Abstract
Anaerobic enrichment cultures with either propionate, succinate, lactate, or valerate and elemental sulfur and inocula from shallow marine or deep-sea sediments were dominated by rod-shaped motile bacteria after three transfers. By application of deep-agar dilutions, five eubacterial strains were obtained in pure culture and designated Kyprop, Gyprop, Kysw2, Gylac, and Kyval. All strains were gram negative and grew by complete oxidation of the electron donors and concomitant stoichiometric reduction of elemental sulfur to hydrogen sulfide. The isolates used acetate, propionate, succinate, lactate, pyruvate, oxaloacetate, maleate, glutamate, alanine, aspartate, and yeast extract. All isolates, except strain Gylac, used citrate as an electron donor but valerate was oxidized only by strain Kyval. Fumarate and malate were degraded by all strains without an additional electron donor or acceptor. Kyprop, Gyprop, and Gylac utilized elemental sulfur as the sole inorganic electron acceptor, while Kysw2 and Kyval also utilized nitrate, dimethyl sulfoxide, or Fe(III)-citrate as an electron acceptor.
Full text
PDF



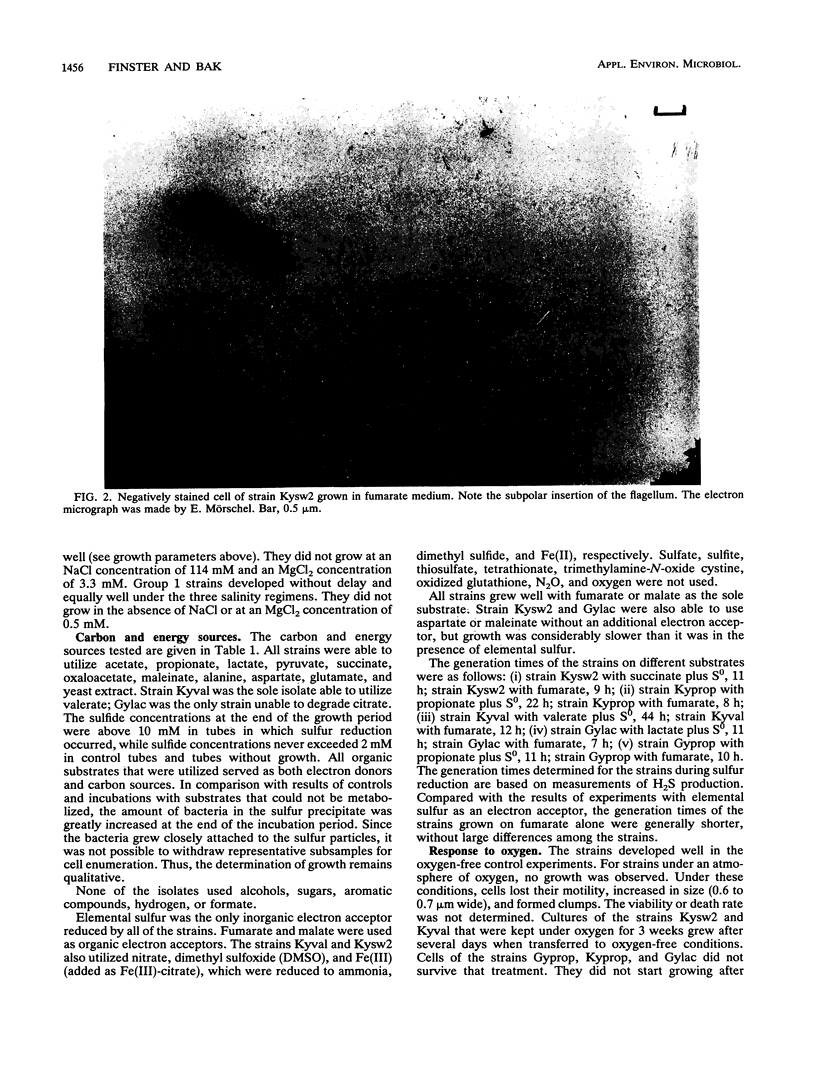


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balashova V. V. Ispol'zovanie molekuliarnoi sery v kachestve okislitelia H2 fakul'tativno-anaerobnym Pseudomonasom. Mikrobiologiia. 1985 Mar-Apr;54(2):324–326. [PubMed] [Google Scholar]
- Biebl H., Pfennig Growth of sulfate-reducing bacteria with sulfur as electron acceptor. Arch Microbiol. 1977 Feb 4;112(1):115–117. doi: 10.1007/BF00446664. [DOI] [PubMed] [Google Scholar]
- Cashion P., Holder-Franklin M. A., McCully J., Franklin M. A rapid method for the base ratio determination of bacterial DNA. Anal Biochem. 1977 Aug;81(2):461–466. doi: 10.1016/0003-2697(77)90720-5. [DOI] [PubMed] [Google Scholar]
- Kröger A. Electron-transport phosphorylation coupled to fumarate reduction in anaerobically grown Proteus rettgeri. Biochim Biophys Acta. 1974 May 22;347(2):273–289. doi: 10.1016/0005-2728(74)90051-6. [DOI] [PubMed] [Google Scholar]
- Laanbroek H. J., Lambers J. T., de Vos W. M., Veldkamp H. L-Aspartate fermentation by a free-living Campylobacter species. Arch Microbiol. 1978 Apr 27;117(1):109–114. doi: 10.1007/BF00689359. [DOI] [PubMed] [Google Scholar]
- Laanbroek H. J., Stal L. H., Veldkamp H. Utilization of hydrogen and formate by Campylobacter spec. under aerobic and anaerobic conditions. Arch Microbiol. 1978 Oct 4;119(1):99–102. doi: 10.1007/BF00407935. [DOI] [PubMed] [Google Scholar]
- Pfennig N., Biebl H. Desulfuromonas acetoxidans gen. nov. and sp. nov., a new anaerobic, sulfur-reducing, acetate-oxidizing bacterium. Arch Microbiol. 1976 Oct 11;110(1):3–12. doi: 10.1007/BF00416962. [DOI] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe R. S., Penning N. Reduction of sulfur by spirillum 5175 and syntrophism with Chlorobium. Appl Environ Microbiol. 1977 Feb;33(2):427–433. doi: 10.1128/aem.33.2.427-433.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinder S. H., Brock T. D. Dimethyl sulfoxide as an electron acceptor for anaerobic growth. Arch Microbiol. 1978 Jan 23;116(1):35–40. doi: 10.1007/BF00408731. [DOI] [PubMed] [Google Scholar]