Abstract
Hepatocyte growth factor/scatter factor (HGF/SF) is a pluripotent growth factor capable of acting as a motogen, a morphogen, and a mitogen. Originally, HGF/SF was found as a blood-borne mitogen for hepatocytes and has since been determined to be very important in liver repair. Previous studies have established that HGF/SF must be proteolytically cleaved to elicit its effects. After liver injury by toxins such as carbon tetrachloride or after surgical resection, partial hepatectomy (PHX), HGF/SF concentrations increase in the blood. The aims of this study were to examine (1) which form of HGF/SF is present in the normal liver, (2) which form is present in the regenerating liver after PHX, and (3) if the HGF/SF used after PHX is derived from existing liver reservoirs. Both single-chain HGF/SF and active two-chain HGF/SF are present in normal liver, with the former being the dominant form. After PHX, the liver can be described as having two phases with regard to the use of endogenous HGF/SF. The first phase from 0 to 3 hours is the consumptive phase and is characterized by a decrease in both single-chain HGF/SF and active two-chain HGF/SF. The second phase is the productive phase. It is characterized by a pronounced reappearance of both single-chain HGF/SF as well as two-chain HGF/SF. The activation index shows a 5-fold increase over sham operations during the productive phase. The use of radiolabeled HGF/SF showed that during the first 3 hours, HGF/SF is used in part from hepatic stores. Furthermore, during the first 3 hours after PHX, only active two-chain HGF/SF is seen in the plasma.
Hepatocyte growth factor/scatter factor (HGF/SF) was initially identified by its motogenic activity on Madin-Darby canine kidney (MDCK) cells1 as well as by its mitogenic activity on hepatocytes.2,3 HGF/SF is a potent mitogenic and motogenic stimulator for primary cultures of either rodent or human hepatocytes. Furthermore, HGF/SF has been shown to have morphogenic properties for a variety of cell systems.4
HGF/SF is an important multifunctional cytokine involved in liver development and liver repair after an injury. In mice, homozygous deletion of either the HGF/SF gene or its tyrosine kinase receptor gene (c-met) is associated with embryonic lethality.5,6 In the case of the HGF/SF gene knockout, the lethality was largely because of an abnormality in placental development. Examination of the livers from these animals revealed them to be grossly underweight with extensive loss of parenchymal cells.6 The HGF/SF knockout embryo can be rescued from lethality by a bolus injection of HGF/SF at day e9.5, but histologic examination of the rescued animal showed that organogenesis, particularly with regard to the liver, was severely impaired.7
HGF/SF is synthesized by mesenchymal cells as a 723-(deleted form) or 728- (full-length) amino acid single-chain polypeptide.8,9 From the N terminus to the C terminus, HGF/SF contains a hairpin loop followed by 4 kringle domains and a pseudo-serine protease domain. HGF/SF is secreted as an inactive single-chain protein, which is proteolyti-cally activated by cleavage at the Arg-Val-Val (aa494–495) site. Two enzymes have been identified that are capable of cleaving inactive single-chain HGF/SF (scHGF/SF) to an active 2-chain HGF/SF (tcHGF/SF): urokinase plasminogen activator (uPA)10,11 and HGF activator (HGFa).12 The heavy chain of HGF/SF (hcHGF/SF) contains the hairpin loop and four kringle domains. Its apparent molecular weight is 65 kd. The light chain of HGF/SF possesses the pseudo-serine protease domain. Its apparent molecular weight is 30 to 34 kd. The HGF/SF receptor (c-Met)13,14 is capable of binding both inactive scHGF/SF as well as active tcHGF/SF with similar avidity, but only tcHGF/SF activates the c-Met tyrosine kinase domain.15,16 Mesenchymal cells are responsible for the production of HGF/SF,17,18 whereas epithelial cells of various organs and tissues including the liver normally express c-Met.19 Therefore, HGF/SF is considered to be an important mediator of mesenchymal-epithelial interaction within the liver as well as in other organs.
After a liver injury by chemicals such as carbon tetrachloride, by surgical resection of two thirds of the liver, partial hepatectomy (PHX), or by antagonistic Fas antibodies, the levels of HGF/SF in the blood increase dramatically.20–23 Administration of HGF/SF neutralizing antibodies to rats before PHX abrogates the normally seen elevation of HGF/SF in the serum, and hepatocyte proliferation is severely inhibited.24 In the PHX model, the relevant HGF/SF activator appears to be uPA.25 uPA activity is detected in as little as 5 minutes after PHX, and the addition of uPA neutralizing antibodies to homogenates of regenerating liver protein will abrogate the normally observed HGF/SF activation associated with the homogenates.26 Also, uPA appears to be the relevant activator in the Fas-mediated liver injury model. When Fas-mediated liver injury is induced in uPA-deficient animals, liver regeneration is severely impaired.23 This further shows the importance of uPA in HGF/SF activation in the regenerating liver.
The liver is the primary organ for the clearance of radiolabeled HGF/SF after intravenous injection.27,28 The clearance kinetics is biphasic representing the two known binding sites for HGF/SF on the cell surface: c-Met and heparin sulfate proteoglycans.29 Because HGF/SF binds with moderate affinity to the interstitial extracellular matrix, the liver has the ability of sequestering large amounts of HGF/SF in its extracellular matrix. This is confirmed by the fact that large quantities of HGF/SF can be eluted from the hepatic biomatrix by perfusing the liver with a high salt solution.30
HGF/SF effects during the liver regeneration process after PHX are important.24 Earlier work examining the phosphorylation state of c-Met during liver regeneration showed that tyrosine phosphorylation of the c-Met receptor reaches a peak within 1 hour after PHX.31 In view of the fact that HGF/SF gene expression is not up-regulated until 3 to 6 hours after PHX,32 the HGF/SF used during the first 3 hours after PHX must be derived predominately from preexisting stores within the body. The first wave of hepatocyte DNA synthesis peaks at 24 hours after PHX and occurs in the periportal region.33 This correlates with the previously reported distribution of HGF/SF within the liver. Immunohistochemistry studies in addition to experiments with radiolabeled HGF/SF showed that the periportal region of the normal liver is the location of the highest concentration of HGF/SF.34
Little is known about which forms of HGF/SF exist in the liver or blood during liver regeneration after PHX. Our current study used Western immunoblots for the detection of scHGF/SF (inactive) and tcHGF/SF (active) after PHX in liver tissue as well as in the blood. In addition, we investigated the fate of preexisting radiolabeled HGF/SF injected into the liver before a partial hepatectomy to determine if HGF/SF stored in the liver is indeed consumed during the early stage of liver regeneration.
