Abstract
Rats with chimeric livers were generated by using the protocol of injecting hepatocytes from dipeptidyl peptidase IV (DPPIV)-positive donors into retrorsine-treated DPPIV-negative recipients subjected to partial hepatectomy. Rats with established chimeric livers were subjected to bile duct ligation, with or without pretreatment with the biliary toxin methylene diamiline (DAPM). Ductules bearing the donor hepatocyte marker DPPIV were seen at 30 days after bile duct ligation. The frequency of the ductules derived from the donor hepatocytes was dramatically enhanced (36-fold) by the pretreatment with DAPM. In conclusion, our results show that hepatocytes can function as facultative stem cells and rescue the biliary epithelium during repair from injury when its proliferative capacity is being compromised.
Hepatocytes and biliary epithelium are the two mature epithelial cells of the adult liver. They both derive from the hepatoblasts of the embryonic liver during embryogenesis. During postnatal life, injury to the liver combined with arrest of proliferation of hepatocytes leads to proliferation of cells known as “oval cells” (in rodent injury models), which transform into hepatocytes and restore the loss of hepatic parenchyma.1–6 The same cell types have been called “ductular hepatocytes” (from studies of histopathology of human disease).7–9 These cells express genes associated with both hepatocytes and biliary cells and appear in many human diseases, from fulminant hepatitis to primary biliary cirrhosis.10–12 Experimental studies with rodent models have shown that oval cells derive from progenitor cells that have properties of biliary epithelium.13,14 The precursors of oval cells may be the cells residing in the canals of Hering. The latter are small ductules lined by biliary epithelium, and they constitute the first defined ductular system carrying newly synthesized bile from the bile canaliculi of the hepatocytes to the portal biliary ductules. Canals of Hering are in close proximity to hepatocytes. Recent studies have defined the length and dimensions of the canals of Hering and have demonstrated that these canals penetrate deep inside the lobules.15–18 Partial hepatectomy followed by administration of N-2 acetyl-aminofluorene (AAF) is a protocol often used to generate large populations of oval cells.2 Partial hepatectomy (PHx) stimulates hepatocyte proliferation, as part of the normal process leading to regeneration of the hepatic tissue. AAF inhibits hepatocyte proliferation. Oval cells appear within 3 to 5 days after PHx in the periportal areas of the hepatic lobule. Studies using this model have shown that, in addition to the canals of Hering, epithelial cells of the biliary ductules may also contribute to the precursor pool of oval cells. Hepatocyte-associated transcription factors appear in the nuclei of biliary epithelial cells of the portal ductules within 1 to 2 days after AAF-PHx, suggesting that these cells actively transdifferentiate to precursors of the oval cells.19 Previous studies from our laboratory have shown that injury to the biliary epithelium by administration of the biliary toxin methylene dianiline (DAPM) before the AAF-PHx protocol inhibits formation of the oval cells.20 This finding also showed that oval cells derive from biliary precursors. Studies from many other laboratories have shown the close connection between oval cells and biliary epithelium. Recent studies by Strain et al. have shown that oval cells in human liver biopsy specimens express an array of biliary markers as well as Bcl-2 and neural adhesion molecules.21 Oval cells produce alpha-fetoprotein and transform into small hepatocytes producing albumin and other hepatocyte-associated markers within 6 to 8 days after PHx. These small hepatocytes incorporate themselves into the histological architecture of the lobule and allow restoration of the lost hepatic mass. The precise timetable of the cellular events varies between studies. Oval cells and their transformation to hepatocytes appear later if chemical injury is administered instead of PHx.22
These studies show that in situations in which hepatocytes are unable to proliferate after loss of hepatic tissue, biliary cells will function as facultative stem cell for hepatocytes and lead to restoration hepatic mass. The opposite pathway, that is, the transdifferentiation of hepatocytes to biliary epithelial cells, has not been clearly shown in whole animal or human disease models. There is suggestive evidence that this occurs from studies of human liver. Strain et al. have shown that OV6, a marker expressed in rodent oval cells as well as the ductal plate cells and hepatoblasts of the human liver, also appears in clusters of human hepatocytes in many hepatic diseases, including biliary atresia and alpha-1-antitrypsin deficiency, primary biliary cirrhosis, and primary sclerosing cholangitis.10,11,21
We recently showed that hepatocytes can convert to biliary epithelial cells in organoid cultures.23 In that study, we used Fisher rats with chimeric livers. In these livers, a part of the hepatocyte population is derived from donor dipeptidyl peptidase IV (DPPIV)-positive Fischer rats, in which hepatocytes express the enzymatic marker DPPIV. This marker is not expressed in the recipient DPPIV-negative Fischer rats, neither in hepatocytes nor in biliary epithelial cells. The histology of the chimeric livers has been extensively described by Laconi et al.24 and Shafritz et al.25,26 Biliary epithelial cells positive for the hepatocyte marker DPPIV were seen in the organoid cultures derived from the chimeric livers, thus providing proof of principle that biliary epithelial cells can derive from hepatocytes.
In the current study, we use the same Fischer rat DP-PIV chimeric liver model, to examine the potential contribution of hepatocytes to formation of biliary epithelium in the whole animal. We used the chronic biliary injury model of bile duct ligation. Previous studies have shown that bile duct ligation is followed by intense proliferation of biliary epithelial cells, forming multiple ductules in the portal triads.27,28 Our results show that when proliferation of biliary epithelial cells is compromised by toxic injury (induced by DAPM), large-scale conversion of hepatocytes occurs adjacent to the portal areas into biliary ductules.
Materials and Methods
Animals
Fisher 344 male rats, DPPIV positive, were obtained from Charles River Laboratories, Frederick, Maryland. A colony of German Fisher 344 DPPIV-negative rats was obtained from founders donated by Dr. Bryon Petersen, University of Florida. The colony is maintained in the Animal Facility of the University of Pittsburgh School of Medicine. The animal husbandry and all procedures performed on the rats employed for these studies were approved under the IACUC protocol #0699068A-1 and conducted according to National Institute of Health guidelines.
Generation of Rats With Chimeric Livers
The protocol established by Laconi et al.24 was employed for the studies. Male German Fisher rats (DPPIV negative), weighing 200 g, were given two intraperitoneal injections with retrorsine, 30 mg/kg body weight, dissolved in water. The injections were given 15 days apart. A month after the last injection, the rats were subjected to PHx, as described by Higgins and Andersen.29 During the PHx operation, the rats were also injected directly into the portal circulation (via a peripheral branch of the superior mesenteric vein) with 3.5 million hepatocytes. These cells were isolated by collagenase perfusion of the liver23 immediately before the operation, from DPPIV-positive Fisher 344 male rats (200 g body weight). The animals were left to recover and were not subjected to any other experimental procedures for the next 3 months. Assessment of the degree of engraftment was made under direct microscopic observation of sections from the chimeric livers, stained for DPPIV. The percentage of DPPIV-positive and negative cells was estimated at 40× magnification in optic fields that included at least one portal triad and one central vein. The percentage of DPPIV-positive cells varied from one lobule to another. The range of engraftment per optic field (as defined above) within each animal varied from 30% to 60%. Variation was also seen from one lobe to another.
Bile Duct Ligation
The animals were subjected to a mid-abdominal incision 3 cm long, under general anesthesia. The common bile duct was ligated in two adjacent positions approximately 1 cm from the porta hepatis. The duct was then severed by incision between the two sites of ligation.
