Abstract
Using the rat vibrissa system, we provide evidence for a novel mechanism for the generation of movement. Like other central pattern generators (CPGs) that underlie many movements, the rhythm generator for whisking can operate without cortical inputs or sensory feedback. However, unlike conventional mammalian CPGs, vibrissa motoneurons (vMNs) actively participate in the rhythmogenesis by converting tonic serotonergic inputs into the patterned motor output responsible for movement of the vibrissae. We find that, in vitro, a serotonin receptor agonist, α-Me-5HT, facilitates a persistent inward current (PIC) and evokes rhythmic firing in vMNs. Within each motoneuron, increasing the concentration of α-Me-5HT significantly increases the both the magnitude of the PIC and the motoneuron’s firing rate. Riluzole, which selectively suppresses the Na+ component of PICs at low concentrations, causes a reduction in both of these phenomena. The magnitude of this reduction is directly correlated with the concentration of riluzole. The joint effects of riluzole on PIC magnitude and firing rate in vMNs suggest that the two are causally related. In vivo, we find that the tonic activity of putative serotonergic pre-motoneurons is positively correlated with the frequency of whisking evoked by cortical stimulation. Taken together, these results support the hypothesized novel mammalian mechanism for movement generation in the vibrissa motor system, where vMNs actively participate in the rhythmogenesis in response to tonic drive from serotonergic pre-motoneurons.
Introduction
The ability to move allows an organism to adapt and respond to its environment. Indeed, a primary goal in processing sensory inputs is to select an appropriate motor act in response to those inputs. Often the patterned activation of muscles required to generate a movement is produced subcortically by neural elements that can operate without patterned inputs from higher brain centers or sensory feedback. In mammals, these critical components of motor networks, or central pattern generators (CPGs), are typically composed of groups of interneurons that generate rhythmic drive to motoneurons that innervate the target muscles (Arshavsky et al. 1997; Stein et al. 1997). The motoneurons themselves are not considered part of the rhythm generating network (Orlovksy et al. 1999). Here, using the rat vibrissa motor system, we provide evidence for an alternative mechanism for the generation of movement in mammals where the motoneurons are involved in the rhythmogenesis.
Rats explore their environment by rhythmically palpating objects with their mystacial vibrissae (Brecht et al. 1997; Vincent 1912; Welker 1964). These rhythmic movements, or whisking, persist after sensory denervation (Welker 1964), cortical ablation (Gao et al. 2003; Semba and Komisaruk 1984), or decerebration (Lovick 1972) suggesting that the whisking motor pattern is produced subcortically by a CPG. We demonstrated previously that serotonin (5-HT) drives vibrissa motoneurons (vMNs) at whisking frequencies, and that infusion of 5-HT receptor antagonists onto vMNs suppresses voluntary whisking (Hattox et al. 2003). Based on these results, we hypothesized that 5-HT is both necessary and sufficient for rhythmic whisking and that, in this system, the motoneurons are part of the rhythm-generating network. The experiments described here were designed to test this hypothesis.
In spinal (Perrier and Hounsgaard 2003) and trigeminal (Hsiao et al. 1998) motoneurons, 5-HT acts as a potent facilitator of inactivation-resistant inward currents. First discovered in cat spinal motoneurons (Schwindt and Crill 1980), these persistent inward currents (PICs) modulate the firing frequencies of motoneurons (Heckman et al. 2003; Schwindt and Crill 1982). Thus, by activating and amplifying PICs, 5-HT is reported to modulate firing in motoneurons (Harvey et al. 2006b; Heckman et al. 2005; Hounsgaard et al. 1988; Hounsgaard and Kiehn 1989; Hsiao et al. 1998). Here, we test the hypothesis that 5-HT is sufficient to generate rhythmic firing in vMNs through a graded facilitation of PICs. In addition, we test the prediction that the tonic output from serotonergic pre-motoneurons is positively correlated with whisking frequencies. Our findings support the hypothesis that vMNs generate the rhythmicity of the whisking motor pattern in response to a graded facilitation of a PIC by 5-HT. Furthermore, they suggest that, in this system, there exists a novel mammalian mechanism for movement generation.
Materials and Methods
In vitro slice preparation
We cut 500 μm thick coronal brainstem slices from P13 to P18 Sprague-Dawley rats (i.e., after the onset of voluntary whisking, Welker 1964). During the isolation of the brainstem and cutting of slices, the tissue was submersed in ice-cold modified artificial cerebrospinal fluid (ACSF) in which sucrose was substituted for NaCl. The sucrose-ACSF was composed of (in mM): 248 sucrose, 26 NaHCO3, 1.25 NaH2PO4, 3 KCl, 5 MgSO4, 1 CaCl2, and 15 glucose. We allowed the slices to recover for 1 hour in a holding chamber containing warmed normal-ACSF (32° C), aerated with 95% O2 and 5% CO2. The normal ACSF was composed of (in mM): 124 NaCl, 25 NaHCO3, 5 N,N-bis(2-hydroxyethyl)-2-aminoethanesul-fonic acid (BES), 3 KCl, 1.3 MgSO4, 2.0 CaCl2, and 15 glucose. For recording, we transferred individual slices to a recording chamber and continuously perfused the slice with normal ACSF (3-4 mL/min).
Whole-cell recordings
Visually guided whole-cell patch clamp recordings were made from motoneurons in the lateral subdivision of the facial nucleus with the use of near-infrared differential interference-contrast microscopy. This region contains motoneurons innervating the intrinsic muscles of the vibrissae (Hattox et al. 2003; Klein and Rhoades 1985; Semba and Egger 1986). Recordings were obtained with an EPC-10 amplifier (HEKA Instruments, Germany), digitized at 20 kHz with an A/D board (ITC-18; Instrutech Corp., Great Neck, NY) using PatchMaster software (HEKA), and stored on PC. The impedance of patch electrodes was 3-5 MΩ. The intracellular recording solution contained (in mM) 120 K-gluconate, 10 KCl, 10 HEPES, 1 MgCl2, 2.5 MgATP, 0.2 Tris-GTP, 0.1 BAPTA, and 5.2 biocytin (pH was adjusted to 7.3 with KOH).
The following pharmacological agents were obtained from Sigma-Aldrich (St. Louis, MO): D(—)-2-amino-5-phosphonopentanoic acid (AP5; applied at 50 μM to the perfusate), 6-cyano-7-nitroquinoxaline-2,3-dione disodium (CNQX; 20 μM), gabazine (10 μM), tetrodotoxin (TTX; 1 μM), α-Me-5HT (1 to 60 μM), riluzole (2 to 5 μM).
Cells were filled with biocytin (Molecular Probes, Eugene, OR) through the recording pipette and slices were fixed overnight in a buffered solution containing 4% paraformaldehyde. To visualize labeled cells, slices were reacted with the Vectastatin ABC kit (1:1000; Vector Labs, Burlingame, CA) and 3-3′ diaminobenzidine (DAB; 0.5 mg/ml), urea H2O2 (0.3 mg/ml), and CoCl2 (0.2 mg/ml) in 0.05 M Tris buffer containing 0.5 M NaCl.
Analysis of PICs
The magnitude and activation voltage of persistent inward currents (PICs) in vMNs were determined using a slow triangular voltage ramp command. The ascending phase of the voltage command consisted of a linearly increasing ramp (speed 8 to 16 mV/s) for 5 seconds. The descending phase returned the membrane potential to -90 mV at the same rate. For each cell, we selected the fastest single ramp speed that minimized the occurrence of breakthrough action potentials in all conditions (control and drug).
All analyses were performed on leak-subtracted currents, generated by subtracting the predicted ohmic leak currents from the measured currents. The leak currents were determined by multiplying the sub-threshold membrane conductance (i.e., the membrane conductance prior to activation of voltage gated channels) by the voltage command. We monitored each cell’s series resistance throughout the experiments and rejected from analysis cells in which the series resistance changed by >10%.
