Abstract
To understand the physiological functions of exogenous hepatocyte growth factor (HGF) on normal adult animals, we delivered human HGF gene into mice by a hydrodynamics-based in vivo gene transfection approach using a naked plasmid vector. Systemic administration of naked plasmid containing HGF cDNA driven under cytomegalovirus promoter (pCMV-HGF) by rapid injection via the tail vein produced a remarkable level of human HGF protein in the circulation, beginning to appear at 4 hours and peaking at 12 hours following injection. Tissue distribution studies identified the liver as the organ with the highest level of transgene expression. Through weekly repeated injections of plasmid vector, we achieved sustained, long-term, high levels of exogenous HGF expression in mice for 8 weeks. Increases of more than 31% and 16% in liver and body weights were found, respectively, in the mice that received pCMV-HGF plasmid compared with that given the control vector for 8 weeks. Expression of exogenous HGF in vivo activated mitogen-activated protein kinases and induced proliferating cell nuclear antigen expression in normal adult liver and kidneys. These data suggest that systemic administration of naked plasmid vector is a convenient, safe, and highly efficient approach to introduce and maintain exogenous HGF gene expression in vivo in a controllable fashion. Our results also indicate that long-term expression of human HGF in mice markedly activates growth-related signal transduction events, promotes cell proliferation, and leads to liver and overall body growth in whole adult animals.
Hepatocyte growth factor (HGF) is a multi-functional cytokine displaying remarkable ability to promote tissue repair and organ regeneration after injury.1–4 HGF is demonstrated to be a potent therapeutic agent for acceleration of tissue regeneration following acute insult as well as for amelioration of tissue fibrosis and dysfunctions in chronic disease conditions.5–9 Despite this, the fundamental aspects of HGF biology, such as the long-term effects of exogenous HGF on normal adult animals, remain poorly understood. Because it is rapidly cleared by the liver in vivo, exogenous HGF is extremely unstable in the blood circulation with a half-life of less than 15 minutes.10,11 This makes it almost impossible to sustain a constantly high level of exogenous HGF in circulation even using repeated injections of HGF protein at short intervals. A logical way to overcome this problem is to develop a gene transfer strategy allowing persistently expressing HGF protein in vivo.12,13 Clearly, efficient and sustainable expression of HGF gene in vivo is an essential step toward understanding its biological functions and evaluating its therapeutic potential in whole animals.
Strategies to deliver a foreign gene in vivo can be divided into two distinct groups: nonviral vector and virus-based vector methods, including retrovirus, adenovirus, and adeno-associated virus vector systems.12–14 Despite being reasonably efficient in transferring foreign genes in vivo, the current virus systems have several major inherent limitations. Besides the labor-intensive procedures for construction and preparation, some viral vectors cannot infect nonproliferating cells, making it impossible for substantial expression of transgene in most normal and diseased tissues where cell turnover is relatively low. Other viral vectors induce severe host immune responses that not only cause short duration of transgene expression but also make the repeated administration extremely difficult, if not impossible.
Direct administration of plasmid vector containing the genes of interest represents a promising new strategy to deliver and express foreign genes in vivo.15,16 This approach has obvious advantages including the easy preparation of a large amount of plasmids as well as its proven safety in vivo. However, transduction of plasmid vectors in vivo generally is far less efficient, and the duration of transgene expression is often transient.17 In spite of that tremendous efforts have been made to increase the expression level of transgene and to sustain the duration of transgene expression,18–20 introduction and expression of exogenous HGF genes in vivo by plasmid vector remain extremely inefficient. For example, a previous report only achieved 0.1 ng/mL of exogenous human HGF in rat circulation by intramuscular injection of HGF expression plasmid with liposomes containing the hemagglutinating virus of Japan (HVJ).20
In this article, we describe a simple hydrodynamics-based in vivo transfection procedure by systemic administration of naked HGF expression plasmid that results in a high level, sustainable expression of exogenous human HGF in mice. Our data also indicate that persistent, long-term expression of HGF in vivo activates mitogen-activated protein kinases and promotes liver and overall body growth in normal adult animals.
MATERIALS AND METHODS
Animals
Male CD-1 mice weighing at 16 to 20 g were purchased from Harlan Sprague Dawley (Indianapolis, IN). They were housed in the animal facilities of the University of Pittsburgh Medical Center with free access to food and water. Animals were treated humanely using approved procedures in accordance with the guidelines of the Institutional Animal Use and Care Committee of National Institutes of Health at the University of Pittsburgh School of Medicine.
Plasmid Injection
The recombinant human HGF expression plasmid (pCMV-HGF) that contains full-length human HGF cDNA driven under a human cytomegalovirus (CMV) promoter was cloned as described previously.21 This clone encompasses nucleotides − 26 to + 2262 of the human placental HGF cDNA. The empty expression plasmid vector pcDNA3 was purchased from Invitrogen (San Deigo, CA). The plasmid vector containing cDNA encoding the mutated version of green fluorescent protein under the control of CMV promoter (pCMV-GFP) was obtained from Quantum Biotechnologies Inc. (Montreal, Quebec, Canada). Plasmid DNA was administrated into mice by a hydrodynamic-based gene transfer technique via rapid injection of a large volume of DNA solution through the tail vein, as recently described by Liu et al.16 Briefly, different amounts of plasmid DNA (10 to 40 μg) was diluted in 1.6 mL of saline unless indicated otherwise, and injected via the tail vein into the circulation within 5 to 10 seconds. For some animals, the pCMV-HGF plasmid was injected intramuscularly at multiple sites as described previously.22
Production of Monoclonal Anti-HGF Antibody
Monoclonal antibodies against human HGF were prepared using a standard protocol of hybridoma technology. Briefly, hybrid cell lines were generated through cell fusion between SP 2/0 myeloma and spleen cells from the mice immunized with purified recombinant human HGF (Genentech, Inc., South San Francisco, CA). Monoclonal antibody H14 in this study was one of 14 antibodies (designated as H1 to H14) established and was shown to specifically recognize human HGF protein through preliminary characterization. H14 was purified using a two-step precipitation method involving 4% caprylic acid followed by 50% ammonium sulfate. Purified antibody was dialyzed against phosphate buffered saline and stored at −80°C with or without 0.1% thimerosal. Under nonreducing conditions, H14 identified human recombinant HGF as low as 5 ng/lane in an immunoblot as a band of ~90 kd, but did not recognize either purified recombinant rat HGF protein at 50 ng/lane or mouse tissue HGF in immuoblots. This antibody could detect human HGF protein, but not rodent HGF in an ELISA and in immunohistochemical staining (see later). H14 antibody did not show any cross-reactivity with other growth factors, cytokines, and other proteins possessing structural similarity to HGF such as plasminogen and plasmin (data not shown).
Determination of HGF Levels by ELISA
The amount of human plasma HGF was measured using an enzyme-linked immunosorbent assay (ELISA) method. This assay utilizes a sandwich method consisting of 3 steps of antigen-antibody reactions. Briefly, the 96-well microtitre plates (Nunc-Immuno Module, Fisher Scientific, Pittsburgh, PA) were incubated with 50 μL of uncoupled monoclonal anti-HGF antibody (H14) per well diluted in 50 mmol/L Tris HCl, pH 8.0 at a final concentration of 1.5 μg/mL at room temperature for 16 hours. The plates were washed 3 times with PBS containing 0.05% Tween 20, pH 7.4, and blocked with 200 μL of blocker solution (PBS containing 1% BSA) at room temperature for 2 hours. The plates were extensively washed for 5 times with PBS containing 0.05% Tween 20 and kept at 4°C. Fifty-microliter aliquots of standard human HGF solution or plasma samples were added to the wells and incubated for 2 hours at room temperature. After extensive washing, a 100-μL aliquot of biotinylated goat antihuman HGF polyclonal antibody (purchased from R & D Systems, Minneapolis, MN) at a dilution of 1:2,000 was added, and the plates were incubated for another 2 hours. Following washing, they were then incubated with 100 μL of horseradish peroxidase (HRP)-conjugated streptavidin (Zymed Laboratories, South San Francisco, CA) at a dilution of 1:20,000, and subsequently with enzyme substrate solution containing 0.1 mg/mL of tetramethylbenzidine and 0.006% H2O2 in 0.1 mol/L sodium citrate, pH 6.0. The plates were allowed to stand for 30 minutes at room temperature, and the reaction was stopped by addition of 50 μL 4N H2SO4. Absorbance was read at 405 nm by an automatic Emax Precision Microplate Reader (Molecular Devices Co., Sunnyvale, CA).
For measurement of tissue HGF level, various tissues from mice were homogenized in the HGF extraction buffer containing 20 mmol/L Tris-HCl, pH 7.5, 2 mol/L NaCl, 0.1% Tween-80, 1 mmol/L EDTA, and 1 mmol/L PMSF, as described previously.23 After centrifugation at 19,000 g for 20 minutes at 4°C, the supernatant was recovered for determination of HGF using the human HGF ELISA as described earlier. Samples were diluted if necessary to give an optical density that was within the linear portion of the standard curve. Total protein levels were determined using a bicinconinic-acid (BCA) protein assay kit (Sigma) with BSA as a standard. The concentration of HGF in organs was expressed as ng/mg total protein.