Administration of DAPM
The biliary toxin DAPM (4,4′-Methylenedianiline, Sigma Chemicals, St. Louis, MO) was dissolved in dimethylsulfoxide at a concentration of 50 mg/mL. A group of rats were given an intra-peritoneal injection of 50 mg/kg 2 days before the bile duct ligation. No added mortality or any evident symptomatology was observed from this treatment.20
Animal Sacrifice and Tissue Processing
The animals were sacrificed at 30 days after bile duct ligation. The livers were removed and preserved for histology. Parts of each lobe were placed in formalin or processed for frozen sectioning.
Immunohistochemistry for CK7 was performed on paraformaldehyde-fixed frozen sections, using primary antibodies from Dako Cytomation, Glostrup, Denmark. OV6 monoclonal antibody, reactive against oval cells and biliary cells, was provided by Dr. Douglas Hixson, Director, Molecular Carcinogenesis Laboratory, and Professor of Pathology, Brown University.
DPPIV Histochemistry
Staining for DPPIV was performed as previously described.23 The tissues were frozen and sectioned by using a cryostat. Sections of 4- to 5-μm thickness were used for histological analysis. Immunostains for proliferating cell nuclear antigen (PCNA) and hepatocyte-specific antigen marker (HEPPAR) were performed as previously described.30
Results
Histology of Chimeric Livers Before Bile Duct Ligation
Chimeric livers of Fisher rats were examined at 3 months after injection of hepatocytes and performance of PHx. The degree of colonization by DPPIV-positive hepatocytes varied from animal to animal within a range of colonization from 30% to 60% of the total lobular surface. Variation was also seen within different areas of the hepatic lobes and from lobule to lobule. DPPIV-positive hepatocytes were arranged in clusters. Portal triads varied in terms of their contact with the DPPIV-positive hepatocyte clusters. Most triads were adjacent to some clusters of DPPIV-positive hepatocytes, with a variation of contact from minimal to being completely surrounded. Others (approximately 25% of the triads) did not have any evident proximity to DPPIV-positive hepatocyte clusters at the plane of section. Portal triads were examined in animals sacrificed without performing bile duct ligation, and the bile ductules in the portal triads were examined by DPPIV histochemistry. Only portal triads that had direct proximity to some DPPIV-positive hepatocyte clusters were included in this part of the study. There was not a single DPPIV-positive bile duct epithelial cell in 45 portal triads examined in sections taken randomly and stained by histochemistry for DPPIV. A typical appearance of the histology of a portal triad in the DPPIV chimeric livers not subjected to bile duct ligation is shown in Fig. 1.
Fig. 1.
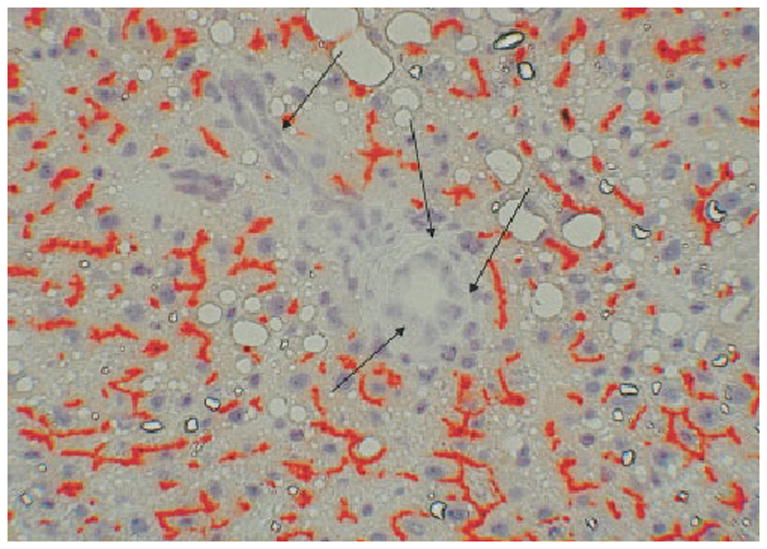
DPPIV histochemistry of chimeric liver before bile duct ligation. Bile canaliculi of donor hepatocytes stain red (positive) for DPPIV. Bile ductules in portal triads of the recipient liver (arrows) are uniformly negative for DPPIV (original magnification ×200).
Effects of Bile Duct Ligation and DAPM at Day 30 of the Protocol
Animals were harvested at 30 days after bile duct ligation. The overall histology, examined by hematoxylineosin stain, was typical of that described in the literature for this model of biliary injury. Expanded portal triads containing multiple bile ductules are shown in Fig. 2A. A higher-power magnification of such ductules is shown in Fig. 2B. Occasional inflammatory cells and some individual hepatocytes are seen, surrounded by the proliferating ductules. There was no noticeable difference in any aspect of histology at 30 days after bile duct ligation between livers of animals that had or had not been treated by DAPM.
Fig. 2.
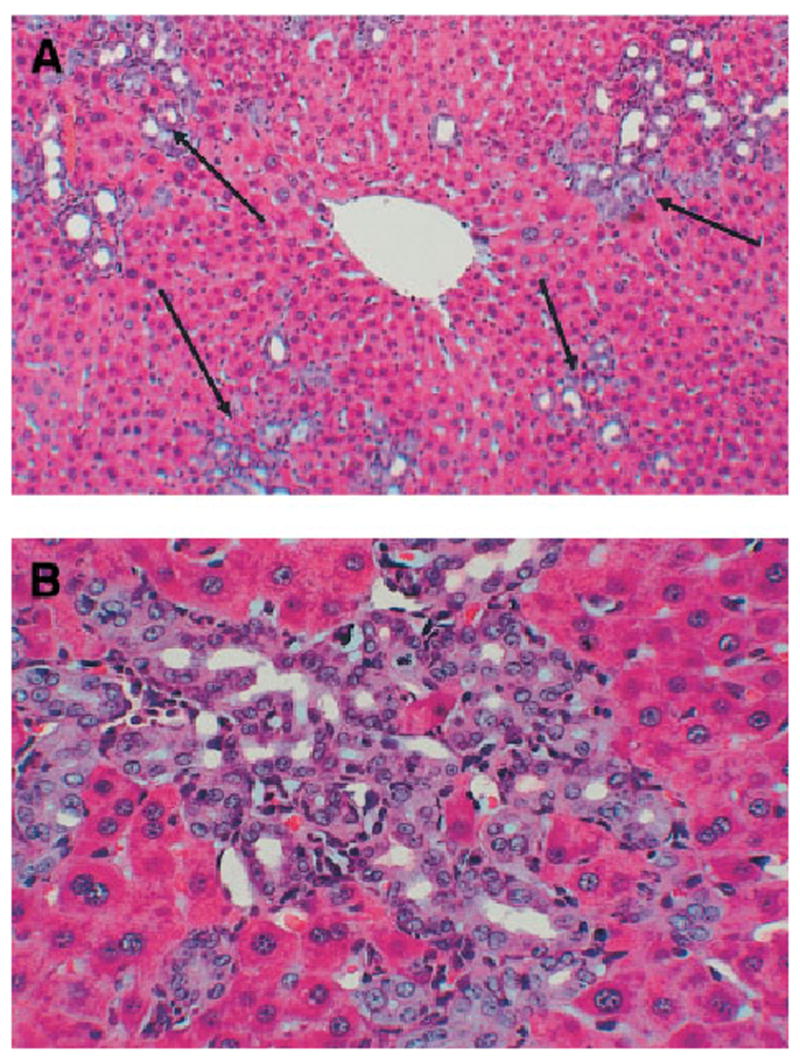
Hematoxylineosin stain of livers at day 30 after bile duct ligation. (A) Portal triads are expanded with numerous bile ductules (arrows). (original magnification ×200). (B) High-power view of a cluster of bile ductules (original magnification ×400). There was no detectable difference in histology between livers exposed to DAPM versus controls.
