Abstract
Research on action simulation identifies brain areas that are active while imagining or performing simple overlearned actions. Are areas engaged during imagined movement sensitive to the amount of actual physical practice? In the present study, participants were expert dancers who learned and rehearsed novel, complex whole-body dance sequences 5 h a week across 5 weeks. Brain activity was recorded weekly by fMRI as dancers observed and imagined performing different movement sequences. Half these sequences were rehearsed and half were unpracticed control movements. After each trial, participants rated how well they could perform the movement. We hypothesized that activity in premotor areas would increase as participants observed and simulated movements that they had learnt outside the scanner. Dancers’ ratings of their ability to perform rehearsed sequences, but not the control sequences, increased with training. When dancers observed and simulated another dancer’s movements, brain regions classically associated with both action simulation and action observation were active, including inferior parietal lobule, cingulate and supplementary motor areas, ventral premotor cortex, superior temporal sulcus and primary motor cortex. Critically, inferior parietal lobule and ventral premotor activity was modulated as a function of dancers’ ratings of their own ability to perform the observed movements and their motor experience. These data demonstrate that a complex motor resonance can be built de novo over 5 weeks of rehearsal. Furthermore, activity in premotor and parietal areas during action simulation is enhanced by the ability to execute a learned action irrespective of stimulus familiarity or semantic label.
Keywords: Action representation, Motor learning, Expertise, Mirror neuron, Skill
Introduction
A little girl who watches The Nutcracker may imagine she can dance like the sugar plum fairy. Once she begins taking dance classes and learns the movements she first saw professional dancers make, how does her cognitive representation of those movements change? It has been proposed that we understand new actions by mapping others’ movements onto our own motor representations, such that there is a close correspondence between the pattern of neural activity recorded while observing, imagining and performing the same action (Rizzolatti and Craighero, 2004; Rizzolatti et al., 2001). We now investigate the extent to which this mapping system requires experience of physically performing actions in order to be engaged while observing and imagining actions.
Action simulation is defined as the internal representation of motor programs without overt movement (Jeannerod, 2001). In brain imaging experiments, imagined movement has long been used as a surrogate marker for simulation. Early positron emission tomography (PET) studies reported premotor and supplementary motor area (SMA) activation, but not primary motor cortical (M1) involvement, during imagined hand movements (Ingvar and Philipson, 1977; Roland et al., 1977, 1980a, b, 1982). Subsequent work performed with functional magnetic resonance imaging (fMRI) and PET demonstrated more detailed functional specificity within brain regions involved in simulation. Specifically, SMA is functionally divisible into subregions, including anterior rostral SMA (SMAr), which is most active during imagined movement, and posterior caudal SMA (SMAc), a subregion most active during action execution (Grafton et al., 1996; Stephan et al., 1995; Tyszka et al., 1994). Additional studies of imagined movement demonstrate involvement of ventral premotor cortex (PMv), the inferior parietal lobule (IPL), the super temporal sulcus (STS), and, rarely, M1 (Binkofski et al., 2000; Decety, 1996; Grafton et al., 1996; Nishitani and Hari, 2000; Rizzolatti et al., 1996b; Stephan et al., 1995). Together, these five areas (SMAr, PMv, IPL, STS, and M1) are implicated as key components in an action simulation circuit.
An enduring question in the study of action simulation is whether the simulation circuit is more active for actions that are embodied, i.e., actions for which the simulator has real physical experience. This would be manifest as greater activity when an individual imagines making movements that are more physically familiar. The present study directly addressed this question by longitudinally comparing simulation of observed movements that participants learned to embody by daily practice to simulation of movements that participants never physically rehearsed. Critically, participants were asked to make judgments about their own ability to perform the observed movements. This self-rating measure was used as a proxy marker of action embodiment. We predicted that simulating more familiar movements would result in increased activity in the simulation circuit, along with higher ratings of ability to perform the observed movements.
One important distinction between the action simulation paradigm used in the present study and those that have been used in the past is that prior simulation studies often asked participants to simply imagine an action without external guidance. For example, subjects have been asked to imagine grasping objects with no visual stimulation (e.g., Stephan et al., 1995; Grafton et al., 1996, Binkofski et al., 2000), but the timing and detail of the imaged action were not controlled. In contrast, we asked participants to observe a dancer’s actions and at the same time imagine themselves performing the actions. Thus, the visual stimulus guides and constrains the motor simulation. For clarity, we refer to our task wherein participants imagine themselves performing an observed action simply as action simulation.
Because the task used in the present study involved action observation, we must also consider how visual stimuli depicting human actions are able to drive motor regions of the brain. Evidence for an action observation/execution matching system was first discovered in the ventral premotor cortex (area F5) of monkeys using single unit recordings, and from this work emerged a new class of neurons that have both visual and motor properties, named ‘mirror neurons’ (Gallese et al., 1996; Rizzolatti et al., 1996a). Since this discovery, attempts to map a corresponding human mirror neuron system have compared the anatomical and functional boundaries of neural systems for action preparation, execution, observation, imitation, and simulation.
A striking and consistent result is that the motor and premotor areas that are classically associated with movement preparation are also active when simply observing the actions of others. This is demonstrated by numerous neuroimaging studies (Buccino et al., 2001; Grafton et al., 1996; Grezes and Decety, 2001; Grezes et al., 2001; Iacoboni et al., 1999; Johnson-Frey et al., 2003; Rizzolatti et al., 1996b). Behavioral studies have demonstrated interactions between action perception and execution (Brass et al., 2000, 2001a,b; Hamilton et al., 2004; Kilner et al., 2003) and lend additional credence to the idea of overlapping neural processes for action observation and execution. Meta-analysis of 26 functional neuroimaging studies on action representations by Grezes and Decety (2001) illustrates that extensive overlap exists between brain regions active during action observation, simulation, and execution, but also highlights the differences in active regions between these tasks. When considering differences solely between action observation and simulation, data from the studies reviewed showed that PMv is most active during action simulation and was not always reported to be active during action observation. In addition, action observation activated more temporal regions, including superior temporal gyrus (STG), that are not active during simulation, a finding the authors attribute to increased visual scene processing demands.
In the present study, we used action observation to guide action simulation. Therefore, the goal of the present study was not to dissociate action simulation from action observation, but instead to measure the effect of embodiment on action simulation constrained by simultaneous observation. Most studies of action observation and simulation have used highly familiar actions, for which there is an established motor representation that can be activated by the imagery or observation task. However, it is also possible to observe movements which are not embodied and cannot be performed. Such movements might be poorly simulated and lead to weaker activations in the simulation circuit than familiar movements. Several studies have begun to address this question and have collectively demonstrated that the action simulation circuit shows the greatest activity when an individual observes an action that he or she is able to perform, compared to observation of physically impossible movements (Costantini et al., 2005; Stevens et al., 2000), movements made by a non-conspecific (Buccino et al., 2004), or unfamiliar dance movements (Calvo-Merino et al., 2005). However, all these studies have used extreme differences in the action stimuli between conditions, comparing actions performed daily to ones which are seldom seen and never performed by the participants. For example, in the dance study, participants watched movies of performers executing a style with which the observer was expertly familiar versus a different dance style that they had never before performed. The study was therefore limited by a cross-population design and differences of physical, visual, and semantic familiarity with the movements.