Western Immunoblotting Analysis
For detection of human HGF protein levels in vivo, total tissue protein extracts as described earlier for ELISA were used for Western immunoblotting. For detection of other proteins such as mitogen-activated protein (MAP) kinases and proliferating cell nuclear antigen (PCNA) by immunoblotting, tissues were homogenized by a polytron homogenizer (Brinkmann) in RIPA lysis buffer (1% NP40, 0.1% SDS, 100 μg/mL PMSF, 0.5% sodium deoxycholate, 1 mmol/L sodium orthovanadate, 2 μg/mL aprotinin, 2 μg/mL antipain, and 2 μg/mL leupeptin in PBS) on ice, and the supernatants were collected after centrifugation at 13,000 g at 4°C for 20 minutes. Protein concentration was determined using a BCA protein assay kit (Sigma, St. Louis, MO), and tissue lysates were mixed with an equal amount 2X SDS loading buffer (100 mmol/L Tris-HCl, 4% SDS, 20% glycerol, and 0.2% bromophenol blue), as described previously.23 Samples were heated at 100°C for 5 to 10 minutes before loading and separated on precasted 10% or 15% SDS-polyacrylamide gels (Bio-Rad, Hercules, CA). The proteins were electrotransferred to a nitrocellulose membrane (Amersham, Arlington Heights, IL) in transfer buffer containing 48 mmol/L Tris-HCl, 39 mmol/L glycine, 0.037% SDS, and 20% methanol at 4°C for 1 hour. Nonspecific binding to the membrane was blocked for 1 hour at room temperature with 5% Carnation nonfat milk in TBS buffer (20 mmol/L Tris-HCl, 150 mmol/L NaCl, and 0.1% Tween 20). The membranes were then incubated for 16 hours at 4°C with various primary antibodies in blocking buffer containing 5% milk. The monoclonal anti-HGF antibody (H14) was diluted at a final concentration of 2 μg/mL. The phospho-specific Erk1/2 antibody that detects Erk1/2 only when phosphorylated at specific sites was obtained from New England BioLabs (Beverly, MA). The Erk1/2 antibody that detects total Erk1/2 (phosphorylation state-independent) was purchased from Sigma (St. Louis, MO). The monoclonal anti-PCNA antibody and the rabbit polyclonal anti-actin antibody were purchased from Santa Cruz Biochemicals (Santa Cruz, CA). Following 3 extensive washes, the membranes were then incubated with horseradish peroxidase-conjugated secondary antibody (Bio-Rad) for 1 hour at room temperature in 1% nonfat milk. The signals were visualized by the enhanced chemiluminescence system (ECL, Amersham). Quantitation was performed by measuring the intensity of the hybridization signals using NIH Image analysis software.24
Frozen Section and Immunofluorescence Microscopy
Tissues from various organs were rapidly frozen in OCT compound (Fisher Scientific) by liquid nitrogen and stored at −80°C. Five-micrometer thick cryosections were prepared in a cryostat by routine procedures. Sections were directly observed on Nikon Eclipse E600 Epi-fluorescence microscope (Melville, NY) for GFP expression. For immunofluorescent staining, cryosections were fixed for 5 minutes in PBS containing 3% paraformaldehyde, and then incubated with primary anti-HGF monoclonal antibody (H14) at a 1:500 dilution in PBS containing 1% BSA overnight at 4°C. Sections were then incubated for 1 hour with fluorescein-conjugated secondary antibodies at a dilution of 1:200 in PBS containing 5% BSA, before being washed extensively with PBS. Slides were mounted with anti-fade mounting media (Vector Laboratories, Burlingame, CA) and examined using fluorescence microscope.
TUNEL Staining
Apoptotic cell death was determined by in situ detection of DNA fragmentation using terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end labeling (TUNEL assay).24 Tissue frozen sections, 5 μm in thickness, were stained according to the protocol specified by the manufacturer (Apoptosis Detection System; Promega, Madison, WI).
Morphological Studies
Tissue sections from the mice were prepared at 4 μm thick by a routine procedure. Sections were stained with hematoxylin/eosin for general histology.
Statistical Analysis
Animals were randomly assigned to control and treatment groups. Statistical analysis of the data was performed by Student’s t-test using SigmaStat software (Jandel Scientific, San Rafael, CA). A P value of less than .05 was considered significant.
RESULTS
In Vivo Exogenous HGF Gene Expression by Systemic Administration of Naked Plasmid
To efficiently deliver exogenous HGF gene, we have adapted an in vivo gene transfection procedure by rapid injection of a large volume of naked plasmid DNA solution via the tail vein.16 As shown in Fig. 1, systemic administration of the pCMV-HGF plasmid resulted in marked expression of human HGF gene in vivo. The levels of human HGF protein in the circulation could reach as high as more than 4 ng/mL at 8 hours following intravenous injection (Fig. 1). The level of HGF gene expression was dependent on the amount of pCMV-HGF plasmid injected. Twenty micrograms of the pCMV-HGF plasmid injected per mouse was sufficient for maximal expression of the HGF gene (Fig. 1A). The effect of injection volume per mouse on the levels of HGF gene expression is shown in Fig. 1B. A large volume of DNA solution to be injected (1.6 mL per mouse) was apparently required for optimal expression of exogenous gene by this gene delivery approach.
Fig. 1.
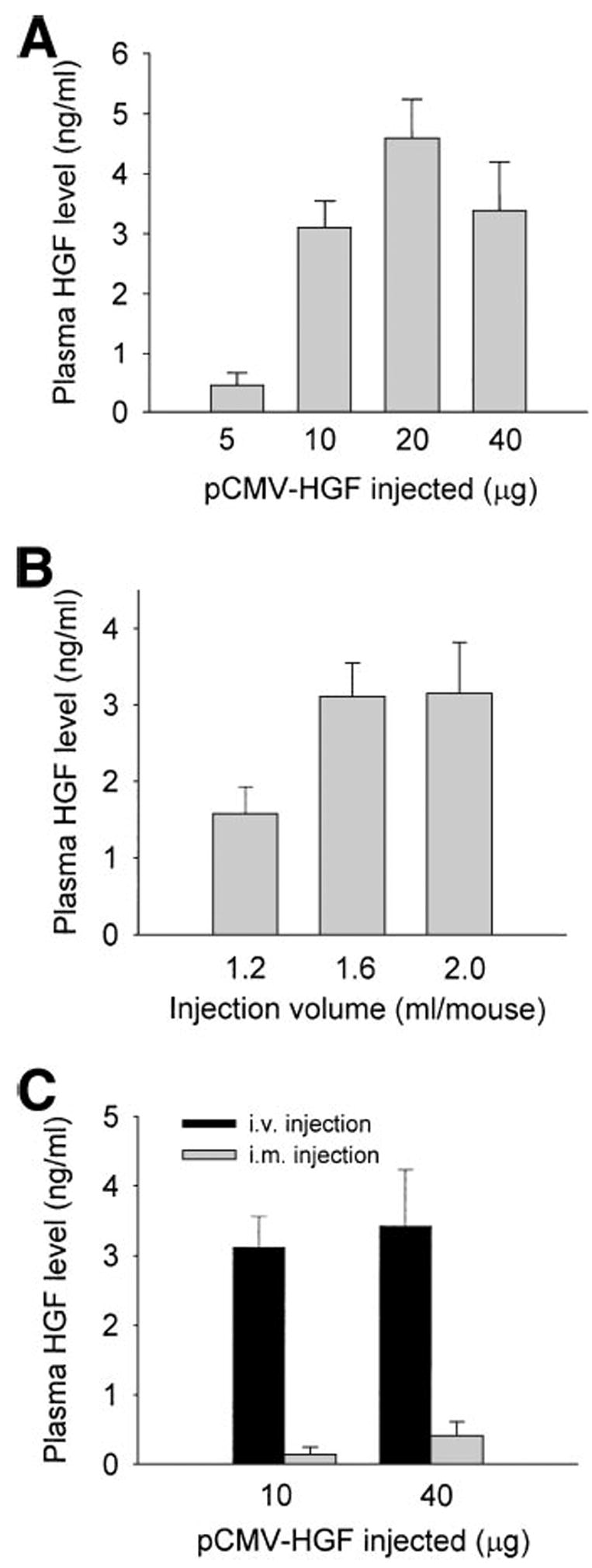
Expression of human HGF in mice following systemic administration of naked plasmid encoding HGF cDNA. The male CD-1 mice were rapidly injected via the tail vein with a large volume of solution containing different amounts of plasmid DNA encoding human HGF cDNA as indicated. The plasma was collected at 8 hours following injection, and the HGF levels were determined by a specific ELISA for human HGF protein. (A) Effect of the amounts of plasmid DNA injected (μg) on the levels of HGF gene expression. (B) Effect of injection volumes on the levels of circulating human HGF protein. (C) Effect of injection routes (i.v. vs. i.m.) on the levels of HGF gene expression. Data are presented as mean ± S.D. (n = 4).
We also compared the gene transfection efficiency of this hydrodynamic-based approach with a direct intramuscular injection method. As shown in Fig. 1C, direct intramuscular injection of naked pCMV-HGF plasmid resulted in very low levels of exogenous HGF protein in the circulation. Therefore, compared to intramuscular injection, the hydrodynamic-based systemic administration of the naked plasmid is a far more efficient gene transfer approach.
Temporal and Spatial Expression of Exogenous HGF Gene Delivered by Naked Plasmid
We next determined the temporal and spatial expression of exogenous HGF gene delivered by naked plasmid injection. As shown in Fig. 2, significant HGF protein was detected in the circulation as early as 4 hours following single injection of pCMV-HGF plasmid via the tail vein. The levels of exogenous HGF protein rapidly increased and reached to more than 8 ng/mL at the peak at 12 hours after injection. Although the circulating level of human HGF began to decline thereafter, significant amount of human HGF protein was still found in the circulation at 6 days following the initial injection of pCMV-HGF plasmid. As expected, injection of empty plasmid vector pcDNA3 in an identical manner produced no human HGF protein in the circulation of the mice (Fig. 2).
Fig. 2.