Many of the otherwise indistinguishable, normal proliferating ductules were positive for the marker DPPIV. As discussed (Fig. 1), in the chimeric livers constructed for this study and following the protocols established in the literature, DPPIV is seen only in hepatocytes and not in biliary epithelium. Examples of DPPIV ductules are shown in Fig. 3. This finding suggests that some of the proliferating ductules induced by the reaction to the bile duct ligation derive from hepatocytes. The distribution of the DPPIV-positive ductules varied between portal triads. In some, all of the ductules were DPPIV positive (Fig. 3A–B) or all negative (Fig. 3D). In most portal triads, however, there was partial replacement of some of the ductules (Fig. 3C)
Fig. 3.
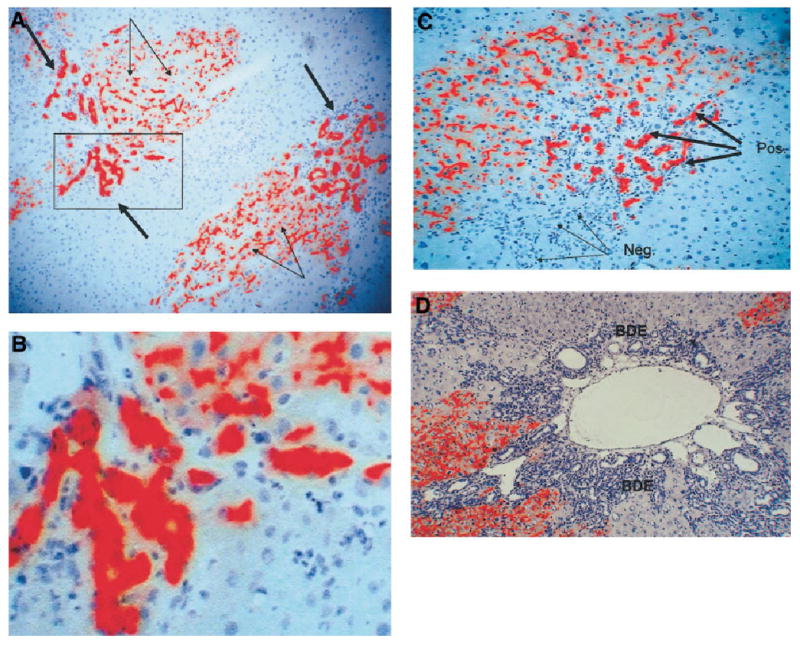
(A) DPPIV histochemistry shows multiple clusters of DPPIV-positive (red) ductules (thick arrows). The canaliculi of the donor hepatocytes also stain positive for DPPIV (thin arrows) (original magnification ×200). The area surrounded by a square is magnified in panel B (original magnification ×400). (C) A portal triad with a portion of the ductules being DPPIV-positive (red) (thick arrows), whereas another portion has ductules that are DPPIV-negative (thin arrows). (original magnification ×300). (D) A portal triad in which all ductules are negative for DPPIV (original magnification ×200). BDE, bile duct epithelium.
Immunohistochemical stain for the hepatocyte-specific antigen marker HEPPAR showed that only hepatocytes were positive, with none of the biliary ductules staining positive for this marker. Two hepatocytes, however, are forming a ductular structure (Fig. 4A). The ductules had nuclei positive for PCNA in the range of 60% to 90% (Fig. 4B). Many hepatocytes were also positive for PCNA, as previously described. All ductules generated by the protocol were indistinguishable by any histological technique other than DPPIV. Figure 4C and 4D demonstrate that two groups of ductules, one positive and one negative for DPPIV, were equally positive for the biliary marker CK7. At the end of the DAPM–bile duct ligation (BDL) protocol (day 30), no differences are seen in cell or nuclear size between the DPPIV-positive and -negative ductules.
Fig. 4.
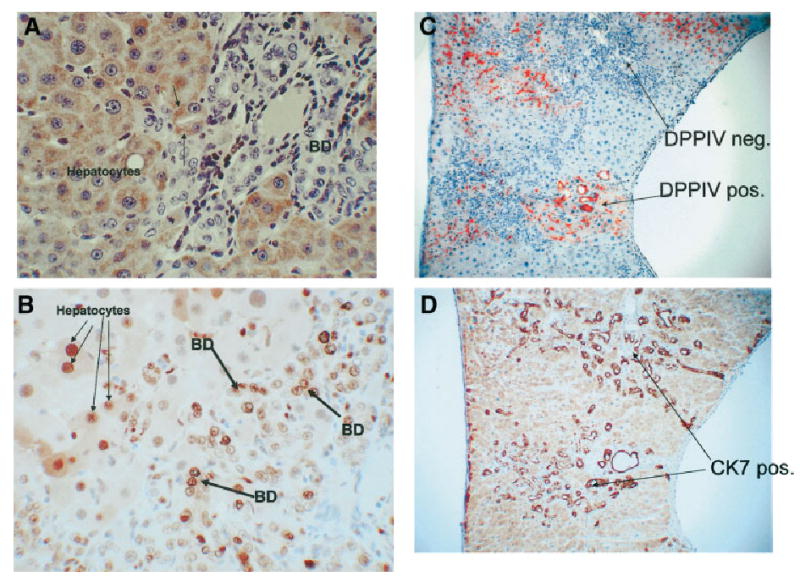
A DAPM-BDL protocol at day 30. Immunohistochemical stain for the hepatocyte marker HEPPAR. (A) All hepatocytes stain positive (brown), whereas biliary ductules are negative. (original magnification ×400). Two hepatocytes, however, are arranged in a ductular configuration (arrows). (B) Day 30 of the DAPM-BDL protocol. Immunohistochemical stain for PCNA, indicating presence of cells in the cell cycle. Numerous nuclei of both hepatocytes (thin arrows) and biliary epithelial cells (bile duct, thick arrows) are positive for PCNA. (original magnification ×400). (C–D) Two groups of bile ductules are shown in panel C, of which one is positive and the other negative for DPPIV. Panel D demonstrates that, regardless of the DPPIV expression, both groups of ductules are indistinguishable in terms of CK7. (original magnification ×200). BD, bile duct.
The percentage of DPPIV-positive biliary cells and of the triads carrying DPPIV-positive ductules in the DAPM-treated and control groups are shown in Fig. 5. DAPM treatment resulted in a striking difference in both assay endpoints. There was a 36-fold increase in DPPIV-positive biliary epithelial cells and portal triads containing DPPIV-positive biliary ductules (DAPM-treated: n = 4, untreated: n = 3).
Fig. 5.