Surgical procedures
We used 9 female Sprague-Dawely rats (225-270 g) for in vivo experiments. All procedures strictly adhered to Institutional and Federal guidelines. Under halothane (2.5%) anesthesia, a small incision was made in the facial skin, and a pair of bipolar EMG electrodes (76 μm Teflon-coated stainless steel wire) was tunneled sub -cutaneously into the deep intrinsic muscles. Prior to insertion, the electrode tips were bent into a hook to prevent shifts in location during movements. Following infusion of local anesthetics to surgical sites, we performed craniotomies over the vibrissa motor cortex (vMCx) and the lateral paragigantocellularis (LPGi) nucleus. The exposed dura was kept from drying by using dental wax to build a saline filled well over the craniotomies. During recordings, rats were maintained at anesthetic level III-2, characterized by an ECoG frequency between 5 to 7 Hz, a respiratory rate of ∼100 breaths/min, and an absence of a withdrawal reflex to noxious stimuli (paw or tail pinch, Friedberg et al. 1999). As the threshold for evoking whisking depended on the depth of anesthesia, care was taken to maintain a consistent and stable level of anesthesia. Body temperature was maintained at 37°C with a servo-controlled heating blanket.
Cortical stimulation
We evoked rhythmic whisking through intracortical microstimulation (ICMS) of the rhythmic subregion (RS) of vMCx as in our previous study (Cramer and Keller 2006). Custom-made platinum-in-quartz electrodes (∼20 μm tip) were lowered to a depth of 1.5 mm, corresponding to layer V of vMCx. The rhythmic subregion (RS) of vMCx was identified by applying low intensity current pulses (50 monophasic pulses@50 Hz, 200 μs pulse width, 25 to 250 μA) to the area of vMCx identified by Haiss and Schwarz (2005). Stimulation in this region evoked rhythmic vibrissae movements, while stimulation outside of this region either generated small vibrissal retractions or failed to produce movements. RS was reliably located at anterioposterior 1.3, mediolateral 1.3 relative to bregma, as previously documented (Cramer and Keller 2006; Haiss and Schwarz 2005).
Recording and analyses
We obtained extracellular recordings using a NeuroData IR183A amplifier (Cygnus Technology, Inc, Delaware Water Gap, PA), digitized at 40 kHz with an A/D board (ITC-18; Instrutech Corp., Great Neck, NY) using software custom written in IGOR (Wave Metrics Inc., Lake Oswego, OR), and stored on PC. Recordings from putative serotonergic pre-motoneurons were obtained from pulled borosilicate glass electrodes (∼ 2 μM tip, 2 to 20 MΩ) stereotaxically guided to the rostral (juxtafacial) lateral paragigantocellularis nucleus (LPGi, anteroposterior 11.4 mm, lateromedial 1.0 mm, relative to bregma).
To analyze the responses of putative pre-motoneurons to ICMS of vMCx, we isolated the action potential waveforms of each unit using Offline Sorter (Plexon Inc., Dallas, TX). The timestamps of individual units along with the corresponding stimulus triggers were imported into IGOR for further analyses.
We defined a pre-motoneuron response as significant if the number of action potentials fired during stimulation exceeded the baseline activity by two standard deviations. The baseline activity for each cell was defined as the number of spikes fired during the one-second interval prior to stimulation, averaged over all trials. To measure response latency, we constructed peristimulus time histograms (PSTHs, bin size = 1 ms) from the pre-motoneuron timestamps and stimulus triggers. The onset latency was defined as the first occurrence of two consecutive bins that exceeded the baseline activity by two standard deviations.
Bouts of rhythmic whisking evoked by ICMS of vMCx were recorded using the implanted vibrissal EMG electrodes. EMG recordings were amplified (Model 1700 Differential AC Amplifier, A-M Systems, Inc., Carlsborg WA) sampled at 40 kHz with an A/D board (ITC-18, Instrutech), filtered (1 Hz to 5 kHz) and stored on a computer running software written in Igor. We defined the whisking frequency as the peak of the power spectral density generated from the rectified, filtered EMG data, sub-sampled to 500 Hz.
Results
5-HT drive determines vMN firing rates
Using a brainstem slice preparation, we first tested the prediction that individual vibrissa motoneurons (vMNs) generate a range of firing frequencies in response to varying levels of serotonergic drive. The in vitro preparation is advantageous because it permits application of pharmacological agents with a greater accuracy than can be achieved in vivo. To simulate endogenous serotonergic drive to vMNs, we bath applied the serotonin type 2 (5-HT2) receptor agonist, α-Me-5HT to the brainstem slices. We focused on this receptor subtype because, when activated, it produces rhythmic firing in vMNs, and its antagonist (metergoline) suppresses rhythmic whisking (Hattox et al. 2003). We examined whole-cell recordings from 50 vMNs in this experiment, all of which lacked spontaneous activity in control conditions. In support of our prediction, vMNs fired rhythmic trains of action potentials at whisking frequencies (1.5 to 17 Hz; Figs. 1 and 4) in response to α-Me-5HT. In each cell tested, these effects of α-Me-5HT persisted in the presence of glutamatergic and GABAergic receptor antagonists (n = 31; see Materials and Methods), suggesting that the firing in vMNs did not result from glutamatergic or GABAergic pre-motoneurons.
Figure 1.
5-HT2 receptor agonist evokes dose-dependent firing in vMNs
A. Current clamp recordings from a representative vMN in the presence of two concentrations of the agonist, α-Me-5HT. This neuron was silent in control conditions and fired at a significantly higher rate (p < 10-3) in the presence of 5μM agonist (5.5 ± 0.2 Hz) than in the presence of 1μM agonist (3.3 ± 0.2 Hz).
B. Time course of the agonist-induced firing for the vMN shown in A. The instantaneous firing frequency of the vMN in each agonist concentration is plotted as a function of time. The horizontal green bar indicates the duration of agonist application. We determined steady-state firing rates from the final third portion of the drug delivery period (red portions of each trace).
C. Histograms (1 ms bin size) of steady state firing frequencies for the motoneuron shown in A. The peak in each histogram corresponds to the mode of the steady state firing frequency. The half width at half max of each histogram corresponds to the variance.
D. Summary data from 15 vMNs with a significant dose-dependent response to the agonist. Each pair of joined data points indicates the mode of the steady state firing rate for a single vMN at the individually determined “high” (2 to 60 μM) and “low” (1 to 40 μM) agonist concentrations. The response of the motoneuron shown in A is highlighted in red. In each vMN, increasing the agonist concentration caused a significant increase in the steady-state firing frequency.
Figure 4.
Riluzole causes a concentration-dependent reduction in agonist induced firing rates of vMNs
A. Representative example of the effects of riluzole on agonist induced firing in a vMN. In control conditions, the vMN did not fire spontaneously. Application of 20 μM α-Me-5HT evoked rhythmic firing that was gradually suppressed by the addition of 5 μM riluzole. After washout, re-application of 20 μM α-Me-5HT again evoked rhythmic firing in the vMN. The times listed under each trace indicate the time elapsed after recording the control trace.
B. Group data for 17 vMNs demonstrating the concentration-dependent effects of riluzole on the agonist induced firing in vMNs. Increasing the concentration of riluzole resulted in a concentration-dependent reduction in the vMN firing frequencies.
To test the effects of different levels of serotonergic drive on vMN firing rates, in 16 vMNs we applied the agonist at two concentrations. The firing frequency evoked in individual neurons by any one concentration of α-Me-5HT varied from cell to cell (Fig. 1), possibly due to variable truncation of the dendritic tree during slice preparation, or to differences in effective drug concentrations near the recorded neurons. Thus, for each cell, we applied a “low” concentration that ranged between 1 to 40 μM and a “high” concentration that ranged between 2 to 60 μM. We randomized the order of application of these drug concentrations.