MATERIALS AND METHODS
Materials
All chemicals were obtained from Sigma Chemical Company (St. Louis, MO) unless otherwise indicated.
Animals
All experiments were performed on male Fisher 344 rats weighing 140 to 150 g (Charles River Laboratories, Wilmington, MA). Animals were allowed access to food and water ad libitum until they were used for the experimental procedures. The Institutional Animal Use and Care Committee of the University of Pittsburgh approved of all housing arrangements and experimental procedures. Metofane (Pitman-Moore, Mundelein, IL) was used to anesthetize animals for surgeries unless otherwise stated.
Partial Hepatectomy and Blood Collection
The rats were given a two-thirds partial hepatectomy as originally described by Higgins and Anderson.35 For controls, a time-matched sham operation was performed. Sham operations involved a laparotomy and resection of the xyphoid process of the sternum. At defined time points, the animals were anesthetized with Nembutal (Abbott, Chicago, IL) and the remaining lobes were removed. All liver samples were promptly frozen in liquid nitrogen and stored at − 80°C. Blood intended for plasma preparation was collected via the inferior vena cava with a syringe containing 50 μ L of 15% EDTA, and the blood was then transferred into a purple top Vacutainer tube (Becton-Dickinson, Franklin Lakes, NJ). The Vacutainer tube was spun at 3,000 rpm for 5 minutes at 4°C. The plasma was removed and AEBSF, a serine protease inhibitor, was added to a final concentration of 0.05 mg/mL. The plasma was frozen until used.
Mesenteric Vein Injections
Rats were operated on under metophane anesthesia. The peritoneal cavity was accessed through midline laparotomy. After visualization of the inferior mesenteric vein by extra-abdominal repositioning of the bowel, 200 μ L of labeled scHGF/SF (gift of Toyobo, Osaka, Japan) were infused into the inferior mesenteric vein via a 1-mL syringe with a 30-gauge needle. Following the injection, manual compression of the injection site with a sterile cotton swab followed the removal of the needle to minimize the risk of bleeding. The animals were then housed for exactly 24 hours until either a PHX or sham operation was performed. The animals were allowed to drink water and eat a standard rat diet ad libitum.
Tissue Homogenization
Buffer used for the homogenization of the liver samples consisted of 20 mmol/L Tris at pH of 8. Included in the buffer was 1% sodium dodecyl sulfate, 5 mmol/L EDTA, 3 mmol/L EGTA, 1 mmol/L DTT, E-64 (0.01 mg/mL), pepstatin A (0.01 mg/mL), leupeptin (0.01 mg/mL), aprotinin (0.02 mg/mL), and AEBSF (0.05 mg/mL). The ratio of buffer to tissue used in the homogenization was always 0.2 mg of liver tissue to 2 mL of homogenization buffer. The tissue was homogenized on ice for 1 to 1.5 minutes at 10,000 rpm with a Polytron homogenizer (Brinkman, Westbury, NY). The samples were then spun for 15 minutes at 5,000 rpm at 4°C in a Beckman J-90 centrifuge (Palo Alto, CA). The supernatant was promptly aliquotted and stored at − 80°C.
Plasma Liquid Chromatography and Extraction
A fixed volume (13 mL) of rat plasma (prepared as described) was diluted 2-fold with Tris-buffered saline containing AEBSF (0.2 mg/mL). The diluted plasma was then loaded onto a 10-mL disposable column (Bio-Rad, Hercules, CA) containing 0.5 mL of heparin sepharose. Before the sample was loaded onto the column, the heparin sepharose column was equilibrated with 20 mL of Tris-buffered saline with 1 mg/mL of protease-free albumin (Boehringer Mannheim, Mannheim, Germany). After sample loading, the column was washed with 3 mL of Tris-buffered saline (0.1 mg/mL AEBSF). The bound proteins were eluted from the column with 1.0 mL of 1.2 mol/L NaCl solution (0.1 mg/mL AEBSF) in a 1.5-mL tube (Sardstedt, Newton, NC). The protein was extracted from the solution by addition of 0.5 mL of Tris-buffered saline saturated phenol. The samples were vortexed for 20 seconds and spun for 1 minute at high speed in a bench-top centrifuge. The upper phase containing the aqueous fraction was discarded and 1 mL of diethyl ether (Mallinckrodt, Chesterfield, MO) was added to the remaining phenol layer. The sample was once again vortexed for 20 seconds and centrifuged at high speed for 1 minute. The upper ether phase was discarded. The addition and removal of ether was repeated.36 The sample was then lyophilized.