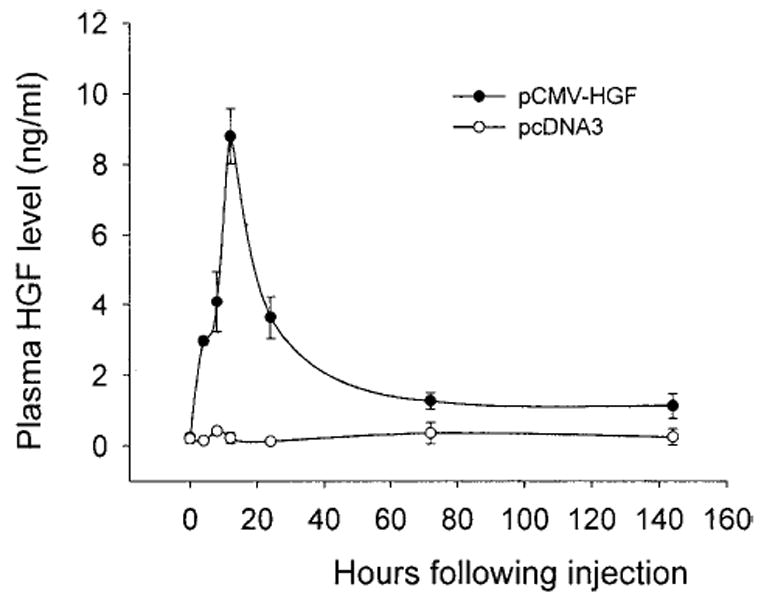
The circulating human HGF levels in mice following a single injection of naked HGF cDNA plasmid. Mice were rapidly injected via the tail vein with a large volume of solution containing 10 μg of either human HGF cDNA plasmid (pCMV-HGF) or empty plasmid (pcDNA3). At different time points as indicated, the plasma was collected, and the HGF levels were determined by a specific ELISA for human HGF protein. Data are presented as mean ± S.D. (n = 4).
We studied tissue distribution of exogenous HGF protein in different organs after injection of HGF expression plasmid vector. Figure 3 shows tissue human HGF protein levels from various organs at 8 hours following a single injection of pCMV-HGF plasmid. We found that the liver is the organ with the highest level of exogenous HGF expression, at least during the initial period of time immediately after plasmid injection. Quantitative ELISA revealed as high as 318 ng of human HGF protein per mg of total tissue protein in the liver (Fig. 3). The kidneys also exhibited a high level of exogenous HGF expression, whereas human HGF protein in other organs such as the lung and spleen were extremely low, ranging from 1 to 2 ng per mg of total tissue protein (Fig. 3). Of note, because the H14 antibody only detects HGF protein specific for human and does not cross-react with endogenous mouse HGF, the level of tissue HGF protein detected by this assay represents only exogenous human HGF derived from transgene expression. These results are independently confirmed by an immunoblotting method. As shown in Fig. 3B, marked expression of exogenous HGF protein was detected in total liver extracts, with the estimated level being 10 ng per 30 μg of total protein (or ~ 330 ng per mg of total protein) using purified human HGF protein as a standard. It is worthwhile to mention that the level of human HGF in the kidney was below the detection limit of immunoblotting when loaded at 30 μg of total protein per lane (Fig. 3B). Consistent with this notion, increase in total tissue protein loaded per lane to 60 μg did detect HGF protein by Western blot in kidney extracts (see later). Of note, no human HGF protein was detected in various tissues from the mice injected with pcDNA3 control plasmid.
Fig. 3.
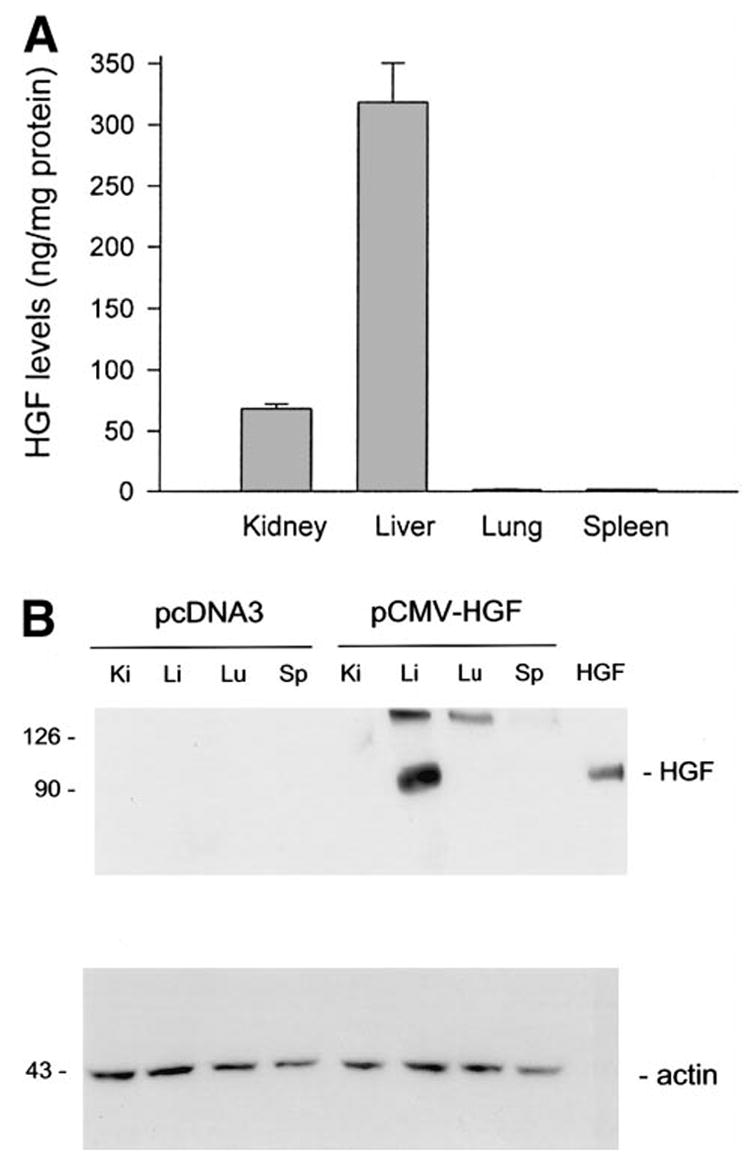
Tissue distribution of exogenous HGF protein in different organs following a single injection of pCMV-HGF plasmid. (A) Various tissues were collected and homogenized from the mice given either human HGF cDNA plasmid (pCMV-HGF) or empty plasmid (pcDNA3) at 8 hours after injection. Tissue HGF protein levels were determined by a specific ELISA. Human HGF levels in various tissues from the mice injected with pcDNA3 plasmid were undetectable (data not shown). Data are presented as mean ± S.D. (n = 4). (B) Western blot shows exogenous human HGF expression in various tissues at 8 hours following injection of plasmid vector. Tissue total protein extracts were separated on a SDS-polyacrylamide gel under nonreducing conditions and immunoblotted with a specific monoclonal antibody (clone H14) against human HGF. Thirty micrograms of tissue total protein was loaded each lane, which represents about 10 ng of exogenous HGF in liver sample, 2 ng in kidney. Purified recombinant human HGF (5 ng) was also loaded in the adjacent lane to confirm the correct size of hybridized signal and to estimate the amounts of HGF protein in each sample. The same blot was stripped and reprobed with actin to confirm equal loading.
To further determine the cellular origin of this robust transgene HGF expression in the liver following plasmid injection, we performed immunohistochemical staining for direct localization of the sites where the transgene is expressed. Immunostaining of the cryosections of liver revealed that exogenous human HGF gene was predominantly expressed in hepatocytes, the most abundant cells in liver (Fig. 4). Expression of exogenous HGF in hepatocytes was still abundant at 3 days after initial administration of the plasmid. Despite significant reduction, HGF expression was definitely detectable as late as 6 days after a single injection, the longest time point that we examined. To exclude the possibility that this pattern of staining may reflect the binding of secreted HGF to hepatocytes rather than in situ expression, we used a nonsecreted protein GFP as a marker gene to confirm the cellular origin of the transgene expression. Thus, a plasmid construct, pCMV-GFP, was injected via tail vein into mice in the identical fashion. A similar pattern of GFP expression was found in the liver following plasmid injection, with hepatocytes as the major type of liver cells expressing GFP transgene (Fig. 4).
Fig. 4.
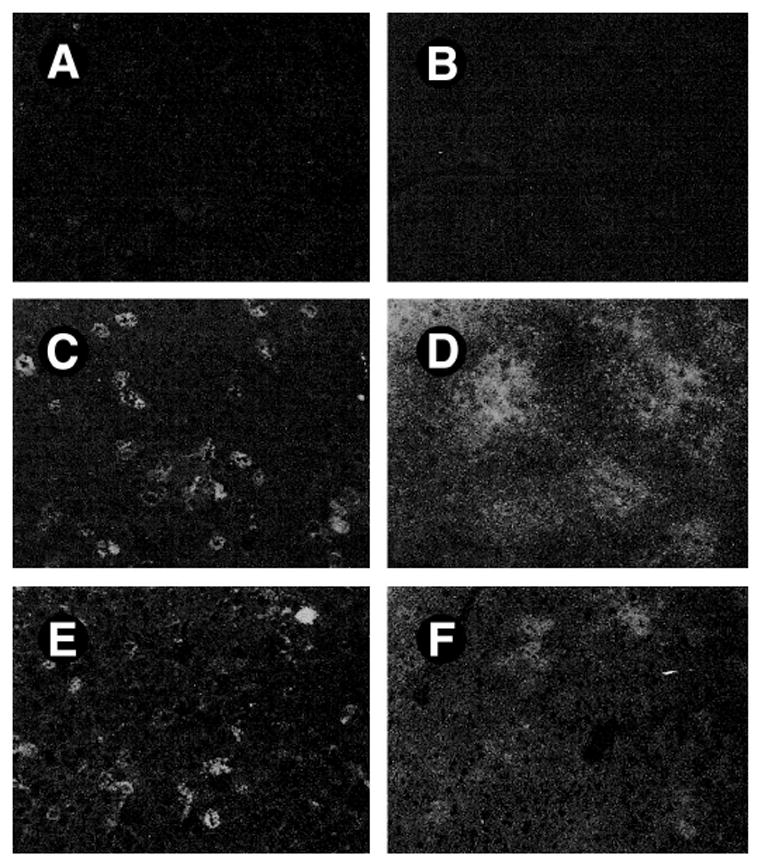
Exogenous gene is predominantly expressed in the hepatocytes of liver. Livers were collected from mice at different time points as indicated following a single injection of pCMV-HGF and pCMV-GFP plasmid vectors, respectively. (A, C, E) Frozen sections were immunostained with antihuman HGF antibody (H14) and observed under fluorescence microscope. (A) No human HGF staining was observed in liver section of control mice. (C) Abundant hepatocytes were HGF–positive in liver sections at 8 hours following injection of pCMV-HGF. (E) Significant expression of HGF was still obvious at 3 days after initial administration of pCMV-HGF. No staining occurs when omitting the primary antibody or replacing it with normal mouse IgG. (B, D, F) Frozen liver sections from the mice injected with pCMV-GFP were directly observed under fluorescence microscope. (B) Control. (D) At 8 hours after injection of pCMV-GFP. (F) At 3 days after injection of pCMV-GFP.