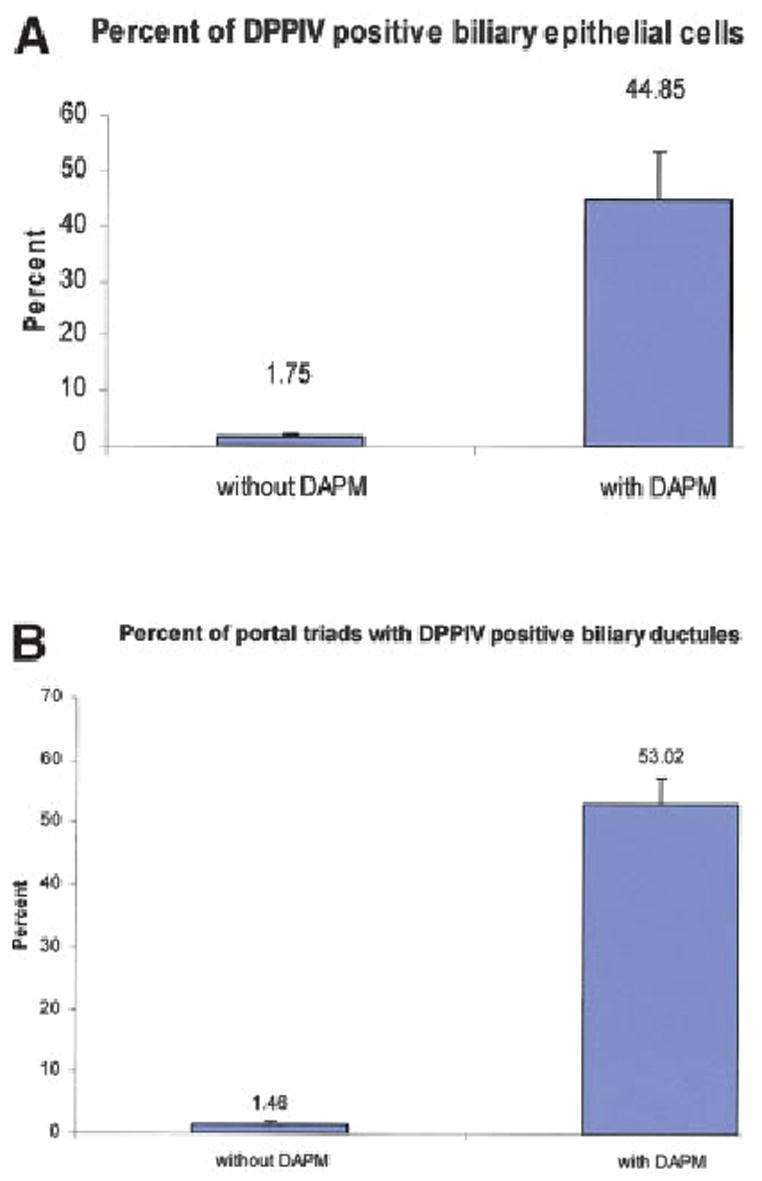
Quantification of (A) the percentage of DPPIV-positive bile duct epithelial cells and (B) the percentage of portal triads bearing DPPIV-positive ductules. Data were derived from 3 rats not treated with DAPM and 4 rats treated with DAPM. Three sections were taken randomly from each rat liver. A total of 50 portal triads per rat liver were randomly assessed to derive the data indicated. Each bar represents the mean (± SE) derived by statistical analysis of the data derived from the animals in each group.
Sequence of Cellular Changes in the Liver During the Experimental Protocol
Biliary toxicity after administration of DAPM: As previously described, administration of DAPM causes necrosis of the biliary epithelium within 1 day after administration. The necrotic phase lasts from day 1 to day 3, with signs of repair appearing after that time. The degree of biliary cell necrosis is not uniform in all the ducts throughout the liver. Some of the ducts were only partially affected. Several ducts ruptured and generated “bile infarcts” at days 2 to 4 after DAPM. Some of the histological changes are shown in Fig. 6. We have shown in previous studies20 that the toxic effects of DAPM last up to 5 to 7 days, with no residual histological damage seen after that point.
Changes in periportal hepatocytes associated with the biliary transformation: These studies were carried out in regular (nonchimeric) rats, subjected to the identical protocol (DAPM followed by BDL) used for the chimeric rats. Small hepatocytes seen in the immediate periportal location assumed expression of biliary markers during the study. Changes recognizable by simple hematoxylineosin stain were seen as early as 7 days after BDL. Some of the hepatocytes were intercalated into ductular/acinar structures populated in part by biliary cells (Fig. 7A). Other small hepatocytes assumed a ductular configuration, as shown in Fig. 7B. The oval cell and biliary marker OV6 was expressed in hepatocytes in the periportal region from day 2 after DAPM and at all times thereafter (Fig. 8A). CK7 (Fig. 8B) was seen in the immediate periportal hepatocytes after day 9 after BDL and was not spread as much into the rest of the lobule as the reactivity for OV6. Overall, there was no expansion of oval cells.
Connections of the hepatocyte-derived ductules: We used the DPPIV marker to trace connections between the newly formed hepatocyte-derived ductules and the other components of the biliary compartment. Connections were readily seen between newly formed ductules and the bile canaliculi of donor-derived DPPIV-positive hepatocytes (Fig. 9A). Connections with portal ductules draining into the biliary system are tentatively established by demonstrating the location of the DPPIV ductules in close proximity to the portal triads (Fig. 9B).
Fig. 6.
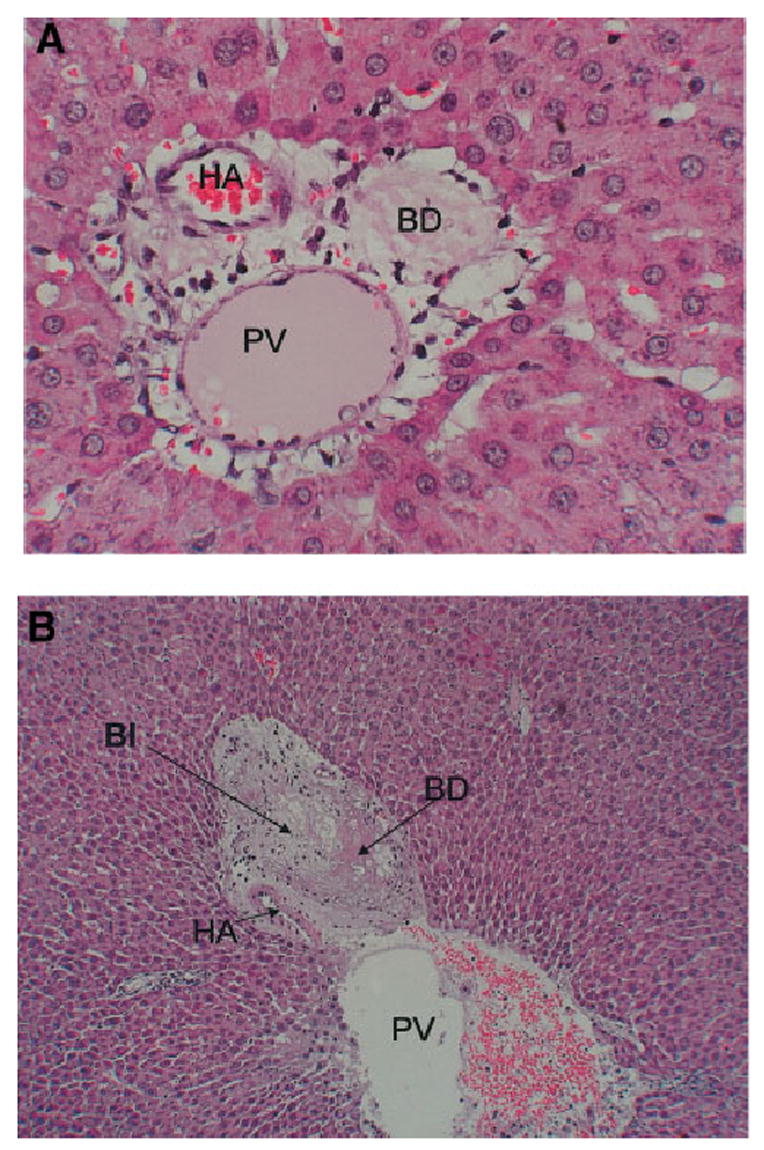
Histological changes after administration of DAPM. (A) Section of a portal triad at day 2 after administration of DAPM. The bile ductule is devoid of biliary epithelium. (original magnification ×400). (B) An area of hepatocyte necrosis (BI) in immediate proximity to a ruptured bile ductule, at day 4 after DAPM. (original magnification ×100). PV, portal vein branch; HA, hepatic artery branch; BD, interlobular bile ductile; BI, bile infarct.