Gel Electrophoresis
The protein concentration of the homogenates was measured by the bicinchoninic acid technique.37 Equal amounts (300 μ g protein) of homogenates were brought up to a uniform volume. A corresponding volume of loading buffer consisting of 62 mmol/L Tris, 2% sodium dodecyl sulfate, 10% glycerol, and 0.14 mmol/L bromphenol blue was added to the homogenates. The column-purified proteins from the plasma samples were resuspended in 15 μ L of Milli-Q water (Millipore, Bedford, MA) and 15 μ L of DTT containing loading buffer. The samples were heated to 90°C for 10 minutes and were analyzed by continuous Tris-tricine gel electrophoresis.38
Immunoblotting
The gels were transferred overnight at 250 mA to an Immobilon-P membrane (Millipore) in a transfer buffer consisting of 50 mmol/L Tris-HCl, 95 mmol/L glycine, and 0.005% sodium dodecyl sulfate. After the transfer, the membranes were soaked for 2 hours in a blotting buffer (blotto) with 5% nonfat dry milk. The blotto contained 20 mmol/L Tris, 150 mmol/L NaCl, 0.1% Tween 20 at a pH of 7.5. The primary antibody used to detect HGF/SF heavy chain and single chain was obtained from the Institute of Immunology (Tokyo, Japan). The monoclonal antibody was used at a concentration of 1:500 in 5% blotto. After a 2-hour incubation at room temperature in the primary antibody, the membranes were washed 3 times in 1% blotto for a total of 30 minutes. The membranes were then placed in 1% blotto containing a horseradish peroxidase–conjugated secondary antibody (Chemicon, Temecula, CA) at a 1:50,000 dilution. After a 1-hour incubation at room temperature, the membranes were washed 5 times with blotto for a total of 50 minutes. The membranes were then soaked for 3 minutes in an enhanced chemiluminescense reagent (Pierce, Rockford, IL) and exposed to film (Kodak, Rochester, NY) and developed.
HGF/SF Iodination
Iodobeads (Pierce, Rockford, IL) were prepared as per the instructions of the manufacturer. Between 2 and 5 μ g of HGF/SF was placed in a tube with 100 mmol/L sodium phosphate reaction buffer (pH 6.8) and 0.5 mCi of sodium iodide (Amersham, Arlington Heights, IL) to a final volume of 100 μ L. The reaction proceeded for 8 minutes and was stopped by removal of the reaction solution from the iodobeads. The excess reactive free iodine was removed by the addition of tyrosine to a final concentration of 5 mmol/L. The reaction mixture was then loaded onto a 5-mL G-25 desalting column (Pierce, Rockford, IL) that was previously equilibrated in Tris-buffered saline containing protease-free albumin, 1 mg/mL, and pH7.4. If the labeled HGF/SF was to be used for injections into rodents, the buffer used was phosphate-buffered saline containing rat albumin (Calbiochem, San Diego, CA), 1 mg/mL, and pH 7.4. The iodinated HGF/SF was evaluated by gel chromatography to ensure that the HGF/SF remained intact. Specific activity was normally 17,000 to 25,000 cpm/ng.
RESULTS
Forms of HGF/SF Present in the Regenerating Liver Following PHX
To examine the forms of HGF/SF present in the regenerating rat liver, livers were harvested from 3 sets of animals (3 separate experiments). Each set included animals subjected to either PHX or a sham operation. In each set, the time points of animal sacrifice ranged from 0 to 72 hours after the operation. The samples were analyzed by polyacrylamide gel electrophoresis (PAGE), and the results from one set are shown in Fig. 1A. Figure 1B and C represents the average results from three sets of experiments. The integrated optical density of the scHGF/SF (inactive) and hcHGF/SF (active) was normalized to the zero time point of each blot from a minimum of 3 separate Western blots. In the normal liver, both forms of HGF/SF were present (Fig. 1A, lane 0), but the majority of HGF/SF appeared to be in the inactive single-chain form. In the sham-operated animals, the amount of scHGF/SF (Fig. 1B) and hcHGF/SF (Fig. 1C) remained relatively constant. During the first hour after PHX, levels of scHGF/SF decreased by 55% relative to the zero time point (Fig. 1A, lanes 0–60; Fig. 1B) while the sham decreased by no more than 10% (Fig. 1B, lanes 0–60; Fig. 1C). By 3 to 6 hours after PHX, the levels of scHGF/SF began to increase and continued to increase through 48 hours (Fig. 1A, lanes 3–48). At 72 hours after PHX, the level of scHGF/SF in the animals subjected to PHX was similar to the levels observed in the sham-operated animals.
Fig. 1.
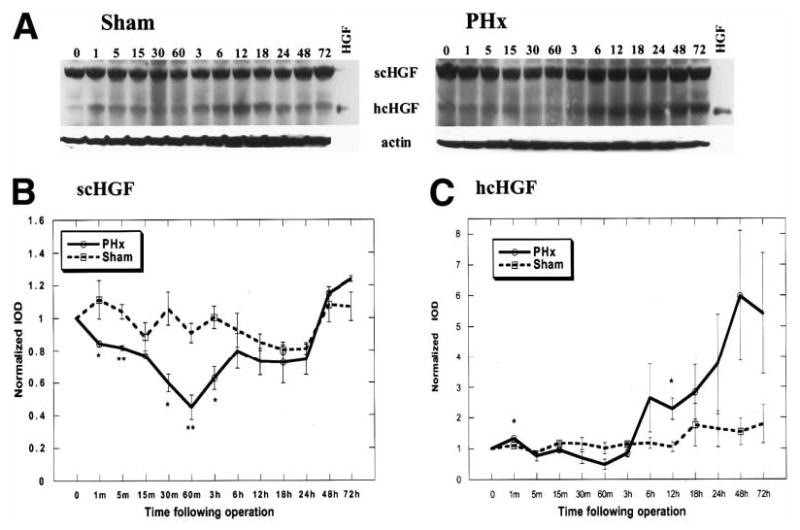
Western blot of endogenous HGF/SF in whole liver homogenates. (A) Animals were subjected to either a PHX or sham operation. Tissue was harvested from the respective animals at indicated times and homogenized as described in the Materials and Methods. Three hundred micrograms of total liver homogenates from different time points were analyzed by 10% Tris-tricine PAGE. Purified rat tcHGF/SF was used as a standard for the hcHGF/SF. Equal loading is confirmed by a Western blot of actin as seen in the lower panel. Following densitometric analysis of three separate Western blot experiments, the integrated optical density (IOD) of bands corresponding to either scHGF/SF or hcHGF/SF for a given time point were normalized to the zero time point from the same blot. The values are expressed as normalized mean (IOD) ± SEM. The comparison between the sham and PHX was done for either the scHGF/SF (B) or the hcHGF/SF (C).