Because induction of human HGF protein in the circulation is relatively transient with a rapid decline after its peak level (Fig. 2), we intended to determine the kinetics of tissue exogenous HGF levels following a single injection of plasmid vector. As shown in Fig. 5, human HGF protein in liver rose rapidly, peaked at 8 hours, and declined thereafter. This pattern largely resembles that of HGF protein in the circulation (Fig. 2), except that HGF peak in liver was several hours earlier than that in the plasma. To our surprise, human HGF protein in the kidney was slightly increased, rather than decreased as expected, after 8 hours following plasmid injection. In fact, at 3 and 6 days after injection, tissue exogenous HGF protein levels in the liver and kidneys of the mice were comparable with each other (Fig. 5). Such results determined by a specific ELISA are further confirmed by the Western immunoblotting data. Shown in Fig. 5B, tissue HGF protein in the kidney was at least sustained, if not increased, to 6 days after initial plasmid injection, whereas human HGF in liver sharply declined during the same period of time.
Fig. 5.
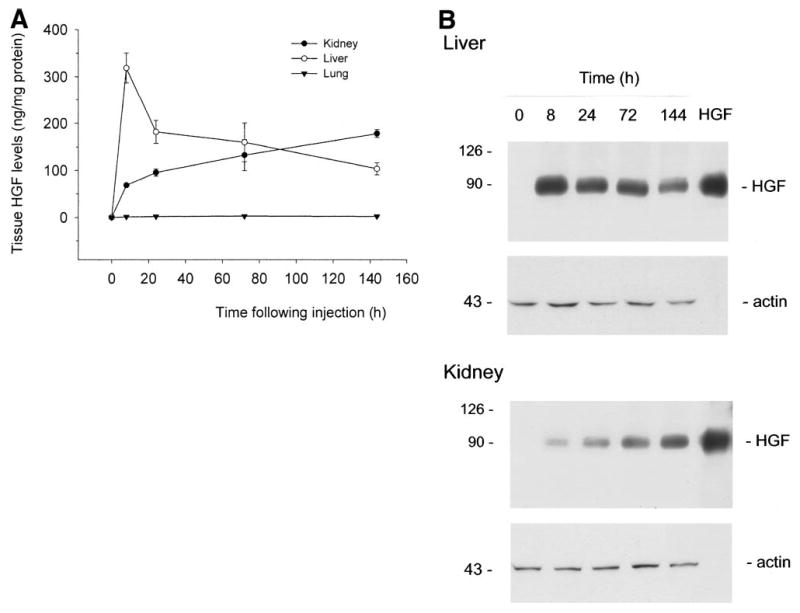
Time course of tissue exogenous HGF levels in mice following a single injection of the plasmid vector. Various tissues were collected from mice at different time points as indicated following a single injection of pCMV-HGF plasmid. Tissue HGF protein levels were determined either by a specific ELISA for human HGF protein (A) or by Western immunoblotting (B). (A) Tissue human HGF levels detected by ELISA are expressed as ng per mg of total protein. Data are presented as mean ± SD. (n = 4). (B) Tissue total protein was immunoblotted with a specific monoclonal antibody (clone H14) for human HGF. Thirty micrograms of liver total protein was loaded into each lane, while 60 μg of total protein was used for kidney samples. Purified recombinant human HGF (10 ng) was also loaded in the adjacent lane to confirm the correct size of hybridized signal and to estimate the amounts of HGF protein in each sample. The same blot was stripped and reprobed with anti-actin antibody to confirm equal loading.
Long-Term Expression of HGF Gene by Repeated Injections of Plasmid Vector
To achieve a long-term expression of exogenous HGF gene in vivo, we explored this issue by repeated injections of naked plasmid DNA into mice. As shown in Fig. 6, repeated weekly injections for 8 weeks produced a repetitive pattern of HGF expression profile in the circulation. The peak levels of exogenous human HGF protein in the circulation were found at 12 hours immediately after each weekly injection (Fig. 6). The levels of HGF protein declined, but still significantly elevated at 6 days following each injection, similar to the kinetics of HGF expression following a single injection as described earlier (Fig. 2). As a negative control, repeated weekly injections of empty plasmid vector pcDNA3 produced no exogenous human HGF protein (Fig. 6). Of interest, there was a tendency that the absolute levels of exogenous HGF protein in the circulation gradually increased both at peak and at the baseline following each weekly injection within the first 5 weeks (Fig. 6). These data indicate that high level, repetitive expression of exogenous HGF gene is achievable through repeated injections of naked plasmid DNA.
Fig. 6.
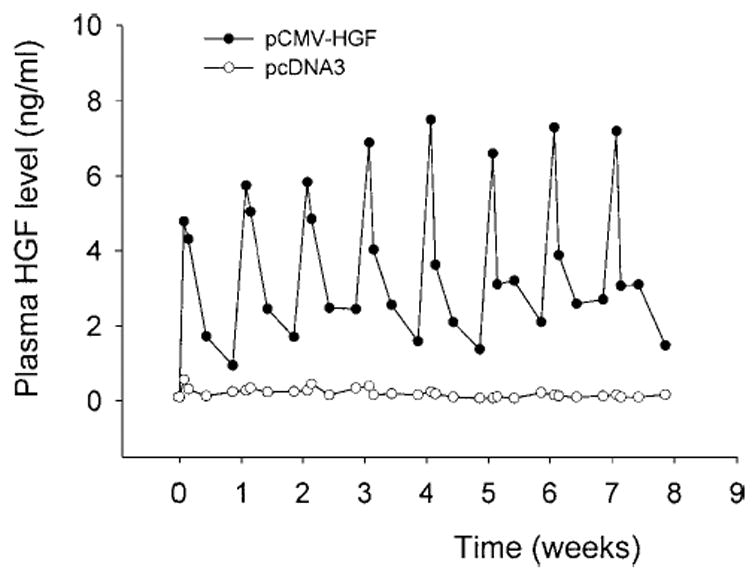
Repetitive expression of HGF gene in mice following repeated injections of naked plasmid DNA. Mice were injected once a week for 8 weeks via the tail vein with a large volume of solution containing 10 μg of either human HGF cDNA plasmid (pCMV-HGF) or empty plasmid (pcDNA3). At different time points (0.5, 1, 3, and 6 days following each injection), the plasma was collected and circulating HGF levels were determined by a specific ELISA for human HGF protein. Data are presented as means of 6 animals (n = 6).
To examine tissue exogenous HGF levels in mice following repeated injections of naked plasmid, we measured the human HGF levels in various tissues at the end point of the first, fourth, and eighth weekly injections, respectively. Because each time point was selected at 6 days following weekly plas-mid injection, based on the circulating HGF protein levels described earlier (Fig. 6), we reasoned that exogenous HGF levels at these time points could represent the lowest level possible in a tissue during the entire 8 weeks experimental scheme. As shown in Fig. 7, tissue human HGF levels in the liver and kidneys were no less than 100 ng per mg of total protein at any time point, as shown by ELISA and by Western blots.
Fig. 7.
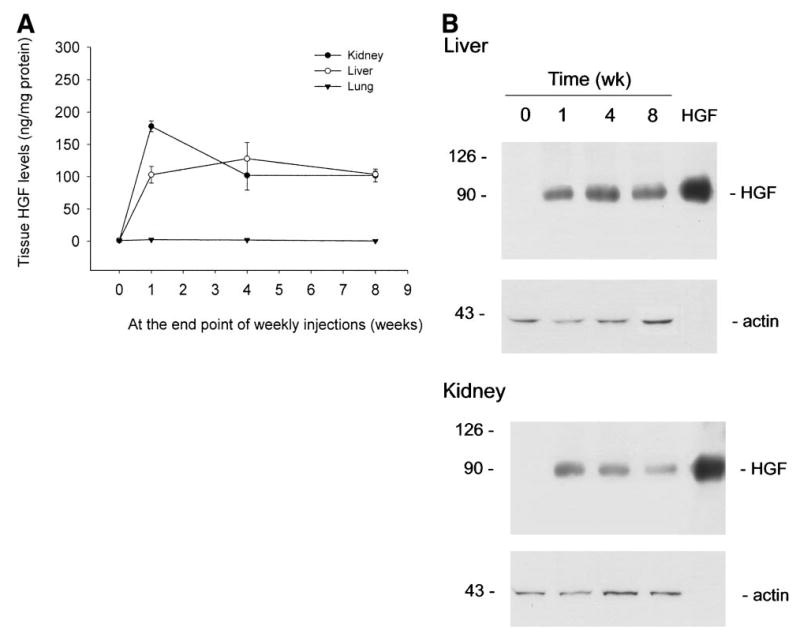
Tissue exogenous HGF levels are sustained in mice following repeated injections of naked plasmid DNA. Mice were injected once a week for 8 weeks via the tail vein with 10 μg of either pCMV-HGF or pcDNA3. Six days following the first, fourth, and eighth injections, various tissues were collected and homogenized from mice and HGF levels determined by ELISA and Western blotting. (A) Human HGF levels in tissues determined ELISA. (B) Western blot showing sustainable expression of human HGF protein in various tissues for 8 weeks. Purified recombinant human HGF (10 ng) was also loaded in the adjacent lane to confirm the correct size of hybridized signal and to estimate the amounts of HGF protein in each sample. The same blot was stripped and reprobed with anti-actin antibody to confirm equal loading.
