Scientific Committee on Animal Health and Animal Welfare) Report on Bovine Herpesvirus 1 (BHV1) marker vaccines and the accompanying tests. European Commission, Sanco/C3/AH/R20/2000. p. 2. October, 25th 2000.
- Ståhl K, Rivera H, Vågsholm I, Moreno-López J. Bulk milk testing for antibody seroprevalences to BVDV and BHV-1 in a rural region of Peru. Prev Vet Med. 2002;56(3):193–202. doi: 10.1016/S0167-5877(02)00161-7. [DOI] [PubMed] [Google Scholar]
- Straub OC. Advances in BHV1 (IBR) research. Dtsch Tierarztl Wochenschr. 2001;108(10):419–422. [PubMed] [Google Scholar]
- Synge BA, Clark AM, Moar JA, Nicolson JT, Nettleton PF, Herring JA. The control of bovine virus diarrhoea virus in Shetland. Vet Microbiol. 1999;64(2–3):223–229. doi: 10.1016/S0378-1135(98)00271-5. [DOI] [PubMed] [Google Scholar]
- Valle PS, Martin SW, Tremblay R, Bateman K. Factors associated with being a bovine-virus diarrhoea (BVD) seropositive dairy herd in the More and Romsdal County of Norway. Prev Vet Med. 1999;40(3–4):165–177. doi: 10.1016/S0167-5877(99)00030-6. [DOI] [PubMed] [Google Scholar]
- van Oirschot JT, Kaashoek MJ, Rijsewijk FA. Advances in the development and evaluation of bovine herpesvirus 1 vaccines. Vet Microbiol. 1996;53(1–2):43–54. doi: 10.1016/S0378-1135(96)01233-3. [DOI] [PubMed] [Google Scholar]
- Viltrop A, Alaots J, Pärn M, Must K. Natural changes in the spread of bovine viral diarrhoea virus (BVDV) among Estonian cattle. J Vet Med B Infect Dis Vet Public Health. 2002;49(6):263–269. doi: 10.1046/j.1439-0450.2002.00560.x. [DOI] [PubMed] [Google Scholar]
- Virakul P, Suadsong S, Suwimonteerabutr J, Singlor J. Prevalence of infectious bobine rhinotracheitis (IBR), bovine viral diarrea (BVD), parainfluenza-3 (PI-3) and bovine respiratory syncytial (BRS) viruses in Thai dairy farms. Thai J Vet Med. 1997;27:295–313. [Google Scholar]
- Waage S, Krogsrud J, Nyberg O. The Norwegian programme for eradication of bovine viral diarrhoea/mucosal desease. 18th World Buitatrics Congress: 26th Congress of the Italian Association of Buiatrics, Bologna, Italy. pp. 773–775. Augusti 29- September 2, 1994.