Fig. 7.
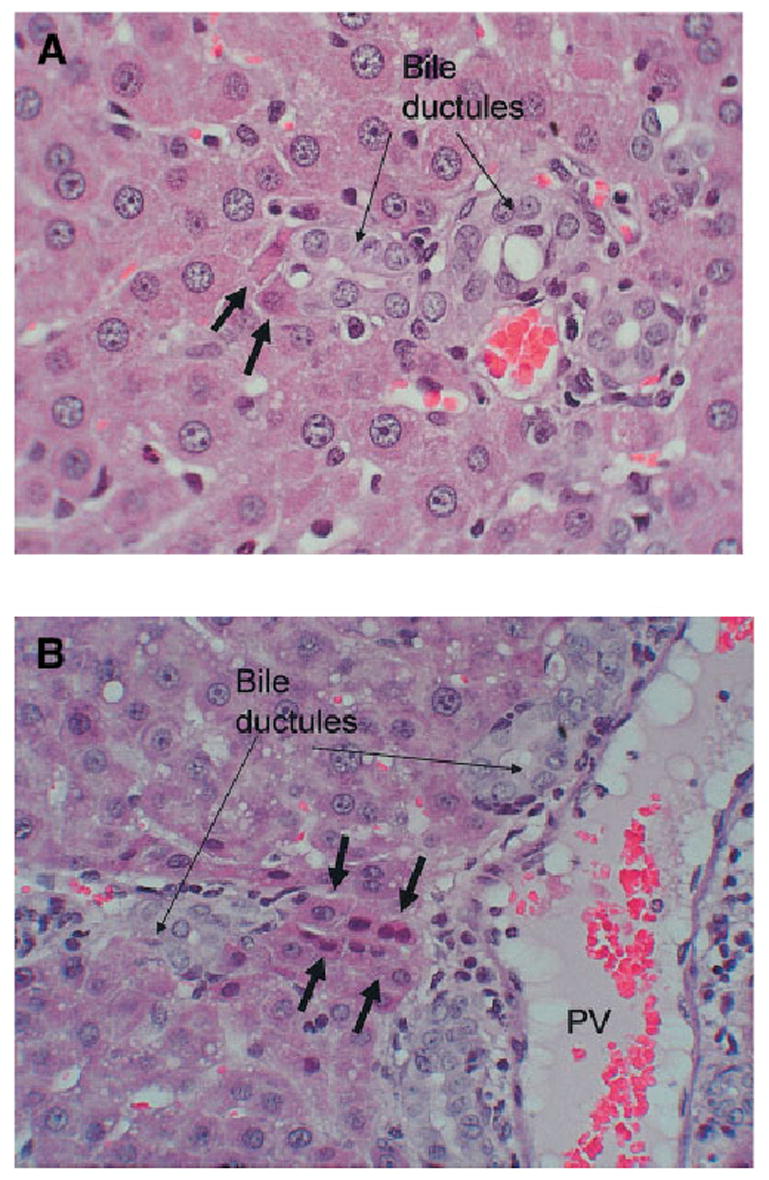
Hematoxylineosin stain of liver sections at day 12 of the DAPM-BDL protocol (original magnification ×200). (A) Two hepatocytes (short arrows) are intercalated into the structure of a bile ductule (long arrows). (B) Hepatocytes are arranged in a ductular configuration (short arrows). PV, portal vein.
Fig. 8.
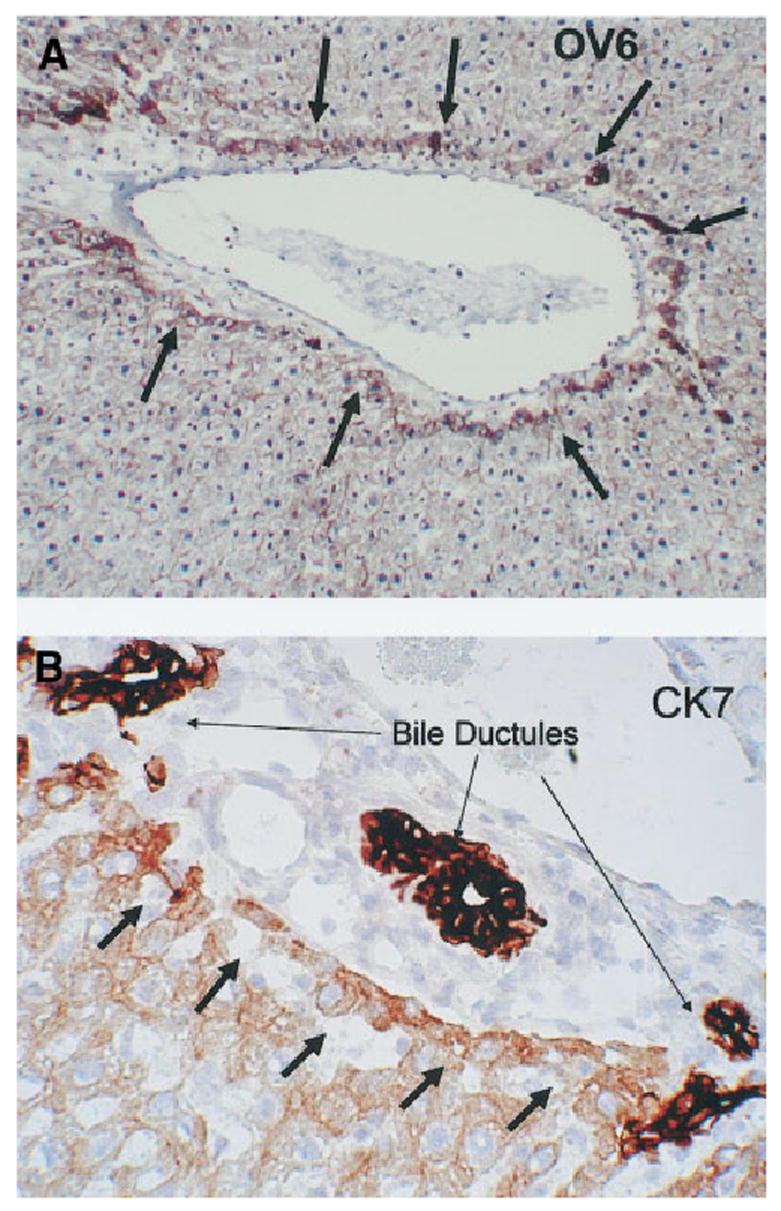
(A) Immunohistochemistry for OV6. This antibody stains positive in oval cells and biliary epithelium. Hepatocytes immediately adjacent to the portal triad (arrows) become positive for OV56 at day 3 of the DAPM-BDL protocol (original magnification ×100). (B) Day 9 of the DAPM-BDL protocol. Hepatocytes immediately adjacent to the portal triad (short arrows) become moderately positive for CK7. The bile ductules of the same triad (long arrows) are intensely positive for CK7 (original magnification ×200).