HGF Gene Expression Promotes Liver and Overall Body Growth
Sustained expression of exogenous HGF gene in mice by repeated injection did not produce any obvious changes in phenotype and behavior when compared with those group-injected with pcDNA3 empty plasmid. However, we found that mice receiving HGF gene transfer grew significantly faster than their littermates. Figure 8 shows the body weights of 2 groups of mice receiving weekly injections of either pCMV-HGF or pcDNA3 plasmids for 8 weeks. Significant difference in body weights between these 2 groups of mice was found as early as 1 week following injection. At the end of 8 weeks, the average body weight of the mice receiving human HGF gene transfer was proximately 16% heavier than that of those receiving empty plasmid vector (43.21 ± 0.69 g versus 37.13 ± 1.31 g, P < .01), suggesting that sustained expression of HGF gene in vivo promotes overall body growth.
Fig. 8.
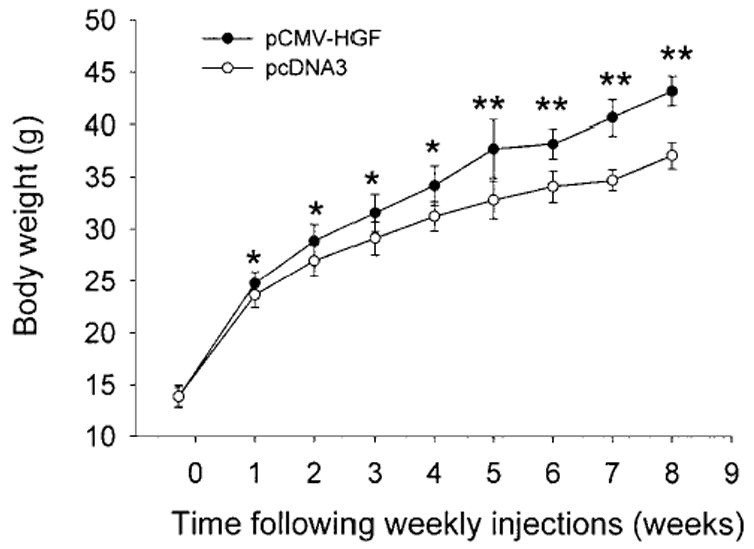
Body weights of mice following repeated injections of naked plas-mid containing HGF gene. Mice were injected once a week for 8 weeks via the tail vein with a large volume of solution containing 10 μg of either pCMV-HGF or pcDNA3. Every week following the injection, the body weights of the mice were recorded. Data are presented as mean ± S.D. (n = 6).
Table 1 shows the weights of major organs in the mice receiving weekly injections of either pCMV-HGF or pcDNA3 plasmid for 4 and 8 weeks, respectively. The absolute weights of major organs such as the liver, kidneys, and spleen were markedly increased in mice receiving HGF gene transfer (Table 1), compared with those receiving empty vector plasmid. However, significant difference of the relative organ weight per body weight was only found for liver and spleen in mice between these 2 groups. These data indicate that sustained expression of exogenous HGF gene in vivo selectively promotes liver and spleen growth.
Table 1.
Body Weight and Organ Weight of the Mice Following Weekly Injections of Naked Plasmid DNA
| Liver
|
Kidney
|
Lung
|
Spleen
|
Heart
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Body Weight (g) | Weight (g) | Ratio (%)* | Weight (g) | Ratio (%) | Weight (g) | Ratio (%) | Weight (g) | Ratio (%) | Weight (g) | Ratio (%) | |
| 4 weeks pcDNA3 n = 6 | 31.30 ± 0.8 | 1.68 ± 0.25 | 5.35 ± 0.79 | 0.47 ± 0.03 | 1.49 ± 0.09 | 0.21 ± 0.02 | 0.66 ± 0.07 | 0.13 ± 0.01 | 0.41 ± 0.03 | 0.16 ± 0.01 | 0.49 ± 0.03 |
| pCMV-HGF n = 6 | 34.11 ±6 1.9‡ | 2.08 ± 0.18† | 6.08 ± 0.29 | 0.54 ± 0.05† | 1.59 ± 0.11 | 0.23 ± 0.01 | 0.67 ± 0.03 | 0.18 ± 0.02† | 0.51 ± 0.15† | 0.16 ± 0.02 | 0.46 ± 0.04 |
| 8 weeks pcDNA3 n = 6 | 37.13 ±6 1.31 | 1.78 ± 0.04 | 4.82 ± 0.17 | 0.50 ± 0.02 | 1.35 ± 0.08 | 0.23 ± 0.01 | 0.61 ± 0.03 | 0.11 ± 0.01 | 0.29 ± 0.02 | 0.22 ± 0.02 | 0.59 ± 0.05 |
| pCMV-HGF n = 6 | 43.21 ±6 0.69‡ | 2.34 ± 0.05‡ | 5.41 ± 0.10‡ | 0.64 ± 0.01‡ | 1.48 ± 0.03 | 0.25 ± 0.01 | 0.57 ± 0.01 | 0.17 ± 0.01‡ | 0.39 ± 0.01‡ | 0.24 ± 0.01 | 0.55 ± 0.04 |
Wet organ weight to body weight ratio.
P < .05.
P < .01.
Activation of Mitogen-Activated Protein Kinase In Vivo After Exogenous HGF Expression
To understand the mechanism underlying organ growth induced by exogenous HGF expression, we investigated the signal transduction events leading to cell proliferation in vivo following injection of HGF expression plasmid vector. As shown in Fig. 9, activation of p44-MAP kinase (Erk1) and p42-MAP kinase (Erk2) was found in the liver and kidneys in vivo at 8 hours following plasmid injection (Fig. 9A). About ninefold induction in the phosphorylated status of these kinases was detected using a phospho-specific antibody. Time course studies revealed that activation of Erk1/2 was peaked at 8 hours in the liver (Fig. 9B), whereas Erk1/2 phosphorylation was maximal at 24 hours in the kidney (Fig. 9C). These distinct kinetics of Erk1/2 activation in the liver and kidneys were closely associated with that of exogenous HGF protein expressed in these organs (Fig. 5). Of note, persistent elevation of phosphorylated Erk1/2 was also observed in liver and kidneys at the end of 4 and 8 weeks following weekly injection of HGF expression plasmid (Fig. 9).
Fig. 9.
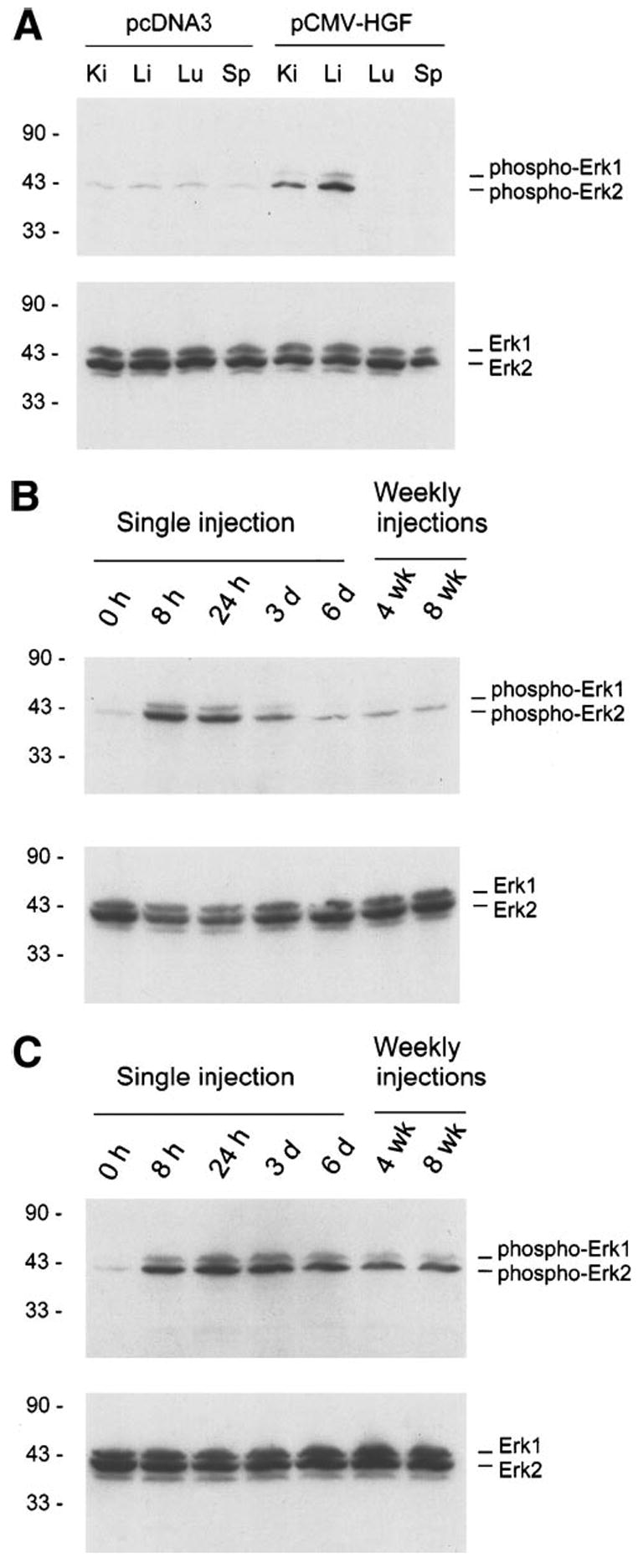
Activation of MAP kinases in liver and kidney following HGF expression. (A) Various tissues were homogenized from the mice given either pCMV-HGF or empty plasmid (pcDNA3) vector. Western blot shows activation of Erk1/2 in liver and kidney at 8 hours following injection. The blot was first probed with a phosphospecific antibody against activated Erk1/2. The same blot was then stripped and reprobed with antibody against total Erk1/2. (B) Time course of Erk1/2 activation in the liver after either single or multiple injections of pCMV-HGF plasmid. (C) Time course of Erk activation in the kidney after either single or multiple injections of pCMV-HGF plasmid. Tissues were collected at different time points as indicated following single injection, or at the 6 days following each weekly injections. The blots were first probed with a phosphospecific antibody against activated Erk1/2, and followed by reprobing with antibody against total Erk1/2.