In the present study, we examine the effects of embodiment on action simulation in greater detail, comparing observation of dance movements that have been recently learned and are physically familiar to observation of unlearned movements in the same style that are not physically familiar. We manipulated participants’ motor experience with 2 sets of complex modern dance sequences and scanned participants once a week for 5 weeks while they learned the movements. By using a within-subject design, we avoid between-population comparisons. The test and control video stimuli were equally visually familiar. Additionally, participants in the present study were learning and observing modern dance sequences that do not have standardized verbal labels attached to the movements. Most forms of dance, including classical ballet, tap, ballroom, capoeira, square dancing, and many others, have specific words associated with individual movements that dancers combine to create sequences. However, the bulk of modern dance does not have a standard, specific, or readily identified movement lexicon. Therefore, we are able to minimize confounds of verbalization.
With these methodological gains, the present study evaluated three hypotheses concerning the modulation of cortical activity within simulation circuits. First, we hypothesized that the simulation regions, SMAr, PMv, IPL, STS, and M1, might demonstrate greater activity during observation and imagination of recently learned movement compared to visually familiar but physically unpracticed movement. Second, we hypothesized that time spent practicing movement subsequent to learning might modulate activity within brain areas involved in action resonance. Third, we predicted that simulation circuits would be modified by self-judgment of performance ability for each of the simulated movements.
Methods
Participants
Participants were 10 members of the Dartmouth Dance Ensemble (8 women, mean age 20.7 ± 2.2 years), a modern dance company comprising expert undergraduate and graduate student dancers. Nine dancers were right hand dominant according to the Edinburgh Handedness Inventory (Oldfield, 1971). All participants had normal vision and no history of neurological or psychiatric disorders and gave their written informed consent to participate in this study in a manner approved by the Committee for the Protection of Human Subjects at Dartmouth College. Mean history of dance training was 12.8 ± 5.6 years, and the dancers spent an average of 11.7 ± 1.8 h per week in dance classes and rehearsals for 4 weeks prior to the scanning and during the 6 weeks of scanning. During the 5 weeks of data collection for this study (scanning sessions 2–6), participants spent an average of 5.2 ± 0.9 h per week learning the novel, highly irregular, and complex movement sequences of Skylight (Dean, 1982).
Stimuli
Stimuli consisted of 36 5 s color video clips of a professional modern dancer performing movement sequences, recorded with a digital video camera. All participants were equally familiar with the model dancer as she taught the piece to participants over the course of the study. Eighteen of the clips were from 5 different sections of Skylight (experimental stimuli). For the first experimental scanning session, participants had begun to learn material from 15 of the 18 video clips (4 of the 5 sections). By the next scanning session, participants had begun to learn material from all 18 experimental video clips. Since behavioral analyses of dancers’ ratings of performance ability for movements within each of the 5 sections revealed no significant differences between section or between section and week (all Ps > 0.05), we did not dwell on divisions between the 5 sections of movements or differences between time of learning the material from each section in our analyses. Henceforth, all experimental stimuli are considered as a whole.
The remaining 18 clips featured the same dancer performing movements that were kinematically similar to Skylight movements with regard to location in space, speed, and body parts used (control stimuli). The dancer performed the movement sequences in a bare studio with neutral colored floors and walls, facing a mirror. The camera was positioned behind the dancer so that both front and back aspects of the movement were captured with use of the mirror. This filming arrangement was specifically chosen to emulate what the dancers saw as they learned and rehearsed the movement in the studio. The video clips’ resolution was reduced for presentation in the scanner so that the dancer’s facial features were not discernable, but all body movements were clearly identifiable.
Behavioral procedure
Participants were scanned at the end of each week for six consecutive weeks. The procedure was identical for each weekly scanning session. Visual stimuli were presented on a Macintosh Powerbook G4 Laptop computer running PsyScope software (Cohen et al., 1993). All stimuli were back projected from an Epson LCD projector (model ELP-7000) onto an adjustable angled mirror mounted at the top of the head coil. Each subject positioned the 4 fingers of his or her right hand on the 4 light-sensitive response keys of a fiber-optic keypad. This apparatus was used to collect responses, which were recorded through the PsyScope button box (New Micros, Dallas, TX).
This study employed an event-related design to measure brain activation differences when participants observe and simulate familiar or unfamiliar dance sequences. Participants completed two experimental runs per scanning session, observing 18 of the 36 video clips in each run. Equal numbers of experimental and control video clips appeared in the two runs and participants saw all control and experimental stimuli once per day of scanning. Stimuli presentation order was randomized. Each run lasted 5 min and 3 s, began with 10 s of fixation, and ended with 20 s of fixation. On each trial, participants saw a video clip for 5 s, followed by 2 s of fixation, and then a question appeared for 3 s. The question read, ‘‘How well could you dance this movement right now?’’, and the four possible responses were: 1—extremely well, 2—well, 3—okay, or 4—not well/need to see again. This question was followed by 5.1 s of fixation, and then a new trial began with presentation of the next video clip. Fig. 1 illustrates the experimental design. Participants were instructed to imagine themselves performing the movement sequences as they observed them and to assess how well they could perform each movement sequence as they watched the videos. Participants were trained outside the scanner with a different set of video clips to become familiar with the response schedule.
Fig. 1.
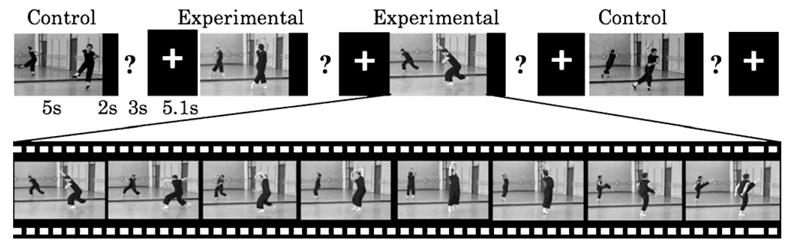
Stimuli and experimental design. The top row of the figure shows an example of part of a movie sequence observed by participants. Control and experimental video clips were randomly presented. After observing and imagining themselves performing the movements in each 5-s video clip, participants responded to a question asking how well they could presently perform the movement just observed.
Imaging procedure
Images were acquired with a 1.5 T GE Signa scanner using a standard birdcage head coil. Head movements were minimized with the use of a foam pillow and padding. Images were acquired continuously during functional scanning using a gradient-echo, echo-planar pulse sequence (TR, 2.5 s; TE, 35 ms; flip angle, 90° ; field of view, 24 cm; 3.75 × 3.75 mm in-plane resolution). The first four volumes of each functional run were discarded to allow for longitudinal magnetization to approach equilibrium, and then an additional 119 volumes of axial images were collected with 25 slices per TR (4.5 mm thickness, 1 mm gap), allowing whole brain coverage. Data were collected and analyzed for 5 weeks of scanning following 1 week of practice scanning. Data for the first session for one subject were accidentally lost, so this subject’s contrasts were calculated on the last 4 weeks of scanning data.
Imaging analysis
Functional data were analyzed with Statistical Parametric Mapping software (SPM2, Wellcome Department of Cognitive Neurology, London, UK; Friston et al., 1995). For each functional run, data were realigned, unwarped, and normalized to the MNI template with a 2 × 2 × 2 mm resolution, which approximates Talairach and Tournoux (1988) atlas space. A 6-mm smoothing kernel was applied to the normalized images. An individualized design matrix was generated and fitted for each subject with each 5 s dance movie trial modeled by a box car convolved with a standard hemodynamic response function. Experimental and control movies were modeled separately, and for each movie, the participant’s rating of his or her ability to perform the movement was included as a parametric modulator. Regressors for self-rating (experimental and control) were center mean normalized across all 5 weeks of data rather than for each week separately. The 3 s question period following each video clip presentation was not specifically modeled.