Fig. 9.
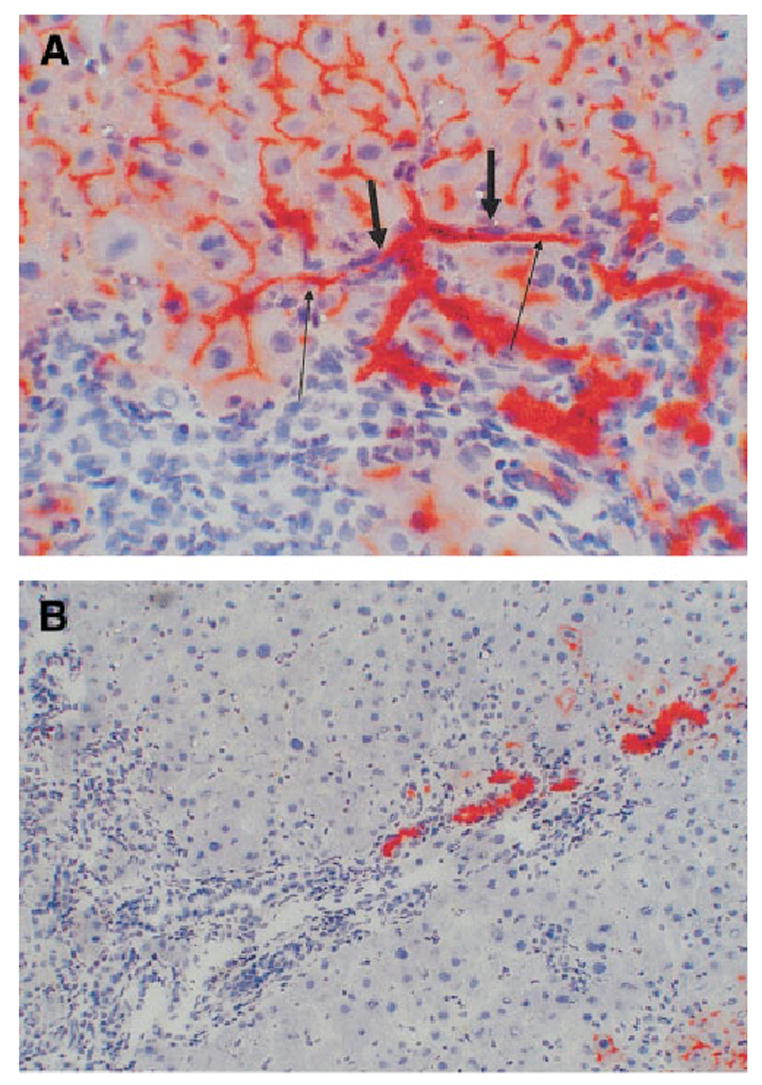
DAPM-BDL protocol at day 30. (A) Connections between the lumen of the bile ductules (short arrows) and bile canaliculi of hepatocytes (long arrows). (original magnification ×400). (B) DPPIV-positive bile ductules (red) are arranged linearly within a complex of DPPIV-negative ductules (blue-gray) draining into a portal triad (original magnification ×100).
Discussion
The data presented are an extension of our recent previous study in which we showed that hepatocytes can transdifferentiate into biliary epithelium in organoid cultures.23 Our current results show that hepatocytes are capable of converting into biliary epithelial cells not only in culture but also in the liver in vivo, in situations of severe biliary injury (BDL), in which the capacity of the biliary cells to repair injury is compromised (by DAPM). Although the conditions used are experimental and thus drastic, in many aspects of human liver disease bile ductules are gradually destroyed, and thus their proliferative capacity is presumably compromised. This is often seen as a result of chronic immune attack, as, for example, in primary biliary cirrhosis or primary sclerosing cholangitis.
Previous studies of human pediatric and adult liver with biliary pathological changes (e.g., primary biliary cirrhosis, primary sclerosing cholangitis) have documented the presence of the oval cell marker OV6 in hepatocytes.10, 11 Expression of bile duct–associated cytokeratins, including CK7, has been noted in cholestatic disease in humans.31 Additional studies with experimental models in the rat (choline-deficient diet or exposure to the biliary toxin ANIT) have demonstrated hepatocytes expressing biliary markers, suggesting a bidirectional differentiation of progenitor cells.32 Studies with experimental injury models using transgenic mice have also suggested that hepatocytes may give rise to cells with the properties of oval cells.33 The described studies have shown evidence that hepatocytes can express biliary cell–associated markers, suggesting that hepatocytes in rodents and humans have the capability to transdifferentiate into biliary epithelial cells.
The protocol (bile duct ligation plus DAPM) used for these studies bears resemblance to the protocol of PHx + AAF used to induce the phenotypic conversion that occurs in the opposite direction, the conversion of biliary epithelial cells into hepatocytes through the oval cell pathway. In situations associated with loss of hepatic parenchyma (e.g., PHx, recovery from CCl4-induced chemical injury), regeneration of the liver proceeds through a limited set of proliferative events in which hepatocytes generate more hepatocytes, biliary cells generate more biliary cells, and so forth.34 The hepatic mass is restored at the end of the regenerative process. When, however, the capacity of hepatocytes to proliferate is impaired (e.g., after administration of AAF, retrorsine, etc.), then oval cells appear in the periportal areas and eventually transdifferentiate into hepatocytes. All evidence suggests that oval cells derive from the biliary compartment. The summary of the evidence is as follows:
Oval cells express markers of biliary cells before converting to small hepatocytes.35,36
Hepatocyte-associated transcription factors become elevated in the biliary ductules soon after PHx plus AAF.19
Administration of the biliary toxin DAPM immediately after PHx plus AAF eliminates the appearance of oval cells.20
The precise location within the biliary tree of the cell population giving rise to oval cells (canals of Hering vs. portal ductules) has been debated. Canals of Hering and intraportal biliary ductules have been considered as non–mutually exclusive alternatives.19,37–40
In our current study, BDL was used as the stimulus to elicit biliary cell proliferation, and DAPM was used to inhibit (via selective toxicity) the proliferation of the biliary epithelial cells. In symmetry with the results of the PHx + AAF protocol (biliary cells transdifferentiate into hepatocytes), we observe that in the BDL + DAPM protocol hepatocytes become biliary cells. The percentage of biliary ductules derived from hepatocytes presented in this study is an underestimate of the total, because only the DAPM-positive ductules can be identified and counted. At least half of the hepatocytes throughout the chimeric livers were DPPIV negative. The recipient-derived DPPIV-negative hepatocytes mayalso contribute to some extent toward formation of biliary ductules, which cannot be traced because of lack of markers. Note, however, that the contribution of the DPPIV-negative (recipient) hepatocytes toward ductular differentiation may be limited by the fact that they had been treated with retrorsine, thus restricting their proliferative potential.24
We examined the histology of the ductules at the end of the study (30 days after bile duct ligation), and we did not observe any differences between the portal ductules that would suggest a dual phenotype, even though (in the DAPM-BDL protocol) almost half were derived from hepatocytes. This is illustrated by studying expression of CK7, in Fig. 4C–D. However, transitional changes were seen in the hepatocytes of the periportal region, and especially in the hepatocytes immediately adjacent to the portal triad. These cells expressed OV6 very early in the protocol, within days after administration of DAPM. Expression of CK7, a biliary marker, was also enhanced in the cells, albeit at a later time, starting at approximately day 7 after BDL. These changes were restricted to the periportal region. We did not see proliferation of cells with oval cell characteristics as a distinct cell population, as seen with the AAF-PHx protocol for generation of oval cells. The findings in Figs. 7 and 8 suggest that the immediately periportal hepatocytes undergo gradual transdifferentiation as some of them convert into biliary epithelial cells. The formation of a ring of cells around the portal triad with phenotypic characteristics of both hepatocytes and biliary cells (Fig. 8A) is reminiscent of the ductal plate observed during embryology.9
It is conceivable that at the time of injection of the DPPIV-positive hepatocytes into the retrorsine-treated and hepatectomized livers, some biliary cells or cells with stem cell properties might have been injected as contaminants. It may also be argued that some of the DPPIV-positive ductules seen in the animals not subjected to DAPM may derive from such contaminant cells. This, however, is not likely to be the case in the animals treated with DAPM. The latter is a well-documented biliary toxin and would have likely caused the death of the contaminant biliary cells present within the lobular areas. The issue of stem cells in the liver is more complex because such cells have not been conclusively shown to exist. Oval cells are the closest identified cell type in liver with progenitor cell properties. We have previously shown that treatment with DAPM eliminates development of oval cells.20 Because oval cells share many phenotypic markers with biliary epithelium (bile ductules or canals of Hering), it is also highly likely that treatment with DAPM would be toxic to these cells as well. The dramatic rise of the number of DPPIV-positive ductules after DAPM administration clearly suggests that the origin of the DP-PIV-positive ductules is not from a small number of potential biliary or oval cell contaminants surviving after injection in the liver lobule; the increase in DPPIV-positive ductules associated with DAPM clearly suggests that these DPPIV-positive ductules must derive from hepatocytes, known to be resistant to the DAPM treatment.