Because one of the functional consequences of Erk1/2 activation is cell proliferation, we examined the tissue expression of proliferating cell nuclear antigen (PCNA) in vivo after administration of pCMV-HGF plasmid. Western blot analyses of whole-tissue lysates using a specific anti-PCNA monoclonal antibody displayed a 3.2-fold increase in PCNA protein expression in the liver beginning at 24 hours following injection, suggesting that exogenous HGF promotes liver-cell proliferation (Fig. 10A). Of significance, the induction of PCNA expression in liver was sustained as late as 8 weeks following weekly injections. This indicates a persistent cell proliferation in liver during the entire experimental scheme, which ultimately leads to an increased liver weight (Table 1). Similar results were obtained by immunostaining with PCNA antibody in the liver tissue sections (Fig. 10C and D). Elevated levels of PCNA protein were also found in the kidney at 3 and 6 days after injection (Fig. 10B). However, the PCNA protein level in the kidney returned to baseline thereafter, despite persistent Erk1/2 activation in this organ after weekly injections.
Fig. 10.
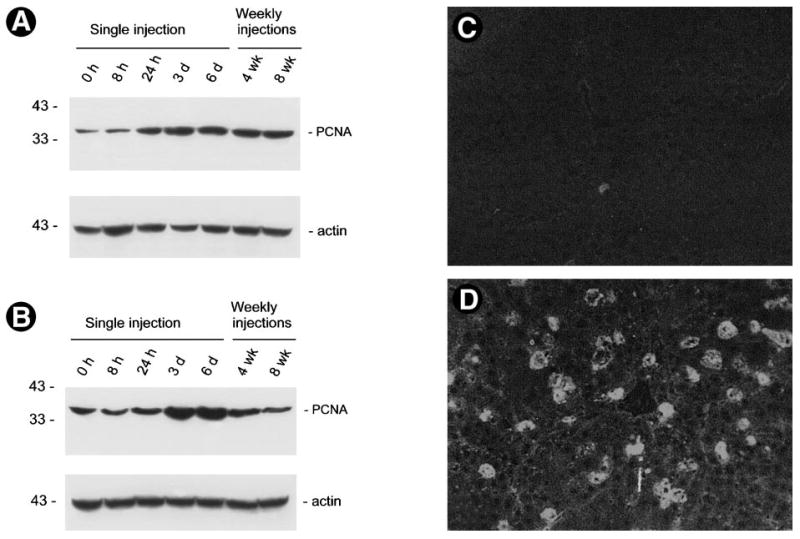
Protein expression of proliferating cell nuclear antigen (PCNA) in whole organs. Liver (A) and kidney (B) were collected from mice at different time points as indicated following either a single or multiple injections of pCMV-HGF plasmid vector. Tissue total protein lysates were immunoblotted with a specific monoclonal antibody against PCNA, a G1-to-S-phase marker. Thirty micrograms of tissue total protein was loaded each lane. Significant elevation of PCNA protein was found in the liver beginning at 24 hours following injection of HGF expression plasmid vector. The PCNA protein levels were transiently increased in the kidney. The same blots were stripped and reprobed with anti-actin antibody to confirm equal loading. (C and D) Immunostaining of PCNA protein expression in liver. (C) Liver cryosection from the mouse at 3 days following injection of pcDNA3. (D) Liver cryosection from the mouse at 3 days after a single injection of pCMV-HGF plasmid.
We also examined the effect of exogenous HGF expression on apoptotic cell death in normal adult animal. TUNEL staining revealed no significant difference in apoptosis in liver sections from the mice receiving either pCMV-HGF or control pcDNA3 plasmid (data not shown).
Tissue Morphology and Functions Following Sustained Expression of HGF Gene In Vivo
We examined the structural and functional consequence of sustained expression of exogenous HGF gene in vivo. Careful observation and anatomy did not reveal any tumor formation and gross abnormality in the mice receiving weekly injections of pCMV-HGF plasmid for 4 and 8 weeks, respectively. As shown in Fig. 11, tissues from major organs such as the liver, kidneys, lung, and spleen at 1 day following injection displayed typically normal morphology, suggesting no acute tissue injury by injection procedure itself or by exogenous HGF expression. Tissues taken from mice receiving weekly repeated injections for 4 and 8 weeks also exhibited no morphological abnormalities (Fig. 11). No significant difference in urine protein was found among these groups (data not shown). These data suggest that either injections of plasmid vector itself (pcDNA3) or sustained expression of exogenous HGF (pCMV-HGF) for the long term did not produce any noticeable, adverse side effects in vivo.
Fig 11.
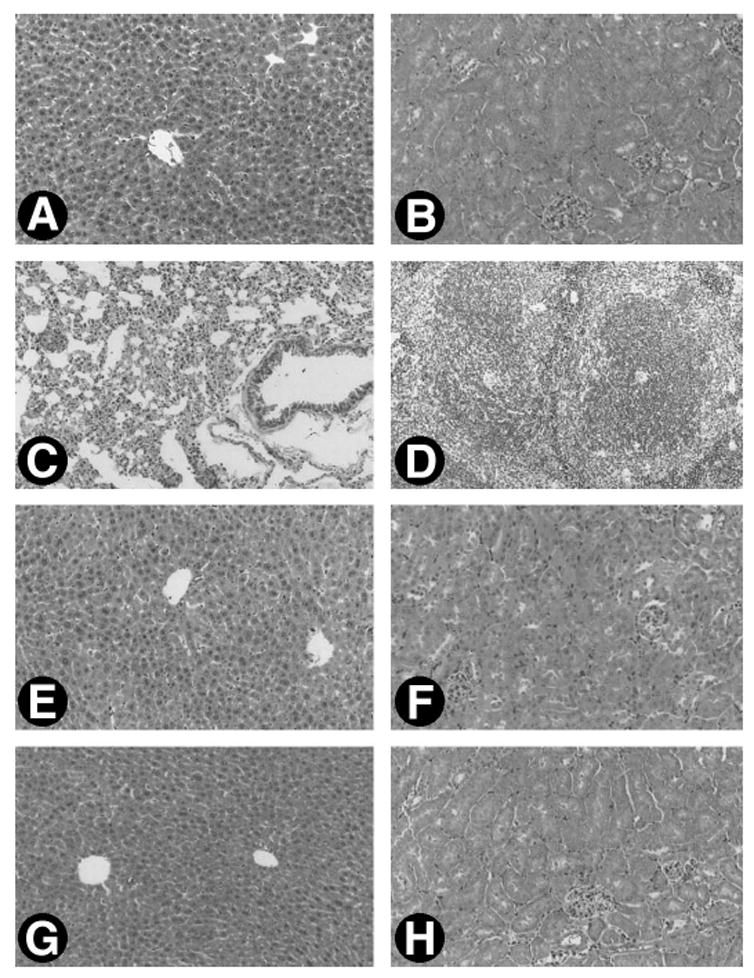
Microscopic morphology of major organs of mice following single or repeated injections of pCMV-HGF plasmid. Mice were sacrificed at 1 day following single injection or at 4 and 8 weeks after weekly injections of either pCMV-HGF or pcDNA3 plasmid. Representative micrograph shows the normal tissue morphology of major organs of the mice following injections. (A–D) Tissue morphology of liver, kidneys, lung, and spleen at 1 day following injection of pCMV-HGF, respectively. (E and F) Morphology of liver and kidney at 4 weeks following weekly injections of pCMV-HGF. (G and H) Morphology of liver and kidney at 8 weeks following weekly injections of pCMV-HGF. Normal tissue morphology was also observed in various organs at different time points following injection of control pcDNA3 plasmid (data not shown).
DISCUSSION
Although delivery of HGF could theoretically be achieved through injections of recombinant HGF protein, there are several major obstacles to administration of HGF via direct injection. First, it is enormously difficult to maintain a constantly high level of exogenous HGF in circulation even using repeated injections at short intervals, as it is rapidly cleared by the liver in vivo. Furthermore, HGF is shown to have therapeutic roles in preventing tissue fibrosis and dysfunctions in a variety of organs.25–28 The progression of these chronic tissue fibrosis diseases is a slow process that often progresses over long periods ranging from years to decades in human.29–32 Given that long-term therapy may be necessary, repeated injection of recombinant HGF is not only inconvenient, but also extremely costly. In addition, the difficulty in making large quantities of biologically active recombinant HGF is also overwhelming. HGF is a large molecule with molecular weight of 95 kd as well as a glycoprotein whose biological activity depends on proper folding and processing in a suitable environment. It is possible that HGF produced in a bacterial vector expressing system is biologically inactive or less active because of the lack of post-translational modification and incorrect folding. Therefore, our results on developing a gene delivery strategy allowing efficient expression of exogenous HGF in vivo provide a simple and effective approach for studying HGF biological functions and for evaluating its therapeutic efficacy in whole animals.