Statistical Nonparametric Mapping software (SnPM3; Nichols and Holmes, 2002/2005) was used for group-level comparisons to most accurately assess results from a small subject group. A random effects contrast was calculated for the main effect of watching dance (both experimental and control movements) compared to rest, and a one-sample pseudo-t test was used to test for a zero-mean effect across participants at P < 0.05 uncorrected. We used a variance smoothing of 10 mm FWHM and performed 1024 permutations of conditions. The same SnPM parameters were used for all subsequent analyses, with the exception of the P value, which was set to P < 0.01 for all remaining analyses. From this first analysis, a region of interest mask (all-dance mask) was created isolating all brain regions active while observing dance.
To evaluate the hypothesis that simulation of complex whole-body movements that have been physically practiced drives action resonance circuits more than simulation of control movements, we performed a pseudo-t test on the main effect of dance type within the all-dance mask. To determine how action simulation is modulated by time (specifically, weeks) spent rehearsing the test movements, a pseudo-t test was performed on the two time by task interactions. These interactions were brain areas increasing over time while observing experimental movements compared to brain areas decreasing over time while observing control movements, and brain areas decreasing over time while watching experimental movements compared to brain areas increasing over time while watching control movements. As a test of the hypothesis that simulation activity will be greater when participants observe movements with which they are familiar and that they judge that they can perform well, a pseudo-t test on the contrast between experimental clips and control clips with self-ratings as the parameter of interest center mean averaged over 5 weeks of training (modulated experimental > modulated control) was carried out within the dance observation mask. This contrast identifies brain areas where a significant positive correlation exists between self-ratings for the experimental stimuli but not the control stimuli over all 5 weeks. Activations at P < 0.01, uncorrected, are reported for each contrast within the all-dance mask. Resultant pseudo-t images were smoothed by 6 mm to enhance visibility and displayed on partially inflated cortical surfaces using the PALS dataset and Caret visualization tools (http://brainmap.wustl.edu/caret).
Results
Behavioral results
Participants’ ratings of their own ability to perform the movements they rehearsed each week indicated that they thought they were improving as testing sessions advanced, while ratings for performance ability for the control movements did not change significantly (Fig. 2). A 5 (testing session) by 2 (dance clip type) repeated measures analysis of variance (ANOVA) revealed an interaction and two significant main effects. Most importantly, there was a significant interaction between session number and type of dance clip, F(1.37,36) = 4.7, P = 0.041, meaning that ratings of ability to perform the experimental movements improved over the 5 weeks of the study, but there was no evidence of improvement in ratings of control movements. There was a main effect of testing session, with a significant effect of training session on judgment of own performance ability, F(1.73,9) = 7.81, P = 0.006, and performance improved linearly from one session to the next, F(1,9) = 18.5, P = 0.002. Another main effect was present for clip type, with participants judging their ability to perform experimental movements better than their ability to perform control movements, F(1,9) = 12.87, P = 0.006.
Fig. 2.
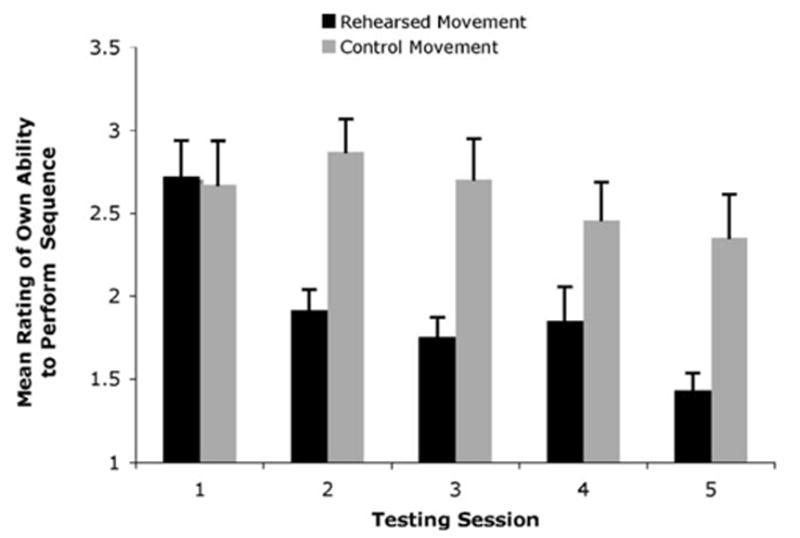
Dancers’ mean rating of their own ability to perform rehearsed and control movements, across scanning session. Ratings were based on a 1–4 scale, with 1 corresponding to the ability to perform the observed movement perfectly and 4 corresponding to being able to perform the observed movement poorly at present. Error bars represent standard error of the mean.
Functional localization
All-dance simulation
First, we defined the brain regions subserving action simulation guided by observation, independent of motor experience or appraised performance ability. This is referred to as the all-dance contrast. For this contrast, we predicted activity within the previously described simulation circuit, including bilateral SMA/CMA, PMv, IPS, STS, and M1. We found unilateral or bilateral activity within all areas of this circuit, including bilateral SMA/CMA, left PMv, bilateral IPS, right STS, and right M1 (Fig. 3). While we did not see clear bilateral activation of all the sites within the predicted circuit, our pattern of activation is still in accord with past action simulation and observation studies, showing preferential activation of left PMv (Binkofski et al., 2000) and right STS (Grossman and Blake, 2002). The one region we could have potentially seen more bilateral activation is M1. Lack of left M1 activity is not surprising because the model included the 3 s question and keypress response period during the baseline against which we compared activity while observing and imagining movement sequences. This contrast also revealed activity in higher-level visual cortex (area V5/MT) and in subcortical motor nuclei, including the caudate and putamen. The locations of all sites showing significant changes in BOLD magnitude while observing dance are summarized in Table 1.
Fig. 3.
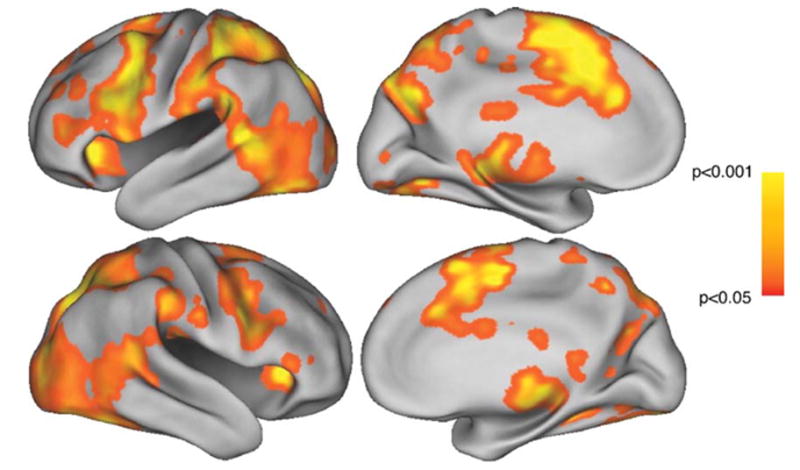
Observation of all-dance, by dancers. Top row: left hemisphere, lateral and medial views. Bottom row: right hemisphere, lateral and medial views. All activations significant at the P < 0.05 level, uncorrected.