The appearance of the hcHGF/SF is indicative of proteolytically activated scHGF/SF. Levels of hcHGF/SF decreased relative to the sham-operated animals, reaching a nadir at 60 minutes (Fig. 1C). Beginning at 60 minutes, the hcHGF/SF levels increased markedly relative to the sham-operated animals and remained elevated through 72 hours after PHX (Fig. 1C).
Fate of Preloaded Radioactive HGF/SF After PHX
Previous work has shown that the liver is very efficient in extracting HGF/SF from the blood. We used this property to explore the consumption of endogenous HGF/SF after PHX.27,34 Iodinated scHGF/SF was injected into the liver via the inferior mesenteric vein. This ensured that the liver rather than other parts of the body would receive the largest amount of the labeled HGF/SF. The total amount of scHGF/SF (800 ng) injected was sufficient to label the liver but was not considered to be a pharmacologic level.39 Because of the fact that iodinated proteins are accompanied by a small percentage of free iodine (after the I-125 HGF/SF administration), the animals were kept under normal housing conditions for 24 hours before performing PHX. This allowed for an equilibrium binding of the injected scHGF/SF to the periportal extracellular matrix as well as for the excretion of any residual free iodine. Initial experiments in which I-125–labeled human scHGF/SF, human tcHGF/SF, or rat tcHGF/SF were used, all showed similar utilization kinetics (data not shown). A representative autoradiogram of a PAGE analysis of the labeled human scHGF/SF used in these experiments is shown in Fig. 2A. Greater than 85% of the labeled HGF/SF was in the inactive single-chain form. Twenty-four hours after the iodinated HGF/SF injection, the livers were harvested between 0 and 3 hours after PHX, and the amount of labeled HGF/SF in the liver tissue was determined for each respective time point by measuring the iodine-associated radioactivity (Fig. 2B). A complete set of time points was always obtained with the use of a single batch of labeled HGF/SF. Since different batches of labeled human scHGF/SF were used, and the data sets were obtained on different days, all data sets were normalized to the zero time point for a given data set. The values plotted are the average of the normalized results for the given time points with the error bars representing the SEM (Fig. 2B). After PHX, the amount of HGF/SF-associated radioactivity in the liver of the PHX group declined faster than the corresponding sham animals (Fig. 2B).
Fig. 2.
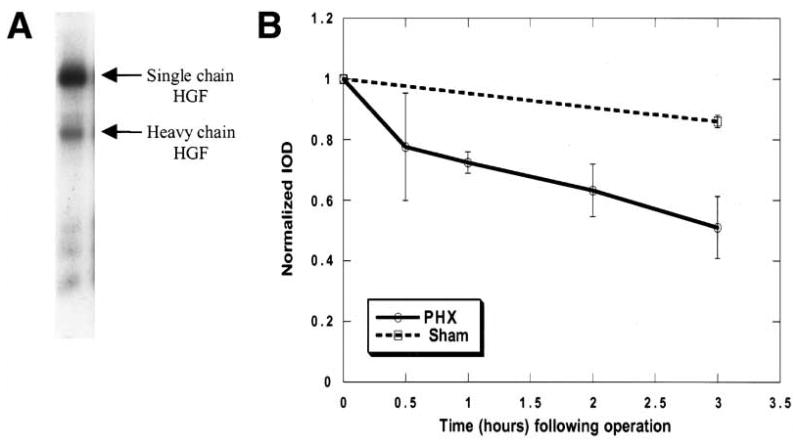
Fate of preloaded HGF/SF after a PHX. (A) A sample (3 ng) of labeled HGF/SF, which was used for labeling of the rat liver, was analyzed by 10% Tris-tricine PAGE. The bands representing the HGF/SF are labeled as scHGF/SF or hcHGF/SF. 125I-HGF/SF was injected into the mesenteric vein 24 hours before PHX. At indicated times after the PHX or sham operation, the liver was harvested, and its specific activity (cpm/μg) was determined. The specific activity data were normalized to the zero time point for each experimental set. (B) The data represent the mean specific activity of the liver from 3 experimental sets of animals ± SEM
Forms of HGF/SF Present in the Blood After PHX
Previous experiments have shown that HGF/SF increases in the blood after PHX. However, the form of HGF/SF present in the blood that accounts for this elevation after PHX has not been clearly identified. To address this question, animals (2 per time point) were subjected to PHX. At the indicated times, blood was drawn, and plasma was prepared as described under Materials and Methods. Identical volumes of plasma (13 mL) were loaded onto heparin agarose columns for each time point ranging from 0 to 3 hours. The bound protein was eluted, extracted, and then lyophilized as described under Materials and Methods. The samples were separated by 10% Tris-tricine PAGE and transferred to a polyvinylidine difluoride membrane. The membrane was visualized by Western blotting. No scHGF/SF was detected at any of the time points. By two hours after PHX, hcHGF/SF was visible, and its levels increased through 3 hours (Fig. 3). A control lane consisting of 20 ng of purified rat tcHGF/SF was used to conclusively show the proper location of the hcHGF/SF in the plasma samples.
Fig. 3.