The response of a vMN to the agonist is shown in the representative current clamp recordings in Figure 1A. This vMN, which was silent in the absence of the agonist, fired at a significantly (p < 10-3) higher frequency in response to the high agonist concentration (5 μM, 5.5 ± 0.2 Hz) compared to the lower agonist concentration (1 μM, 3.3 ± 0.2 Hz). The time course of the neuron’s responses to each agonist concentration is plotted in Figure 1B. The delay between the beginning of drug application and onset of firing may primarily reflect the time required for the drug to diffuse through the tissue to reach the cell. We computed firing rates from the last third of the drug application period (highlighted in red in Fig. 1B) by constructing histograms (bin size = 0.25 Hz) of instantaneous firing frequencies from this period, which we defined as the steady state (Fig. 1C). We defined the magnitude of responses at steady state as the peak of these histograms and identified the whisking frequency at which this peak occurred. We defined the half width at half maximum of each histogram as the standard deviation of the steady state firing rate. Similar results were found for 15 of 16 vMNs tested, as shown in Fig. 1D. For these cells, the higher agonist concentration evoked a significantly higher firing rate (Student’s t-test, p’s < 10-3). In one cell only, the firing rates at the two concentrations were not significantly different (p = 1). These findings support the hypothesis that vMNs generate the whisking frequency in response to a varying degree of drive from serotonergic pre-motoneurons.
vMNs express PICs
The firing rate of neurons, including motoneurons, is closely related to the magnitude of inward current reaching the soma (Powers and Binder 2001). Neuromodulators that regulate membrane currents can alter this current-frequency relationship and are thus capable of regulating the cell’s firing frequency (Powers and Binder 2001; Rekling et al. 2000). One important neuromodulator is 5-HT, which, acting through 5-HT2 receptors, potently facilitates slowly or non-inactivating voltage-dependent inward currents (Harvey et al. 2006a; Heckman et al. 2005; Perrier and Hounsgaard 2003). These persistent inward currents (PICs) have a significant impact on the firing rates of some motoneurons (Prather et al. 2001). We therefore hypothesized that, in vMNs, 5-HT produces firing by activating PICs. Because the firing rate of vMNs is determined by the magnitude of 5-HT2 receptor activation (see above), we further predicted that the regulation of vMN firing is achieved through a graded facilitation of these PICs.
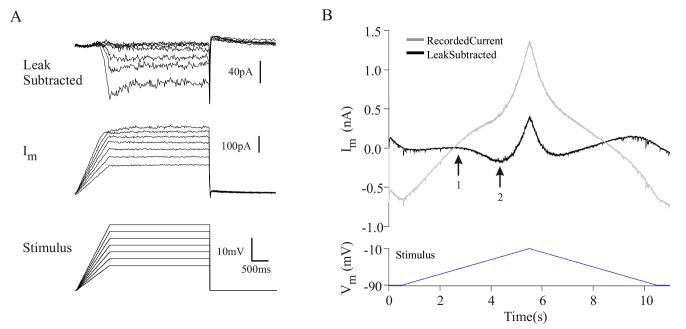
The presence of a PIC in a vMN is demonstrated by the data depicted in Figure 2A. To better isolate the persistent currents, we used protocols with slow voltage ramps that minimize the activation of potentially confounding transient Na+ currents (Lee and Heckman 1998). Starting from a holding potential of -60 mV, we ramped the membrane potential to -49 mV and held the cell at this potential for three seconds. We examined the voltage dependence of the currents activated by this protocol by increasing the holding potential in 3 mV increments. At more depolarized membrane potentials, the increase in net outward current was reduced (Fig. 2A, middle). Examination of the leak subtracted currents (top panel) reveals that this reduction resulted from a slowly or non-inactivating inward current that lasted for the duration of the voltage command (3 sec). Currents with these kinetics and voltage-dependence are defined as PICs (Schwindt and Crill 1982).
Figure 2.
vMNs express PICs
A. A ramp and hold protocol reveals the existence of a PIC in a representative vMN in control conditions. Note the nearly uniform reduction in the recorded membrane current (Im) at more depolarized holding potentials. Leak subtracted currents reveal that the observed reduction in Im resulted from a voltage-dependent PIC.
B. Protocol for measuring the PIC magnitude and activation voltage. The membrane currents (grey) generated in response to a triangular voltage command (16 mV/s) are dominated by an outward current. The downward inflections in the recorded membrane current traces during both the ascending and descending phases result from a PIC, seen more clearly in the leak-subtracted current trace (black). The PIC activation voltage was defined as voltage where the slope of the leak subtracted current trace first becomes negative (1). The peak PIC magnitude was measured at the peak of the inward inflection during the ascending phase of the voltage command (2).
To quantify these PICs, we used a slowly increasing (8 to 16 mV/s) triangular voltage command. Like the ramp-and-hold protocol described above, this slow-ramp protocol suppressed activation of fast Na+ spikes. In addition, the slow-ramp is advantageous in that, using a single voltage command, it provides information simultaneously about both the PIC activation voltage and magnitude. A representative recording using this protocol is shown in Figure 2B. The bottom panel depicts the triangular voltage command, while the top panel shows the recorded membrane current (gray) and leak-subtracted (see Materials and Methods) current (black). During the ascending phase of the voltage ramp, the currents were dominated by an outward leak current; however, at -58 mV, an inward current was activated (arrow “1”). As the voltage ramp continued to ascend, outward currents dominated. During the descending phase of the voltage ramp, a second inflection was visible, suggesting that the current had remained active.
We defined the PIC activation voltage as the membrane potential where the slope of leak-subtracted current first becomes negative (Fig. 2B, arrow “1”). We defined the PIC magnitude as the peak of the leak-subtracted inward current during the ascending phase of the voltage command (Fig. 2B, arrow “2”). Cells without a net inward leak-subtracted current were considered to not have a PIC. We observed a similar PIC in 26 of 34 vMNs tested, indicating that most vMNs express PICs, even in the absence of agonists. The magnitude of the PIC averaged -120 ± 60 pA (median, -150 pA) and had an activation voltage of -53 ± 6 mV. These PICs persisted in the presence of antagonists of fast glutamatergic and GABAergic receptors (n = 20, see Materials and Methods), indicating that the PICs are an intrinsic property of vMNs.
PICs are facilitated by 5-HT2 receptors
To examine the effects of α-Me-5HT on the PICs, we focused on currents evoked during the ascending phase of the voltage ramps, and compared, in each cell, the leak-subtracted activation voltage and current magnitude before and after drug application (1 to 30 μM). For the 26 vMNs that expressed a PIC in control conditions, the average PIC magnitude in the presence of α-Me-5HT increased from -120 ± 60 pA to -500 ± 200 pA (median, -500 pA). α-Me-5HT also significantly shifted the PIC activation voltage to a more hyperpolarized value of -57 ± 5 mV (p = 0.029, Kolmogorov-Smirnov Test). In the eight vMNs that did not express a PIC in control conditions, the agonist evoked a PIC of -360 ± 140 pA with an average activation threshold of -56 ± 5 mV.
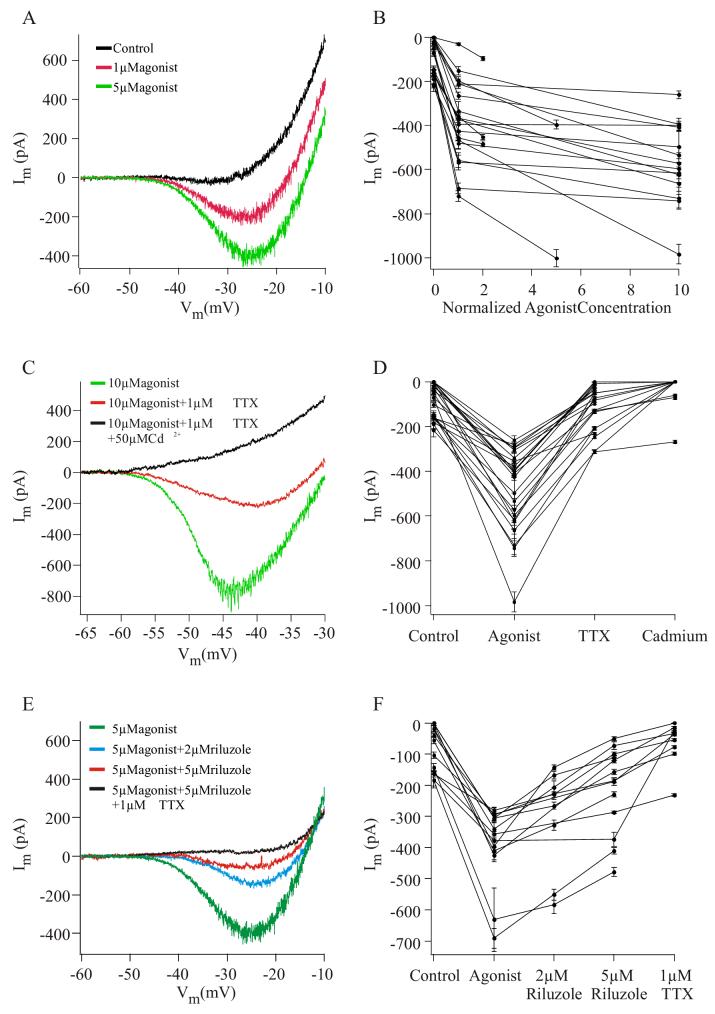
To examine the effects of increases in serotonergic drive on the facilitation of PIC magnitude, we applied multiple agonist concentrations (1 to 30 μM) to 19 of these cells. A representative example is shown in Figure 3A. This vMN had a PIC in control conditions (black trace) with a peak magnitude of -19 ± 10 pA. Application of 1 μM agonist (red trace) enhanced the PIC magnitude to -198 ± 20 pA. Increasing the agonist concentration to 5 μM (green trace) further enhanced the PIC magnitude to -396 ± 21 pA. The impact of the agonist on the PIC magnitude for all 19 motoneurons is shown in Figure 3B, where the peak PIC magnitude is plotted as a function of agonist concentration normalized to the lowest concentration applied to each cell. In every neuron, the increase in PIC magnitude from control to low concentration was significant (p < 10-3). Increasing the concentration of the agonist resulted in an additional, significant increase in PIC magnitude in 18 of 19 cells (p < 10-3). These results indicate that, through the graded facilitation of PICs, 5-HT can modulate the magnitude of inward currents in vMNs, thereby generating the full range of whisking frequencies.