Table 1.
Localization of averaged BOLD magnitude during dance observation, relative to baseline, across testing sessions 1–5
| MNI coordinates
|
||||||
|---|---|---|---|---|---|---|
| Region | BA | x | y | z | Functional name | P value |
| Predicted areas/Areas of interest | ||||||
| L/R superior frontal gyrus | 6 | 0 | −6 | 57 | SMAr | 0.002 |
| L superior frontal gyrus | 6 | −3 | 6 | 54 | Pre-SMA | 0.002 |
| R cingulate gyrus | 32 | −9 | 12 | 42 | CMA | 0.002 |
| L inferior precentral sulcus | 6 | −36 | 0 | 45 | PMv | 0.002 |
| L inferior frontal gyrus | 44 | −48 | 12 | 21 | PMv | 0.002 |
| L inferior frontal gyrus | 44 | −54 | 6 | 33 | PMv | 0.003 |
| R superior parietal gyrus | 7 | 27 | −69 | 54 | IPS | 0.004 |
| L superior parietal gyrus | 5 | −33 | −48 | 66 | IPS | 0.006 |
| R superior temporal sulcus | 41 | 45 | −48 | 18 | STS | 0.008 |
| R precentral gyrus | 4 | 66 | −15 | 30 | M1 | 0.013 |
| R precentral gyrus | 4 | 63 | −18 | 39 | M1 | 0.023 |
| Other areas | ||||||
| L caudal postcentral gyrus | 2 | −36 | −36 | 42 | S1 | 0.001 |
| R insula | 47 | 36 | 24 | 0 | VLPFC | 0.002 |
| R middle occipital gyrus | 19 | 33 | −87 | 21 | V5/MT | 0.003 |
| L superior occipital gyrus | 17 | −18 | −99 | −3 | Peristriate area V1 | 0.003 |
| L anterior insula | 47 | −30 | 24 | 0 | VLPFC | 0.003 |
| L caudate/putamen | −18 | 12 | 0 | 0.006 | ||
| L putamen | −18 | 3 | 0 | 0.007 | ||
| R middle occipital gyrus | 19 | 36 | −75 | −21 | V5/MT | 0.007 |
| L hippocampus | −18 | −30 | −3 | 0.012 | ||
| R postcentral sulcus | 2 | 57 | −30 | 36 | S1 | 0.014 |
| R superior temporal gyrus | 42 | 51 | −45 | 9 | 0.017 | |
| R superior temporal gyrus | 42 | 57 | −36 | 21 | 0.033 | |
Significance at all sites was tested by a one-sample pseudo-t test on beta values averaged over each voxel in the cluster, uncorrected P < 0.05. Coordinates are from the MNI template and use the same orientation and origin as found in the Talairach and Tournoux (1988) atlas. BA: Brodmann’s area; R: right; L: left, SMAr: rostral portion, supplementary motor area; CMA: cingulate motor area; PMv: ventral premotor cortex; IPS: intraparietal sulcus; STS: superior temporal sulcus; M1: primary motor cortex; S1: primary somatosensory cortex; VLPFC: ventrolateral prefrontal cortex.
Dance simulation, modulated by physical experience
Next, we evaluated the main effect of motor practice by determining brain regions that were more active when participants observed and simulated movement they had practiced compared to control, non-rehearsed movements. We applied a thresholding mask from the all-dance group contrast to the data to test that this effect was occurring in areas that had also shown the main effect of all-dance > rest. Brain areas that were more active when participants simulated rehearsed movements compared to control movements are illustrated in Fig. 4. Within the action simulation circuit, observation and simulation of rehearsed movements were associated with activity in STS, PMv/pars opercularis, and SMA/CMA, all within the left hemisphere. All brain areas revealed by this contrast are summarized in Table 2. This contrast has commonality with the main finding of Calvo-Merino et al.’s (2005), who compared observation of familiar versus unfamiliar dance. Although the current study also required subjects to imagine generating the movements, both studies demonstrate recruitment of the STS and intraparietal sulcus (IPS), as identified in Table 2.
Fig. 4.
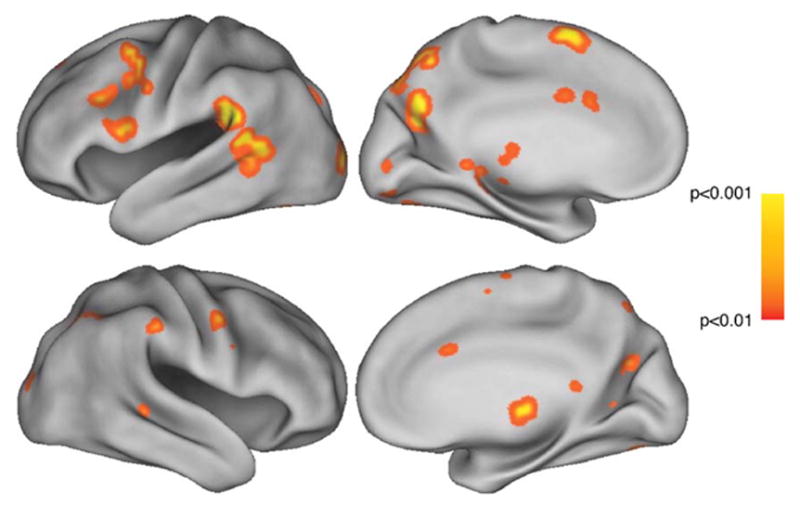
Observation of rehearsed movement, compared to non-rehearsed, control movement, masked by all-dance activations (Fig. 3). Top row: left hemisphere, lateral and medial views. Bottom row: right hemisphere, lateral and medial views. All activations significant at the P < 0.01 level, uncorrected.
Table 2.