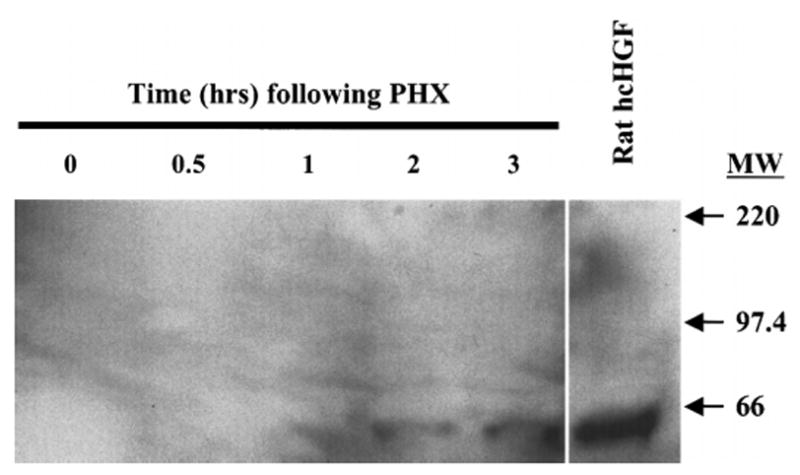
Form of endogenous HGF/SF in the blood after PHX. Blood was harvested from two animals at indicated times after PHX and plasma was prepared as described in the Materials and Methods. Plasma totaling 13 mL for each time point was loaded onto a heparin agarose column. The bound protein was eluted with 1.2 mol/L NaCl and extracted as described in the Materials and Methods. The sample was then resolved on a 10% Tris-tricine acrylamide gel and blotted to a polyvinylidine difluoride membrane. The reference lane consists of purified rat tcHGF/SF and the hcHGF/SF is labeled.
DISCUSSION
The aim of this study was to characterize the forms of HGF/SF present during the liver regeneration process after PHX. Our data provide new information regarding the relationship between liver regeneration and the biotransformation of scHGF/SF to its active form, tcHGF/SF. The findings show two distinct phases, which are characterized by HGF/SF-related changes. The first phase (from 0 to 3 hours) is the consumptive phase. It is characterized by a decline of both scHGF/SF as well as active tcHGF/SF in the total liver homogenates. Although one would expect that a decrease in the scHGF/SF would be accompanied by a commensurate increase in the active tcHGF/SF, this does not appear to be the case in our experiments. This probably reflects the fact that the active tcHGF/SF binds to its receptor c-Met, and subsequently, it becomes internalized and destroyed. This prevents it from accumulating in the liver. Several studies support this conclusion. Our laboratory has shown that after PHX, there is increased tyrosine phosphorylation of c-Met seen as early as 5 minutes after PHX and peaking at 60 minutes after PHX.31 In vitro experiments that examined the metabolic fate of c-Met showed that, on binding to tcHGF/SF, c-Met is rapidly internalized and destroyed by the ubiquitin-proteasome degradation pathway.40 Other in vitro work that examined the metabolic fate of radiolabeled tcHGF/SF showed similar results. tcHGF/SF is rapidly internalized and degraded.41
The second phase is the productive phase (3 to 72 hours). It is characterized by an increase in the levels of scHGF/SF and active tcHGF/SF. Previous studies, including work from this laboratory, have shown that an increase in HGF/SF messenger RNA (mRNA) is not detectable until 3 hours after PHX and peaks at 12 hours after PHX.32,42 This correlates well with what was observed in our Western blot experiments. At 3 hours, concentrations of both scHGF/SF and tcHGF/SF increase relative to the 60-minute time point in the total liver homogenates (Fig. 1A), paralleling the kinetics of the increase in HGF/SF mRNA,32 and supporting the notion that new synthesis of HGF/SF is occurring.
BecauseHGF/SFtranscriptsdonotappreciablyincreasepreceding the 3-hour time point after PHX, the source of the HGF/SF during the initial consumptive phase of HGF/SF-related changes must be either from preexisting stores or from an increase in HGF/SF synthesis from preexisting HGF/SF mRNA pools. It is unlikely that the preexisting RNA pools are responsible for producing HGF/SF for two reasons. First, Northern blot analysis on normal liver shows that the amount of HGF/SF mRNA is barely detectable. Second, the amount of scHGF/SF declines during the first 3 hours relative to the zero time point (Fig. 1A) implying that no new synthesis from existing HGF/SF transcripts is occurring. The previously described increase in HGF/SF mRNA would predict that scHGF/SF levels should begin to rise at 3 hours. In our Western blots, this is indeed when the levels of scHGF/SF began to increase.
In Fig. 4, the ratio of (hcHGF/SF)/(scHGF/SF) (activation ratio) was used as an index of HGF/SF activation due to ambient processes. Earlier work from this laboratory showed that an increase in uPA activity (a known HGF/SF activator) is detectable within 5 minutes after PHX.26 Although the activation ratio showed a transient increase at 1 minute after PHX (Fig. 4), overall the amounts of scHGF/SF and hcHGF/SF relative to the zero time point declined in parallel during the first 60 minutes after PHX (Fig. 1C and D). One would expect that the scHGF/SF decrease would be accompanied by a corresponding increase in tcHGF/SF. Several reasons may explain why this did not occur. First, the scHGF/SF may indeed be activated but is released into the blood, thus appearing as a decrease in hcHGF/SF in the liver tissue. Second, the active tcHGF/SF is likely to bind to the HGF/SF receptor, c-met, and thus be subjected to intracellular degradation. After the 3-hour time point, a striking difference was seen in the activation ratio between the PHX and sham-operated animals.
Fig. 4.
