Abstract
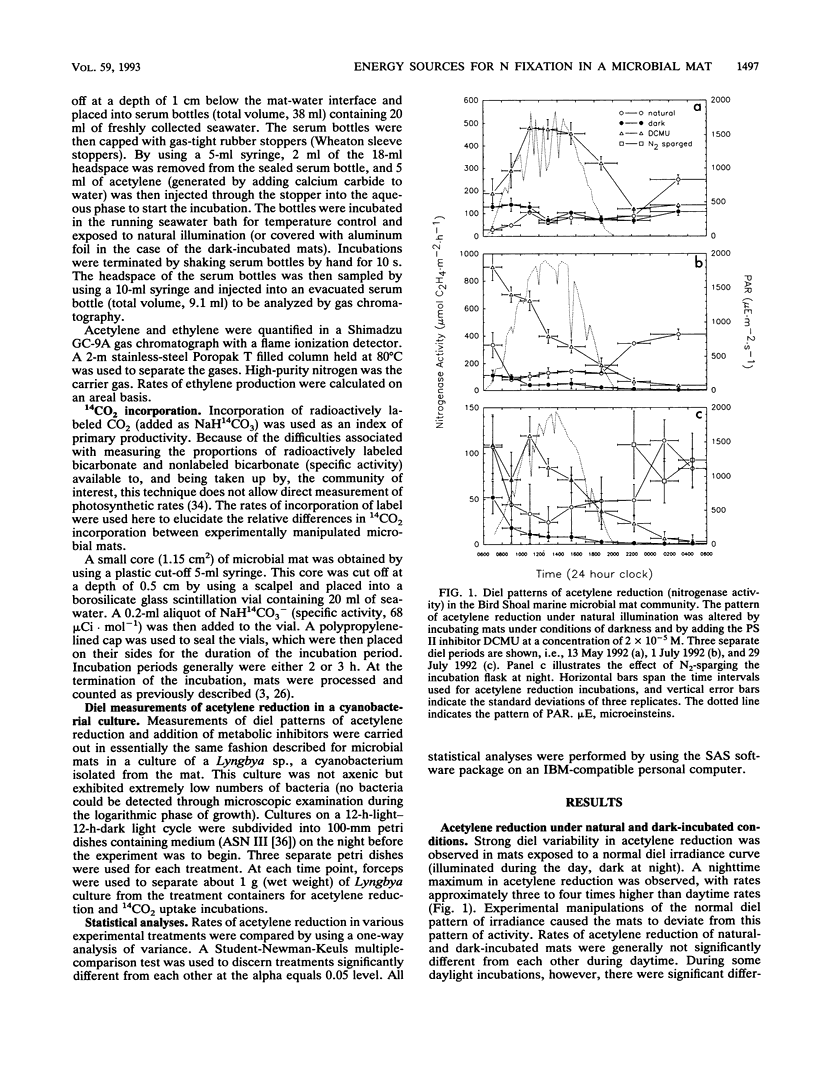
Experimental manipulations of a microbial mat community were performed to determine sources of energy and reductant used for nitrogen fixation and to physiologically characterize the responsible diazotrophs. The dominant photolithotrophic members of this community were nonheterocystous cyanobacteria, but other potential nitrogen-fixing microorganisms were also present. Pronounced diel variability in rates of acetylene reduction was observed, with nighttime rates a factor of three to four higher than daytime rates. Acetylene reduction measured at night was dependent upon the occurrence of oxygenic photosynthesis the preceding day; mats incubated in the dark during the daytime reduced acetylene at rates comparable to those of light-incubated mats but were not able to reduce acetylene at the normally high rates the following night. The addition of various exogenous carbon compounds to these dark-incubated mats did not elicit nighttime acetylene reduction. Nighttime acetylene reduction apparently proceeds under anoxic conditions in these mats; the highest rates of acetylene reduction occur late at night. Additions of 3-(3,4-dichlorophenyl)-1,1-dimethylurea (an inhibitor of oxygenic photosynthesis) to mats resulted in a pronounced stimulation of acetylene reduction during the day, but acetylene reduction the next night proceeded at greatly reduced rates (relative to untreated mats). This daytime stimulation, under the 3-(3,4-dichlorophenyl)-1,1-dimethylurea-induced anoxic conditions in the experimentally treated mats, was light dependent. These results suggest that nitrogen fixation in these mats may be attributed to the activities of nonheterocystous cyanobacteria utilizing storage products of oxygenic photosynthesis under anoxic conditions at night.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bebout B. M., Paerl H. W., Crocker K. M., Prufert L. E. Diel interactions of oxygenic photosynthesis and n(2) fixation (acetylene reduction) in a marine microbial mat community. Appl Environ Microbiol. 1987 Oct;53(10):2353–2362. doi: 10.1128/aem.53.10.2353-2362.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstein T., Henis Y., Cavari B. Z. Nitrogen Fixation by the Photosynthetic Sulfur Bacterium Chlorobium phaeobacteroides from Lake Kinneret. Appl Environ Microbiol. 1981 Feb;41(2):542–544. doi: 10.1128/aem.41.2.542-544.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. M., Fay P. Special aspects of nitrogen fixation by blue-green algae. Proc R Soc Lond B Biol Sci. 1969 Apr 1;172(1029):357–366. doi: 10.1098/rspb.1969.0026. [DOI] [PubMed] [Google Scholar]