Localization of averaged BOLD magnitude for main effect of simulation of rehearsed movements > simulation of control movements, masked by all-dance contrast
| MNI coordinates
|
||||||
|---|---|---|---|---|---|---|
| Region | BA | x | y | z | Functional name | P value |
| Predicted areas/Areas of interest | ||||||
| L posterior superior temporal sulcus* | 22/42 | −48 | −39 | 15 | STS | 0.0005 |
| L superior temporal sulcus* | 22 | −48 | −42 | 9 | STS | 0.0005 |
| L inferior frontal gyrus | 44 | −42 | 18 | 15 | PMv/opercularis | 0.0005 |
| L anterior cingulate cortex | 24 | −3 | 15 | 30 | CMA | 0.0005 |
| R intraparietal sulcus* | 7 | 27 | −54 | 33 | IPS | 0.0005 |
| L superior frontal gyrus | 6 | −6 | 9 | 69 | SMA/pre-SMA | 0.0005 |
| L inferior frontal gyrus | 44 | −51 | 9 | 36 | PMv/opercularis | 0.0015 |
| L precentral gyrus | 6 | −57 | 6 | 12 | PMv | 0.0015 |
| R superior temporal sulcus | 22 | 48 | −51 | 21 | STS | 0.0015 |
| L inferior frontal gyrus | 44 | −42 | 18 | 27 | PMv/opercularis | 0.0015 |
| L inferior frontal gyrus | 44 | −54 | 12 | 33 | PMv/opercularis | 0.0020 |
| Non-predicted areas | ||||||
| L lingual gyrus | 18 | −12 | −87 | −12 | 0.0005 | |
| L perirhinal cortex | 35 | −9 | −27 | −18 | 0.0005 | |
| L calcarine cortex | 17 | −12 | −96 | −6 | 0.0005 | |
| L lingual gyrus | 19 | −9 | −48 | −3 | 0.0005 | |
| R posterior thalamus | 18 | −24 | 6 | 0.0005 | ||
| L parieto-occipital sulcus | 31 | −18 | −66 | 21 | 0.0005 | |
| R precuneus | 31 | 21 | −60 | 24 | 0.0005 | |
| L ventral posterior cingulate cortex | 23 | −9 | −18 | 33 | 0.0005 | |
| R occipitotemporal junction | 19 | 36 | −72 | −6 | 0.0015 | |
| R putamen | 21 | 0 | 9 | 0.0015 | ||
| R middle occipital gyrus | 19 | 24 | −87 | 15 | 0.0015 | |
| L precuneus | 7 | −6 | −66 | 57 | 0.0015 | |
| R cerebellum | 12 | −75 | −33 | 0.0015 | ||
| L cerebellum | −42 | −69 | −24 | 0.0015 | ||
| R cerebellum | 30 | −81 | −24 | 0.002 | ||
| R postcentral gyrus | 2 | 60 | −21 | 48 | 0.003 | |
| L precuneus | 7 | −9 | −81 | 45 | 0.004 | |
Significance at all sites was tested by a one-sample pseudo-t test on beta values averaged over each voxel in the cluster, uncorrected P < 0.01. Stars (*) indicate same regions found in similar analysis by Calvo-Merino et al. (2005). IPL: inferior parietal lobule; all other labeling and abbreviation conventions as in Table 1.
Dance simulation, modulated by weeks spent rehearsing and prior physical experience
We assessed the effect of practice accrual on action simulation. This analysis determined what sites increased or decreased across the 5 weeks of movement rehearsal, independent of participants’ judgments of their own performance abilities. We calculated both increasing and decreasing brain activations while participants observed and simulated experimental and control movements. No areas within the all-dance mask survived in the contrast that examined areas that decreased over time (P < 0.01). For the contrast that evaluated brain areas whose activation profiles were increasing over time while observing and simulating the rehearsed movements compared to the unrehearsed movements, three regions within the mask were significant at the P < 0.01 level. One region was in the left posterior cingulate cortex (reported in MNI atlas space: x = −12, y = −30, z = 24; P = 0.001). A second was in the right fusiform gyrus (x = 30, y = −75, z = −21; P = 0.008), and the third was in the right parahippocampal cortex (x = 21, y = −33, z = −9; P = 0.0098).
Dance simulation, modulated by perceived ability and prior physical experience
The critical analysis compared areas of activation that are greater when participants observed and simulated movements that they have rehearsed and judge that they can perform well, compared to movements that they have no motor experience with, but judge that they can perform well. With this analysis, we identified brain areas that were preferentially recruited when the participant was familiar with and confident of his or her ability to execute the observed movement over all 5 weeks of training, as was evidenced by positive slope for the beta weights in the experimental condition and a negative slope for the beta weights in the control condition. We calculated the interaction of motor experience (rehearsed movements vs. unrehearsed movements) by self-rating of ability within the all-dance mask at the P < 0.01 (uncorrected) level.
Activations were found in two left hemisphere regions within the classic simulation circuit, specifically the IPS/IPL and PMv. A cluster within the left parahippocampal gyrus also emerged. The brain areas revealed by this contrast are summarized in Table 3 and displayed in Fig. 4. These areas are activated specifically by the interaction of physical experience with judged ability, demonstrating that both these factors contributed to motor simulations (Fig. 5).
Table 3.
Localization of averaged BOLD magnitude for main interaction contrast: modulated observation of rehearsed movements > modulated observation of control movement, masked by all-dance observation contrast
| MNI coordinates
|
||||||
|---|---|---|---|---|---|---|
| Region | BA | x | y | z | Functional name | P value |
| L inferior frontal gyrus | 44/6 | −51 | 6 | 30 | PMv | 0.001 |
| L parahippocampal cortex | 36 | −21 | −33 | −12 | 0.002 | |
| L inferior parietal | 40 | −57 | −27 | 36 | IPL (rostral) | 0.005 |
Significance at all sites was tested by a one-sample pseudo-t test on beta values averaged over each voxel in the cluster, uncorrected P < 0.01. All other labeling and abbreviation conventions as in Table 1.
Fig. 5.
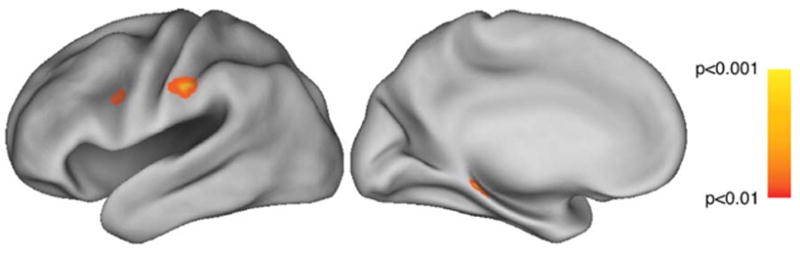
Observation of rehearsed movement > observation of non-rehearsed movement, modulated by ratings of performance ability and masked by all-dance activations (Fig. 3). Figure depicts lateral and medial views of the left hemisphere, respectively. All activations significant at the P < 0.01 level, uncorrected.
Discussion
The primary aim of the present study was to evaluate changes in the action simulation circuit that track with participants’ objective and subjective experience with movements. The first level of findings addressed the question of what brain areas are active when dancers observed and simulated another dancer’s complex whole-body movements, regardless of physical experience or perceived expertise with the movements. We reported activations within a large complement of cortical and subcortical neural regions. Importantly, we found activity within the 5 regions of the purported simulation circuit, including SMAr, PMv, IPL, STS, and M1. We also found a main effect of simulation of rehearsed movement compared to non-rehearsed movement, with more pronounced activity in STS, PMv, IPS, and SMAr.
Next, we distinguished between the influences of time (weeks) spent rehearsing the movements and participants’ self-assessed ratings of movement embodiment. We did not find a linear effect of weeks spent rehearsing on activity within the simulation circuit. This could be because we began scanning the dancers 1 week after they started learning the movement and thus lacked power in the analysis or because the effects of rehearsal may not be linear. Future studies should be able to establish more precise effects of learning time for complex actions by including a pre-training scan and scanning at closer intervals, such as every 24 h, instead of every 7 days, as in the present study. However, we did find an interaction between motor experience and judged ability in three regions. One was the left parahippocampal cortex, which is known to be involved in various elements of spatial and multimodal associative learning (see Squire et al., 2004, for a review). However, we will limit our discussion to the predicted areas within the simulation circuit since we had no a priori predictions about the hippocampal activity. The other two regions fell within the predicted simulation circuit. Specifically, activity in left IPL and PMv was positively correlated with participants’ self-rated sense of action competency, but only for the rehearsed movements.