Figure 3.
5-HT2 receptor agonist facilitates a Na+ based PIC in a concentration-dependent manner.
A. Concentration-dependent facilitation of PIC magnitudes in a representative vMN. The magnitude of the PIC in control conditions (black trace, -19 ± 10 pA) was enhanced by addition of 1μM α-Me-5HT (red trace, -198 ± 20 pA). Application of 5μM agonist caused an additional increase in PIC magnitude (green trace, -396 ± 21 pA).
B. PIC magnitude as a function of normalized agonist concentration for 19 vMNs. Agonist concentrations were normalized to the lowest concentration applied to each cell. The increase in PIC magnitude from control conditions (concentration of zero) to the lowest agonist concentration was significant in each cell. Increasing the agonist concentration caused an additional significant increase in 18 vMNs. Here and in similar figures, data are presented as mean ± SD.
C. PICs in vMN have a prominent Na+ component. Application of 1 μM TTX (red trace) caused a significant reduction in the agonist facilitated PIC (green trace) in a representative vMN. Subsequent addition of Cd2+, a calcium channel blocker, completely eliminated the remaining PIC (black trace).
D. Effect of TTX and Cd2+ on facilitated PIC magnitudes, recorded from 23 vMNs. Application of 1μM TTX significantly reduced the PIC magnitude in each motoneuron. The subsequent application of 50 to 200μM Cd2+ to 13 vMNs completely suppressed the remaining PIC in 10 neurons, and significantly reduced it in the remaining 3.
E. Response of a representative vMN to riluzole. Riluzole (2 μM, blue trace; 5 μM red trace) caused a concentration-dependent reduction in the magnitude of the agonist facilitated PIC (green trace). Application of TTX completely abolished the PIC in this vMN (black trace).
F. Group data for 13 vMNs demonstrating the concentration-dependent effects of riluzole on the magnitude of the agonist facilitated PIC. In the nine vMNs tested at both concentrations, riluzole significantly reduced the PIC magnitude in a concentration-dependent manner. A similar reduction was observed in three of four vMNs tested with only 5 μM riluzole. In the nine vMNs in which it was applied, TTX caused an additional significant reduction in PIC magnitude.
PICs are largely composed of sodium currents
PICs in spinal (Harvey et al. 2006a), hypoglossal (Powers and Binder 2003), and trigeminal (Hsiao et al. 1998) motoneurons consist of Na+ and Ca2+ currents. To determine if Na+ currents contribute to PICs in vMNs, we suppressed Na+ conductances with TTX (1 μM). A representative example is depicted in Figure 3C. In response to application of 10 μM α-Me-5HT (green trace), this motoneuron had a PIC magnitude of -741 ± 31 pA. Co-application of 1 μM TTX (red trace) reduced the PIC magnitude to -210 ± 6 pA. Group data for 23 motoneurons is shown in Figure 3D. Following application of TTX, the agonist-facilitated PIC was reduced in magnitude by 79 ± 16% (median=81%). In each case, the reduction was significant (p < 10-3, Student’s t-test).
In 13 of these motoneurons, we subsequently applied 50 to 200 μM cadmium (Cd2+) to determine whether the TTX-insensitive component resulted from Ca2+ currents. For the motoneuron shown in Figure 3C, application of 50 μM Cd2+ (black trace) completely suppressed the TTX-insensitive component of the PIC. We recorded a similar complete suppression of PICs in 10 of 13 vMNs (Fig. 3D). In the remaining three neurons Cd2+ application significantly (p < 10-3), but not completely (38 ± 21%), further suppressed PIC amplitudes. Thus, in vMNs, PICs are predominantly composed of sodium currents, suggesting that a persistent Na+ current (INaP) plays a central role in generating firing in these cells.
Riluzole modulates PIC magnitudes in vMNs
Because INaP accounted for most of the PIC in the vMNs, we focused on these currents in the remaining experiments. At low concentrations (2 to 5 μM), riluzole selectively antagonizes this current without significantly affecting fast Na+ currents that underlie action potentials (Wu et al. 2005). We took advantage of this specificity to test the hypothesis that INaP underlies the ability of vMNs to generate a range of whisking frequencies. Injection of depolarizing current pulses in current clamp recordings confirmed that 5 μM riluzole had no effect on the generation of fast spikes.
The effects of riluzole are illustrated for a representative motoneuron in Figure 3E. Riluzole (2 μM) significantly (Student’s t-test, p < 10-3) decreased the magnitude of the agonist-facilitated PIC from -396 ± 21 pA to -143 ± 10 pA. Increasing the concentration of riluzole to 5 μM further suppressed the PIC to -50 ± 7 pA. The subsequent addition of 1 μM TTX completely abolished the PIC in this motoneuron. Group data for nine neurons tested at both riluzole concentrations, and four vMNs tested only at the 5 μM concentration, are shown in Figure 3F. In all 13 vMNs, riluzole significantly suppressed PIC magnitudes. The amount of suppression with 5 μM riluzole was significantly (p ≤10-3, n = 9) greater than the suppression in the presence of 2 μM riluzole. Subsequent addition of TTX suppressed the agonist facilitated PIC by a total of 80 ± 20% (n = 9), eliminating the majority of the PIC. We obtained similar results in the presence of ionotropic glutamatergic and GABAergic antagonists (see Materials and Methods), suggesting that riluzole acted directly on the recorded vMNs. These findings strengthen the premise that INaP is a major component of PICs in vMNs, and that its activity may underlie the generation of the whisking rhythms by vMNs.
Riluzole modulates firing in vMNs
The postulated role of INaP on regulating the firing rate of vMNs is demonstrated in the current clamp recordings shown in Figure 4A. In control conditions (top trace) the motoneuron was silent; however, application of 20 μM α-Me-5HT caused the vMN to fire regularly at approximately 2 Hz. Following the addition of 5 μM riluzole, the firing frequency began to decline and was eventually completely suppressed. Riluzole’s effects were reversible: After washout with artificial cerebrospinal fluid (ACSF) for 22 minutes, re-application of 20 μM α-Me-5HT evoked firing in the vMN near 2 Hz. Group data for eight vMNs tested at both 2 and 5 μM riluzole and nine vMNs tested only at 5 μM are shown in Figure 4B. In the eight cells tested at both concentrations, 2 μM riluzole completely suppressed firing in three vMNs, and significantly reduced the firing frequency by 28 ± 17% (p < 10-3) in the remaining five neurons. Subsequent application of 5 μM riluzole to these five vMNs caused a complete suppression of firing in four cells and an additional significant 59% reduction in the remaining vMN (p < 10-3). In the nine vMNs tested only at 5 μM riluzole, firing was completely suppressed in six cells and significantly reduced by 35 ± 21% in the remaining three (p < 10-3). Washout was successful in seven out of eight cells tested.