- Fay P., Cox R. M. Oxygen inhibition of nitrogen fixation in cell-free preparations of blue-green algae. Biochim Biophys Acta. 1967;143(3):562–569. doi: 10.1016/0005-2728(67)90061-8. [DOI] [PubMed] [Google Scholar]
- Fay P. Factors influencing dark nitrogen fixation in a blue-green alga. Appl Environ Microbiol. 1976 Mar;31(3):376–379. doi: 10.1128/aem.31.3.376-379.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay P. Oxygen relations of nitrogen fixation in cyanobacteria. Microbiol Rev. 1992 Jun;56(2):340–373. doi: 10.1128/mr.56.2.340-373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fründ C., Cohen Y. Diurnal Cycles of Sulfate Reduction under Oxic Conditions in Cyanobacterial Mats. Appl Environ Microbiol. 1992 Jan;58(1):70–77. doi: 10.1128/aem.58.1.70-77.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirshtein J. D., Paerl H. W., Zehr J. Amplification, cloning, and sequencing of a nifH segment from aquatic microorganisms and natural communities. Appl Environ Microbiol. 1991 Sep;57(9):2645–2650. doi: 10.1128/aem.57.9.2645-2650.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paerl H. W., Prufert L. E., Ambrose W. W. Contemporaneous N(2) Fixation and Oxygenic Photosynthesis in the Nonheterocystous Mat-Forming Cyanobacterium Lyngbya aestuarii. Appl Environ Microbiol. 1991 Nov;57(11):3086–3092. doi: 10.1128/aem.57.11.3086-3092.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paerl H. W., Prufert L. E. Oxygen-poor microzones as potential sites of microbial n(2) fixation in nitrogen-depleted aerobic marine waters. Appl Environ Microbiol. 1987 May;53(5):1078–1087. doi: 10.1128/aem.53.5.1078-1087.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson R. L., Postgate J. R. Oxygen and hydrogen in biological nitrogen fixation. Annu Rev Microbiol. 1980;34:183–207. doi: 10.1146/annurev.mi.34.100180.001151. [DOI] [PubMed] [Google Scholar]
- Stewart W. D., Fitzgerald G. P., Burris R. H. In situ studies on N2 fixation using the acetylene reduction technique. Proc Natl Acad Sci U S A. 1967 Nov;58(5):2071–2078. doi: 10.1073/pnas.58.5.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]