The level of exogenous HGF expression achieved in this report via systemic administration of plasmid vector is quite impressive. At 8 hours following single injection of 10 μg of HGF-expressing plasmid vector, human HGF level reached to more than 300 ng per mg of total protein, representing approximately 50 μg of HGF protein per gram of liver. This level of exogenous HGF expression is comparable with that of luciferase marker gene expression recently reported using the same gene delivery approach.16 Of note, previous studies using intramuscular injection of HGF expression plasmid with HVJ liposomes only achieved 0.1 ng/mL of human HGF in rat circulation, although the level of exogenous HGF protein in the rat tissues was not examined in that study.20 Thus, the current approach described in this article establishes a highly efficient way to introduce and express HGF gene in vivo that results in the highest level of HGF transgene expression ever achieved in whole animals. More importantly, we have achieved sustained, long-term, high level of HGF gene expression in vivo by repeated injections of plasmid in a controllable fashion. Although the long-term expression of exogenous HGF in mice is terminated at the end of 8 weeks in the current study, this kind of approach to allow sustainable expression of HGF by weekly injections of plasmid vector could presumably be carried on during the entire lifespan of the mice. This markedly distinguishes it from the approach of HGF gene delivery via high dose of adenoviral vector,33 in which it is extremely difficult to sustain HGF gene expression by repeated injections because of induction of the well-known adverse immune responses.
The liver and kidneys appear to be the organs with high levels of transgene HGF expression, whereas the lung and spleen display a low level of exogenous gene expression. These results are mostly likely due to the characteristic nature of the hydrodynamic and anatomical flow of the injected plas-mid DNA solution of this procedure. As discussed previously,16 when the injection rate exceeds the cardiac output, the injected plasmid DNA solution likely accumulates in the inferior vena cava, leading to a high hydrostatic pressure in that area. This pressure will force the flow of DNA solution into those organs that are directly connected to the inferior vena cava, such as the liver and kidney.16 In agreement with this notion, we found that abundant hepatocytes express transgene following a single injection of a large volume of GFP expression plasmid in vivo (Fig. 4).
While human HGF protein in liver is typically peaked at 8 hours, the peak of exogenous HGF protein in the circulation occurs at 12 hours following initial injection of HGF expression plasmid (Fig. 2). This time lag implies that the circulating HGF protein is secreted from various tissues, most likely from the liver where exogenous HGF is robustly expressed. Of interest, although liver produces a large amount of exogenous HGF protein shortly following the injection, the level of human HGF protein within liver rapidly declines. In contrast, HGF protein levels in other organs do not fall (Fig. 5). In the kidney, the exogenous HGF at 24 hours is even higher than that at 8 hours following injection, probably due to the accumulation of expressed protein. The rapid decline of exogenous HGF in liver is unlikely caused by a sudden loss of HGF production, because significant expression of exogenous genes including GFP still takes place as late as 6 days following plasmid injection. Rather, it is probably a result of the rapid clearance of HGF protein by liver.10,11 Another notable factor contributing to the fall of HGF level in liver could be the rapid release and secretion of HGF into circulation. This notion is supported by the concomitant fall and rise of human HGF protein in liver and blood circulation, respectively.
The high-level, long-term expression of exogenous HGF for 8 weeks in whole animals not only markedly augments the absolute liver weight, but also significantly increases the liver/body ratio. This indicates that HGF, as its name implies, indeed promotes liver growth in normal adult animals. Of great interest, expression of exogenous HGF activates growth-related signal transduction events and persistently induces the expression of PCNA, an indicative marker of cell proliferation (Figs. 9 and 10). Of note, previous studies suggest that short-term infusion of exogenous HGF into animals only promote hepatocyte replication in injury-primed liver but not in normal intact liver.34,35 This discrepancy is probably a result of the extremely short half-life of exogenous HGF in liver as well as the low amount of HGF infused. Indeed, when we increased HGF half-life in the circulation by mixture with dextran sulfate and by continuous delivery using an osmotic mini-pump, exogenous HGF did promote liver growth in normal animals.36 Although Erk1/2 activation also leads to promotion of cell survival, we do not find significant difference in apoptotic cell death by TUNEL staining following exogenous HGF gene expression in adult mice. This suggests that the increase in liver weight after HGF gene transfer is mostly mediated by enhanced cell proliferation, rather than by decreasing cell death. It should be noted that HGF expression also causes an increase in spleen weight (Table 1). The reason underlying this phenomenon is unknown. One simple explanation for the enlargement of spleen could be a result of the immune response to human HGF in mice. Further experiments using plasmid vector containing mouse HGF cDNA are needed to clarify this issue.
Although the fact that long-term HGF expression in whole animals promotes liver growth is not surprising, it is quite interesting to find that HGF facilitates overall body growth of the animals. As early as 6 days following a single injection of HGF expression plasmid vector, significant difference in the body weight is found in 2 groups of mice that received either HGF plasmid or empty vector (Fig. 8). This suggests that short-term exposure to high level of exogenous HGF is sufficient to enhance the metabolism of the animals. Given that the specific receptor for HGF, c-met, is widely expressed in various organs,37,38 it is not difficult to speculate that the increased levels of systemic HGF protein following injections can target diverse tissues in vivo, which eventually lead to overall growth in whole animals.
It is worthwhile to point out that the plasmid injection procedure itself as well as long-term expression of exogenous HGF has not produced any adverse side effects. Despite apparent activation of mitogen-activated protein kinases and increased cell proliferation in liver and kidneys, no tumor formation and other lesions are found in these organs. This suggests that long-term administration of HGF for therapeutic purposes is probably safe and unlikely results in severe adverse effects in vivo. This notion is further supported by the phenotypic characterization of several HGF transgenic mouse lines recently developed independently in different laboratories.39–41 However, phenotypes with tumorigenesis and chronic renal failure are also reported in a particular strain of HGF transgenic mice in which HGF is driven under metallothionein promoter.42 This phenotypic difference could be because of the inherent nature of de novo HGF expression and/or random chromosomal insertions associated with transgenic model. Nevertheless, we cannot exclude the possibility that the mice receiving HGF plasmid injections could develop tumors at late time points beyond the 8 weeks that we follow-up, or display to be more susceptible to carcinogenic challenges.
In summary, we have described in this article a convenient, safe, and efficient approach to introduce and express a high level of exogenous HGF in vivo by systemic injection of a large volume of naked plasmid solution. By repeated injections, we have achieved sustained, long-term expression of HGF gene in a controllable fashion. Expression of HGF transgene activates MAP kinases and promotes cell proliferation that ultimately leads to liver and overall body growth. Although current technology may not be directly applied to human HGF gene therapy for immediate usage in a clinical setting, this approach may open a wide window for investigating the biological functions, evaluating the therapeutic efficacy, and studying the transcriptional regulation in vivo of not only HGF, but also any gene of interest, in whole animals.
Acknowledgments
The authors thank Dr. Vivian Lui for providing expert advice on the technique of plasmid injection. The authors are also grateful to Dr. Chunsun Dai for his help with TUNEL staining.
Abbreviations
- HGF
hepatocyte growth factor
- CMV
cytomegalovirus
- ELISA
enzyme-linked immunosorbent assay
- MAP kinase
mitogen-activated protein kinase
- Erk1/2
extracellular signal-regulated kinase1/2
- PCNA
proliferating cell nuclear antigen
- PBS
phosphate-buffered saline
- GFP
green fluorescent protein
Footnotes
Supported by the National Institutes of Health grants DK-02611, DK-54922 (Y.L.), DK-44935, DK-54552 (L.H.), CA-35373 (G.K.M.), and National Natural Science Foundation of China grant 39825508 (Y.L.). J.Y. was supported in part by the Pathology Postdoctoral Research Training Grant from the Department of Pathology at the University of Pittsburgh School of Medicine.