Injections of current pulses (200 ms) during the peak effects of riluzole confirmed that the neurons (n = 15) were still capable of generating trains of fast action potentials. This ensured that the fast sodium channels underlying the action potentials had not been affected by the riluzole. Together, the effects of riluzole on the magnitude of INaP and its impact on the agonist-induced firing in vMNs support the hypothesis that the facilitation of a PIC by 5-HT is necessary and sufficient for vMNs to generate whisking over a range of frequencies.
Activity in putative serotonergic pre-motoneurons is correlated with whisking frequency
The results of our in vitro experiments support the hypothesis that vMNs generate rhythmic whisking in response to a graded facilitation of a PIC by 5-HT. In the intact animal, serotonergic drive to vMNs originates from a subset of serotonergic neurons in the brainstem that both project to vMNs in the lateral facial nucleus and receive projections from the vibrissa motor cortex (vMCx, Hattox et al. 2003; Hattox et al. 2002). Thus, these serotonergic pre-motoneurons likely serve as the endogenous source of the 5-HT employed by vMNs to generate whisking rhythms. As the frequency of the motor pattern generated by vMNs varies with the level of serotonergic drive they receive, we predict that the output from these serotonergic pre-motoneurons should correlate positively with whisking frequencies.
To test this prediction, we evoked rhythmic whisking by applying intracortical microstimulation (ICMS) to vMCx, as previously described (Cramer and Keller 2006; Haiss and Schwarz 2005), while recording extracellularly from putative serotonergic pre-motoneurons. Our attempts to unambiguously identify the pre-motoneurons by evoking antidromic action potentials following stimulation of the facial nucleus were unsuccessful. This is most likely due to the difficulty in activating small diameter, non-myelinated fibers characteristic of serotonergic neurons (Azmitia and Gannon 1983) and the close proximity of the recording and stimulation sites. As the electrophysiological properties (e.g. spontaneous activity and spike shape indices) of serotonergic neurons show considerable variation (Zhang et al. 2006), we concluded that these criteria cannot be used reliably to identify serotonergic neurons. We therefore accepted for analysis, and defined as putative serotonergic pre-motoneurons, all cells recorded in the rostral lateral (juxtafacial) pargigantocellularis nucleus (LPGi) according to stereotaxic coordinates. This nucleus is composed predominantly of serotonergic neurons (Bowker and Abbott 1990; Darnall et al. 2005) that both receive direct inputs from vMCx and project to vMNs in the lateral facial nucleus (Hattox et al. 2003; Hattox et al. 2002).
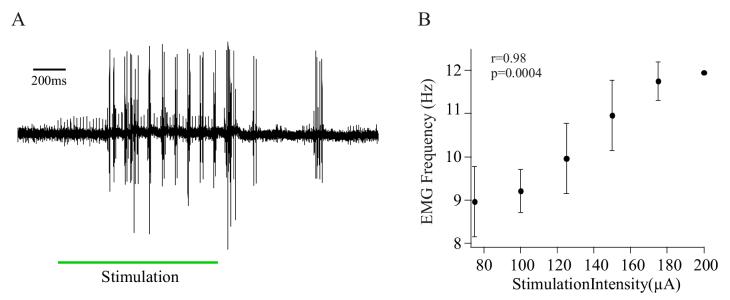
An example of ICMS-evoked whisking, monitored by recording EMG activity from the whisker pad, is shown in Figure 5A. As we reported previously, whisking frequency was positively correlated with ICMS intensity (r = 0.98, p = 0.0004; Fig. 5B), enabling us to control whisking frequency in the anesthetized animal. This allowed us to investigate the relationship between whisking frequency and activity in serotonergic pre-motoneurons.
Figure 5.
The intensity of intracortical microstimulation (ICMS) determines whisking frequency.
A. Example of EMG activity recorded during ICMS-evoked whisking. Following a significant delay, stimulation of vMCx evoked rhythmic whisking, recorded as bursts of activity in the EMG. The small, regularly spaced spikes that occur throughout the stimulation are the stimulus artifacts. Note that the EMG activity outlasts the stimulus (green bar).
B. The frequency of ICMS-evoked EMG activity is positively correlated with the intensity of cortical stimulation.
Figure 6A shows the response of putative serotonergic pre-motoneuron to ICMS (50 Hz, 125 μA, 1 s duration) of vMCx. This neuron responded to vMCx stimulation with a latency of 6 ± 2 ms (Fig. 6B). Increasing ICMS intensities resulted in an increase in the number of spikes evoked by this neuron (Fig. 6C), without affecting response latency. Group data for 16 putative serotonergic pre-motoneurons is shown in Figure 6D, where the pre-motoneuron spike count and stimulus intensity have been normalized to their threshold values. The activity in these pre-motoneurons was positively correlated with the ICMS intensity (r = 0.92, p = 10-8).
Figure 6.
The activity of putative serotonergic pre-motoneurons is positively correlated with ICMS intensity
A. Action potentials (*) evoked in a putative serotonergic pre-motoneuron by ICMS of vMCx. The pre-motoneuron reliably followed cortical stimulation (indicated by arrows). Stimulus artifacts have been truncated for clarity.
B. PSTH (1 ms bins) constructed from the response of the pre-motoneuron shown in A to repeated ICMS of vMCx. The red line depicts the 95.4% confidence interval. This neuron responded with a latency of 6 ms.
C. Like the frequency of ICMS-evoked whisking, the number of spikes evoked in the pre-motoneuron was positively correlated with the stimulation intensity.
D. Plot of the number of evoked spikes versus stimulus intensity for 16 putative serotonergic pre-motoneurons. These two parameters, normalized to their threshold values, are positively correlated.
The findings that ICMS intensity is positively correlated with whisking frequency (Figure 5, Cramer and Keller 2006), and that ICMS intensity is correlated also with spike counts of putative serotonergic pre-motoneurons, support the conjecture that spiking output of these pre-motoneurons is causally related to whisking frequencies. We were able to test this directly for four pre-motoneurons. This small sample size reflects the difficulty in obtaining stable recordings under the relatively light anesthesia necessary for ICMS-evoked whisking (Cramer and Keller 2006; Haiss and Schwarz 2005). In these neurons, we recorded vibrissal EMG simultaneously with the neuronal recordings. The relationship between ICMS evoked-activity in a representative putative serotonergic pre-motoneuron and the simultaneously recorded EMG is shown in Figure 7A. Increasing ICMS intensity increased both the number of spikes generated by the pre-motoneuron and the whisking frequency, revealing a positive correlation between these latter parameters (r = 0.94, p = 0.006). Group data for all pre-motoneurons/EMG pairs are shown in Figure 7B where whisking frequency and pre-motoneuron activity were normalized to their threshold values. Whisking frequency was positively correlated with the level of activity in the putative pre-motoneuron (r = 0.89, p = 10-7). These findings support the hypothesis that LPGi pre-motoneurons provide the tonic serotonergic drive employed by vMNs to generate the whisking rhythm.
Figure 7.

The activity of putative serotonergic pre-motoneurons is positively correlated with whisking frequency.
A. Plot of the ICMS-evoked whisking frequency versus the number of spikes evoked in a pre-motoneuron. The color of each data point corresponds to the stimulation intensity. Increasing ICMS intensity resulted in an increased output from the pre-motoneuron and a corresponding increase in EMG frequency. Data are shown as mean ± SD.
B. Group data for four pre-motoneuron/EMG pairs. The color of each data point represents the ICMS intensity. All parameters have been normalized to their respective values at the threshold intensity. The strong correlation between the ICMS-evoked EMG frequency and output from the putative serotonergic pre-motoneurons suggests the two phenomena are causally related.