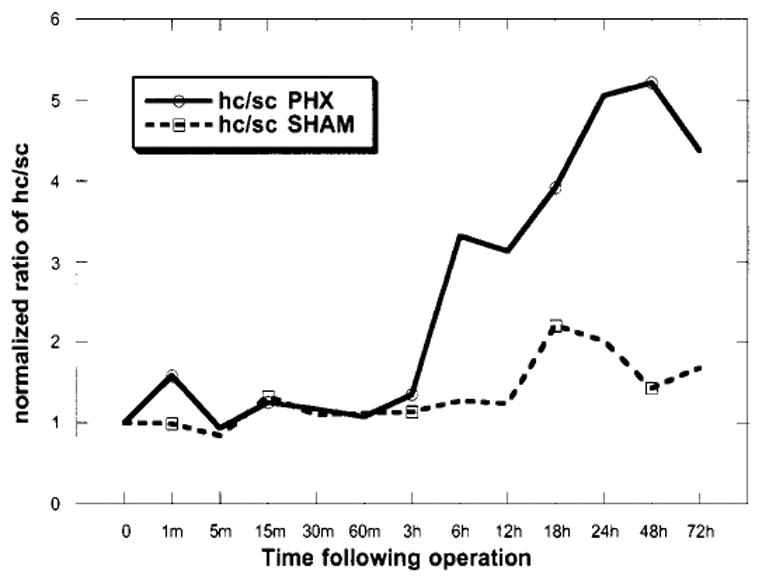
Activation index of endogenous HGF/SF. The ratio of hcHGF/SF to scHGF/SF was compared between PHX and sham-operated animals. The activation index was constructed from the normalized values in Fig. 1B and C.
The liver has been shown to be very efficient at removing HGF/SF from the blood.34 To ensure that the radiolabeled HGF/SF had equal access to all lobes, the injections of labeled HGF/SF were performed through the mesenteric vein, which supplies the portal vein. This allowed for an equilibration of the labeled HGF/SF with the blood during vascular flow before the blood entered the liver. The rate of disappearance of I-125 scHGF/SF from the liver during the first hour after PHX was more than twice the rate of the sham-operated animals. This indicates that the HGF/SF consumed during the initial hour after a PHX is in part from hepatic reservoirs. After the first hour, the rate of disappearance of HGF/SF in the PHX animals slowed but was still somewhat faster than that observed in the sham-operated animals.
The functions of the active tcHGF/SF in the two different phases may be distinct. Earlier experiments have conclusively shown that the plasma of rats that have undergone PHX stimulates DNA synthesis in hepatocytes.43,44 More recent studies showed that the total blood exchange at 6 and 12 hours after PHX with blood from a normal rat significantly suppressed the early stage of liver regeneration.45 Although multiple factors rise in the plasma after PHX (HGF/SF, norepinephrine, interleukin 6, tumor necrosis factor α, and others), it is likely that one of the key differences in blood between normal animals and animals subjected to PHX is the significantly increased amount of HGF/SF in the plasma after PHX. Previous studies from this laboratory have shown that c-Met tyrosine phosphorylation peaks at 60 minutes after PHX.31 Thus, the HGF/SF mobilized from preexisting stores and/or rising in the blood must be exerting a biological effect on the regenerating liver. In this study, we show that the only detectable form of circulating HGF/SF in the plasma during the consumptive phase (first phase) is active tcHGF/SF. Inactive scHGF/SF was not detectable. This finding enhances the possibility that the circulating active tcHGF/SF after PHX has biologic effects on end-target tissues, which are primed to respond.46 This is consistent with previous findings from the literature in which infusion of small39 or large47 amounts of active tcHGF/SF into the peripheral circulation was capable of stimulating measurable amounts of DNA synthesis in hepatocytes of normal animals after a “priming” of the liver with collagenase. The role of the newly synthesized scHGF/SF and tcHGF/SF during the second phase needs to be determined. The prolonged appearance of the tcHGF/SF may suggest that the processing of the active HGF/SF has changed in some way. The elevated scHGF/SF and tcHGF/SF may be important for sustaining proliferation of hepatocytes throughout the regenerative process and/or for providing mitogenic, motogenic, or morphogenic stimuli for other hepatic cell types responsive to HGF/SF (endothelial cells, biliary epithelial cells, etc.).
Abbreviations
- HGF/SF
hepatocyte growth factor/scatter factor
- sc
single chain
- tc
two chain
- uPA
urokinase plasminogen activator
- hc
heavy chain
- PHX
partial hepatectomy
- PAGE
polyacrylamide gel electrophoresis
- mRNA
messenger RNA
Footnotes
Research leading to this work was supported by NIH grants CA30241 and CA35373 (P.I.:G.K.M.).