References
- 1.Zarnegar R, Michalopoulos GK. The many faces of hepatocyte growth factor: from hepatopoiesis to hematopoiesis. J Cell Biol. 1995;129:1177–1180. doi: 10.1083/jcb.129.5.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsumoto K, Nakamura T. Emerging multipotent aspects of hepatocyte growth factor. J Biochem. 1996;119:591–600. doi: 10.1093/oxfordjournals.jbchem.a021283. [DOI] [PubMed] [Google Scholar]
- 3.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 4.Vargas GA, Hoeflich A, Jehle PM. Hepatocyte growth factor in renal failure: promise and reality. Kidney Int. 2000;57:1426–1436. doi: 10.1046/j.1523-1755.2000.00987.x. [DOI] [PubMed] [Google Scholar]
- 5.Kosai K, Matsumoto K, Funakoshi H, Nakamura T. Hepatocyte growth factor prevents endotoxin-induced lethal hepatic failure in mice. HEPATOLOGY. 1999;30:151–159. doi: 10.1002/hep.510300102. [DOI] [PubMed] [Google Scholar]
- 6.Miller SB, Martin DR, Kissane J, Hammerman MR. Hepatocyte growth factor accelerates recovery from acute ischemic renal injury in rats. Am J Physiol. 1994;266:F129–F134. doi: 10.1152/ajprenal.1994.266.1.F129. [DOI] [PubMed] [Google Scholar]
- 7.Ueda T, Takeyama Y, Hori Y, Shinkai M, Takase K, Goshima M, Yamamoto M, et al. Hepatocyte growth factor increases in injured organs and functions as an organotrophic factor in rats with experimental acute pancreatitis. Pancreas. 2000;20:84–93. doi: 10.1097/00006676-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Hammerman MR, Safirstein R, Harris RC, Toback FG, Humes HD. Acute renal failure. III. The role of growth factors in the process of renal regeneration and repair. Am J Physiol Renal Physiol. 2000;279:F3–F11. doi: 10.1152/ajprenal.2000.279.1.F3. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Rajur K, Tolbert E, Dworkin LD. Endogenous hepatocyte growth factor ameliorates chronic renal injury by activating matrix degradation pathways. Kidney Int. 2000;58:2028–2043. doi: 10.1111/j.1523-1755.2000.00375.x. [DOI] [PubMed] [Google Scholar]
- 10.Kawaida K, Matsumoto K, Shimazu H, Nakamura T. Hepatocyte growth factor prevents acute renal failure and accelerates renal regeneration in mice. Proc Natl Acad Sci U S A. 1994;91:4357–4361. doi: 10.1073/pnas.91.10.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu ML, Mars WM, Zarnegar R, Michalopoulos GK. Uptake and distribution of hepatocyte growth factor in normal and regenerating adult rat liver. Am J Pathol. 1994;144:129–140. [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson WF. Human gene therapy. Nature. 1998;392(Suppl):25–30. doi: 10.1038/32058. [DOI] [PubMed] [Google Scholar]
- 13.Kelley VR, Suhatme VP. Gene transfer in the kidney. Am J Physiol. 1999;276:F1–F9. doi: 10.1152/ajprenal.1999.276.1.F1. [DOI] [PubMed] [Google Scholar]
- 14.Imai E, Isaka Y. New paradigm of gene therapy: skeletal-muscle-targeting gene therapy for kidney disease. Nephron. 1999;83:296–300. doi: 10.1159/000045420. [DOI] [PubMed] [Google Scholar]
- 15.Li K, Welikson RE, Vikstrom KL, Leinwand LA. Direct gene transfer into mouse heart. J Mol Cell Cardiol. 1997;29:1499–1504. doi: 10.1006/jmcc.1997.0389. [DOI] [PubMed] [Google Scholar]
- 16.Liu F, Song YK, Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999;6:1258–1266. doi: 10.1038/sj.gt.3300947. [DOI] [PubMed] [Google Scholar]
- 17.Crystal RG. Transfer of genes to humans: early lessons and obstacles to success. Science. 1995;270:404–410. doi: 10.1126/science.270.5235.404. [DOI] [PubMed] [Google Scholar]
- 18.Mann MJ, Gibbons GH, Hutchinson H, Poston RS, Hoyt EG, Robbins RC, Dzau VJ. Pressure-mediated oligonucleotide transfection of rat and human cardiovascular tissues. Proc Natl Acad Sci U S A. 1999;96:6411–6416. doi: 10.1073/pnas.96.11.6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ueda H, Sawa Y, Matsumoto K, Kitagawa-Sakakida S, Kawahira Y, Nakamura T, Kaneda Y, et al. Gene transfection of hepatocyte growth factor attenuates reperfusion injury in the heart. Ann Thorac Surg. 1999;67:1726–1731. doi: 10.1016/s0003-4975(99)00279-9. [DOI] [PubMed] [Google Scholar]
- 20.Ueki T, Kaneda Y, Tsutsui H, Nakanishi K, Sawa Y, Morishita R, Matsumoto K, et al. Hepatocyte growth factor gene therapy of liver cirrhosis in rats. Nat Med. 1999;5:226–230. doi: 10.1038/5593. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Centracchio JN, Lin L, Sun AM, Dworkin LD. Constitutive expression of HGF modulates renal epithelial cell phenotype and induces c-met and fibronectin expression. Exp Cell Res. 1998;242:174–185. doi: 10.1006/excr.1998.4107. [DOI] [PubMed] [Google Scholar]
- 22.Tsurumi Y, Takeshita S, Chen D, Kearney M, Roissow S, Passeri J, Horowitz J, Symes J. Direct intramuscular gene transfer of naked DNA encoding vascular endothelial growth factor augments collateral development and tissue perfusion. Circulation. 1996;94:3281–3290. doi: 10.1161/01.cir.94.12.3281. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Tolbert EM, Lin L, Thursby MA, Sun AM, Nakamura T, Dworkin LD. Up-regulation of hepatocyte growth factor receptor: an amplification and targeting mechanism for hepatocyte growth factor action in acute renal failure. Kidney Int. 1999;55:442–453. doi: 10.1046/j.1523-1755.1999.00267.x. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y. Hepatocyte growth factor promotes renal epithelial cell survival by dual mechanisms. Am J Physiol Renal Physiol. 1999;277:F624–F633. doi: 10.1152/ajprenal.1999.277.4.F624. [DOI] [PubMed] [Google Scholar]
- 25.Yasuda H, Imai E, Shiota A, Fujise N, Morinaga T, Higashio K. Antifibrogenic effect of a deletion variant of hepatocyte growth factor on liver fibrosis in rats. HEPATOLOGY. 1996;24:636–642. doi: 10.1053/jhep.1996.v24.pm0008781336. [DOI] [PubMed] [Google Scholar]
- 26.Mizuno S, Kurosawa T, Matsumoto K, Mizuno-Horikawa Y, Okamoto M, Nakamura T. Hepatocyte growth factor prevents renal fibrosis and dysfunction in a mouse model of chronic renal disease. J Clin Invest. 1998;101:1827–1834. doi: 10.1172/JCI1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yaekashiwa M, Nakayama S, Ohnuma K, Sakai T, Abe T, Satoh K, Matsumoto K, et al. Simultaneous or delayed administration of hepatocyte growth factor equally represses the fibrotic changes in murine lung injury induced by bleomycin: a morphologic study. Am J Respir Crit Care Med. 1997;156:1937–1944. doi: 10.1164/ajrccm.156.6.9611057. [DOI] [PubMed] [Google Scholar]
- 28.Matsumoto K, Mizuno S, Nakamura T. Hepatocyte growth factor in renal regeneration, renal disease and potential therapeutics. Curr Opin Nephrol Hypertens. 2000;9:395–402. doi: 10.1097/00041552-200007000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Eddy AA. Molecular insights into renal interstitial fibrosis. J Am Soc Nephrol. 1996;7:2495–2508. doi: 10.1681/ASN.V7122495. [DOI] [PubMed] [Google Scholar]
- 30.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–2250. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 31.Kliem V, Johnson RJ, Alpers CE, Yoshimura A, Couser WG, Koch KM, Floege J. Mechanisms involved in the pathogenesis of tubulointerstitial fibrosis in 5/6-nephrectomized rats. Kidney Int. 1996;49:666–678. doi: 10.1038/ki.1996.95. [DOI] [PubMed] [Google Scholar]
- 32.Hostetter TH. Progression of renal disease and renal hypertrophy. Annu Rev Physiol. 1995;57:263–278. doi: 10.1146/annurev.ph.57.030195.001403. [DOI] [PubMed] [Google Scholar]
- 33.Gao C, Jokerst R, Gondipalli P, Cai SR, Kennedy S, Ponder KP. Intramuscular injection of an adenoviral vector expressing hepatocyte growth factor facilitates hepatic transduction with a retroviral vector in mice. Hum Gene Ther. 1999;10:911–922. doi: 10.1089/10430349950018319. [DOI] [PubMed] [Google Scholar]
- 34.Webber EM, Godowski PJ, Fausto N. In vivo response of hepatocytes to growth factor requires an initial priming stimulus. HEPATOLOGY. 1994;19:489–497. [PubMed] [Google Scholar]
- 35.Liu ML, Mars WM, Zarnegar R, Michalopoulos GK. Collagenase pretreat ment and the mitogenic effects of hepatocyte growth factor and transforming growth factor-alpha in adult rat liver. HEPATOLOGY. 1994;19:1521–1527. [PubMed] [Google Scholar]
- 36.Roos F, Ryan AM, Chamow SM, Bennett GL, Schwall RH. Induction of liver growth in normal mice by infusion of hepatocyte growth factor/scatter factor. Am J Physiol. 1995;268:G380–386. doi: 10.1152/ajpgi.1995.268.2.G380. [DOI] [PubMed] [Google Scholar]
- 37.Chan AML, King HWS, Deakin EA, Tempest PR, Hilkens J, Kroezen V, Edwards DR, et al. Characterization of the mouse met proto-oncogene. Oncogene. 1988;2:595–599. [PubMed] [Google Scholar]
- 38.Liu Y, Tolbert E, Sun AM, Dworkin LD. Primary structure of rat HGF receptor and induced expression in glomerular mesangial cells. Am J Physiol. 1996;271:F679–F688. doi: 10.1152/ajprenal.1996.271.3.F679. [DOI] [PubMed] [Google Scholar]
- 39.Shiota G, Wang TC, Nakamura T, Schmidt EV. Hepatocyte growth factor in transgenic mice: effects on hepatocyte growth, liver regeneration and gene expression. HEPATOLOGY. 1994;19:962–972. [PubMed] [Google Scholar]
- 40.Bell A, Chen Q, DeFrances MC, Michalopoulos GK, Zarnegar R. The five amino acid-deleted isoform of hepatocyte growth factor promotes carcinogenesis in tansgenic mice. Oncogene. 1999;18:887–895. doi: 10.1038/sj.onc.1202379. [DOI] [PubMed] [Google Scholar]
- 41.Garcia-Ocana A, Takane KK, Syed MA, Philbrick WM, Vasavada RC, Stewart AF. Hepatocyte growth factor overexpression in the islet of transgenic mice increases beta cell proliferation, enhances islet mass, and induces mild hypoglycemia. J Biol Chem. 2000;275:1226–1232. doi: 10.1074/jbc.275.2.1226. [DOI] [PubMed] [Google Scholar]
- 42.Takayama H, LaRochelle WJ, Sharp R, Otsuka T, Kriebel P, Anver M, Aaronson SA, Merlino G. Diverse tumorigenesis associated with aberrant development in mice overexpressing hepatocyte growth factor/scatter factor. Proc Natl Acad Sci U S A. 1997;94:701–706. doi: 10.1073/pnas.94.2.701. [DOI] [PMC free article] [PubMed] [Google Scholar]