While it is true that we did not formally evaluate dancers’ competency with performing the movements they observed, we can surmise that sense of competency matches actual embodiment of the newly acquired motor skill. This is a reasonable inference because most trained athletes and dancers have an accurate ability to judge relevant performance in self and others, and post hoc verbal reporting of performance by highly trained performers has been validated by the expert performance literature (Ericsson and Lehmann, 1996; Ericsson and Simon, 1993). Our findings demonstrate the sensitivity of the IPL and PMV regions within the simulation circuit to physical embodiment, not just to movement that is familiar through weeks of practice or visual experience. In the following discussion, we will examine both the relationship between action embodiment and activity within IPL and PMv/pars opercularis and how these findings inform and extend upon prior research.
Activation was found in left IPL and PMv when participants observed movements that they had practiced weekly and judged they could perform well, compared to observing movement that they never practiced and judged their performance ability as poor. These two regions make up the human mirror system as defined by Rizzolatti and Craighero (2004). Neuroimaging research conducted with human subjects supports the notion that a functional relationship exists between inferior parietal and premotor areas, including PMv (Binkofski et al., 2000; Creem-Regehr and Lee, 2005; Wise et al., 1997).
More specifically, the IPL is activated when humans observe, prepare, or simulate actions, and this activation is specific for movement of the limbs, but not the eyes (Deiber et al., 1991; Krams et al., 1998). This area has been shown to be involved with mediating motor attention processes (Rushworth et al., 2001) and is strongly implicated as the primary area of cortex responsible for the visuomotor transformations necessary for imitation or reproduction of observed movement (Grezes et al., 1998; Zentgraf et al., 2005). Evidence from work with non-human primates further informs our knowledge of the role of IPL in action understanding (Fogassi et al., 2005; Gallese et al., 2002). Fogassi and colleagues recorded responses from individual neurons within the convexity of IPL and demonstrated that, not only do many of these neurons show mirror neuron properties for observed and executed actions, but they also code for the specific goals or intentions of motor acts (Fogassi et al., 2005). Here, we have shown that activity in human IPL is greatest when participants simulate actions with which they have physical experience and judge that they can perform. Thus, mirror activity in IPL is related to the degree to which an action is embodied.
Our finding of PMv/pars opercularis activation under these same conditions is in accord with the pattern of results reported from a number of other neuroimaging works on action observation or imagined action (e.g., Buccino et al., 2004; Calvo-Merino et al., 2005; Decety, 1996; Grezes and Decety, 2001). Although the PMv activation seen in the present study is less robust than the IPL activation, it nonetheless has valuable implications. Animal data indicate that the non-human primate homologue of PMv, area F5, is primarily composed of sensorimotor neurons that code for specific action goals, such as reaching or grasping (Rizzolatti and Fadiga, 1998). Work conducted with humans shows that PMv is more involved in covert action stages, such as action observation and imagination, than in actual action execution (Schubotz and von Cramon, 2004). In humans, the main motor area present in caudal inferior frontal gyrus (IFG) is BA 44/pars opercularis, directly adjacent to premotor cortex (BA6). Schubotz and von Cramon (2004) suggest that this caudal motor area of IFG might be responsible for performing higher-level organization of actions, while the adjacent section of inferior premotor cortex (inferior BA 6) is involved with the organization of simpler, lower-level action representations. Such an explanation is in accord with our finding of activity mainly within BA 44/pars opercularis while simulating complex dance sequences.
While it is true that the BA44 is also involved in language processing, it is unlikely that activation in this area in the present study is being driven by an increased ability to verbalize observed and imagined movements. First, we do not see activation of other middle temporal areas involved with semantic categorization (Vandenberghe et al., 1996). In addition, several studies have demonstrated clear activation of this area in action tasks where speech was not involved, suggesting that BA44/PMv can have an action component independent of language processing (Carey et al., 1997; Decety et al., 1997; Iacoboni et al., 1999; Nishitani and Hari, 2000; Rizzolatti et al., 1996a; Schubotz and von Cramon, 2004). As discussed earlier, we specifically chose to study the learning of modern dance movements that are not associated with standardized verbal labels. While it is possible that participants could have assigned arbitrary labels to the movements, this would almost certainly have been done at an implicit level as post hoc interviews revealed that dancers did not rely on specific verbal labels or descriptions while simulating (or learning) movement.
There are several aspects of the present study that are best understood by comparison to other relevant studies in the field. First, we consider a recent study that investigated how acquired motor skills influence the perception of another individual’s actions in ballet dancers, capoeira dancers, and inexpert control subjects (Calvo-Merino et al., 2005). All participants passively viewed ballet and capoeira dance clips while being scanned. The authors reported activity within the action resonance circuit, including the 5 sites in the simulation circuit, when the participants observed the movement style they had expertise in performing. This indicates that brain regions involved in action resonance processes are sensitive to movement familiarity. Our results replicate these findings in a within-subjects design with a single class of movements, all of the same style and differing only in motor experience. We showed that, when the same participants observe movement they had rehearsed, areas within the action resonance circuit show greater activity than when observing never-rehearsed movements. We extend Calvo-Merino et al.’s findings further through use of externally guided movement simulation, instead of passive movement observation, to begin to address the issue of movement embodiment. Thus, areas involved in action observation and imagination are sensitive to prior physical experience.
An additional issue broached by the Calvo-Merino et al. study that we have addressed is the role of verbal familiarity. Confounds of verbal familiarity cannot be ruled out with certainty in the Calvo-Merino et al. study as both dance styles investigated have well-established movement lexicons associated with the component movements. The present study has extended this work by investigating the neural processes subserving action simulation in an active context, guided by visual input, with less of a chance of engagement of language processes. Within this framework, we have established that time spent practicing the movements and visual and physical familiarity with the movements is not enough to drive core areas of the simulation circuit. Instead, it is one’s own ability to actually generate the movement that has the greatest influence on further increasing activity within action understanding areas.
Another study that informs the findings of the present study was conducted by Creem-Regehr and Lee on the degree to which tools or graspable non-tool objects stimulate the action simulation circuit (Creem-Regehr and Lee, 2005). In this fMRI study, participants viewed 3D images of tools or other graspable, non-tool objects and were asked to simply view the items or to view and imagine grasping them. Data revealed that, when participants imagined grasping either type of object, a consistent pattern of premotor (including PMv) and posterior parietal activity was present, with stronger activations in the left hemisphere. This pattern of activity was far more robust and encompassed more parietal and premotor areas when participants were imagining grasping the tools they were viewing compared to grasping the non-tool objects. The grasping simulation was in response to a visually presented object and the task relied upon the object’s features to create the simulation. A question that arises from this approach is whether participants were actually simulating the movement or simply recalling overlearned functional knowledge of familiar objects, as has been shown to occur when participants view tools compared to non-tool objects (e.g., Chao et al., 1999; Chao and Martin, 2000). The issue of nameability is present in their study as well since tools have readily accessed names that non-tool shapes do not necessarily have. This difference might be partially responsible for some of the differences in IFG activation between the tool and non-tool conditions in this study. The present study extends this work by looking at how unnamed perceptual motor processes change with learning when simulation is not just externally triggered but is externally guided as well.