Discussion
Using the rodent vibrissa motor system, we provided evidence for a novel mammalian mechanism of rhythm generation that underlies movements. Like CPGs, this system does not rely on cortical inputs (Gao et al. 2003; Lovick 1972; Semba and Komisaruk 1984) or sensory feedback (Welker 1964) to generate rhythmic output. However, whereas mammalian CPGs are typically composed of networked interneurons that generate the motor pattern and subsequently drive motoneurons, in the vibrissa system, our results suggest that rhythmic movements are generated by the motoneurons themselves in response to tonic input from serotonergic pre-motoneurons. The involvement of vMNs in the rhythmogenesis, the necessity of 5-HT for generating movements and sufficiency of 5-HT to generate the motor pattern are unique to this mammalian model of motor control.
In support of the postulate that 5-HT is necessary for rhythmic whisking are our findings that 5-HT receptor antagonists suppress both voluntary whisking (Hattox et al. 2003) as well as ICMS-evoked whisking (Cramer and Keller 2006). The finding that the activity of putative 5-HT pre-motoneurons co-varies with whisking frequency (Fig. 7) is also in agreement with this postulate. Available evidence suggests that 5-HT interactions with vMNs are also sufficient for generating rhythmic whisking: 5-HT (or its receptor agonists) applied at different concentrations evoke a range of rhythmic firing rates in vMNs (Hattox et al. 2003, Figure 1), an effect that is suppressed by 5-HT receptor antagonists (Hattox et al. 2003). These pharmacological manipulations are resistant to suppression of glutamatergic and GABAergic receptors, suggesting that these serotonergic effects are specific to vMNs. This effect appears unique to vMNs as an earlier investigation into the effects of 5-HT on facial motoneurons, that did not restrict sampling to any particular sub-nucleus, did not observe 5-HT evoked rhythmic firing (McCall and Aghajanian 1979). Similarly, 5-HT does not evoke rhythmic firing in guinea pig trigeminal motoneurons (Hsiao et al. 1997). Thus, whereas in other motor systems the actions of 5-HT on motoneurons is described as modulatory, capable of shaping an on-going rhythm but not responsible for the rhythm generation itself (Heckman et al. 2004), in the vibrissa motor system 5-HT is both necessary and sufficient for generating the whisking motor pattern and thus capable of generating rhythmic whisking.
Our results indicate that 5-HT acts through a graded facilitation of a persistent inward current (PIC) within vMNs. PICs are voltage dependent membrane currents that resist inactivation (Heckman et al. 2005; Schwindt and Crill 1980). In many motoneurons, including vMNs (Figs. 2 and 3) these currents are facilitated by 5-HT, frequently through the activation of 5-HT2 receptors (Harvey et al. 2006a; Heckman et al. 2005; Perrier and Hounsgaard 2003). Although these currents are considered to modulate ongoing firing in other motoneurons, in vMNs their presence is associated with the generation of rhythmic firing. We found that concentrations of a 5-HT2 receptor agonist that produced a graded facilitation in PIC magnitude also generated a progressive increase in firing rates in vMNs, suggesting a causal relationship between the two phenomena. The action of riluzole on the agonist-induced firing rates supports this causal relationship. Riluzole, which selectively antagonizes persistent sodium currents at low concentrations (2 to 5 μM, Wu et al. 2005) caused a similarly graded suppression in both PIC magnitude and firing rate of vMNs. Together, these data support the hypothesis that vMNs generate whisking rhythms in response to serotonergic drive.
ICMS-evoked whisking displayed a relatively long onset latency (Fig. 7). This delay may reflect the kinetics of the transduction cascades initiated following activation of metabotropic 5-HT2 receptors on vMNs. In addition, 5-HT axon terminals in the facial nucleus, as in other brain regions, are thought not to form classical chemical synapses. Rather, activation of 5-HT receptors is thought to occur through the slow diffusion of 5-HT and the activation of extrasynaptic receptors (De-Miguel and Trueta 2005). We have recently reported that increasing ICMS intensity significantly decreases whisking onset latency, most likely by increasing the output from 5-HT pre-motoneurons (Cramer and Keller 2006), consistent with the postulate that extrasynaptic transmission contributes to the delayed whisking. It is pertinent that the long latencies between cortical activation and EMG onset are consistent with our previous studies in behaving rats (Friedman et al. 2006).
The mechanisms responsible for terminating whisking remain to be determined. Inhibitory inputs are effective in rapidly terminating PICs (Heckman et al. 2005), suggesting that inhibitory inputs to vMNs may be involved. Indeed, we have previously found that both the onset and offset of a whisking epoch is preceded by a brief increase in activity in vMCx (Friedman et al. 2006), suggesting vMCx sends commands to both start and stop a whisking epoch.
Exploratory whisking occurs at frequencies between 5 to 15 Hz (Berg and Kleinfeld 2003), a range that is encompassed by the agonist induced firing rates (Figs. 1 and 4). The correspondence between vMN firing rates in vitro and whisking frequencies in vivo suggests that, during voluntary whisking, vMNs fire a single action potential per whisk. Previously, we demonstrated that some vMNs do indeed fire in a one-to-one manner during ICMS-evoked whisking (Cramer and Keller 2006). Some vMNs, however, fire bursts of action potentials per whisk. The failure to evoke a similar bursting firing pattern in vMNs in vitro may result from the reduced nature of this preparation. In particular, the partial truncation of the dendritic tree, where the channels that carry PICs are thought to reside (Heckman et al. 2003), might impact the ability of the vMNs to burst in vitro. In addition, non-serotonergic inputs present in the intact animal but missing in vitro may be essential for bursting in vMNs.
vMNs in the lateral facial nucleus are densely innervated by serotonergic neurons, many of which receive direct projections from the vibrissa motor cortex (vMCx, Hattox et al. 2003; Hattox et al. 2002). The highest density of serotonergic inputs to vMNs appears to originate from the rostral (juxtafacial) lateral paragigantocellularis nucleus (LPGi, Hattox et al. 2002), and stimulation of these neurons produces vibrissa movements (Hattox et al. 2003). These observations suggest that serotonergic LPGi neurons are strategically placed to act as the source of 5-HT used by vMNs to generate whisking. Indeed, our results indicate that the activity of neurons within this nucleus is positively correlated with whisking frequency. However, because LPGi contains a subset of non-5-HT neurons (Bellintani-Guardia et al. 1996), we cannot conclusively determine the identity of the neurons we recorded from and therefore referred to them as putative serotonergic pre-motoneurons.
The circuits described above suggest that the endogenous source of 5-HT used by vMNs can be activated and regulated by vMCx. In support of this we found that during rhythmic whisking evoked by ICMS of vMCx, the activity of putative serotonergic pre-motoneurons was positively correlated with the whisking frequency (Fig. 7). We have shown previously that this stimulation-evoked whisking is suppressed by 5-HT receptor antagonists (Cramer and Keller 2006), further supporting the hypothesis that voluntary control of the whisking rhythm is achieved by actions of vMCx on 5-HT pre-motoneurons.
Additional lines of evidence support the role of vMCx in regulating whisking through a 5-HT-dependent mechanism. The activity of vMCx neurons does not co-vary with whisking frequency, suggesting that vMCx does not directly drive vMNs (Carvell et al. 1996). Increased vMCx activity does, however, precede both the onset of whisking and changes in whisking kinematics (Friedman et al. 2006). These observations are consistent with vMCx modulating the activity of a subcortical structure, such as serotonergic pre-motoneurons, to initiate and modulate whisking. A role for the motor cortex as a coordinator of movement patterns is supported by studies in primates, demonstrating that the motor cortex may control higher-order movement parameters, such as ethologically relevant motor behaviors, rather than activation of individual muscles or movements (Graziano 2006).