References
- 1.Stoker M, Perryman M. An epithelial scatter factor released by embryo fibroblasts. J Cell Sci. 1985;77:209–223. doi: 10.1242/jcs.77.1.209. [DOI] [PubMed] [Google Scholar]
- 2.Zarnegar R, Michalopoulos G. Purification and biological characterization of human hepatopoietin A, a polypeptide growth factor for hepatocytes. Cancer Res. 1989;49:3314–3320. [PubMed] [Google Scholar]
- 3.Naldini L, Weidner KM, Vigna E, Gaudino G, Bardelli A, Ponzetto C, Narsimhan RP, et al. Scatter factor and hepatocyte growth factor are indistinguishable ligands for the MET receptor. EMBO J. 1991;10:2867–2878. doi: 10.1002/j.1460-2075.1991.tb07836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montesano R, Matsumoto K, Nakamura T, Orci L. Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell. 1991;67:901–908. doi: 10.1016/0092-8674(91)90363-4. [DOI] [PubMed] [Google Scholar]
- 5.Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature. 1995;376:768–771. doi: 10.1038/376768a0. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschiesche W, Sharpe M, Gherardi E, et al. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373:699–702. doi: 10.1038/373699a0. [DOI] [PubMed] [Google Scholar]
- 7.Uehara Y, Mori C, Noda T, Shiota K, Kitamura N. Rescue of embryonic lethality in hepatocyte growth factor/scatter factor knockout mice [letter] Genesis. 2000;27:99–103. [PubMed] [Google Scholar]
- 8.Miyazawa K, Tsubouchi H, Naka D, Takahashi K, Okigaki M, Arakaki N, Nakayama H, et al. Molecular cloning and sequence analysis of cDNA for human hepatocyte growth factor. Biochem Biophys Res Commun. 1989;163:967–973. doi: 10.1016/0006-291x(89)92316-4. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura T, Nishizawa T, Hagiya M, Seki T, Shimonishi M, Sugimura A, Tashiro K, et al. Molecular cloning and expression of human hepatocyte growth factor. Nature. 1989;342:440–443. doi: 10.1038/342440a0. [DOI] [PubMed] [Google Scholar]
- 10.Naldini L, Tamagnone L, Vigna E, Sachs M, Hartmann G, Birchmeier W, Daikuhara Y, et al. Extracellular proteolytic cleavage by urokinase is required for activation of hepatocyte growth factor/scatter factor. EMBO J. 1992;11:4825–4833. doi: 10.1002/j.1460-2075.1992.tb05588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mars WM, Zarnegar R, Michalopoulos GK. Activation of hepatocyte growth factor by the plasminogen activators uPA and tPA. Am J Pathol. 1993;143:949–958. [PMC free article] [PubMed] [Google Scholar]
- 12.Shimomura T, Miyazawa K, Komiyama Y, Hiraoka H, Naka D, Morimoto Y, Kitamura N. Activation of hepatocyte growth factor by two homologous proteases, blood-coagulation factor XIIa and hepatocyte growth factor activator. Eur J Biochem. 1995;229:257–261. doi: 10.1111/j.1432-1033.1995.tb20463.x. [DOI] [PubMed] [Google Scholar]
- 13.Bottaro DP, Rubin JS, Faletto DL, Chan AM, Kmiecik TE, Vande Woude GF, Aaronson SA. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science. 1991;251:802–804. doi: 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- 14.Naldini L, Vigna E, Narsimhan RP, Gaudino G, Zarnegar R, Michalopoulos GK, Comoglio PM. Hepatocyte growth factor (HGF) stimulates the tyrosine kinase activity of the receptor encoded by the proto-oncogene c-MET. Oncogene. 1991;6:501–504. [PubMed] [Google Scholar]
- 15.Lokker NA, Mark MR, Luis EA, Bennett GL, Robbins KA, Baker JB, Godowski PJ. Structure-function analysis of hepatocyte growth factor: identification of variants that lack mitogenic activity yet retain high affinity receptor binding. EMBO J. 1992;11:2503–2510. doi: 10.1002/j.1460-2075.1992.tb05315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naka D, Ishii T, Yoshiyama Y, Miyazawa K, Hara H, Hishida T, Kidamura N. Activation of hepatocyte growth factor by proteolytic conversion of a single chain form to a heterodimer. J Biol Chem. 1992;267:20114–20119. [PubMed] [Google Scholar]
- 17.Noji S, Tashiro K, Koyama E, Nohno T, Ohyama K, Taniguchi S, Nakamura T. Expression of hepatocyte growth factor gene in endothelial and Kupffer cells of damaged rat livers, as revealed by in situ hybridization. Biochem Biophys Res Commun. 1990;173:42–47. doi: 10.1016/s0006-291x(05)81018-6. [DOI] [PubMed] [Google Scholar]
- 18.Maher JJ. Cell-specific expression of hepatocyte growth factor in liver. Upregulation in sinusoidal endothelial cells after carbon tetrachloride. J Clin Invest. 1993;91:2244–2252. doi: 10.1172/JCI116451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prat M, Narsimhan RP, Crepaldi T, Nicotra MR, Natali PG, Comoglio PM. The receptor encoded by the human c-MET oncogene is expressed in hepatocytes, epithelial cells and solid tumors. Int J Cancer. 1991;49:323–328. doi: 10.1002/ijc.2910490302. [DOI] [PubMed] [Google Scholar]
- 20.Lindroos PM, Zarnegar R, Michalopoulos GK. Hepatocyte growth factor (hepatopoietin A) rapidly increases in plasma before DNA synthesis and liver regeneration stimulated by partial hepatectomy and carbon tetrachloride administration. HEPATOLOGY. 1991;13:743–750. [PubMed] [Google Scholar]
- 21.Kinoshita T, Tashiro K, Nakamura T. Marked increase of HGF mRNA in non-parenchymal liver cells of rats treated with hepatotoxins. Biochem Biophys Res Commun. 1989;165:1229–1234. doi: 10.1016/0006-291x(89)92733-2. [DOI] [PubMed] [Google Scholar]
- 22.Hamanoue M, Kawaida K, Takao S, Shimazu H, Noji S, Matsumoto K, Nakamura T. Rapid and marked induction of hepatocyte growth factor during liver regeneration after ischemic or crush injury. HEPATOLOGY. 1992;16:1485–1492. doi: 10.1002/hep.1840160626. [DOI] [PubMed] [Google Scholar]