A third study to consider is the seminal work by Buccino and colleagues on imitation learning in the context of learning to play chords on the guitar (Buccino et al., 2004). In this imaging study, musically naive participants completed multiple sessions of four experimental conditions. In the imitate condition, participants observed an experienced guitar-playing model play a chord then imitated the same chord after a pause. In the non-imitative condition, participants observed the same thing, but this time performed a different, non-chord-related hand action after the pause. In the observe condition, participants simply watched the model play a chord, and, in the execute condition, participants performed a chord of their choice without visual guidance from a model. These authors found that IPL and PMv became active as participants observed a model play the chords that they had to imitate after the pause and suggest that the relay of sensorimotor information between these two mirror neuron-rich areas is an essential component of imitation learning. This result is in accord with our findings that these same areas are activated when participants observe and imagine performing actions that they have physically embodied and are able to perform.
The present study establishes a role of physical embodiment in action simulation. We replicated past findings that reveal a general mechanism for action resonance and action simulation that encompasses parietal, premotor, and subcortical areas. This general mechanism has been shown by prior work to be sensitive to different features including familiarity, conceptual knowledge, and physical plausibility. Within this network, IPL and PMv/pars opercularis are demonstrated to be the two distinct regions that are sensitive to perceived physical competency. This finding is in accord with the theory that motor vocabularies are stored within these two brain regions, an idea that is supported by studies with apraxic patients (Buxbaum et al., 2003; Fukutake, 2003) and animal studies (Rizzolatti and Craighero, 2004; Rizzolatti et al., 1996a). This implies a close relationship between the substrates of action and physical embodiment.
Acknowledgments
The authors would like to thank the Dartmouth Dance Ensemble for their tireless efforts, Catherine Hynes for technical assistance, Raymundalo Mar for scanning assistance, and two anonymous reviewers for helpful comments. Supported by the James S. McDonnwell Foundation and Public Health Service grant NS33504.
References
- Binkofski F, Amunts K, Stephan KM, Posse S, Schormann T, Freund HJ, et al. Broca’s region subserves imagery of motion: a combined cytoarchitectonic and fmri study. Hum Brain Mapp. 2000;11 (4):273–285. doi: 10.1002/1097-0193(200012)11:4<273::AID-HBM40>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass M, Bekkering H, Wohlschlager A, Prinz W. Compatibility between observed and executed finger movements: comparing symbolic, spatial, and imitative cues. Brain Cogn. 2000;44 (2):124–143. doi: 10.1006/brcg.2000.1225. [DOI] [PubMed] [Google Scholar]
- Brass M, Bekkering H, Prinz W. Movement observation affects movement execution in a simple response task. Acta Psychol (Amst) 2001a;106 (1–2):3–22. doi: 10.1016/s0001-6918(00)00024-x. [DOI] [PubMed] [Google Scholar]
- Brass M, Zysset S, von Cramon DY. The inhibition of imitative response tendencies. NeuroImage. 2001b;14 (6):1416–1423. doi: 10.1006/nimg.2001.0944. [DOI] [PubMed] [Google Scholar]
- Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V. Action observation activates premotor and parietal areas in a somatotopic manner: an fmri study. Eur J Neurosci. 2001;13 (2):400–404. [PubMed] [Google Scholar]
- Buccino G, Lui F, Canessa N, Patteri I, Lagravinese G, Benuzzi F, et al. Neural circuits involved in the recognition of actions performed by nonconspecifics: an fmri study. J Cogn Neurosci. 2004;16 (1):114–126. doi: 10.1162/089892904322755601. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Sirigu A, Schwartz MF, Klatzky R. Cognitive representations of hand posture in ideomotor apraxia. Neuropsychologia. 2003;41 (8):1091–1113. doi: 10.1016/s0028-3932(02)00314-7. [DOI] [PubMed] [Google Scholar]
- Calvo-Merino B, Glaser DE, Grezes J, Passingham RE, Haggard P. Action observation and acquired motor skills: an fmri study with expert dancers. Cereb Cortex. 2005;15 (8):1243–1249. doi: 10.1093/cercor/bhi007. [DOI] [PubMed] [Google Scholar]
- Carey D, Perrett D, Oram M. Recognizing, understanding and reproducing action. In: Boller F, Grafman J, editors. Handbook of Neuropsychology. Vol. 11. Elsevier; Amsterdam: 1997. pp. 111–129. [Google Scholar]
- Chao LL, Martin A. Representation of manipulable man-made objects in the dorsal stream. NeuroImage. 2000;12 (4):478–484. doi: 10.1006/nimg.2000.0635. [DOI] [PubMed] [Google Scholar]
- Chao LL, Haxby JV, Martin A. Attribute-based neural substrates in temporal cortex for perceiving and knowing about objects. Nat Neurosci. 1999;2 (10):913–919. doi: 10.1038/13217. [DOI] [PubMed] [Google Scholar]
- Cohen J, Macwhinney B, Flatt M, Provost J. PsyScope: a new graphic interactive environment for designing psychological experiments. Behav Res Methods Instrum Comput. 1993;25:257–271. [Google Scholar]
- Costantini M, Galati G, Ferretti A, Caulo M, Tartaro A, Romani GL, et al. Neural systems underlying observation of humanly impossible movements: an fmri study. Cereb Cortex. 2005:1761–1767. doi: 10.1093/cercor/bhi053. [DOI] [PubMed] [Google Scholar]
- Creem-Regehr SH, Lee JN. Neural representations of graspable objects: are tools special? Brain Res Cogn Brain Res. 2005;22 (3):457–469. doi: 10.1016/j.cogbrainres.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Dean L. Skylight. 1982. Artist. [Dance] [Google Scholar]
- Decety J. Do imagined and executed actions share the same neural substrate? Brain Res Cogn Brain Res. 1996;3 (2):87–93. doi: 10.1016/0926-6410(95)00033-x. [DOI] [PubMed] [Google Scholar]
- Decety J, Grezes J, Costes N, Perani D, Jeannerod M, Procyk E, et al. Brain activity during observation of actions. Influence of action content and subject’s strategy. Brain. 1997;120 (Pt 10):1763–1777. doi: 10.1093/brain/120.10.1763. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Passingham RE, Colebatch JG, Friston KJ, Nixon PD, Frackowiak RS. Cortical areas and the selection of movement: a study with positron emission tomography. Exp Brain Res. 1991;84 (2):393–402. doi: 10.1007/BF00231461. [DOI] [PubMed] [Google Scholar]
- Ericsson KA, Lehmann AC. Expert and exceptional performance: evidence of maximal adaptation to task constraints. Annu Rev Psychol. 1996;47:273–305. doi: 10.1146/annurev.psych.47.1.273. [DOI] [PubMed] [Google Scholar]
- Ericsson KA, Simon HA. Protocol Analysis: Verbal Reports as Data. MIT Press; Cambridge: 1993. [Google Scholar]
- Fogassi L, Ferrari PF, Gesierich B, Rozzi S, Chersi F, Rizzolatti G. Parietal lobe: from action organization to intention understanding. Science. 2005;308 (5722):662–667. doi: 10.1126/science.1106138. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Poline JB, Frith CD, Heather JD, Frackowaik RSJ. Spatial registration and normalization of images. Hum Brain Mapp. 1995;2:1–25. [Google Scholar]