During voluntary whisking, vibrissae often move in unison (Carvell and Simons 1990; Gao et al. 2001). Although the synchrony between vibrissae in the same whisker pad (Sachdev et al. 2002) and bilaterally (Towal and Hartmann 2006) does not occur during all behaviors, the prevalence of such synchrony suggests the presence of underlying coordinating mechanisms. Since the facial nucleus is thought not to contain interneurons (Courville 1966), and since facial motoneurons do not have axon collaterals (Fanardjian et al. 1983), unilateral synchrony may be achieved through electrical coupling. Electrical coupling through gap junctions enhances synchronous activity in neurons (Connors and Long 2004) and is important for generating coordinated output from motoneuron pools (Kiehn and Tresch 2002; Tresch and Kiehn 2000). The presence of gap junction proteins in the facial nucleus (Rohlmann et al. 1993) further suggests that the unilateral synchronous movements of vibrissae results from the coordinated discharge of electrically coupled vMNs. Such a mechanism is supported by findings in the developing mouse hindbrain, where 5-HT generates widespread synchronous activity that is abolished by gap junction blockers (Hunt et al. 2006). Alternatively, synchronous whisking, both unilateral and bilateral, may be achieved through the action of pre-motoneurons. These may include pre-motoneurons in LPGi, as these project bilaterally to vMNs (Hattox et al. 2003; Hattox et al. 2002), or pre-motoneurons in one of the numerous nuclei that project to vMNs (Hattox et al. 2002).
Many of the nuclei that project to vMNs contain non-serotonergic neurons, whose role in the regulation of whisking remains to be established (Hattox et al. 2002). Within these nuclei may reside a more classically composed whisking CPG that delivers rhythmic inputs to the motoneurons. vMNs also receive direct, albeit sparse inputs from the vMCx (Grinevich et al. 2005), and although vMCx is not necessary for whisking (Gao et al. 2003; Lovick 1972; Semba and Komisaruk 1984), it has been proposed that vMCx is capable of generating the whisking rhythm itself on a cycle-by-cycle basis (Berg and Kleinfeld 2003). Additionally, in some pathological states vMCx activity is phased-locked to individual whisks (Castro-Alamancos 2006). In light of these observations, it is unlikely that any one mechanism operates in isolation to generate the full range of vibrissal movements. Nevertheless, our findings support the hypothesis that vMNs require only serotonergic inputs to generate the whisking motor pattern. The involvement of vMNs in rhythmogenesis and the necessary and sufficient role of 5-HT in generating the whisking motor pattern, establish this network as a novel mechanism for the generation of movements in mammals.
Acknowledgments
We are grateful to Drs. Danny Weinreich, Eve Marder, Phillip Zeigler, Radi Masri, Ms. Wendy Friedman and Mrs. Marie Hemelt for their critical reading of the manuscript. We are also grateful to Dr. Larisa Sellers for her expert technical assistance.
Footnotes
Funding
This work was supported by NIH grant NS-35360 (AK) and NIH NRSA NS-053306 (NPC).
References
- Arshavsky YI, Deliagina TG, Orlovsky GN. Pattern generation. Curr Opin Neurobiol. 1997;7:781–789. doi: 10.1016/s0959-4388(97)80136-5. [DOI] [PubMed] [Google Scholar]
- Azmitia E, Gannon P. The ultrastructural localization of serotonin immunoreactivity in myelinated and unmyelinated axons within the medial forebrain bundle of rat and monkey. J Neurosci. 1983;3:2083–2090. doi: 10.1523/JNEUROSCI.03-10-02083.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellintani-Guardia B, Schweizer M, Herbert H. Analysis of projections from the cochlear nucleus to the lateral paragigantocellular reticular nucleus in the rat. Cell Tissue Res. 1996;283:493–505. doi: 10.1007/s004410050560. [DOI] [PubMed] [Google Scholar]
- Berg RW, Kleinfeld D. Vibrissa movement elicited by rhythmic electrical microstimulation to motor cortex in the aroused rat mimics exploratory whisking. J Neurophysiol. 2003;90:2950–2963. doi: 10.1152/jn.00511.2003. [DOI] [PubMed] [Google Scholar]
- Bowker R, Abbott L. Quantitative re-evaluation of descending serotonergic and non-serotonergic projections from the medulla of the rodent: evidence for extensive co-existence of serotonin and peptides in the same spinally projecting neurons, but not from the nucleus raphe magnus. Brain Res. 1990;512:15–25. doi: 10.1016/0006-8993(90)91164-c. [DOI] [PubMed] [Google Scholar]
- Brecht M, Preilowski B, Merzenich MM. Functional architecture of the mystacial vibrissae. Behav Brain Res. 1997;84:81–97. doi: 10.1016/s0166-4328(97)83328-1. [DOI] [PubMed] [Google Scholar]
- Carvell G, Simons DJ. Biometric analyses of vibrissal tactile discrimination in the rat. J Neurosci. 1990;10:2638–2648. doi: 10.1523/JNEUROSCI.10-08-02638.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvell GE, Miller SA, Simons DJ. The relationship of vibrissal motor cortex unit activity to whisking in the awake rat. Somatosens Motor Res. 1996;13:115–127. doi: 10.3109/08990229609051399. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA. Vibrissa myoclonus (rhythmic retractions) driven by resonance of excitatory networks in motor cortex. J Neurophysiol. 2006;96:1691–1698. doi: 10.1152/jn.00454.2006. [DOI] [PubMed] [Google Scholar]
- Connors BW, Long MA. Electrical synapses in the mammalian brain. Annu Rev Neurosci. 2004;27:393–418. doi: 10.1146/annurev.neuro.26.041002.131128. [DOI] [PubMed] [Google Scholar]
- Courville J. The nucleus of the facial nerve: The relation between cellular groups and peripheral branches of the nerve. Brain Res. 1966;1:338–354. doi: 10.1016/0006-8993(66)90126-0. [DOI] [PubMed] [Google Scholar]
- Cramer NP, Keller A. Cortical control of a whisking central pattern generator. J Neurophysiol. 2006;96:209–217. doi: 10.1152/jn.00071.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnall RA, Harris MB, Gill WH, Hoffman JM, Brown JW, Niblock MM. Inhibition of serotonergic neurons in the nucleus paragigantocellularis lateralis fragments sleep and decreases rapid eye movement sleep in the piglet: Implications for sudden infant death syndrome. J Neurosci. 2005;25:8322–8332. doi: 10.1523/JNEUROSCI.1770-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De-Miguel FF, Trueta C. Synaptic and extrasynaptic secretion of serotonin. Cell Mol Neurobiol. 2005;25:297–312. doi: 10.1007/s10571-005-3061-z. [DOI] [PubMed] [Google Scholar]
- Fanardjian VV, Manvelyan LR, Kasabyan SA. Mechanisms regulating the activity of facial nucleus motoneurones-1. Antidromic activation. Neurosci. 1983;9:815–822. doi: 10.1016/0306-4522(83)90270-1. [DOI] [PubMed] [Google Scholar]
- Friedberg MH, Lee SM, Ebner FF. Modulation of receptive field properties of thalamic somatosensory neurons by the depth of anesthesia. J Neurophysiol. 1999;81:2243–2252. doi: 10.1152/jn.1999.81.5.2243. [DOI] [PubMed] [Google Scholar]
- Friedman WA, Jones LM, Cramer NP, Kwegyir-Afful EE, Zeigler HP, Keller A. Anticipatory activity of motor cortex in relation to rhythmic whisking. J Neurophysiol. 2006;95:1274–1277. doi: 10.1152/jn.00945.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, Bermejo B, Zeigler P. Whisker deafferentation and rodent whisking patterns: behavioral evidence for a central pattern generator. J Neurosci. 2001;21:5374–5380. doi: 10.1523/JNEUROSCI.21-14-05374.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, Hattox AM, Jones LM, Keller A, Zeigler HP. Whisker motor cortex ablation and whisker movement patterns. Somatosens Mot Res. 2003;20:191–198. doi: 10.1080/08990220310001622924. [DOI] [PubMed] [Google Scholar]
- Graziano M. The organization of behavioral repertoire in motor cortex. Ann Review Neurosci. 2006;29:105–134. doi: 10.1146/annurev.neuro.29.051605.112924. [DOI] [PubMed] [Google Scholar]