In summary, our findings have implications for regenerative biology of the liver. Several studies have aimed to identify committed stem cells in adult rodent liver, with the expectation that these stem cells would be available constantly and on a stand-by basis to replenish hepatocytes or biliary epithelium, as the need arose. Our results suggest that the most likely regenerative scheme in nonstandard regenerative conditions is that hepatocytes and biliary epithelium are facultative stem cells for each other. The term “facultative stem cells”16 is used to denote cellular populations who, under conditions of tissue quiescence, have a specific function related to the mission of the tissue. These cells, however, are capable of transdifferentiating into other cell types to provide progenitor cells and replace cell populations incapable of doing so on their own. The canals of Hering are thought to be a source of such facultative stem cells for hepatocytes. Canals of Hering are the vanguard of biliary epithelium in contact with hepatocytes, and they have been shown to penetrate deeply into the lobule.15,40 Our studies show that adult hepatocytes can also function as facultative stem cells for the biliary epithelium. We presume that these hepatocytes are likely to reside in the periportal areas, but the possibility of hepatocytes in other areas potentially contributing to this process cannot be excluded.
Abbreviations
- AAF
N-2 acetyl-aminofluorene
- PHx
2/3 partial hepatectomy
- DAPM
methylene dianiline
- DPPIV
dipeptidyl peptidase IV
- PCNA
proliferating cell nuclear antigen
- HEPPAR
hepatocyte-specific antigen marker
- BDL
bile duct ligation
Footnotes
Conflict of interest: Nothing to report.
Supported by NIH grants CA35373 and CA103958 and by the Rangos Fund for Enhancement of Research in Pathology.
References
- 1.Alison M, Golding M, Lalani EN, Nagy P, Thorgeirsson S, Sarraf C. Wholesale hepatocytic differentiation in the rat from ductular oval cells, the progeny of biliary stem cells. J Hepatol. 1997;26:343–352. doi: 10.1016/s0168-8278(97)80051-7. [DOI] [PubMed] [Google Scholar]
- 2.Evarts RP, Nagy P, Marsden E, Thorgeirsson SS. A precursor-product relationship exists between oval cells and hepatocytes in rat liver. Carcinogenesis. 1987;8:1737–1740. doi: 10.1093/carcin/8.11.1737. [DOI] [PubMed] [Google Scholar]
- 3.Evarts RP, Nagy P, Nakatsukasa H, Marsden E, Thorgeirsson SS. In vivo differentiation of rat liver oval cells into hepatocytes. Cancer Res. 1989;49:1541–1547. [PubMed] [Google Scholar]
- 4.Evarts RP, Hu Z, Omori N, Omori M, Marsden ER, Thorgeirsson SS. Precursor-product relationship between oval cells and hepatocytes: comparison between tritiated thymidine and bromodeoxyuridine as tracers. Carcinogenesis. 1996;17:2143–2151. doi: 10.1093/carcin/17.10.2143. [DOI] [PubMed] [Google Scholar]
- 5.Golding M, Sarraf CE, Lalani EN, Anilkumar TV, Edwards RJ, Nagy P, et al. Oval cell differentiation into hepatocytes in the acetylaminofluorene-treated regenerating rat liver. Hepatology. 1995;22:1243–1253. doi: 10.1016/0270-9139(95)90635-5. [DOI] [PubMed] [Google Scholar]
- 6.Lazaro CA, Rhim JA, Yamada Y, Fausto N. Generation of hepatocytes from oval cell precursors in culture. Cancer Res. 1998;58:5514–5522. [PubMed] [Google Scholar]
- 7.Fiel MI, Antonio LB, Nalesnik MA, Thung SN, Gerber MA. Characterization of ductular hepatocytes in primary liver allograft failure. Mod Pathol. 1997;10:348–353. [PubMed] [Google Scholar]
- 8.Haque S, Haruna Y, Saito K, Nalesnik MA, Atillasoy E, Thung SN, et al. Identification of bipotential progenitor cells in human liver regeneration. Lab Invest. 1996;75:699–705. [PubMed] [Google Scholar]
- 9.Haruna Y, Saito K, Spaulding S, Nalesnik MA, Gerber MA. Identification of bipotential progenitor cells in human liver development. Hepatology. 1996;23:476–481. doi: 10.1002/hep.510230312. [DOI] [PubMed] [Google Scholar]
- 10.Crosby HA, Hubscher SG, Joplin RE, Kelly DA, Strain AJ. Immunolocalization of OV-6, a putative progenitor cell marker in human fetal and diseased pediatric liver. Hepatology. 1998;28:980–985. doi: 10.1002/hep.510280412. [DOI] [PubMed] [Google Scholar]
- 11.Crosby HA, Hubscher S, Fabris L, Joplin R, Sell S, Kelly D, et al. Immunolocalization of putative human liver progenitor cells in livers from patients with end-stage primary biliary cirrhosis and sclerosing cholangitis using the monoclonal antibody OV-6. Am J Pathol. 1998;152:771–779. [PMC free article] [PubMed] [Google Scholar]
- 12.Vandersteenhoven AM, Burchette J, Michalopoulos G. Characterization of ductular hepatocytes in end-stage cirrhosis. Arch Pathol Lab Med. 1990;114:403–406. [PubMed] [Google Scholar]
- 13.Paku S, Schnur J, Nagy P, Thorgeirsson SS. Origin and structural evolution of the early proliferating oval cells in rat liver. Am J Pathol. 2001;158:1313–1323. doi: 10.1016/S0002-9440(10)64082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paku S, Nagy P, Kopper L, Thorgeirsson SS. 2-acetylaminofluorene dose-dependent differentiation of rat oval cells into hepatocytes: confocal and electron microscopic studies. Hepatology. 2004;39:1353–1361. doi: 10.1002/hep.20178. [DOI] [PubMed] [Google Scholar]
- 15.Crawford JM. Development of the intrahepatic biliary tree. Semin Liver Dis. 2002;22:213–226. doi: 10.1055/s-2002-34508. [DOI] [PubMed] [Google Scholar]
- 16.Falkowski O, An HJ, Ianus IA, Chiriboga L, Yee H, West AB, et al. Regeneration of hepatocyte ’buds’ in cirrhosis from intrabiliary stem cells. J Hepatol. 2003;39:357–364. doi: 10.1016/s0168-8278(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 17.Saxena R, Theise N. Canals of Hering: recent insights and current knowledge. Semin Liver Dis. 2004;24:43–48. doi: 10.1055/s-2004-823100. [DOI] [PubMed] [Google Scholar]