- 23.Shimizu M, Hara A, Okuno M, Matsuno H, Okada K, Ueshima S, Matsuo O, et al. Mechanism of retarded liver regeneration in plasminogen activator–deficient mice: impaired activation of hepatocyte growth factor after Fas-mediated massive hepatic apoptosis. HEPATOLOGY. 2001;33:569–576. doi: 10.1053/jhep.2001.22650. [DOI] [PubMed] [Google Scholar]
- 24.Burr AW, Toole K, Chapman C, Hines JE, Burt AD. Anti-hepatocyte growth factor antibody inhibits hepatocyte proliferation during liver regeneration. J Pathol. 1998;185:298–302. doi: 10.1002/(SICI)1096-9896(199807)185:3<298::AID-PATH88>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 25.Roselli HT, Su M, Washington K, Kerins DM, Vaughan DE, Russell WE. Liver regeneration is transiently impaired in urokinase-deficient mice. Am J Physiol. 1998;275:G1472–G1479. doi: 10.1152/ajpgi.1998.275.6.G1472. [DOI] [PubMed] [Google Scholar]
- 26.Mars WM, Liu ML, Kitson RP, Goldfarb RH, Gabauer MK, Michalopoulos GK. Immediate early detection of urokinase receptor after partial hepatectomy and its implications for initiation of liver regeneration. HEPATOLOGY. 1995;21:1695–1701. [PubMed] [Google Scholar]
- 27.Liu KX, Kato Y, Narukawa M, Kim DC, Hanano M, Higuchi O, Nakamura T, Sugiyama Y. Importance of the liver in plasma clearance of hepatocyte growth factors in rats. Am J Physiol. 1992;263:G642–649. doi: 10.1152/ajpgi.1992.263.5.G642. [DOI] [PubMed] [Google Scholar]
- 28.Appasamy R, Tanabe M, Murase N, Zarnegar R, Venkataramanan R, Van Thiel DH, Michalopoulos GK. Hepatocyte growth factor, blood clearance, organ uptake, and biliary excretion in normal and partially hepatectomized rats. Lab Invest. 1993;68:270–276. [PubMed] [Google Scholar]
- 29.Liu KX, Kato Y, Kato M, Kaku TI, Nakamura T, Sugiyama Y. Existence of two nonlinear elimination mechanisms for hepatocyte growth factor in rats. Am J Physiol. 1997;273:E891–E897. doi: 10.1152/ajpendo.1997.273.5.E891. [DOI] [PubMed] [Google Scholar]
- 30.Masumoto A, Yamamoto N. Sequestration of a hepatocyte growth factor in extracellular matrix in normal adult rat liver. Biochem Biophys Res Commun. 1991;174:90–95. doi: 10.1016/0006-291x(91)90489-t. [DOI] [PubMed] [Google Scholar]
- 31.Stolz DB, Mars WM, Petersen BE, Kim TH, Michalopoulos GK. Growth factor signal transduction immediately after two-thirds partial hepatectomy in the rat. Cancer Res. 1999;59:3954–3960. [PubMed] [Google Scholar]
- 32.Zarnegar R, DeFrances MC, Kost DP, Lindroos P, Michalopoulos GK. Expression of hepatocyte growth factor mRNA in regenerating rat liver after partial hepatectomy. Biochem Biophys Res Commun. 1991;177:559–565. doi: 10.1016/0006-291x(91)92020-k. [DOI] [PubMed] [Google Scholar]
- 33.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 34.Liu ML, Mars WM, Zarnegar R, Michalopoulos GK. Uptake and distribution of hepatocyte growth factor in normal and regenerating adult rat liver. Am J Pathol. 1994;144:129–140. [PMC free article] [PubMed] [Google Scholar]
- 35.Higgins GM, Anderson RM. Experimental pathology of the liver. I. Restoration of the liver of the white rat following partial surgical removal. Arch Pathol. 1934;12:186–202. [Google Scholar]
- 36.Sauve DM, Ho DT, Roberge M. Concentration of dilute protein for gel electrophoresis. Anal Biochem. 1995;226:382–383. doi: 10.1006/abio.1995.1242. [DOI] [PubMed] [Google Scholar]
- 37.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, et al. Measurement of protein using bicinchoninic acid [published erratum appears in Anal Biochem 1987 May 15;163(1):279] Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 38.Schagger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 39.Liu ML, Mars WM, Zarnegar R, Michalopoulos GK. Collagenase pretreatment and the mitogenic effects of hepatocyte growth factor and transforming growth factor-alpha in adult rat liver. HEPATOLOGY. 1994;19:1521–1527. [PubMed] [Google Scholar]
- 40.Jeffers M, Taylor GA, Weidner KM, Omura S, Vande Woude GF. Degradation of the Met tyrosine kinase receptor by the ubiquitin-proteasome pathway. Mol Cell Biol. 1997;17:799–808. doi: 10.1128/mcb.17.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naka D, Shimomura T, Yoshiyama Y, Sato M, Ishii T, Hara H. Internalization and degradation of hepatocyte growth factor in hepatocytes with down-regulation of the receptor/c-Met. FEBS Lett. 1993;329:147–152. doi: 10.1016/0014-5793(93)80212-d. [DOI] [PubMed] [Google Scholar]
- 42.Mitsue S, Hamanoue M, Tanabe G, Ogura Y, Yoshidome S, Aikou T, Nakamura T. Expression of HGF and TGF-beta 1 mRNA after partial hepatectomy in rats with liver cirrhosis. Surg Today. 1995;25:237–243. doi: 10.1007/BF00311534. [DOI] [PubMed] [Google Scholar]
- 43.Moolten FL, Buchner NL. Regenaration of rat liver: transfer of humoral agent by cross circulation. Science. 1967;158:272–274. doi: 10.1126/science.158.3798.272. [DOI] [PubMed] [Google Scholar]
- 44.Grisham JW, Leong GF, Hole BV. Heterotropic partial autotransplantation of rat liver. Technique demonstration of structure and function of the graft. Cancer Res. 1964;24:1474–1482. [PubMed] [Google Scholar]
- 45.Eguchi S, Sugiyama N, Kawazoe Y, Kawashita Y, Fujioka H, Furui J, Kanematsu T. Total blood exchange suppresses the early stage of liver regeneration following partial hepatectomy in rats. Artif Organs. 1998;22:847–853. doi: 10.1046/j.1525-1594.1998.06166.x. [DOI] [PubMed] [Google Scholar]
- 46.Fausto N. Liver regeneration. J Hepatol. 2000;32:19–31. doi: 10.1016/s0168-8278(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 47.Patijn GA, Lieber A, Schowalter DB, Schwall R, Kay MA. Hepatocyte growth factor induces hepatocyte proliferation in vivo and allows for efficient retroviral-mediated gene transfer in mice. HEPATOLOGY. 1998;28:707–716. doi: 10.1002/hep.510280317. [DOI] [PubMed] [Google Scholar]