- Grinevich V, Brecht M, Osten P. Monosynaptic pathway from rat vibrissa motor cortex to facial motor neurons revealed by lentivirus-based axonal tracing. J Neurosci. 2005;25:8250–8258. doi: 10.1523/JNEUROSCI.2235-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haiss F, Schwarz C. Spatial segregation of different modes of movement control in the whisker representation of rat primary motor cortex. J Neurosci. 2005;25:1579–1587. doi: 10.1523/JNEUROSCI.3760-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PJ, Li X, Li Y, Bennett DJ. 5-HT2 receptor activation facilitates a persistent sodium current and repetitive firing in spinal motoneurons of rats with and without chronic spinal cord injury. J Neurophysiol. 2006a;96:1158–1170. doi: 10.1152/jn.01088.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PJ, Li X, Li Y, Bennett DJ. Endogenous monoamine receptor activation is essential for enabling persistent sodium currents and repetitive firing in rat spinal motoneurons. J Neurophysiol. 2006b;96:1171–1186. doi: 10.1152/jn.00341.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattox AM, Li Y, Keller A. Serotonin regulates rhythmic whisking. Neuron. 2003;39:342–352. doi: 10.1016/s0896-6273(03)00391-x. [DOI] [PubMed] [Google Scholar]
- Hattox AM, Priest CA, Keller A. Functional circuitry involved in the regulation of whisker movements. J Comp Neurol. 2002;442:266–276. doi: 10.1002/cne.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Gorassini MA, Bennett DJ. Persistent inward currents in motoneuron dendrites: implications for motor output. Muscle Nerve. 2005;31:135–156. doi: 10.1002/mus.20261. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Kuo JJ, Johnson MD. Synaptic integration in motoneurons with hyper-excitable dendrites. Can J Physiol Pharmacol. 2004;82:549–555. doi: 10.1139/y04-046. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Lee RH, Brownstone RM. Hyperexcitable dendrites in motoneurons and their neuromodulatory control during motor behavior. Trends Neurosci. 2003;26:688–695. doi: 10.1016/j.tins.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Bistability of alpha-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J Physiol. 1988;405:345–367. doi: 10.1113/jphysiol.1988.sp017336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Serotonin-induced bistability of turtle motoneurones caused by a nifedipine-sensitive calcium plateau potential. J Physiol (Lond) 1989;414:265–282. doi: 10.1113/jphysiol.1989.sp017687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao C, Trueblood P, Levine M, Chandler S. Multiple effects of serotonin on membrane properties of trigeminal motoneurons in vitro. j Neurophysiol. 1997;77:2910–2924. doi: 10.1152/jn.1997.77.6.2910. [DOI] [PubMed] [Google Scholar]
- Hsiao CF, Del Negro CA, Trueblood PR, Chandler SH. Ionic basis for serotonin-induced bistable membrane properties in guinea pig trigeminal motoneurons. J Neurophysiol. 1998;79:2847–2856. doi: 10.1152/jn.1998.79.6.2847. [DOI] [PubMed] [Google Scholar]
- Hunt PN, Gust J, McCabe AK, Bosma MM. Primary role of the serotonergic midline system in synchronized spontaneous activity during development of the embryonic mouse hindbrain. J Neurobiol. 2006;66:1239–1252. doi: 10.1002/neu.20259. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Tresch MC. Gap junctions and motor behavior. Trends Neurosci. 2002;25:108–115. doi: 10.1016/s0166-2236(02)02038-6. [DOI] [PubMed] [Google Scholar]
- Klein BG, Rhoades RW. Representation of whisker follicle intrinsic musculature in the facial motor nucleus of the rat. J Comp Neurol. 1985;232:55–69. doi: 10.1002/cne.902320106. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in persistent inward currents. J Neurophysiol. 1998;80:583–593. doi: 10.1152/jn.1998.80.2.583. [DOI] [PubMed] [Google Scholar]
- Lovick TA. The behavioral repertoire of precollicular decerebrate rats. J Physiol (Lond) 1972;224:4–6. [PubMed] [Google Scholar]
- McCall RB, Aghajanian GK. Serotonergic facilitation of facial motoneuron excitation. Brain Res. 1979;169:11–27. doi: 10.1016/0006-8993(79)90370-6. [DOI] [PubMed] [Google Scholar]
- Orlovksy GN, Deliagina TG, Grillner S. Neuronal control of locomotion: from mollusc to man. Oxford University Press; New York: 1999. [Google Scholar]
- Perrier JF, Hounsgaard J. 5-HT2 receptors promote plateau potentials in turtle spinal motoneurons by facilitating an L-type calcium current. J Neurophysiol. 2003;89:954–959. doi: 10.1152/jn.00753.2002. [DOI] [PubMed] [Google Scholar]
- Powers RK, Binder MD. Input-output functions of mammalian motoneurons. Rev Physiol Biochem Pharmacol. 2001;143:137–263. doi: 10.1007/BFb0115594. [DOI] [PubMed] [Google Scholar]
- Powers RK, Binder MD. Persistent sodium and calcium currents in rat hypoglossal motoneurons. J Neurophysiol. 2003;89:615–624. doi: 10.1152/jn.00241.2002. [DOI] [PubMed] [Google Scholar]
- Prather JF, Powers RK, Cope TC. Amplification and linear summation of synaptic effects on motoneuron firing rate. J Neurophysiol. 2001;85:43–53. doi: 10.1152/jn.2001.85.1.43. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL. Synaptic central of motoneuronal excitability. Physiol Rev. 2000;80:767–852. doi: 10.1152/physrev.2000.80.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlmann A, Laskawi R, Hofer A, Dobo E, Dermietzel R, Wolff JR. Facial nerve lesions lead to increased immunostaining of the astrocytic gap junction protein (connexin 43) in the corresponding facial nucleus of rats. Neurosci Lett. 1993;154:206–108. doi: 10.1016/0304-3940(93)90208-3. [DOI] [PubMed] [Google Scholar]
- Sachdev RNS, Sato T, Ebner FF. Divergent movement of adjacent whiskers. J Neurophysiol. 2002;87:1440–1448. doi: 10.1152/jn.00539.2001. [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Crill WE. Factors influencing motoneuron rhythmic firing: results from a voltage-clamp study. J Neurophysiol. 1982;48:875–890. doi: 10.1152/jn.1982.48.4.875. [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Crill WE. Properties of a persistent inward current in normal and TEA-injected motoneurons. J Neurophysiol. 1980;43:1700–1724. doi: 10.1152/jn.1980.43.6.1700. [DOI] [PubMed] [Google Scholar]
- Semba K, Egger MD. The facial “motor” nerve of the rat: control of vibrissal movement and examination of motor and sensory components. J Comp Neurol. 1986;247:144–158. doi: 10.1002/cne.902470203. [DOI] [PubMed] [Google Scholar]
- Semba K, Komisaruk BR. Neural substrates of two different rhythmical vibrissal movements in the rat. Neurosci. 1984;12:761–774. doi: 10.1016/0306-4522(84)90168-4. [DOI] [PubMed] [Google Scholar]
- Stein PSG, Grillner S, Selverston AL, Stuart DG. Neurons, Networks, and Motor Behavior. The MIT Press; Cambridge: 1997. p. 305. [Google Scholar]
- Towal RB, Hartmann MJ. Right-left asymmetries in the whisking behavior of rats anticipate head movements. J Neurosci. 2006;26:8838–8846. doi: 10.1523/JNEUROSCI.0581-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresch MC, Kiehn O. Motor coordination without action potentials in the mammalian spinal cord. Nat Neurosci. 2000;3:593–599. doi: 10.1038/75768. [DOI] [PubMed] [Google Scholar]
- Vincent SB. The function of the vibrissae in the behavior of the white rat. Behav Monographs. 1912;1:7–86. [Google Scholar]
- Welker WI. Analysis of sniffing of the albino rat. Behavior. 1964;22:223–244. [Google Scholar]
- Wu N, Enomoto A, Tanaka S, Hsiao CF, Nykamp DQ, Izhikevich E, Chandler SH. Persistent sodium currents in mesencephalic v neurons participate in burst generation and control of membrane excitability. J Neurophysiol. 2005;93:2710–2722. doi: 10.1152/jn.00636.2004. [DOI] [PubMed] [Google Scholar]
- Zhang L, Sykes KT, Buhler AV, Hammond DL. Electrophysiological heterogeneity of spinally projecting serotonergic and nonserotonergic neurons in the rostral ventromedial medulla. J Neurophysiol. 2006;95:1853–1863. doi: 10.1152/jn.00883.2005. [DOI] [PubMed] [Google Scholar]