- 18.Hytiroglou P, Tobias H, Saxena R, Abramidou M, Papadimitriou CS, Theise ND. The canals of Hering might represent a target of methotrexate hepatic toxicity. Am J Clin Pathol. 2004;121:324–329. doi: 10.1309/5HR9-0TNC-4Q4J-RXWX. [DOI] [PubMed] [Google Scholar]
- 19.Nagy P, Bisgaard HC, Thorgeirsson SS. Expression of hepatic transcription factors during liver development and oval cell differentiation. J Cell Biol. 1994;126:223–233. doi: 10.1083/jcb.126.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen BE, Zajac VF, Michalopoulos GK. Bile ductular damage induced by methylene dianiline inhibits oval cell activation. Am J Pathol. 1997;151:905–909. [PMC free article] [PubMed] [Google Scholar]
- 21.Strain AJ, Crosby HA, Nijjar S, Kelly DA, Hubscher SG. Human liver-derived stem cells. Semin Liver Dis. 2003;23:373–384. doi: 10.1055/s-2004-815563. [DOI] [PubMed] [Google Scholar]
- 22.Petersen BE, Zajac VF, Michalopoulos GK. Hepatic oval cell activation in response to injury following chemically induced periportal or pericentral damage in rats. Hepatology. 1998;27:1030–1038. doi: 10.1002/hep.510270419. [DOI] [PubMed] [Google Scholar]
- 23.Michalopoulos GK, Bowen WC, Mule K, Lopez-Talavera JC, Mars W. Hepatocytes undergo phenotypic transformation to biliary epithelium in organoid cultures. Hepatology. 2002;36:278–283. doi: 10.1053/jhep.2002.34858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laconi E, Oren R, Mukhopadhyay DK, Hurston E, Laconi S, Pani P, et al. Long-term, near-total liver replacement by transplantation of isolated hepatocytes in rats treated with retrorsine. Am J Pathol. 1998;153:319–329. doi: 10.1016/S0002-9440(10)65574-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dabeva MD, Laconi E, Oren R, Petkov PM, Hurston E, Shafritz DA. Liver regeneration and alpha-fetoprotein messenger RNA expression in the retrorsine model for hepatocyte transplantation. Cancer Res. 1998;58:5825–5834. [PubMed] [Google Scholar]
- 26.Oren R, Dabeva MD, Petkov PM, Hurston E, Laconi E, Shafritz DA. Restoration of serum albumin levels in nagase analbuminemic rats by hepatocyte transplantation. Hepatology. 1999;29:75–81. doi: 10.1002/hep.510290147. [DOI] [PubMed] [Google Scholar]
- 27.Sirica AE, Williams TW. Appearance of ductular hepatocytes in rat liver after bile duct ligation and subsequent zone 3 necrosis by carbon tetrachloride. Am J Pathol. 1992;140:129–136. [PMC free article] [PubMed] [Google Scholar]
- 28.Polimeno L, Azzarone A, Zeng QH, Panella C, Subbotin V, Carr B, et al. Cell proliferation and oncogene expression after bile duct ligation in the rat: evidence of a specific growth effect on bile duct cells. Hepatology. 1995;21:1070–1078. [PMC free article] [PubMed] [Google Scholar]
- 29.Higgins GM, Anderson RM. Experimental pathology of the liver, 1: Restoration of the liver of the white rat following partial surgical removal. Arch Pathol. 1931;12:186–202. [Google Scholar]
- 30.Michalopoulos GK, Bowen WC, Mule K, Stolz DB. Histological organization in hepatocyte organoid cultures. Am J Pathol. 2001;159:1877–1887. doi: 10.1016/S0002-9440(10)63034-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Eyken P, Sciot R, Desmet VJ. A cytokeratin immunohistochemical study of cholestatic liver disease: evidence that hepatocytes can express ’bile duct-type’ cytokeratins. Histopathology. 1989;15:125–135. doi: 10.1111/j.1365-2559.1989.tb03060.x. [DOI] [PubMed] [Google Scholar]
- 32.Roskams T, De Vos R, Van Eyken P, Myazaki H, Van Damme B, Desmet V. Hepatic OV-6 expression in human liver disease and rat experiments: evidence for hepatic progenitor cells in man. J Hepatol. 1998;29:455–463. doi: 10.1016/s0168-8278(98)80065-2. [DOI] [PubMed] [Google Scholar]
- 33.Braun KM, Sandgren EP. Cellular origin of regenerating parenchyma in a mouse model of severe hepatic injury. Am J Pathol. 2000;157:561–569. doi: 10.1016/S0002-9440(10)64566-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 35.Yin L, Sun M, Ilic Z, Leffert HL, Sell S. Derivation, characterization, and phenotypic variation of hepatic progenitor cell lines isolated from adult rats. Hepatology. 2002;35:315–324. doi: 10.1053/jhep.2002.31355. [DOI] [PubMed] [Google Scholar]
- 36.Yin L, Lynch D, Ilic Z, Sell S. Proliferation and differentiation of ductular progenitor cells and littoral cells during the regeneration of the rat liver to CCl4/2-AAF injury. Histol Histopathol. 2002;17:65–81. doi: 10.14670/HH-17.65. [DOI] [PubMed] [Google Scholar]
- 37.Hixson DC, Chapman L, McBride A, Faris R, Yang L. Antigenic phenotypes common to rat oval cells, primary hepatocellular carcinomas and developing bile ducts. Carcinogenesis. 1997;18:1169–1175. doi: 10.1093/carcin/18.6.1169. [DOI] [PubMed] [Google Scholar]
- 38.Yang L, Faris RA, Hixson DC. Phenotypic heterogeneity within clonogenic ductal cell populations isolated from normal adult rat liver. Proc Soc Exp Biol Med. 1993;204:280–288. doi: 10.3181/00379727-204-43664. [DOI] [PubMed] [Google Scholar]
- 39.Faktor VM, Radaeva SA. [The formation of oval-cell ducts during hepatic carcinogenesis in mice. Its relationship to the pre-existing canals of Hering] Ontogenez. 1992;23:407–418. [PubMed] [Google Scholar]
- 40.Theise ND, Saxena R, Portmann BC, Thung SN, Yee H, Chiriboga L, et al. The canals of Hering and hepatic stem cells in humans. Hepatology. 1999;30:1425–1433. doi: 10.1002/hep.510300614. [DOI] [PubMed] [Google Scholar]