- Fukutake T. Apraxia of tool use: an autopsy case of biparietal infarction. Eur Neurol. 2003;49 (1):45–52. doi: 10.1159/000067027. [DOI] [PubMed] [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119 (Pt 2):593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- Gallese V, Fogassi L, Fadiga L, Rizzolatti G. Action representation in the inferior parietal lobule. In: Prinz W, Hommel B, editors. Attention and Performance XIX: Common Mechanisms in Perception and Action. Oxford Univ. Press; Oxford: 2002. [Google Scholar]
- Grafton ST, Arbib MA, Fadiga L, Rizzolatti G. Localization of grasp representations in humans by positron emission tomography: 2. Observation compared with imagination. Exp Brain Res. 1996;112 (1):103–111. doi: 10.1007/BF00227183. [DOI] [PubMed] [Google Scholar]
- Grezes J, Decety J. Functional anatomy of execution, mental simulation, observation, and verb generation of actions: a meta-analysis. Hum Brain Mapp. 2001;12 (1):1–19. doi: 10.1002/1097-0193(200101)12:1<1::AID-HBM10>3.0.CO;2-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grezes J, Costes N, Decety J. Top–down effect of strategy on the perception of human biological motion: a pet investigation. Cogn Neuropsychol. 1998;15:553–582. doi: 10.1080/026432998381023. [DOI] [PubMed] [Google Scholar]
- Grezes J, Fonlupt P, Bertenthal B, Delon-Martin C, Segebarth C, Decety J. Does perception of biological motion rely on specific brain regions? NeuroImage. 2001;13 (5):775–785. doi: 10.1006/nimg.2000.0740. [DOI] [PubMed] [Google Scholar]
- Grossman ED, Blake R. Brain areas active during visual perception of biological motion. Neuron. 2002;35 (6):1167–1175. doi: 10.1016/s0896-6273(02)00897-8. [DOI] [PubMed] [Google Scholar]
- Hamilton A, Wolpert D, Frith U. Your own action influences how you perceive another person’s action. Curr Biol. 2004;14 (6):493–498. doi: 10.1016/j.cub.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999;286 (5449):2526–2528. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- Ingvar DH, Philipson L. Distribution of cerebral blood flow in the dominant hemisphere during motor ideation and motor performance. Ann Neurol. 1977;2:230–237. doi: 10.1002/ana.410020309. [DOI] [PubMed] [Google Scholar]
- Jeannerod M. Neural simulation of action: a unifying mechanism for motor cognition. NeuroImage. 2001;14:S103–S109. doi: 10.1006/nimg.2001.0832. [DOI] [PubMed] [Google Scholar]
- Johnson-Frey SH, Maloof FR, Newman-Norlund R, Farrer C, Inati S, Grafton ST. Actions or hand–object interactions? Human inferior frontal cortex and action observation. Neuron. 2003;39 (6):1053–1058. doi: 10.1016/s0896-6273(03)00524-5. [DOI] [PubMed] [Google Scholar]
- Kilner JM, Paulignan Y, Blakemore SJ. An interference effect of observed biological movement on action. Curr Biol. 2003;13 (6):522–525. doi: 10.1016/s0960-9822(03)00165-9. [DOI] [PubMed] [Google Scholar]
- Krams M, Rushworth MF, Deiber MP, Frackowiak RS, Passingham RE. The preparation, execution and suppression of copied movements in the human brain. Exp Brain Res. 1998;120 (3):386–398. doi: 10.1007/s002210050412. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15 (1):1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitani N, Hari R. Temporal dynamics of cortical representation for action. Proc Natl Acad Sci U S A. 2000;97 (2):913–918. doi: 10.1073/pnas.97.2.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L. Grasping objects and grasping action meanings: the dual role of monkey rostroventral premotor cortex (area f5) Novartis Found Symp. 1998;218:81–95. doi: 10.1002/9780470515563.ch6. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Gallese V, Fogassi L. Premotor cortex and the recognition of motor actions. Brain Res Cogn Brain Res. 1996a;3 (2):131–141. doi: 10.1016/0926-6410(95)00038-0. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Matelli M, Bettinardi V, Paulesu E, Perani D. Localization of grasp representations in humans by pet: 1. Observation versus execution. Exp Brain Res. 1996b;111 (2):246–252. doi: 10.1007/BF00227301. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nat Rev, Neurosci. 2001;2 (9):661–670. doi: 10.1038/35090060. [DOI] [PubMed] [Google Scholar]
- Roland PE, Skinhøj E, Larsen B, Lassen NA. The role of different cortical areas in organization of voluntary movements. Acta Neurol Scand, Suppl. 1977;64:542–543. [PubMed] [Google Scholar]
- Roland PE, Larsen B, Lassen NA, Skinhøj E. Supplementary motor area and other cortical areas in organization of voluntary movements in man. J Neurophysiol. 1980a;43 (1):118–136. doi: 10.1152/jn.1980.43.1.118. [DOI] [PubMed] [Google Scholar]
- Roland PE, Skinhøj E, Lassen NA, Larsen B. Different cortical areas in man in organization of voluntary movements in extrapersonal space. J Neurophysiol. 1980b;43 (1):137–150. doi: 10.1152/jn.1980.43.1.137. [DOI] [PubMed] [Google Scholar]
- Roland PE, Meyer E, Shibasaki T, Yamamoto YL, Thompson CJ. Regional cerebral blood flow changes in cortex and basal ganglia during voluntary movements in normal human volunteers. J Neurophysiol. 1982;48 (2):467–480. doi: 10.1152/jn.1982.48.2.467. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Ellison A, Walsh V. Complementary localization and lateralization of orienting and motor attention. Nat Neurosci. 2001;4 (6):656–661. doi: 10.1038/88492. [DOI] [PubMed] [Google Scholar]
- Schubotz RI, von Cramon DY. Sequences of abstract nonbiological stimuli share ventral premotor cortex with action observation and imagery. J Neurosci. 2004;24 (24):5467–5474. doi: 10.1523/JNEUROSCI.1169-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Stephan KM, Fink GR, Passingham RE, Silbersweig D, Ceballos-Baumann AO, Frith CD, et al. Functional anatomy of the mental representation of upper extremity movements in healthy subjects. J Neurophysiol. 1995;73 (1):373–386. doi: 10.1152/jn.1995.73.1.373. [DOI] [PubMed] [Google Scholar]
- Stevens JA, Fonlupt P, Shiffrar M, Decety J. New aspects of motion perception: selective neural encoding of apparent human movements. NeuroReport. 2000;11 (1):109–115. doi: 10.1097/00001756-200001170-00022. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Thieme; New York: 1988. [Google Scholar]
- Tyszka JM, Grafton ST, Chew W, Woods RP, Colletti PM. Parceling of mesial frontal motor areas during ideation and movement using functional magnetic resonance imaging at 1.5 Tesla. Ann Neurol. 1994;35 (6):746–749. doi: 10.1002/ana.410350617. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Price C, Wise R, Josephs O, Frackowiak RS. Functional anatomy of a common semantic system for words and pictures. Nature. 1996;383 (6597):254–256. doi: 10.1038/383254a0. [DOI] [PubMed] [Google Scholar]
- Wise SP, Boussaoud D, Johnson PB, Caminiti R. Premotor and parietal cortex: corticocortical connectivity and combinatorial computations. Annu Rev Neurosci. 1997;20:25–42. doi: 10.1146/annurev.neuro.20.1.25. [DOI] [PubMed] [Google Scholar]
- Zentgraf K, Stark R, Reiser M, Kunzell S, Schienle A, Kirsch P, et al. Differential activation of pre-sma and sma proper during action observation: effects of instructions. NeuroImage. 2005;26 (3):662–672. doi: 10.1016/j.neuroimage.2005.02.015. [DOI] [PubMed] [Google Scholar]


